Abstract
Patient: Male, 82
Final Diagnosis: Intrahepatic squamous cell carcinoma
Symptoms: None
Medication: —
Clinical Procedure: —
Specialty: Oncology
Objective:
Rare disease
Background:
Cholangiocarcinoma is a rare, aggressive biliary tract malignancy. On histopathology, most tumors are adenocarcinomas, while squamous cell carcinoma of the biliary tract is extremely rare.
Case Report:
An 82-year-old male was admitted due to the detection of a space-occupying lesion at S6 of the liver. On abdominal dynamic computed tomography, there was an irregular mass with inhomogeneous density associated with mild delayed enhancement in the tumor’s peripheral zone, measuring approximately 22×25 mm, at S6, with secondary dilated biliary ducts of B6. Endoscopic retrograde cholangiopancreatography showed a severe stricture at B6. Brush cytology of B6 was positive for both adenocarcinoma and squamous cell carcinoma. Furthermore, mucous brushing cytology of the papilla of Vater was also positive for adenocarcinoma. Finally, the preoperative diagnosis of primary intrahepatic cholangiocarcinoma, combined with a cancer of the papilla of Vater, was made. The patient underwent both extended right lobectomy and pancreaticoduodenectomy. Histological examination showed that the majority (>99%) of this liver tumor was composed of keratinizing squamous cell carcinoma.
Conclusions:
Squamous cell carcinoma of the biliary tree is very rare, since the majority of biliary tree tumors are adenocarcinomas. Cholangiocarcinoma containing a squamous cell component has a poor prognosis. To the best of our knowledge, this is the first case report of a primary intrahepatic squamous cell carcinoma that presented as a solid tumor showing clear histological collision between adenocarcinoma and squamous cell carcinoma and was successfully treated with hepatic resection and achieved disease-free survival of more than one year.
MeSH Keywords: Adenocarcinoma; Carcinoma, Squamous Cell; Cholangiocarcinoma; Liver
Background
Cholangiocarcinomas are bile duct tumors arising from biliary tree epithelium. The incidence of cholangiocarcinoma varies by geographic region, ranging from 0.4–3.4 per 100 000 person-years in North America and Europe to 1–85 per 100 000 person-years in Asia [1]. Although cholangiocarcinoma is a relatively rare cancer, its incidence has been increasing worldwide over the past decade [1]. Cholangiocarcinoma is an aggressive malignancy. Only complete surgical resection can be curative, but the majority of patients are diagnosed with unresectable advanced disease, including metastatic disease and locally advanced tumor, and they usually die within a year of diagnosis [2–4]. Overall, 5% of patients with cholangiocarcinoma have multifocal lesions, 50% have lymph node metastases, and 10–20% have distant metastases at the time of presentation, so that long-term survival cannot be achieved with surgical treatment [5]. From the clinicopathological view, the majority of these tumors are adenocarcinomas, and squamous cell carcinoma (SCC) of the biliary system is extremely rare. Patients with cholangiocarcinoma consisting of a squamous cell component have a poor prognosis due to its aggressive nature, such as early distant or lymph node metastases and cancer invasion into liver parenchyma [6]. Therefore, the clinicopathological characteristics of this cancer are still unclear. The first case of primary intrahepatic SCC (IHSCC) with clear histological collision with adenocarcinoma and SCC is reported.
Case Report
An 82-year-old male with a past medical history of autoimmune pancreatitis, treated by a low-dose immunosuppressive drug, including steroid (5 mg/day×84 months), was admitted to our department with a space-occupying lesion at segment 6 (S6) of the liver, demonstrated on follow-up abdominal computed tomography (CT) for autoimmune pancreatitis. On admission, he had no significant symptoms, including vomiting, jaundice, fever, chills, or abdominal distension. He had a history of autoimmune pancreatitis, with no history of alcohol drinking, smoking, or surgery. On physical examination, the abdomen was soft, without tenderness, rebound tenderness, or a palpable abdominal mass. The following were the laboratory data at that time: hemoglobin 11.5 g/dL; white blood cell count 7900/mm3; platelets 22×104/μL; prothrombin time (test/normal control) 14.4/12.7 sec; international normalized ratio 1.13; albumin 3.6 g/dL; direct bilirubin 0.2 mg/dL: total bilirubin 0.7 mg/dL; aspartate aminotransferase 26 IU/L; ala-nine aminotransferase 14 IU/L; alkaline phosphatase 602 U/L; blood urea nitrogen 22 mg/dL; creatinine 1.01 mg/dL; and C-reactive protein 1.31 mg/L. The serum levels of carcinoembryonic antigen (CEA) (normal <5 ng/mL), alpha-fetoprotein (normal <15 ng/mL), and carbohydrate antigen 19-9 (CA19-9) (normal <37 U/mL) were 3.4 ng/mL, 4 ng/mL, and 38 U/mL, respectively. The plasma retention rate of indocyanine green at 15 min was 30.2%. Subsequent abdominal ultrasonography showed a mixed echoic mass measuring about 20×20 mm occupying S6 of the right lobe of the liver (data not shown). On abdominal dynamic CT, there was an irregular mass with inhomogeneous density associated with mild delayed enhancement in the tumor’s peripheral zone that measured approximately 22×25 mm in its greatest dimensions, with dilated secondary biliary ducts of B6 (Figure 1A, 1B). Fortunately, no intrahepatic metastatic lesions were detected. Taken together, these diagnostic imaging findings were suspicious for intrahepatic cholangiocarcinoma. Endoscopic retrograde cholangiopancreatography (ERCP) showed a severe stricture at B6 of the right hepatic duct. A biliary stent was inserted for the relief of bile obstruction at B6 (Figure 1C). To identify distant metastases, positron-emission tomography-computed tomography (PET-CT) was performed. PET-CT clearly demonstrated a hotspot (SUV max: 8.9) at the same location as the tumor detected by contrast-enhanced CT, but no hot spots for distant metastases were observed (Figure 1D). To confirm the definitive diagnosis of this liver tumor at S6, both bile duct brushing for cytopathology and bile duct biopsy were performed. Papanicolaou staining was done on a ThinPrep slide (Hologic, Japan). On cytopathological examination, cell clusters that consisted of adenocarcinoma cells were detected (Figure 2A). Surprisingly, the bile smears showed sheets, clusters, and scattered squamoid cells with well to moderate atypia, hyperchromatic nuclei, and scanty dense cytoplasm. The tumor cells were orangeophilic on Papanicolaou staining. These findings led to a cytologic diagnosis of SCC (Figure 2B). On histopathological examination, an adenocarcinoma lesion, stained with hematoxylin and eosin (H&E), was detected following B6 bile duct biopsy (Figure 2C). Furthermore, a small abnormal mucous lesion was found at the papilla of Vater, and mucous brushing of the papilla of Vater was performed. Cytopathological examination showed adenocarcinoma of the papilla of Vater (Figure 2D). The patient underwent an extensive workup to search for a primary SCC, including chest CT, colonoscopy, and upper gastroesophageal endoscopy, all of which were negative. Taken together, the preoperative diagnosis of primary intrahepatic cholangiocarcinoma composed of adenocarcinoma and SCC, combined simultaneously with an early cancer of the papilla of Vater, was made. The patient underwent both extended right lobectomy (right lobe and S1 of the liver) and cholecystectomy to remove the liver mass, as well as pancreaticoduodenectomy with reconstruction of the digestive tract by the modified Child Method to resect the cancer of the papilla of Vater (i.e., right hepatopancreatoduodenectomy). For pancreatojejunostomy, we used the modified Blumgart method. For hepaticojejunostomy, we performed hand-sewn continuous over-and-over anastomosis with absorbable monofilament suture (i.e., PDS®II, Ethicon, Inc., NJ, USA). In this procedure, a stapled anastomosis is used for gastrojejunostomy and Braun anastomosis [7,8]. On intraoperative examination, the liver was non-cirrhotic, and there was a 36×21 mm, white, hard tumor mass located at S5 and S6 (mainly involving S6). For the cancer of the papilla of Vater, no palpable tumors located at the head of the pancreas were observed. The specimen had a 1-cm negative surgical margin without atypical cells or cancer cells on its cut surface.
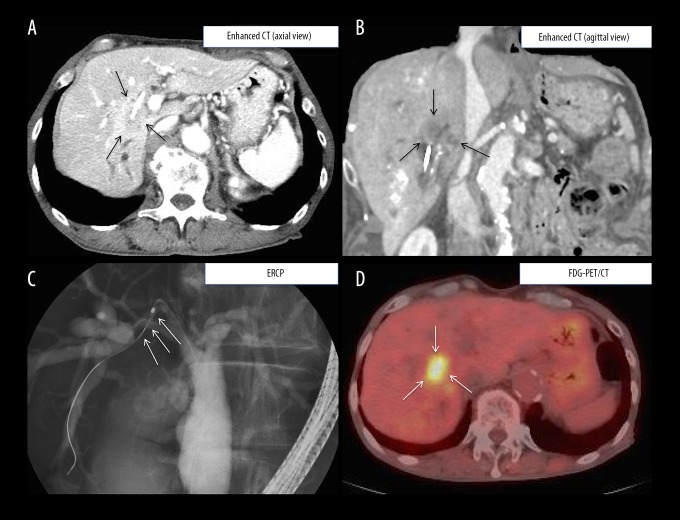
Figure 1.
Selected diagnostic imaging findings of dynamic CT, ERCP, and PET-CT. (A) axial view and (B) sagittal view: abdominal dynamic CT shows an irregular mass (arrows) with inhomogeneous density and mild delayed enhancement in the peripheral zone of the tumor and dilated secondary biliary ducts of the B6 segment. (C) ERCP shows a severe stricture at B6 of the right hepatic duct (arrows). (D) PET-CT shows a hotspot (SUV max: 8.9) at the same location as that detected by contrast-enhanced CT. CT – computed tomography; ERCP – endoscopic retrograde cholangiopancreatography; PET-CT – positron-emission tomography-computed tomography.
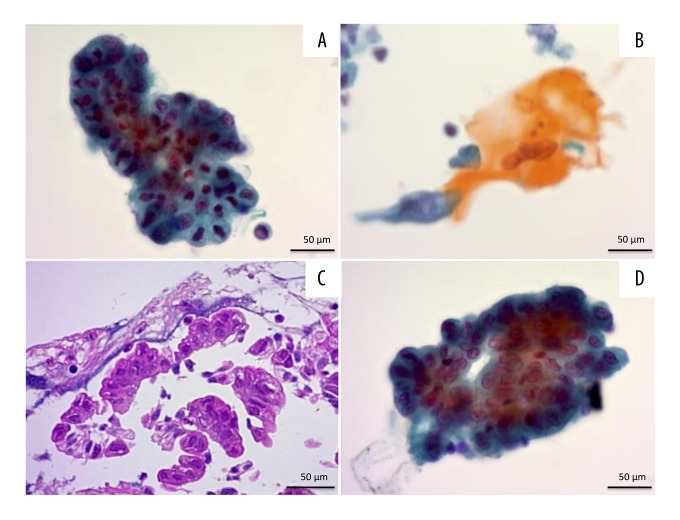
Figure 2.
Cytopathological findings obtained by ERCP. (A) Bile duct brush cytology of the B6 segment shows adenocarcinoma cells (Papanicolaou staining). (B) The bile smears show organeophilic squamoid cells with well to moderate atypia (Papanicolaou staining). (C) Bile duct biopsy of the B6 segment shows an adenocarcinoma lesion (H&E staining). (D) Brush cytology of the papilla of Vater shows adenocarcinoma cells (Papanicolaou staining). ERCP – endoscopic retrograde cholangiopancreatography; H&E – hematoxylin and eosin.
The patient recovered well and was discharged 74 days postoperatively. The final diagnosis was primary IHSCC and early cancer of the papilla of Vater. Routine follow-up was performed in the oncology outpatient clinic with periodic abdominal CT and tumor markers, including CEA, CA19-9, and SCC. At present, no further evidence of recurrence of the tumor has been noted over 1 year after the curative operation.
Microscopic examination showed that the majority (>99%) of this liver tumor was composed of well-differentiated SCC (Figure 3A–3C). As shown in Figure 3C depicting H&E staining, squamous cells with keratinization clearly formed cancer pearls, marked by black arrows. To confirm squamous differentiation, an immunohistochemical study was performed. As shown in Figure 3D, immunostaining for cytokeratin 5/6 (CK 5/6) [9] was strongly positive, and keratin pearls were observed. To further improve the diagnostic accuracy for SCC, immunostaining for p40 molecule, which is a recently introduced antibody that recognizes p63 protein and has shown superior results labeling SCC along with p63, desmocollin 3, and SOX2 [10,11], was performed. A clear nuclear staining pattern was found (Figure 3E). As described, the majority of this liver tumor was composed of SCC, and adenocarcinoma tissue was detected by bile duct biopsy at B6. Therefore, the near part of the B6 bile duct lumen was assessed histopathologically (Figure 4A).
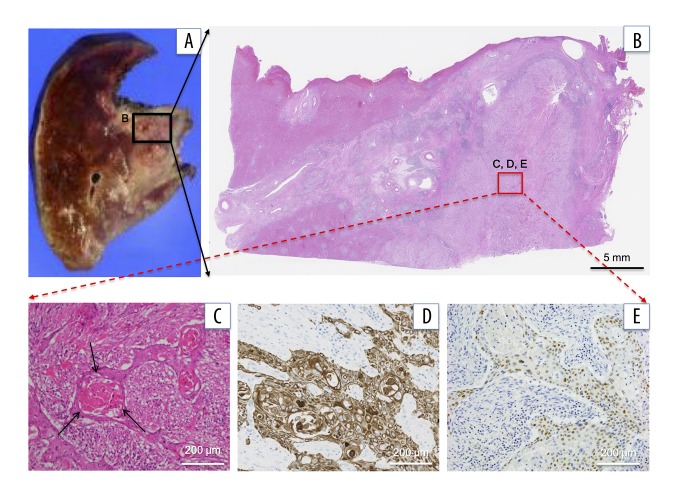
Figure 3.
Immunohistopathological findings of primary liver tumor specimens. (A) The resected specimens of the liver: (B) low power field and (C) high power field. Microscopic findings of resected tumor. Most (>99%) of this liver tumor is composed of well-differentiated squamous cells. The tumor is composed of squamous cells with keratinization that forms cancer pearls (H&E staining, arrows). (D) Squamous cells show diffusely positive CK5/6 staining immunohistochemically. (E) Squamous cells show positive p40 staining immunohistochemically. H&E – hematoxylin and eosin.
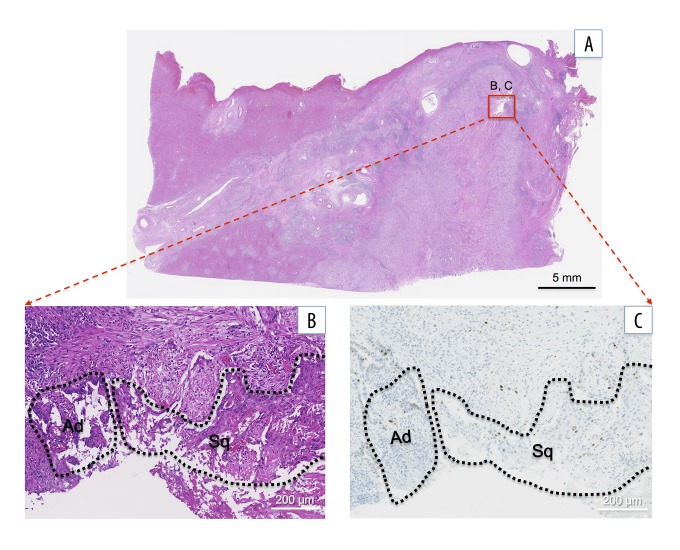
Figure 4.
Histological findings of the collision lesion of adenocarcinoma and squamous cell carcinoma (SCC): (A) low power field and (B) high power field. Microscopically, adenocarcinoma (Ad) coexists closely with the SCC (Sq) lesion (H&E staining). (C) Both adenocarcinoma and SCC lesions are negative for p53 staining. H&E – hematoxylin and eosin.
As shown in Figure 4B, adenocarcinoma coexisted closely with the SCC lesion. To further analyze the genetic background of both the adenocarcinoma and squamous carcinoma cells, p53 immunoreactivity was assessed. As shown in Figure 4C, negative nuclear labeling for p53 was demonstrated in both adenocarcinoma and squamous carcinoma lesions. However, no significant correlations with carcinogenesis were identified between p53 labeling and the development of either SCC or adenocarcinoma in the bile ducts. Accordingly, further studies are needed to prove the precise mechanism of the development of SCC in the bile ducts.
Discussion
Cholangiocarcinoma is a relatively rare and aggressive malignancy of the biliary tract. Although most of these tumors are adenocarcinomas, SCC of the biliary tree (i.e., primary IHSCC) is very rare. Though several theories have been suggested for the pathogenesis of primary IHSCC, the exact mechanism responsible for SCC developing in the bile ducts is poorly understood. Based on the previous literature, the major etiological factors appear to be chronic inflammation of the bile duct, liver cysts associated with infection and/or stones, and congenital cysts of the biliary tract [12–17]. As shown in Table 1 [4,15,18–26], a literature search identified only 12 cases that were identical to the present case in the following respects: i) solid liver tumors composed of SCC; and ii) curative resection was performed, the first dating back to 1984 [18].
Table 1.
Summary of 12 cases of solid liver tumors composed of squamous cell carcinoma undergoing curative resection.
| No. | Year | Age (Y.O.) | Gender (M/F) | Tumor size (cm) | Tumor number | Tumor location | Comorbidities | Preoperative diagnosis | Preoperative detection of cancer cells | Operation | Prognosis (survival) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1984 [16] | 43 | M | 13 | Single | Left lobe (S3) | Hepatolithiasis | N | By: N.D. Cy: N.D. | Lobectomy | Dead (6 mo) |
| 2 | 1991 [13] | 51 | F | 6 | Multiple | Right lobe (S8) | None | Y | Open By (+: Sq) Cy: N.D. | Lobectomy | Dead (10 days) |
| 3 | 2003 [17] | 60 | M | 4 | Single | Right lobe (S5) | None | N | By: N.D. Cy: N.D. | Subsegmentectomy | N/A |
| 4 | 2006 [4] | 40 | M | 10 | Single | Right lobe (S5) | None | N | By: N.D. Cy: N.D. | Extended lobectomy | Survive (9 mo) |
| 5 | 2009 [18] | 56 | M | 3 | Single | Left lobe (S4) | None | Y | By (+: Sq) Cy: N.D. | Extended lobectomy | Survive (6 yr) |
| 6 | 2011 [19] | 72 | M | 10 | Single | Right lobe (S5, 6) | Hepatolithiasis | Y | By (+: Sq) Cy: N.D. | Lobectomy | Survive (7 yr) |
| 7 | 2012 [20] | 46 | F | 6 | Single | Right lobe (S5) | Hepatolithiasis | N | By: N.D. Cy: N.D. | Lobectomy | Survive (19 mo) |
| 8 | 2013 [21] | 55 | F | 2.3 | Single | Right lobe (S8) | Colon cancer | N | By: N.D. Cy: N.D. | Subsegmentectomy + right colectomy | Dead (17 mo) |
| 9 | 2015[22] | 50 | M | 7 | Single | Left lobe (S2, 3, 4) | Hepatolithiasis | N | By: N.D. Cy: N.D. | Lobectomy + choledochojejunostomy | Dead (18 mo) |
| 10 | 2015 [23] | 64 | M | 13.5 | Single | Right lobe (S5) | None | N | By: N.D. Cy (+: atypical cell) | Lobectomy + colon resection | N/A |
| 11 | 2016 [24] | 70 | F | 8 | Single | Both loves (S4, 5, 8) | None | N | By: N.D. Cy: N.D. | Central bisegmentectomy | Survive (2 yr) |
| 12 | 2017 [Our case] | 82 | M | 4 | Single | Right lobe (S6) | Cancer of Vater’s papilla | Y | By (+: ad) Cy (+: ad and Sq) | HPD (lobectomy + PD) | Survive (1 yr) |
N/A – detailed clinical information was not available; HPD – hepatopancreatoduodenectomy; By – biopsy; Cy – bile cytology; N.D. – not done; ad – adenocarcinoma; Sq – squamous cell carcinoma.
Secondary squamous metaplasia may be promoted by chronic inflammation of the bile duct and may lead to malignant transformation [12–17,22]. These hypotheses fit with our cases in which 4 patients had long histories of intra-hepatolithiasis (Table 1). Thus, inflammation-cancer transformation may develop in certain patients harboring bile duct stones. It is important to note that 2 cases, including the present case, had concomitant cancers: 1 case had colon cancer (No. 8, Table 1), and the present case (No. 12, Table 1) had cancer of the papilla of Vater. Such findings suggest that squamous metaplasia of adenocarcinoma cells has significant potential to differentiate into various cell types, with the possibility of primary IHSCC developing from adenocarcinoma [23]. These hypotheses may be supported by the findings of histological collision of adenocarcinoma and SCC. Moreover, nuclear labeling for p53 immunoreactivity of both adenocarcinoma and SCC was negative in the present case, whereas cholangiocarcinomas are pathologically heterogeneous. Taken together, the pathological findings of the present case may support the hypotheses described above.
The diagnosis of primary IHSCC is based on the combination of clinical presentation, imaging studies, including CT, ultrasound, and MRI, tumor markers (CEA, CA19-9, and SCC), and histology [27]. However, in the clinical presentation of this disease, no specific symptom distinguished this disease from other liver tumors. Furthermore, no specific serum markers of primary IHSCC have yet been identified. Thus, it remains very difficult to diagnose primary IHSCC. ERCP is used to obtain brush cytology and/or biopsy of biliary ducts. ERCP cytology has relatively low sensitivity (20–30%) but high specificity. The sensitivity of brush cytology increases (40–70%) [27] when combined with biopsy. Fortunately, both adenocarcinoma and SCC were detected preoperatively by ERCP cytology and biopsy in the present case. Among the 12 cases reported in the literature, 4, including the present case, were diagnosed preoperatively as primary IHSCC by ERCP cytology and/or biopsy of the bile ducts (Table 1). The preoperative diagnosis may provide the opportunity for curative resection of the primary liver lesions, and adjuvant treatments may be started before and then continued after surgery.
Patients with primary IHSCC have an extremely poor prognosis, even after extensive surgery of liver tumors to achieve radical resection [24]. Among the 12 reported cases undergoing curative surgical resection, 7 cases, including the present case, survived over 1 year. Of particular note, 2 cases achieved long-term survival of over 5 years (Table 1). On the other hand, of the 13 reported cases undergoing palliative treatment, only 2 survived more than 12 months [24]. From our case, it was evident that radical surgery led to significantly longer overall survival than palliative treatment. Therefore, for this tumor, we can strongly recommend complete surgical resection over partial excision, transcatheter arterial chemoembolization or radiation therapy.
Finally, primary IHSCC may seem to originate mostly from chronic inflammation of the bile duct due to hepatolithiasis, but the carcinogenesis of such tumors needs to be further studied, such as with genetic analysis using laser microdissection. Radical resection should be recommended as initial treatment, although the prognosis might be poor.
Conclusions
SCC of the biliary tree is extremely rare. The first case of primary IHSCC presenting as a solid liver tumor with clear collision of adenocarcinoma and SCC, assessed with p53 immunostaining, was presented in this study. The patient was successfully treated by complete surgical resection. He survived for over 1 year with no tumor recurrence and no distant metastases.
Acknowledgments
The authors would like to thank Luba Wolchuk, MD, MHSc, CCFP, associated with Forte Science Communications (Tokyo, Japan), for the careful reading and editing of this manuscript.
Footnotes
Conflicts of interest
None.
References:
- 1.Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European network for the study of cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–80. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 2.Bloustein PA, Silverberg SG. Squamous cell carcinoma originating in a hepatic cyst. Case report with a review of the hepatic cyst-carcinoma association. Cancer. 1976;38:2002–5. doi: 10.1002/1097-0142(197611)38:5<2002::aid-cncr2820380523>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Nagorney DM, Donohue JH, Farnell MB, et al. Outcomes after curative re-sections of cholangiocarcinoma. Arch Surg. 1993;128:871–79. doi: 10.1001/archsurg.1993.01420200045008. [DOI] [PubMed] [Google Scholar]
- 4.Lee HL, Liu YY, Yeh CN, et al. Primary squamous cell carcinoma of the liver: A successful surgically treated case. World J Gastroenterol. 2006;12:5419–21. doi: 10.3748/wjg.v12.i33.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai JQ, Cai SW, Cong WM, et al. Diagnosis and treatment of cholangiocarcinoma: a consensus from surgical specialists of China. Chinese Chapter of International hepato Pancreato Biliary Association; Liver Surgery Group, Surgical Branch of the Chinese Medical Association. J Huazhong Univ Sci Technolog Med Sci. 2014;34:469–75. doi: 10.1007/s11596-014-1301-5. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima T, Kondo Y. A clinicopathologic study of intrahepatic cholangio-carcinoma containing a component of squamous cell carcinoma. Cancer. 1990;65:1401–4. doi: 10.1002/1097-0142(19900315)65:6<1401::aid-cncr2820650626>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Yang YL, Xu XP, Wu GQ, et al. Prevention of pancreatic leakage after pancreaticoduodenectomy by modified Child pancreaticojejunostomy. Hepatobiliary Pancreat Dis Int. 2008;7:426–29. [PubMed] [Google Scholar]
- 8.Kleespies A, Rentsch M, Seeliger H, et al. Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head re-section. Br J Surg. 2009;96:741–50. doi: 10.1002/bjs.6634. [DOI] [PubMed] [Google Scholar]
- 9.Khayyata S, Yun S, Pasha T, et al. Value of P63 and CK5/6 in distinguishing squamous cell carcinoma from adenocarcinoma in lung fine-needle aspiration specimens. Diagn Cytopathol. 2009;37:178–83. doi: 10.1002/dc.20975. [DOI] [PubMed] [Google Scholar]
- 10.Tatsumori T, Tsuta K, Masai K, et al. p40 is the best marker for diagnosing pulmonary squamous cell carcinoma: Comparison with p63, Cytokeratin 5/6, Desmocollin-3, and Sox2. Appl Immunohistochem Mol Morphol. 2014;22:377–82. doi: 10.1097/PAI.0b013e3182980544. [DOI] [PubMed] [Google Scholar]
- 11.Lilo MT, Allison D, Wang Y, et al. Expression of P40 and P63 in lung cancers using fine needle aspiration cases. Understanding clinical pitfalls and limitations. J Am Soc Cytopathol. 2016;5:123–32. doi: 10.1016/j.jasc.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weimann A, Klempnauer J, Gebel M, et al. Squamous cell carcinoma of the liver originating from a solitary non-parasitic cyst case report and review of the literature. HPB Surg. 1996;10:45–49. doi: 10.1155/1996/97680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banbury J, Conlon KC, Ghossein R, Brennan MF. Primary squamous cell carcinoma within a solitary nonparasitic hepatic cyst. J Surg Oncol. 1994;57:210–12. doi: 10.1002/jso.2930570316. [DOI] [PubMed] [Google Scholar]
- 14.Nieweg O, Slooff MJ, Grond J. A case of primary squamous cell carcinoma of the liver arising in a solitary cyst. HPB Surg. 1992;5:203–8. doi: 10.1155/1992/32474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roediger WE, Dymock RB. Primary squamous carcinoma of the liver: Clinical and histopathological features. Aust NZJ Surg. 1991;61:720–22. doi: 10.1111/j.1445-2197.1991.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 16.Yagi H, Ueda M, Kawachi S, et al. Squamous cell carcinoma of the liver originating from non-parasitic cysts after a 15 year follow-up. Eur J Gastroenterol Hepatol. 2004;16:1051–56. doi: 10.1097/00042737-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Gresham GA, Rue LW., 3rd Squamous cell carcinoma of the liver. Hum Pathol. 1985;16:413–16. doi: 10.1016/s0046-8177(85)80234-3. [DOI] [PubMed] [Google Scholar]
- 18.Song E, Kew MC, Grieve T, et al. Primary squamous cell carcinoma of the liver occurring in association with hepatolithiasis. Cancer. 1984;53:542–46. doi: 10.1002/1097-0142(19840201)53:3<542::aid-cncr2820530328>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Tsuneyama K, Kaizaki Y, Doden K, et al. Combined hepatocellular and cholangiocarcinoma with marked squamous cell carcinoma components arising in non-cirrhotic liver. Pathol Int. 2003;53:90–97. doi: 10.1046/j.1440-1827.2003.01443.x. [DOI] [PubMed] [Google Scholar]
- 20.Naik S, Waris W, Carmosino L, et al. Primary squamous cell carcinoma of the liver. J Gastrointestin Liver Dis. 2009;18:487–89. [PubMed] [Google Scholar]
- 21.Spaggiari M, Di Benedetto F, Ballarin R, et al. Primary squamous cell carcinoma of the liver associated with Caroli’s disease: A case report. Onkologie. 2011;34:193–95. doi: 10.1159/000326999. [DOI] [PubMed] [Google Scholar]
- 22.Zhu KL, Li DY, Jiang CB. Primary squamous cell carcinoma of the liver associated with hepatolithiasis: A case report. World J Gastroenterol. 2012;18:5830–32. doi: 10.3748/wjg.v18.i40.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morito K, Kai K, Miyoshi A, et al. Primary squamous cell carcinoma of the liver concomitant with primary colon cancer: Report of a case. Clin J Gastroenterol. 2013;6:134–38. doi: 10.1007/s12328-012-0341-2. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XF, Du ZQ, Liu XM, Lv Y. Primary squamous cell carcinoma of liver: Case series and review of literatures. Medicine (Baltimore) 2015;94:e868. doi: 10.1097/MD.0000000000000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubana SS, Singh N, Seligman B, et al. First reported case of primary intrahepatic cholangiocarcinoma with pure squamous cell histology: A case report. Am J Case Rep. 2015;16:438–44. doi: 10.12659/AJCR.894609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikuchi I, Sato T, Wakabayashi T, et al. A case of granulocyte colony-stimulating factor (G-CSF) producing squamous cell carcinoma arising from intrahepatic bile duct. J Jpn Bil Assoc. 2016;30:251–58. [Google Scholar]
- 27.Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33–42. doi: 10.1038/ncpgasthep0389. [DOI] [PubMed] [Google Scholar]