Abstract
Lagenaria siceraria is an economically important cucurbitaceous crop, but suitable reference genes (RGs) to use when the plants are infected by cucumber green mottle mosaic virus (CGMMV) have not been determined. Sixteen candidate RGs of both leaf and fruit and 18 candidate RGs mostly from separate RNA-Seq datasets of bottle gourd leaf or fruit were screened and assessed by RT-qPCR. The expression stability of these genes was determined and ranked using geNorm, NormFinder, BestKeeper and RefFinder. Comprehensive analysis resulted in the selection of LsCYP, LsH3, and LsTBP as the optimal RGs for bottle gourd leaves, and LsP4H, LsADP, and LsTBP for fruits. LsWD, LsGAPDH, and LsH3 were optimal for use in both leaves and fruits under the infection of CGMMV. Isopentenyl transferase (IPT) and DNA-directed RNA polymerase (DdRP) were used to validate the applicability of the most stable identified RGs from bottle gourd in response to CGMMV. All the candidate RGs performed in RT-qPCR consistently with the data from the transcriptome database. The results demonstrated that LsWD, LsGAPDH and LsH3 were the most suitable internal RGs for the leaf, and LsH3, LsGAPDH, LsP4H and LsCYP for the fruit.
Keywords: RT-qPCR, Reference gene, Transcriptome, Bottle gourd, CGMMV
Introduction
Lagenaria siceraria (Molina) Standl. is a specie belongs to Cucurbitaceae family, which was cultivated widely in tropical and temperate regions of the world, it is commonly known as bottle gourd that has good edible, medicinal and horticultural value (Wang et al., 2018; Decker-Walters et al., 2004). It could be routinely used as one rootstock source for watermelon and other cucurbit crops in both Japan and Korea in order to reduce the incidence of soil-borne diseases and promote the vigor of the root system of the crops in low temperature conditions (Yetisir & Sari, 2013; Cho et al., 2017; Spalholz & Kubota, 2017). Medicinally, L. siceraria extract has radioprotective potential in radiation-induced gastrointestinal injury (Sharma, Goel & Chauhan, 2016), and its latex sap exhibits potent lectin activity to mitigate neoplastic malignancy by targeting neovasculature and cell death (Vigneshwaran et al., 2016). Recently, a dedicated database named GourdBase was developed, which promoted the study of biological traits and molecular breeding in the bottle gourd (Wang et al., 2018). Zhejiang province has a long history of cultivating bottle gourd as an important economic crop. In 2011, the leaves of a bottle gourd plant which were brittle and had severe mosaic mottling were shown to be infected with cucumber green mottle mosaic virus (CGMMV) using reverse transcription-polymerase chain reaction (RT-PCR) and ELISA (Zheng et al., 2015). Since CGMMV could pose a great threaten to bottle gourd production, it attracted our attention.
CGMMV (genus Tobamovirus, family Virgaviridae) causes serious diseases in cucurbit crops. The virus is easily transmitted on the outside of seeds, pollen, and other propagation materials. It produces severe mosaic symptoms on the leaves of infected plants and causes fruit deformation, resulting in reduced yield and low market value (Ugaki et al., 1991; Sano et al., 1997; Tan et al., 2000; Zheng et al., 2015; Ali, Mohammad & Khattab, 2012). It has a worldwide distribution, and has been reported from many countries, including Israel, China, Greece, USA, Saudi Arabia and Russia (Ali, Mohammad & Khattab, 2012; Zheng et al., 2015; Slavokhotova et al., 2007; Antignus et al., 1990; Varveri, Vassilakos & Bem, 2002; Ali, Natsuaki & Okuda, 2004; Amer, 2015).
In view of the significant economic losses to cucurbit crops caused by CGMMV, most research has focused on its detection and control. The interaction between CGMMV and its hosts has gained increasing attention recently, but knowledge about it is still limited. Several studies have focused on identifying novel and conserved microRNAs in response to CGMMV infection or virus-derived siRNAs in a CGMMV infected host and exploring the pathogenic mechanisms from the perspective of protein expression levels in its hosts (Liu et al., 2015; Li et al., 2016; Sun, Niu & Fan, 2017). Internal changes in the host involve the host-virus interaction system, which is often mediated at the transcriptional level, thereby altering gene expression and possibly indirectly affecting plant performance. Quantitative RT-PCR (RT-qPCR) has become the most common method for quantifying and comparing gene expression levels during virus infection because of its rapidity, sensitivity, and specificity (Radonić et al., 2014; Huggett et al., 2005; Ceelen, De Craene & De Spiegelaere, 2014). Reference genes (RGs) are used to minimize experimental errors and normalize the experimental data but these are not universal; different RGs are needed under different experimental conditions. In cucurbits, only a few RGs with different traits have been established, and there are no reports of RGs suitable for use with CGMMV-infected bottle gourd.
Several reference genes have been utilized for reliable RT-qPCR in cucurbit crops, including actin (ACT), elongation factor 1 alpha subunit (EF1α), glyceraldehyde-3-phosphate (GAPDH), serine/threonine-protein phosphatase PP2A catalytic subunit (PP2A), Ran-GTPase (RAN), 40S ribosomal protein S15-4 (RPS15), tubulin alpha (TUA), peptidyl-prolyl cis-trans isomerase (CYP), 60S ribosomal protein L23 (RPL23), ADP-ribosylation factor (ADP), ubiquitin-60S ribosomal protein L40 (UBA) and transcription initiation factor TFIID TATA-box-binding protein (TBP) (Kong et al., 2016; Kong et al., 2014a; Wan et al., 2010; Wang et al., 2014; Warzybok & Migocka, 2013; Sestili et al., 2014; Wu et al., 2016; Kong et al., 2014b; Kong et al., 2015). In Nicotiana benthamiana, PP2A, F-Box and L23 are known to be the most stable RGs for exploring plant-virus interactions (Liu et al., 2012).
In this study, traditional reference genes were screened as candidate RGs and new, previously unreported, RGs were also sought. Systematic transcriptome analyses, including RNA-Seq and DNA microarray, have been widely used in the study of host-virus interaction recently. Because transcriptome data provide a valuable resource that can be used to determine appropriate RGs (Kudo et al., 2016; Guo, Jiang & Xia, 2016; Marcolino-Gomes et al., 2015; Zhang et al., 2014; Liu et al., 2018), we screened potential internal RGs from the transcriptome database of bottle gourd infected by CGMMV. We set the corresponding screening parameters to select the candidate genes from the bottle gourd transcriptome database. 11 candidate RGs from leaves and 22 from fruits were selected, including a histon H3 gene (LsH3) and a tryptophan and aspartic acid (WD)-repeat protein (LsWD), which matched the screening parameters for both leaves and fruits.
The stability and suitability of all selected candidate RGs expression was estimated using several algorithms: geNorm, NormFinder, BestKeeper and RefFinder. These algorithms together provide an approach to identify the most stably transcribed new genes (i.e., in addition to the traditional reference genes). Because there is little information on RGs that can be used to normalize gene expression data in CGMMV-infected bottle gourd, we evaluated the selected candidate genes by RT-qPCR, focusing on novel reference gene selection and analysis in CGMMV-infected leaves and fruits. Moreover, parallel analyses on the expression profiles of an Isopentenyl transferase (IPT) gene and a DNA-directed RNA polymerase (DdRP) gene normalized by the identified RGs were performed to demonstrate the reliability of these identified RGs.
Materials & Methods
Plant preparation, virus inoculation
The cultivation and management of bottle gourd (L. siceraria, accession “Hangzhou Gourd”) were performed as follow: after soaking and germination, the seeds were first transplanted into 10 cm nutrient preparations with soil rich in organic matter, and when the seedlings grew to two and a half leaf stage, transplanted them into 20 L PVC drums (1 per barrel). The mixed substrate used was peat: vermiculite: perlite: organic fertilizer = 4:4:1:1, The pH of the culture substrate was about 7.0 and the water content was maintained at about 70% relative humidity. A “flower-free” nutrient solution was used once a week (N:P:K = 20:20:20) (Shanghai Yongtong Chemical Co., Ltd., Shanghai, China). The greenhouse conditions were daily temperature 25–28 °C, night temperature 18–20 °C; photoperiod 14 h/d (light intensity is greater than 87.5 µmol m−2 s−1). Scaffolding, topping, pruning and pollination were carried out according to routine management. The fruits were harvested 10 days after pollination.
CGMMV inoculum (CGMMV-ZJ) was sap from L. siceraria plants with typical symptoms that had been infected with a CGMMV infectious clone 14 d earlier (Zheng et al., 2015). At least six plants were inoculated with CGMMV-infected sap at the two and a half leaf stage on the two expanding leaves. Approximately 1 g of plant tissue was homogenized in 20 volumes of inoculation buffer (0.1 M phosphate buffer, pH 7.5, 0.2% sodium sulfite and 0.01 M 2-mercaptoethanol), while the mock plants were only inoculated with buffer.
RNA sequencing
According to the protocol of TruSeq Small RNA Sample Prep Kits (Illumina, San Diego, CA, USA), the total RNA of about 5 µg was extracted for the preparation of small RNA library. Sequencing of the RNA-Seq libraries was carried out on an Illumina Hiseq2500 at LC-BIO (Hangzhou, China) following the manufacturer’s protocol.
RNA and first strand cDNA preparation
Three replicate samples of flesh tissue of the ripe fruits and newly expanding leaves from both inoculated and control plants were collected for RNA extraction. Total RNA was extracted from each sample using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA quantity and quality from each sample was evaluated by denaturing agarose gel electrophoresis and microfluidic capillary electrophoresis with the Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Only RNA samples with a complete band and A260/A280 ratio in the range 1.8–2.0 were used for the next step. All RNA samples were stored at −70 °C. For virus detection, the first strand cDNA was synthesized using ReverTra Ace-α-® kit (TOYOBO, Japan) following the product’s protocol. The infection of CGMMV in the tissues was confirmed by CGMMV specific primers, and the primers of ZYMV and WMV were also used to monitor the presence of these two common viruses occurred in cucurbit crops (Heeju et al., 2015). For RT-qPCR, first strand cDNA was synthesized from 1 µg total RNA using PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The negative controls without PrimeScript RT Enzyme Mix I were analyzed in parallel to detect the presence of genomic DNA contamination in the RNA samples.
Selection of candidate RGs
Partial candidate RGs in leaves and fruits were selected from publicly available references, but most were from our RNA sequencing data. To obtain RGs that are stably and moderately or highly expressed in CGMMV-infected leaves, we kept the Reads Per Kilobases per Million reads (RPKM), ratio of the maximum to the minimum RPKM (RPKMmax/min), and coefficient of variation (CV) to >40, <2.0, and <0.3 at p < 0.05, respectively. In fruit, the RPKM, RPKMmax/min, and CV were maintained at >40, <2.0, and <0.2 at p < 0.05, respectively. All selected internal RGs have only one transcript and were ranked from small to large according to their RPKMmax/min values.
To select better RGs for both leaves and fruits, the RPKM and RPKMmax/min were kept at >40 and <2.0 for the RGs commonly used in cucurbit plants in keeping with the RNA-seq data. The RGs for leaves and fruits were screened and analyzed simultaneously with 14 common RGs of cucurbit crops in previous studies. A total of 16 RGs from bottle gourd leaves and fruits were screened and analyzed.
Primer design and verification of selected gene amplicons
The fourteen common RGs were amplified according to the references or based on primers designed by Primer-BLAST of the RNA sequence data of leaves and fruits (Table 1). Specific primers for the candidate RGs from our RNA-sequencing data were designed using Primer 3 (http://primer3.ut.ee/) (Table 2). All PCR amplicon lengths were between 80–200 bp. All primers were synthesized by a commercial supplier (Biosune, Hangzhou, China).
Table 1. Primer sequences and PCR amplification characteristics for commonly-used candidate reference genes, LsIPT and LsDdRP.
| Gene name | Description | NCBIHomolog locus | GourdbaseHomolog locus | Forward primer sequence/reserve primer sequenc (5′–3′) | Product size (bp) | Tm (°C) | Leaf | Fruit | ||
|---|---|---|---|---|---|---|---|---|---|---|
| E (%) | R2 | E (%) | R2 | |||||||
| LsH3a,b | histone H3 | LOC103494422 | BG_GLEAN_10002693 | CAAACTGCCCGTAAGTCCAC/ GGCTTCTTCACTCCTCCTGT | 101 | 81.9 | 110.06 | 0.9980 | 103.67 | 0.9986 |
| LsWDa,b | WD repeat-containing protein | LOC101218407 | BG_GLEAN_10012213 | TCTGTGGTACTCGAGAAGGC/ GAGAAATCTCCGGTGTGTCGT | 95 | 82.5 | 106.96 | 0.9987 | 103.13 | 0.9937 |
| LsACT | actin-7 | LOC103499652 | BG_GLEAN_10004800 | GGCAGTGGTTGTGAACATGT/ CCCATGCTATCCTCCGTCTT | 98 | 82.1 | 90.11 | 0.9989 | 97.26 | 0.9999 |
| LsUBA52 | ubiquitin-60S ribosomal protein L40 | LOC101205082 | – | AAGTGTGGACACAGCAACCA/ GGGAAAGAGCCAAAAATAGG | 255 | 79.3 | 94.86 | 0.9990 | 116.23 | 0.9816 |
| LsRPL23a | 60S ribosomal protein L23 | LOC101203845 | BG_GLEAN_10013074 | CATGACCATATCACCAACACAA/ CGACAATACAGGAGCTAAGAA | 100 | 77.3 | 98.16 | 0.9978 | 97.15 | 0.9942 |
| LsRAN | Ran-GTPase | LOC111496316 | BG_GLEAN_10011129 | TCTACTGTTGGGATACCGCT/ CAGAGATCACGATGCCATGTT | 145 | 80.6 | 103.26 | 0.9976 | 90.81 | 0.9963 |
| LsPP2Aa,b | serine/threonine-protein phosphatase PP2A catalytic subunit | LOC103502598 | BG_GLEAN_10010727 | GGCAGATAACTCAAGTTTATGGA/ GCTGTAAGAGGTAAATAATCAAAGAGG | 109 | 75.0 | 92.68 | 0.9993 | 110.62 | 0.9933 |
| LsGAPDHb | Glyceraldehyde-3-phosphate dehydrogenase | LOC103496285 | – | CCCAGGGGATATCTGCAGGG/ CATGGTGTTTTCAATGGAACCA | 109 | 85.2 | 100.97 | 0.9986 | 91.06 | 0.9933 |
| LsEF1αa | Elongation factor 1- α | LOC101215193 | – | CTGCTTGCTCCTGCGTGAAA/ CCACGATGTTGATGTGAATCTTCTC | 118 | 83.9 | 105.42 | 0.9977 | 103.34 | 0.9962 |
| LsADPa,b | ADP ribosylation factor | LOC101217563 | – | ATATTGCCAACAAGGCGTAGA/ TGCCCGTAAACAATGGACAAA | 92 | 80.2 | 95.04 | 0.9966 | 93.69 | 0.9968 |
| LsTUAb | tubulin alpha | LOC103502708 | BG_GLEAN_10002510 | AGGACTGGGACGTACCGACA/ CGGCTAATTTTCGCACTCGG | 145 | 83.9 | 103.16 | 0.9901 | 107.20 | 0.9963 |
| LsTBPa,b | transcription initiation factor TFIID TATA-box-binding protein | LOC103492411 | BG_GLEAN_10001318 | AAACTCTTCCCGCTTCCTCA/ AGCCTTGATCTGCCATTCCT | 143 | 81.3 | 93.79 | 0.9987 | 96.90 | 0.9986 |
| LsRPS15a | 40S ribosomal protein S15-4 | LOC101217711 | BG_GLEAN_10016309 | AGTCCTCTTCTTCGGCACTC/ TCCACTCGAAACCCTAGCAG | 135 | 80.2 | 90.16 | 0.9988 | 92.14 | 0.9999 |
| LsCYP20 | peptidyl-prolyl cis-trans isomerase CYP20-1 | LOC101213040 | BG_GLEAN_10005366 | TTTACCCTCGGCGATGGAAG/ TGTGAACCATTTGTATCTGGA | 134 | 80.8 | 96.15 | 0.9976 | 89.90 | 0.9966 |
| LsCYPa | peptidyl-prolyl cis-trans isomerase 1-like | LOC101206458 | BG_GLEAN_10006142 | CACACCGGCCCTGGTATTTT/ CATCCATGCC TTCAACGACT | 139 | 83.5 | 107.09 | 0.9914 | 95.00 | 0.9941 |
| LsL23A | 60S ribosomal protein L23a | LOC101220073 | BG_GLEAN_10025920 | AAGGATGCCGTGAAGAAGATGT/ GCATCGTAGTCAGGAGTCAACC | 110 | 82.2 | 93.99 | 0.9997 | 98.49 | 0.9958 |
| LsIPT | adenylate isopentenyltransferase (cytokinin synthase) | LOC101204427 | BG_GLEAN_10016404 | GCACTCCAATGGCTCGTTTA/ GGTCGATGGTGGATTTGTCG | 89 | 83.0 | 107.39 | 0.9972 | 93.23 | 0.9951 |
| LsDdRP | DNA-directed RNA polymerase subunit | LOC101215872 | BG_GLEAN_10015299 | AAACTCCCTTTCAGCCTCGA/ AGATGTGGCCCTGTTGAGAA | 174 | 81.6 | 95.45 | 0.9984 | 96.07 | 0.9971 |
Notes.
Bottle gourd gene ID in the NCBI Database (https://www.ncbi.nlm.nih.gov/) and GourdBase (http://www.gourdbase.cn/) were listed. The two genes labeled as aqua green were selected from RNA-seq data which met the criteria (RPKM >40, RPKMmax/min <2.0) to be candidate RGs for both leaves and fruits. The fourteen genes labeled as light gray were selected from the traditional RG used in Cucurbitaceae crops.
indicated the candidate reference genes selected for following analysis in bottle gourd leaves.
indicated the candidate reference genes selected for following analysis in bottle gourd fruits.
Table 2. Primer sequences and PCR amplification characteristics for candidate reference genes selected from bottle gourd RNA-seq database.
| Gene name | Description | NCBIHomolog locus | GourdbaseHomolog locus | Forward primer sequence/reserve primersequenc (5′–3′) | Productsize (bp) | Tm (°C) | E(%) | R2 |
|---|---|---|---|---|---|---|---|---|
| Primers and amplicon characteristics for candidate internal control genes from bottle gourd leaves | ||||||||
| LsARL | ADP-ribosylation factor-like | LOC103504673 | BG_GLEAN_10014274 | GCTGGTCGAAAGTTGACTCC/GTCAAGGCCAAAGAGTAGGCA | 109 | 82.7 | 106.80 | 0.9988 |
| LsTPT | triose phosphate/phosphate translocator | LOC103483570 | BG_GLEAN_10024475 | ACCACCTACGATTGGCAGAAG/GTCTGGGAAAAGTGGCGGTAT | 140 | 81.7 | 102.65 | 0.9838 |
| LsSRK2I | serine/threonine-protein kinase SRK2I | LOC101206398 | BG_GLEAN_10004502 | TTGACCACTACCCATCTTGCA/GCGAGCCTCATCCTCACTAA | 102 | 81.7 | 90.54 | 0.9945 |
| LsCNX | calnexin homolog | LOC101207554 | BG_GLEAN_10001815 | TCGCTCTCTCATCCCAATCC/GTGCGCATTCTCATTGATGGG | 139 | 86.5 | 101.26 | 0.9940 |
| LsPDI | protein disulfide-isomerase-like | LOC103504071 | BG_GLEAN_10010057 | AGGCCCACTTTGCTTCTTCAA/GAGCAGTCATGACCCTCCAAT | 199 | 82.2 | 98.27 | 0.9967 |
| LsSK | shaggy-related protein kinase | LOC103490499 | BG_GLEAN_10005566 | CTTGCTTCACGTCTGCTTCAA/GTTGTTAGGGAGGCGGACATT | 114 | 81.7 | 95.34 | 0.9935 |
| LsUBC | ubiquitin C | LOC111803940 | BG_GLEAN_10016554 | CACTTGGTGCTTCGTCTCAG/TCGATCGTGTCAGAGCTCTC | 98 | 81.7 | 98.75 | 0.9957 |
| LsGAD | glutamate decarboxylase | LOC103501361 | BG_GLEAN_10014712 | TGTCATAGGGCTTGCCTTCAG/CATTGGGTGATGCTGAGACG | 129 | 84.6 | 99.77 | 0.9966 |
| LsRNC | ribonuclease III domain-containing protein RNC1 | LOC111803262 | BG_GLEAN_10017046 | TACATCTTCAAGTTGCCTGCGT/CCAGAAGTGTACCGGGTTCT | 93 | 81.0 | 94.31 | 0.9976 |
| Primers and amplicon characteristics for candidate internal control genes from bottle gourd fruits | ||||||||
| Ls ARIA | arm repeat protein interacting ABF2 | LOC103483725 | BG_GLEAN_10016382 | CTCCCCAATGCAAAAGCTGAC/GAGGTGCTGTTCGACCCTTAA | 82 | 83.9 | 102.99 | 0.9869 |
| LsP4H | prolyl 4-hydroxylase | LOC103485167 | BG_GLEAN_10016694 | AGAGAGAGAGAGGCCTTGGA/CCTGTGTTTC GCCATGGAAAC | 126 | 82.0 | 91.04 | 0.9951 |
| LsXRN1 | 3′–5′exoribonuclease 1 | LOC101214656 | BG_GLEAN_10010883 | ACCTTCCAGATCACACCAGG/AGGCCTCACAGTTCCTCTTC | 129 | 81.2 | 111.01 | 0.9928 |
| LsPARP | inactive poly [ADP-ribose] polymerase | LOC103503572 | BG_GLEAN_10013282 | TTGGAGTCTTCAGGGAGCTG/TCCTCTTGAACGTGGGGTAC | 143 | 81.3 | 93.05 | 0.9970 |
| LsYpgQ | uncharacterized protein YpgQ | LOC103500350 | BG_GLEAN_10019270 | ATGGCGAAAAGAGAAACGGTG/GAAGGATCATGTGACGCGTC | 83 | 84.1 | 115.47 | 0.9986 |
| LsEIF5 | eukaryotic translation initiation factor 5-like | LOC103488416 | BG_GLEAN_10010896 | GCAGCCAATAGTCTCAGCAC/GTAGTTCAAAGTGGAGGGCGT | 142 | 81.6 | 94.77 | 0.9939 |
| LsVAMP | vesicle-associated membrane protein 72 | LOC103502784 | BG_GLEAN_10013195 | AACCTTCGATCTCAGGCACAA/CGCCGCAGACAGACAAAATGA | 145 | 84.4 | 104.32 | 0.9947 |
| LsPL | Phospholipase-like | LOC101210853 | BG_GLEAN_10006169 | CGAATGGGACTCTGCTTTGG/TATTCCGACGAAATCCATCCG | 131 | 83.1 | 96.42 | 0.9925 |
| LsISCA | iron-sulfur assembly protein IscA-like 1 | LOC103489389 | BG_GLEAN_10023226 | ATGGCAGCTTCTTCGTCTTCC/TGGCGCTGTTGAAGAAGTTGT | 127 | 82.7 | 97.43 | 0.9929 |
| LsclpC | ATP-dependent Clp protease ATP-binding subunit ClpC | LOC101207209 | BG_GLEAN_10001965 | TGTGGATGTTGATTCTGATGGA/ACAGGTTACACAGGAATAGCATC | 90 | 79.2 | 94.59 | 0.9971 |
| LsCRCK3 | calmodulin-binding receptor-like cytoplasmic kinase 3 | LOC103487406 | BG_GLEAN_10008798 | ACCGACTGTCCCTTTCACTTG/GTGGCGGATTTTGGATTTGCAA | 83 | 85.0 | 93.72 | 0.9986 |
Notes.
Bottle gourd gene ID in the NCBI Database (https://www.ncbi.nlm.nih.gov/) and GourdBase (http://www.gourdbase.cn/) were listed.
To check the specificity of all primers, the cDNA of each sample was amplified by PCR, and the amplified products were separated by electrophoresis on 3% agarose gel and purified using a QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, and cloned into pEASY-Blunt zero (Transgen, Beijing, China) followed by sequencing.
The quantification cycle (Cq) values obtained by qRT-PCR on a standard curve generated from a fourfold dilution series of one sample at six dilution points for three technical replicates were used to draw the standard curve to get R2 and slope values. The PCR amplification efficiency of each primer was calculated using the equation: E(Efficiency)% = (10 [−1∕slope] −1) ×100%.
Quantitative real-time PCR
qRT–PCR was carried out in 384-well plates using the QuantStudio 6 Flex real-time PCR detection system (ABI, USA). Each reaction mixture consisted of 5 µL SYBR Green Realtime PCR Master Mix (TaKaRa, Dalian, China), 0.5 µL cDNA diluted fivefold, 0.5 µL (10 mM) each of forward and reverse primers, and 3.5 µL RNA-free H2O, equating to a final volume of 10 µL in each well. The qPCR reaction was as follows: initial denaturation at 95 °C for 5 min and 40 cycles of amplification (95 °C 15 s, 58 °C 20 s and 72 °C 20 s). Subsequently, fluorescence acquisition was performed after each cycle. A melting curve was generated after 40 cycles of amplification by heating at 65–95 °C. Cq values and baseline were set automatically by the QuantStudio Real-Time PCR Software v1.2 (ABI, USA) using default parameters.
Gene expression stability analysis
The programs geNorm (Vandesompele et al., 2002), NormFinder (Andersen, Jensen & Orntoft, 2004), BestKeeper (Pfaffl et al., 2004) and RefFinder (http://150.216.56.64/referencegene.php?type=reference) were used to analyze the stability of the candidate RGs under CGMMV infection conditions. All software packages were used according to the manufacturer’s instructions.
Validation of the selected RGs
LsIPT and LsDdRP genes in CGMMV-infected bottle gourd leaf and fruit tissue were selected to detect the effectiveness of these identified RGs. Primers for the two genes were designed as described above and listed in Table 1. The best RGs identified by the algorithms above were used for normalization.
Results
Transcriptome analysis of Lagenaria siceraria under CGMMV infection based on RNA-seq
Bottle gourd leaves and fruits infected by CGMMV were collected from three replicate virus-inoculated plants, and the presence of CGMMV in each sample was further confirmed by RT-PCR and western blot, and the contamination of ZYMV and WMV was excluded by RT-PCR (Fig. S1). The control leaves and fruits samples were in parallel collected from three mock bottle gourds. The analysis of bottle gourd transcripts before and after CGMMV infection showed 639 and 3,930 non-differentially expressed genes (— log2 fold_change — <1, P ≤ 0.05) in the leaves and fruits of bottle gourd, respectively (Tables S1 and S2). And these non-differentially expressed genes were used as the source of candidate RGs from the RNA-Seq dataset.
Selection of candidate RGs
In the present study, 11 and 86 candidate RGs respectively from bottle gourd leaves and fruits were screened from our RNA-Seq dataset by setting up a series of conditions (Tables S3 and S4). Only two genes, LsH3 and LsWD (Table S5), met the criteria to be candidate RGs for both leaves and fruits. The primers were designed based on the gene sequences in the database. To select candidate RGs that could be used in both bottle gourd leaves and fruits, these two novel genes with other 14 traditional candidate RGs were used to compare their expression stability. To select candidate RGs that could be used in bottle gourd leaves or fruits, separately, the commonly used reference gene sequences were then compared with the bottle gourd transcriptome data (Table S6). All the 11 candidate RGs of bottle gourd leaves screened from our RNA-Seq data, and seven traditional candidate RGs, LsPP2A, LsADP, LsEF1α, LsCYP, LsRPS15, LsTBP, and LsRPL23 screened from transcriptome comparison data were selected as candidate RGs of bottle gourd leaves. Of the 86 genes screened from the bottle gourd fruit transcriptome data, the first 11 (based on the ratio of RPKMmax/min) were selected for further analysis in addition to LsH3 and LsWD, and five traditional candidate RGs, LsPP2A, LsADP, LsTBP, LsTUA, and LsGAPDH screened from transcriptome comparison data were selected as candidate RGs of bottle gourd fruits. Therefore, a total of 16 common RGs of both bottle gourd leaves and fruits (Table 1) and 18 RGs of bottle gourd leaves and fruits separately were screened and analyzed (Tables 1 and 2).
Evaluation of target specificity and amplification efficiency in RT-qPCR reactions
Preliminary evaluation of candidate reference gene primers was performed by evaluating primer specificity and efficiency. The single peak in melting curve analyses following RT-qPCR confirmed the specific amplification of each gene (Fig. S2). Each amplicon was detected by agarose gel electrophoresis, only a single fragment of the expected size (80–200 bp) was observed (Fig. S3). Further sequencing results showed all genes sequences were exactly 100% identical to those of the corresponding genes in bottle gourd transcriptome databases. Amplification efficiencies of bottle gourd leaves ranged from 90.1% to 110.1%, whereas those of the fruits ranged from 89.9% to 116.2% (Tables 1 and 2). Furthermore, the standard curves showed good linear relationships (>0.981) between the Cq values and the log-transformed copy numbers of all candidated RGs (Tables 1 and 2). There was no band detected in the negative controls, indicating that the genomic DNA contamination does not exist (Fig. S3).
Expression intensity of candidate RGs
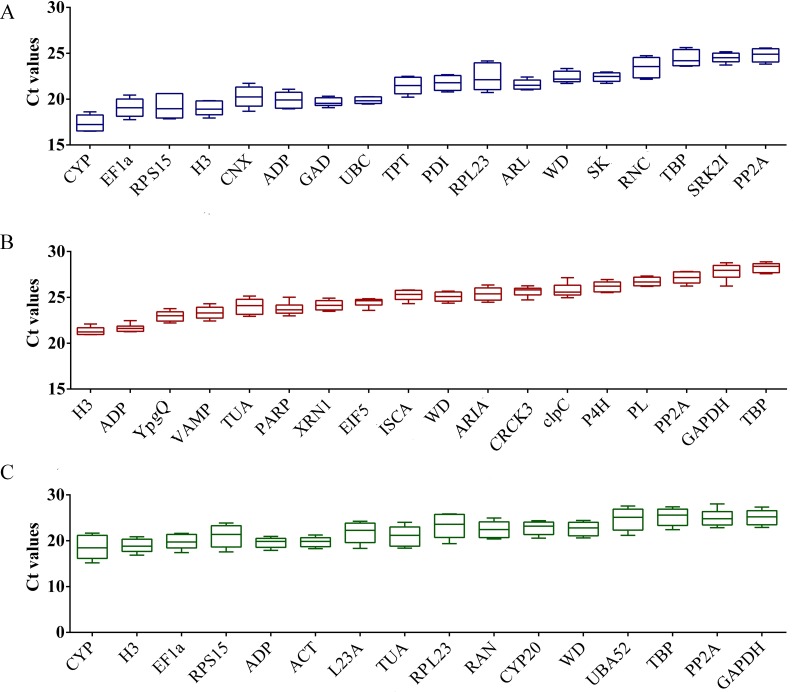
In order to fully understand the relative expression intensity of all candidate RGs in bottle gourd, three biological and three technical replicates (n = 9 for each gene) were used to determine the Cq values for all RGs. From the graph for bottle gourd leaves, the mean Cq values of these candidate genes we selected ranged from 16.51 (LsCYP) to 25.63 (LsTBP) (Fig. 1A), which represented the highest and lowest accumulation levels, respectively. The minimal variation in gene expression was LsUBC (<0.81 cycles) in bottle gourd leaves. The lowest and highest median Cq value of the mRNA accumulation levels in the fruits of bottle gourd was 20.94 (LsH3) and 28.88 (LsTBP), respectively (Fig. 1B). LsPLA expression exhibited the least amount of variation (<1.14 cycles) in bottle gourd fruits. The Cq value 15.18 of LsCYP in leaves was the lowest and 28.04 of LsPP2A in fruits was the highest (Fig. 1C). The minimal variation in gene expression observed in both leaves and fruits were 2.95 cycles (LsACT), 3.06 cycles (LsADP) and 3.78 cycles (LsWD). However, only the comparison of raw Cq values is not sufficient to evaluate the expression stability of candidate RGs, a further intensive statistical analysis was required for more accurate assessment.
Figure 1. Expression intensity of candidate RGs in healthy control and CGMMV-infected samples of bottle gourd.
(A) Bottle gourd leaves indicated in blue color; (B) bottle gourd fruits indicated in red color; (C) bottle gourd leaves and fruits indicated in green color. Values are given as Cq (mean of triplicate samples) and are inversely proportional to the amount of template. The box indicates the 25th and 75th percentiles. Whiskers represent the maximum and minimum values. The thin line within the box marks the median.
Comparison of the expression stability of the universal traditional candidate RGs and novel candidate RGs in both bottle gourd leaves and fruits
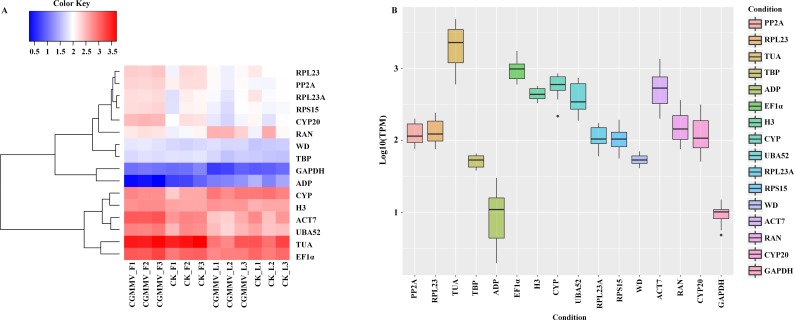
To select universal candidate RGs expressed stably both in bottle gourd leaves and fruits infected by CGMMV, we screened from our RNA-Seq dataset by setting up a series of conditions (RPKMmax/min < 2, and CV < 0.3, p < 0.05, RPKM min > 40). Only two genes, LsH3 and LsWD, met the criteria to be candidate RGs and we then compared the expression stability of these two novel genes with 14 other commonly-used cucurbit RGs. The expression profiles (Fig. 2A) and the variations of the 16 genes (Fig. 2B; Tables S5 and S6) in bottle gourd suggested that LsWD, LsH3 and LsTBP and LsGAPDH were expressed more stably (CV < 0.3), and the variation in respective expression levels was the lowest among all the genes during CGMMV infection, indicating that these genes, especially the two novel genes LsWD and LsH3, may be more suitable for normalization than other traditional candidate RGs. In addition, LsWD, LsH3 and LsGAPDH each had only one transcript, which could facilitate primer design and ensure the accuracy and reliability of the RT-qPCR compared to other genes.
Figure 2. Characteristic expression of the universal genes in bottle gourd under CGMMV infection.
(A) A heatmap was used to visualize the expression pattern of the two novel candidate RGs selected from RNA-Seq data and 14 commonly used RGs under CGMMV infection. (B) Expression levels and variations of the 16 common RGs under CGMMV infection.
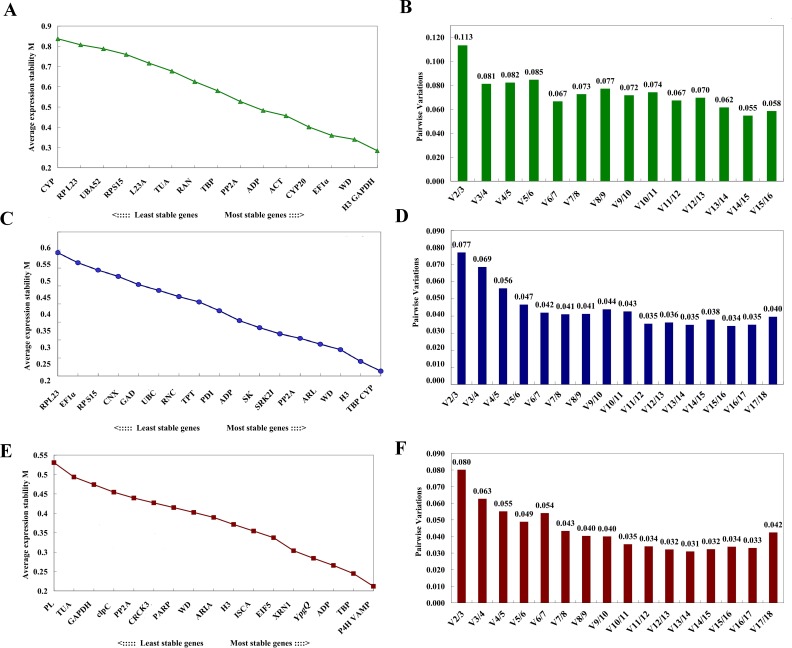
The stability of these 16 potential RGs was further evaluated with two statistical methods. By geNorm analysis, the average gene expression stability (M) of all of the universal candidate RGs were compared, among them, LsH3 and LsGAPDH showed the lowest M value (M = 0.284) in both leaves and fruits, followed by LsWD (M = 0.340) (Fig. 3A), indicating that these genes displayed the most stable profiles. Pairwise variation Vn∕n+1 was less than 0.15 in all leaf and fruit samples (Fig. 3B), indicating that adding other RGs was not necessary, and demonstrating that at least two reference genes were required for more reliable normalization, the top two gene were LsH3 and LsGAPDH. The raw Cq values were also transformed into Q values for NormFinder analysis. The lowest stability value of NormFinder analysis indicates the most stably expressed gene. By NormFinder analysis, the best three universal RGs in both leaf and fruit were LsTBP, LsWD and LsH3 (Fig. 4A). So, both geNorm and NormFinder analysis suggested that the two novel candidate RGs LsWD and LsH3 were suitable to evaluate the gene expression stability of bottle gourd leaves and fruits infected by CGMMV.
Figure 3. Expression stability of the candidate RGs analyzed by geNorm.
M represents the stability value. M of RGs screened from RNA-Seq in both bottle gourd leaves and fruits (A), and Vn/Vn+1 of the universal RGs in both bottle gourd leaves and fruits (B); M of RGs screened from RNA-Seq in bottle gourd leaves (C), and Vn/Vn+1 of RGs screened from the RNA-Seq in bottle gourd leaves (D), M of RGs screened from RNA-Seq in bottle gourd fruits (E), and Vn/Vn+1 of the universal RGs in bottle gourd fruits (F).
Figure 4. Expression stability of the candidate RGs analyzed by NormFinder.
The samples were divided into two subgroups according to the method of leaf and fruit set. The histogram displays the intergroup variation. The error bars represent the intragroup variation. M represents the stability value. Asterisks indicate the best genes. NormFinder analysis of candidate RGs screened from the RNA-Seq and the traditional RGs in both bottle gourd leaves and fruits (A), from the RNA-Seq in bottle gourd leaves (B), and in bottle gourd fruits (C).
Expression stability of candidate RGs in CGMMV infected leaves and fruits of bottle gourd separately from transcriptome analysis
To further select more suitable candidate RGs expressed stably in bottle gourd leaves or fruits infected by CGMMV separately, 18 RGs of bottle gourd leaves or fruits separately obtained from the RNA-Seq dataset were compared using different algorithms. By geNorm analysis, the average gene expression stability of the 18 candidate RGs of bottle gourd leaves and fruits screened from the RNA-Seq were all less than 1.5, respectively (Figs. 3C and 3E; Table 2). For all the tested leaf samples, LsTBP and LsCYP showed the lowest M value (M = 0.213) (Fig. 3C) of all the candidate RGs while in fruit samples, LsP4H and LsVAMP had the lowest M value (M = 0.212) (Fig. 3E). The pairwise variation Vn∕n+1 of each sample was also less than 0.15 (Figs. 3D and 3F), and the top two reference genes those had the lowest M value were needed for more reliable normalization at least. Similarly, by NormFinder analysis, the best three genes screened from the RNA-Seq dataset were LsCYP, LsH3 and LsPP2A in leaves (Fig. 4B), and LsTBP, LsP4H and LsXRN1 in fruits (Fig. 4C).
For further clarification, the expression stability of these candidate RGs was examined by two more algorithms. BestKeeper software can only compare the expression levels of up to 10 internal control genes in 100 samples, so only the top ten genes identified by geNorm and NormFinder were selected for subsequent assessment. Of these, the top three candidate internal RGs were LsCYP (r = 0.995, p-value =0.001), LsH3 (r = 0.984, p-value =0.001) and LsTBP (r = 0.964, p-value =0.002) in leaf samples, and LsP4H (r = 0.978, p-value =0.001), Ls VAMP (r = 0.975, p-value =0.001) and LsTBP (r = 0.959, p-value =0.002) in fruit samples (Table S7). The results of BestKeeper were therefore broadly consistent with geNorm and NormFinder. We also compared and ranked the tested candidate RGs based on a web-based comprehensive analysis tool, RefFinder, which suggested that the top three candidate RGs screened from the RNA-Seq dataset in bottle gourd leaves were LsCYP, LsH3 and LsTBP, while those in bottle gourd fruits were LsP4H, LsADP, and LsTBP (Table S7). These should therefore be the best RGs to use in RT-qPCR.
Validation of the candidate RGs
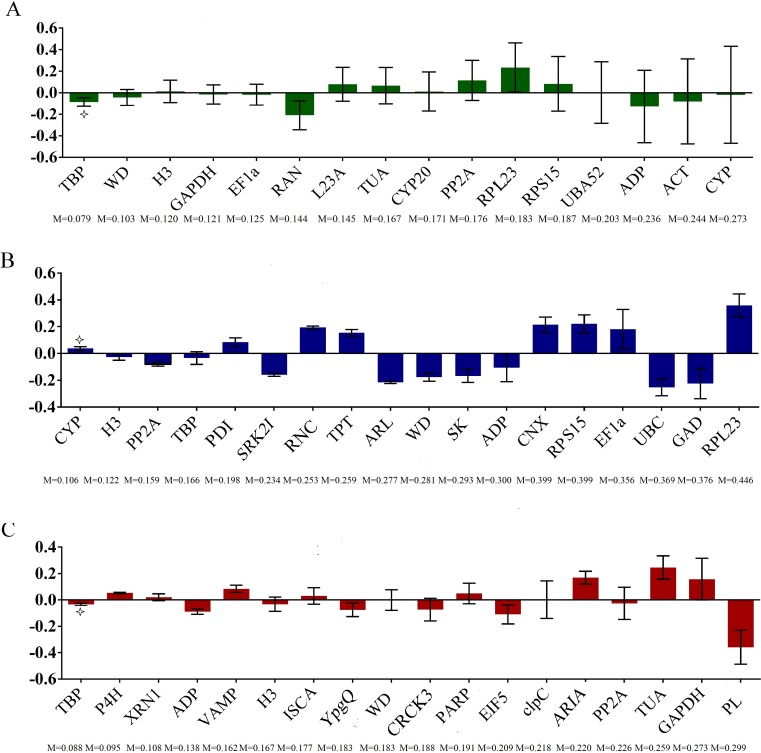
According to the transcriptional data, expression of the IPT and DdRP genes of L. siceraria changed significantly in response to CGMMV. LsIPT increased 1.78 fold in leaves and decreased 1.2 fold in fruits compared with their mock-inoculated tissues, while LsDdRP increased 1.4 fold in leaves and increased 1.63 fold in fruits (Table S8). These genes were therefore chosen to evaluate the reliability of the top candidate RGs as indicated by the previous analysis. The top rank RGs LsH3, LsGAPDH, LsWD, LsCYP, LsTBP and LsP4H selected by geNorm and RefFinder were used as candidate RGs. Among them, LsTBP, LsWD, LsH3, LsGAPDH and LsCYP were selected to use as RGs in leaves, and all these genes together with LsP4H for fruit.
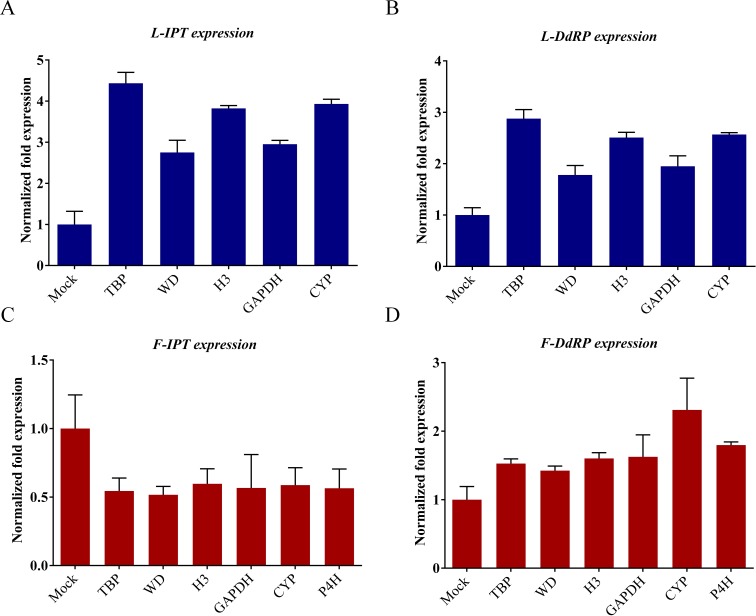
LsIPT increased 2.75 fold when LsWD was used as the reference gene in leaves, with 2.95, 3.82, 3.93 and 4.43 fold increases using LsGAPDH, LsH3, LsCYP and LsTBP respectively. The values of LsIPT normalized fold expression in fruits were 0.54 (LsTBP), 0.52 (LsWD), 0.60 (LsH3), 0.57 (LsGAPDH), 0.59 (LsCYP) and 0.56 (LsP4H) (Fig. 5). LsDdRP increased 1.78, 1.95, 2.51, 2.57 and 2.88 fold in leaves when LsWD, LsGAPDH, LsH3, LsCYP and LsTBP were the internal RGs respectively, while the corresponding values in fruits were 1.42 (LsWD), 1.53 (LsTBP), 1.60 (LsH3), 1.62 (LsGAPDH), 1.80 (LsP4H) and 2.31 (LsCYP) (Fig. 5). The RT-qPCR results showed that all candidate RGs gave results consistent with the data from the transcriptome database. Overall, the most suitable internal RGs chosen for use in leaves were LsWD, LsGAPDH and LsH3 and those for fruits were LsH3, LsGAPDH, LsP4H and LsCYP.
Figure 5. Relative quantification of LsIPT and LsDdRP expression in gourd leaf and fruit infected by CGMMV with RGs selected.
LsTBP (leaf and fruit), LsWD (leaf and fruit), LsH3 (leaf and fruit), LsGAPDH (leaf and fruit), LsCYP (leaf and fruit) and LsP4H (fruit only) were used as RGs. Error bars represent mean standard error calculated from three biological replicates. A control mock-inoculated sample was used as the calibrator (= 1). (A) LsIPT expression of gourd leaves with selected RGs. (B) LsDdRP expression of gourd leaves with selected RGs. (C) LsIPT expression of gourd fruits with selected RGs. (D) LsDdRP expression of gourd fruits with selected RGs. L, leaf; F, fruit.
Discussion
RT-qPCR, as one of the most commonly used and important tools for gene expression analysis, is characterized by rapidity and efficiency, responsiveness, simplicity in operation, high throughput, and specificity (Huggett et al., 2005; Liu et al., 2012). RT-qPCR can be used for qualitative or quantitative analysis of gene expression differences, and the appropriate internal RGs for relative quantitative analysis of the expression of genes are essential. Recent studies indicate that there is no single internal reference gene that is absolutely stable and therefore the choice of internal reference gene depends upon the various experimental conditions (Radonić et al., 2014; Ceelen, De Craene & De Spiegelaere, 2014; Kong et al., 2014a; Kong et al., 2014b). The ideal internal reference gene should be stably expressed under the corresponding experimental conditions, and its expression level should not be too high or too low; moreover, it should not be a pseudogene and its expression level should not be associated with the cell cycle. With continuous improvement in RT-qPCR requirements, researchers choose two or more RGs for gene analysis in order to reduce the error and obtain more reliable results (Liu et al., 2012; Kong et al., 2016).
In recent years, transcriptome sequencing technology has been widely used in various fields of molecular biology. The rapid development of transcriptome technology provides a better understanding of gene expression in plant samples from specific tissue, at different developmental stages, or under stress conditions. Transcriptome analysis based on high throughput sequencing can help us to quickly understand the differences in gene expression levels in plant tissue under specific conditions and can also be used to analyze the expression abundance of transcripts, identify the variable splicing of genes, determine the location of transcription, investigate gene fusion events, and discover new transcripts and other important information. In the screening of plant internal RGs, transcriptome sequencing analysis also provides us with a new screening pathway (Huggett et al., 2005).
We performed RNA-Seq for CGMMV-infected bottle gourd, and the genes that were not differentially expressed were selected as the candidate RGs based on the transcriptome data. Certain parameters were set to screen the stable expression genes as candidate RGs from RNA-seq data, and some traditional RGs were also compared to identify the most suitable candidate RGs for leaves or fruits, separately. Among them, LsH3 and LsWD were selected from the RNA-seq as candidate RGs in the CGMMV-infected leaves and fruits of bottle gourd. H3 is one of the most important constituents of chromatin, and its amino acid sequence is highly conserved. Methylation and acetylation of Histone H3 play an important role in the growth and development of plants (Bortoluzzi et al., 2017; Wollmann et al., 2017; Ingouff et al., 2010). Tryptophan and aspartic acid (WD)-repeat protein is a class of proteins that contain multiple highly conserved WD motifs and are strongly conserved. It is the Gb subunit of heterotrimeric G proteins, which forms a tight dimer (Gbg) with Gg subunits and plays an important role in signal transduction, protein transport, and RNA processing (Smith et al., 1999; Li et al., 2014; Van Nocker & Ludwig, 2003; Gachomo et al., 2014). Both LsH3 and LsWD genes were then compared with 14 genes used traditionally in cucurbitaceous crops to select the most suitable RGs in different bottle gourd tissues under CGMMV infection. Both geNorm and NormFinder analysis suggested that the two novel genes LsWD and LsH3 selected from our RNA-Seq data are suitable candidates to use in evaluating the gene expression stability in bottle gourd leaves and fruits infected by CGMMV. The further RefFinder analysis suggested that LsWD, LsGAPDH and LsH3 were the best three common optimal RGs for both leaves and fruits whether infected by CGMMV or not. Of the commonly used traditional RGs, LsGAPDH was the most stable in both leaves and fruits under CGMMV infection, but the novel LsWD reference gene ranked in first place.
Several other novel RGs selected from the RNA-seq data and some traditional RGs were also compared to identify the most suitable candidate RGs for leaves or fruits, separately. geNorm and NormFinder analysis, and the BestKeeper analysis based on these two algorithms, were consistent with each other with a slight difference, and a web-based comprehensive analysis tool RefFinder combined these analyses and suggested that the top three candidate RGs screened from bottle gourd leaves were LsCYP, LsH3 and LsTBP, while those in fruits were LsP4H, LsADP, and LsTBP. These should be the best RGs to use in RT-qPCR.
IPT is an important rate-limiting enzyme in the synthesis of cytokinin (CTK), catalyzing the decomposition of isopentenyl pyrophosphate and adenosine monophosphate to produce isoforms as precursors of CTK (i.e., monopentenyl AMP, iAMP), which can promote the increase in CTK content in plant cells (Hwang & Sakakibara, 2006; Zhu et al., 2012). The expression of the IPT gene in plants can improve stress resistance (Reguera et al., 2013; Žižková et al., 2015), delay leaf senescence, and improve defence against insect pests (Smigocki et al., 1993; Novák et al., 2013). DdRP is an essential enzyme for the replication of transcription systems in a variety of organisms and plays an important role in controlling transcription during gene expression (Wnendt et al., 1990; Knopf, 1998). These two genes were selected to validate the applicability of the screened RGs from bottle gourd in response to CGMMV. According to the comprehensive analysis, LsTBP, LsWD, LsH3, LsGAPDH and LsCYP were selected to as RGs in leaf, and all these genes with LsP4H were used to analyze IPT and DdRP expression in fruit. RT-qPCR results combined with transcriptome analysis showed a consistent trend of expression, which indicated that the candidate RGs were stable. Among these genes, LsWD, LsGAPDH and LsH3 were most suitable as internal RGs in the leaf, and LsH3, LsGAPDH, LsP4H and LsCYP as those for the fruit. Therefore, the novel genes LsH3 and LsWD were more stable both in leaves and in fruits under CGMMV infection than the previous reference genes, such as CYP, GAPDH, and TBP, although among the traditional RGs, GAPDH showed its superiority both in leaves and in fruits under CGMMV infection.
For the limitation that the RNA-Seq data was only from one bottle gourd variety infected with CGMMV, we further analyzed the existence of these RGs in different bottle gourd genotypes and found all these RGs are existed in about 50 different type of bottle gourd with different fruit shape according to the resequencing data we could access. Also, in the single nucleotide polymorphism (SNP) analysis about the sequences of these RGs amplified with the primers we designed, most are very conserved with no variation, only four RGs (LsXRN1, LsUBC, LsCYP, LsTUA) had slight variation. The SNP analysis further suggest the conservation of these RGs in different bottle gourd type. These selected RGs for bottle gourd leaves and fruits lay the foundation for further related research.
Conclusions
In this study, 16 candidate RGs of both leaf and fruit and 18 candidate RGs mostly from separate RNA-Seq datasets of bottle gourd leaf or fruit were assessed for their potential use as RGs in bottle gourd. Reliable normalized analysis by geNorm, NormFinder, BestKeeper and RefFinder indicated that LsWD, LsGAPDH and LsH3 were the most optimal RGs for bottle gourd leaves, and LsH3, LsGAPDH, LsP4H and LsCYP for the fruit. The candidate RGs provided in this study could be used to normalize the target genes in bottle gourd leaves and fruits to improve the accuracy and reliability of gene expression studies and the further related studies.
Supplemental Information
(A) The typical green mottle mosaic symptom on CGMMV-infected leaves 14 days after inoculation (right panel), while not symptom on mock leaves (left panel). (B) No obvious symptoms of virus on the fruit of CGMMV-infected bottle gourd (the bottom panel) and the mock fruits (the upper panel). (C) Detection of CGMMV (the upper panel), ZYMV (the middle panel) and WMV (the bottom panel) on L. siceraria by RT-PCR. (D) Detection of CGMMV by western blot. Photos by Chenhua Zhang.
(A) Dissolution curves of candidate RGs on both leaf and fruit of bottle gourd. (B) Dissolution curves of candidate RGs on leaf of bottle gourd. (C) Dissolution curves of candidate RGs on fruit of bottle gourd.
(A) Dissolution curves of candidate RGs on both leaf and fruit of bottle gourd. (B) Dissolution curves of candidate RGs on leaf of bottle gourd. (C) Dissolution curves of candidate RGs on fruit of bottle gourd.
(A) Dissolution curves of candidate RGs on both leaf and fruit of bottle gourd. (B) Dissolution curves of candidate RGs on leaf of bottle gourd. (C) Dissolution curves of candidate RGs on fruit of bottle gourd.
The upper line “C”, indicates the cDNA template, the bottom line “G”, indicates the gDNA template. “M” indicates the DNA ladder marker. (A) PCR amplification products of all RGs in L. siceraria leaf. (B) PCR amplification products of all RGs in L. siceraria fruit.
The RPKM > 40, RPKMmax/min < 2.0, and CV < 0.3, p < 0.05. All the selected internal RGs have only one transcript.
The RPKM > 40, RPKMmax/min < 2.0, and CV < 0.2, p < 0.05. All the selected internal RGs have only one transcript, and are ranked from small to large according to their RPKMmax/min values.
The RPKM > 4 0, RPKMmax/ min < 2.0, and CV < 0.2, p < 0.05. All the selected internal RGs have only one transcript.
Acknowledgments
We would like to thank Prof. M. J. Adams, Rothamsted Research, Harpenden, Herts, UK for correcting the English of the manuscript.
Funding Statement
This work was financially supported by Special Fund for Agro-Scientific Research in the Public Interest (201303028), Science and Technology Program (Public Service Technology Application) of Zhejiang Province (2017C32040), the National Nature Science Foundation of China (31500124) and Science and technology fund of Putian City (2016S3001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Hongying Zheng, Email: zhenghongyinghz@163.com.
Fei Yan, Email: fei.yan@mail.zaas.ac.cn.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Chenhua Zhang performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Hongying Zheng conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Xinyang Wu and Xiaohua Wu performed the experiments, approved the final draft.
Heng Xu, Jiejun Peng and Guojing Li analyzed the data, approved the final draft.
Kelei Han, Yuwen Lu and Lin Lin contributed reagents/materials/analysis tools, approved the final draft.
Pei Xu approved the final draft.
Jianping Chen authored or reviewed drafts of the paper, approved the final draft.
Fei Yan conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.
References
- Ali, Mohammad & Khattab (2012).Ali A, Mohammad O, Khattab A. Distribution of viruses infecting cucurbit crops and isolation of potential new virus-like sequences from weeds in Oklahoma. Plant Disease. 2012;96:243–248. doi: 10.1094/PDIS-05-11-0419. [DOI] [PubMed] [Google Scholar]
- Ali, Natsuaki & Okuda (2004).Ali A, Natsuaki T, Okuda S. Identification and molecular characterization of viruses infecting cucurbits in Pakistan. Journal of Phytopathology. 2004;152:677–682. doi: 10.1111/j.1439-0434.2004.00915.x. [DOI] [Google Scholar]
- Amer (2015).Amer MA. Biological and molecular characterization of cucumber green mottle mosaic virus affecting bottle gourd and watermelon plants in Saudi Arabia. International Journal of Agriculture and Biology. 2015;17:748–754. doi: 10.17957/IJAB/14.0015. [DOI] [Google Scholar]
- Andersen, Jensen & Ørntoft (2004).Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- Antignus et al. (1990).Antignus Y, Pearlsman M, Ben-Yoseph R, Cohen S. Occurrence of a variant of cucumber green mottle mosaic virus in Israel. Phytoparasitica. 1990;18:50–56. doi: 10.1007/BF02980826. [DOI] [Google Scholar]
- Bortoluzzi et al. (2017).Bortoluzzi A, Amato A, Lucas X, Blank M, Ciulli A. Structural basis of molecular recognition of helical histone H3 tail by PHD finger domains. Biochemical Journal. 2017;474:1633–1651. doi: 10.1042/BCJ20161053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceelen, De Craene & De Spiegelaere (2014).Ceelen L, De Craene J, De Spiegelaere W. Evaluation of normalization strategies used in real-time quantitative PCR experiments in HepaRG cell Line studies. Clinical Chemistry. 2014;60:451–454. doi: 10.1373/clinchem.2013.209478. [DOI] [PubMed] [Google Scholar]
- Cho et al. (2017).Cho SH, Joung YH, Karna S, Lee HE, Kim JH, Kim JH, Kim JH, Kim DS, Ahn YK. The development of cold resistance rootstock using Agrobacterium-mediated transformation of Arabidopsis CBF3/DREB1A, in bottle gourd (Lageneraria siceraria, Standl.) Scientia Horticulturae. 2017;214:141–146. doi: 10.1016/j.scienta.2016.11.017. [DOI] [Google Scholar]
- Decker-Walters et al. (2004).Decker-Walters DS, Wilkins-Ellert M, Chung SM, Staub JE. COVER ARTICLE: discovery and genetic assessment of wild gottle gourd (Lagenaria Siceraria (Mol.) Standley; Cucurbitaceae) from zimbabwe. Economic Botany. 2004;58:501–508. doi: 10.1663/0013-0001(2004)058[0501:DAGAOW]2.0.CO;2. [DOI] [Google Scholar]
- Gachomo et al. (2014).Gachomo EW, Jimenez-Lopez JC, Baptiste LJ, Kotchoni SO. GIGANTUS1 (GTS1), a member of Transducin/WD40 protein superfamily, controls seed germination, growth and biomass accumulation through ribosome-biogenesis protein interactions in Arabidopsis thaliana. BMC Plant Biology. 2014;14:37. doi: 10.1186/1471-2229-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Jiang & Xia (2016).Guo H, Jiang L, Xia Q. Selection of reference genes for analysis of stress-responsive genes after challenge with viruses and temperature changes in the silkworm Bombyx mori. Molecular Genetics and Genomics. 2016;291:999–1004. doi: 10.1007/s00438-015-1125-4. [DOI] [PubMed] [Google Scholar]
- Heeju et al. (2015).Heeju L, Mikyeong K, Sanggyu L, Changsun C, Hongsoo C, Haeryun K, Gugseoun C, Changhoo C. Physiological characteristics of melon plants showing leaf yellowing symptoms caused by CABYV infection. Korean Journal of Horticultural Science and Technology. 2015;33(2):210–218. doi: 10.7235/hort.2015.14149. [DOI] [Google Scholar]
- Huggett et al. (2005).Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes and Immunity. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- Hwang & Sakakibara (2006).Hwang I, Sakakibara H. Cytokinin biosynthesis and perception. Physiologia Plantarum. 2006;126:528–538. doi: 10.1111/j.1399-3054.2006.00665.x. [DOI] [Google Scholar]
- Ingouff et al. (2010).Ingouff M, Rademacher S, Holec S, Šoljić L, Xin N, Readshaw A, Foo SH, Lahouze B, Sprunck S, Berger F. Zygotic resetting of the HISTONE 3 variant repertoire participates in epigenetic reprogramming in Arabidopsis. Current Biology. 2010;20:2137–2143. doi: 10.1016/j.cub.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Knopf (1998).Knopf CW. Evolution of viral DNA-dependent RNA polymerases. Virus Genes. 1998;16:47–58. doi: 10.1023/A:1007997609122. [DOI] [PubMed] [Google Scholar]
- Kong et al. (2016).Kong Q, Gao L, Cao L, Liu Y, Saba H, Huang Y, Bie Z. Assessment of suitable reference genes for quantitative gene expression studies in melon fruits. Frontiers in Plant Science. 2016;7:e70603. doi: 10.3389/fpls.2016.01178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong et al. (2015).Kong Q, Yuan J, Gao L, Zhao L, Cheng F, Huang Y, Bie Z. Evaluation of appropriate reference genes for gene expression normalization during watermelon fruit development. PLOS ONE. 2015;10:e0130865. doi: 10.1371/journal.pone.0130865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong et al. (2014b).Kong Q, Yuan J, Gao L, Zhao S, Jiang W, Huang Y, Bie Z. Identification of suitable reference genes for gene expression normalization in qRT-PCR analysis in watermelon. PLOS ONE. 2014b;9:e90612. doi: 10.1371/journal.pone.0090612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong et al. (2014a).Kong Q, Yuan J, Niu P, Xie J, Jiang W, Huang Y, Bie Z. Screening suitable reference genes for normalization in reverse transcription quantitative real-time PCR analysis in melon. PLOS ONE. 2014a;9:e87197. doi: 10.1371/journal.pone.0087197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo et al. (2016).Kudo T, Sasaki Y, Terashima S, Matsuda-Imai N, Takano T, Saito M, Kanno M, Ozaki S, Suwabe K, Suzuki G, Watanabe M, Matsuoka M, Takayama S, Yano K. Identification of reference genes for quantitative expression analysis using large-scale RNA-seq data of Arabidopsis thaliana and model crop plants. Genes & Genetic Systems. 2016;2:72–74. doi: 10.1266/ggs.15-00065. [DOI] [PubMed] [Google Scholar]
- Li et al. (2016).Li J, Zheng H, Zhang C, Han K, Wang S, Peng J, Lu Y, Zhao J, Xu P, Wu X, Li G, Chen J, Yan F. Different virus-derived siRNAs profiles between leaves and fruits in cucumber green mottle mosaic virus-infected Lagenaria siceraria plants. Frontiers in Microbiology. 2016;7 doi: 10.3389/fmicb.2016.01797. Article 1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2014).Li Q, Zhao P, Li J, Zhang C, Wang L, Ren Z. Genome-wide analysis of the WD-repeat protein family in cucumber and Arabidopsis. Molecular Genetics and Genomics. 2014;289:103–124. doi: 10.1007/s00438-013-0789-x. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2012).Liu D, Shi L, Han C, Yu J, Li D, Zhang Y. Validation of reference genes for gene expression studies in virus-infected Nicotiana benthamiana using quantitative real-time PCR. PLOS ONE. 2012;7:e46451. doi: 10.1371/journal.pone.0046451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2015).Liu H, Luo L, Liang C, Jiang N, Liu P, Li J. High-throughput sequencing identifies novel and conserved cucumber (Cucumis sativus L.) microRNAs in response to cucumber green mottle mosaic virus infection. PLOS ONE. 2015;10:e0129002. doi: 10.1371/journal.pone.0129002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2018).Liu X, Guan H, Song M, Fu Y, Han X, Lei M, Ren J, Guo B, He W, Wei Y. Reference gene selection for qRT-PCR assays in Stellera chamaejasme subjected to abiotic stresses and hormone treatments based on transcriptome datasets. PeerJ. 2018;6:e4535. doi: 10.7717/peerj.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcolino-Gomes et al. (2015).Marcolino-Gomes J, Rodrigues FA, Fuganti-Pagliarini R, Nakayama TJ, Reis RR, Farias JRB, Harmon FG, Molinari HBC, Molinari MDC, Nepomuceno A. Transcriptome-wide identification of reference genes for expression analysis of soybean responses to drought stress along the day. PLOS ONE. 2015;10:e0139051. doi: 10.1371/journal.pone.0139051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák et al. (2013).Novák J, Pavlů J, Novák O, Nožková-Hlaváčková V, Špundová M, Hlavinka J, Koukalová Š, Skalák J, ČernýBřetislav Brzobohatý M. High cytokinin levels induce a hypersensitive-like response in tobacco. Annals of Botany. 2013;112:41–55. doi: 10.1093/aob/mct092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl et al. (2004).Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-excel-based tool using pair-wise correlations. Biotechnology Letters. 2004;26:509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- Radonić et al. (2014).Radonić A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochemical and Biophysical Research Communications. 2014;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- Reguera et al. (2013).Reguera M, Peleg Z, Abdel-Tawab YM, Tumimbang EB, Delatorre CA, Blumwald E. Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiology. 2013;163:1609–1622. doi: 10.1104/pp.113.227702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano et al. (1997).Sano Y, Inoue H, Kajiwara K, Hiragi Y, Isoda S. Structural analysis of A-protein of cucumber green mottle mosaic virus and tobacco mosaic virus by synchrotron small-angle X-ray scattering. Journal of Protein Chemistry. 1997;16:151–159. doi: 10.1023/A:102639821. [DOI] [PubMed] [Google Scholar]
- Sestili et al. (2014).Sestili S, Sebastiani MS, Belisario A, Ficcadenti N. Reference gene selection for gene expression analysis in melon infected by Fusarium oxysporum, f.sp. melonis. Journal of Plant Biochemistry and Biotechnology. 2014;23:238–248. doi: 10.1007/s13562-013-0207-9. [DOI] [Google Scholar]
- Sharma, Goel & Chauhan (2016).Sharma D, Goel HC, Chauhan S. Radioprotective potential of Lagenaria siceraria extract against radiation induced gastrointestinal injury. Applied Physiology, Nutrition, and Metabolism. 2016;41:1248–1254. doi: 10.1139/apnm-2016-0136. [DOI] [PubMed] [Google Scholar]
- Slavokhotova et al. (2007).Slavokhotova AA, Andreeva EN, Shijan AN, Odintsova TI, Pukhalskij VA. Specifics of the coat protein gene in Russian strains of the cucumber green mottle mosaic virus. Russian Journal of Genetics. 2007;43:1221–1226. doi: 10.1134/S1022795407110038. [DOI] [PubMed] [Google Scholar]
- Smigocki et al. (1993).Smigocki A, Neal JW, McCanna I, Douglass L. Cytokinin-mediated insect resistance in Nicotiana plants transformed with the ipt gene. Plant Molecular Biology. 1993;23:325–335. doi: 10.1007/BF00029008. [DOI] [PubMed] [Google Scholar]
- Smith et al. (1999).Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: a common architecture for diverse functions. Trends in Biochemical Sciences. 1999;24:181–185. doi: 10.1016/S0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- Spalholz & Kubota (2017).Spalholz H, Kubota C. Rootstock affected in-and poststorage performance of grafted watermelon seedlings at low temperature. Horttechnology. 2017;27:93–98. doi: 10.21273/HORTTECH03577-16. [DOI] [Google Scholar]
- Sun, Niu & Fan (2017).Sun Y, Niu X, Fan M. Genome-wide identification of cucumber green mottle mosaic virus-responsive microRNAs in watermelon. Archives of Virology. 2017;162:2591–2602. doi: 10.1007/s00705-017-3401-6. [DOI] [PubMed] [Google Scholar]
- Tan et al. (2000).Tan SH, Nishiguchi M, Murata M, Motoyoshi F. The genome structure of kyuri green mottle mosaic tobamovirus and its comparison with that of cucumber green mottle mosaic tobamovirus. Archives of Virology. 2000;145:1067–1079. doi: 10.1007/s007050070. [DOI] [PubMed] [Google Scholar]
- Ugaki et al. (1991).Ugaki M, Tomiyama M, Kakutani T, Hidaka S, Kiguchi T, Nagata R, Sato T, Motoyoshi F, Nishiguchi M. The complete nucleotide sequence of cucumber green mottle mosaic virus (SH strain) genomic RNA. Journal of General Virology. 1991;72:1487–1495. doi: 10.1099/0022-1317-72-7-1487. [DOI] [PubMed] [Google Scholar]
- Van Nocker & Ludwig (2003).Van Nocker S, Ludwig P. The WD-repeat protein superfamily in Arabidopsis: conservation and divergence in structure and function. BMC Genomics. 2003;4:50. doi: 10.1186/1471-2164-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele et al. (2002).Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Boil. 2002;3:research0034.1-0034.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varveri, Vassilakos & Bem (2002).Varveri C, Vassilakos N, Bem F. Characterization and detection of cucumber green mottle mosaic virus, in Greece. Phytoparasitica. 2002;30:493–501. doi: 10.1007/BF02979754. [DOI] [Google Scholar]
- Vigneshwaran et al. (2016).Vigneshwaran V, Thirusangu P, Madhusudana S, Krishna V, Pramod SN, Prabhakar BT. The latex sap of the ‘Old World Plant’ Lagenaria siceraria with potent lectin activity mitigates neoplastic malignancy targeting neovasculature and cell death. International Immunopharmacology. 2016;39:158–171. doi: 10.1016/j.intimp.2016.07.024. [DOI] [PubMed] [Google Scholar]
- Wan et al. (2010).Wan H, Zhao Z, Qian C, Sui Y, Malik AA, Chen J. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Analytical Biochemistry. 2010;399:257–261. doi: 10.1016/j.ab.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang L, Li P, Wang Z, Liu J, Hu J, Li J. Identification and validation of suitable internal reference genes for SYBR-GREEN qRT-PCR studies during cucumber development. Journal of Horticultural Science & Biotechnology. 2014;89:312–320. doi: 10.1080/14620316.2014.11513085. [DOI] [Google Scholar]
- Wang et al. (2018).Wang Y, Xu P, Wu X, Wu X, Wang B, Huang Y, Hu Y, Lin J, Lu Z, Li G. GourdBase: a genome-centered multi-omics database for the bottle gourd (Lagenaria siceraria), an economically important cucurbit crop. Scientific Reports. 2018;8:3604. doi: 10.1038/s41598-018-22007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warzybok & Migocka (2013).Warzybok A, Migocka M. Reliable reference genes for normalization of gene expression in cucumber grown under different nitrogen nutrition. PLOS ONE. 2013;8:e72887. doi: 10.1371/journal.pone.0072887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wnendt et al. (1990).Wnendt S, Hartmann RK, Ulbrich N, Erdmann VA. Isolation and physical properties of the DNA-directed RNA polymerase from Thermus thermophilus HB8. FEBS Journal. 1990;191:467–472. doi: 10.1111/j.1432-1033.1990.tb19145.x. [DOI] [PubMed] [Google Scholar]
- Wollmann et al. (2017).Wollmann H, Stroud H, Yelagandula R, Tarutani Y, Jiang D, Jing L, Jamge B, Takeuchi H, Holec S, Nie X, Kakutani T, Jacobsen SE, Berger F. The histone H3 variant H3.3 regulates gene body DNA methylation in Arabidopsis thaliana. Genome Boil. 2017;18 doi: 10.1186/s13059-017-1221-3. Article 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2016).Wu W, Deng Q, Shi P, Yang J, Hu Z, Zhang M. Identification of appropriate reference genes for normalization of miRNA expression in grafted watermelon plants under different nutrient stresses. PLOS ONE. 2016;11:e0164725. doi: 10.1371/journal.pone.0164725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetisir & Sari (2013).Yetisir H, Sari N. Effect of different rootstock on plant growth yield and quality of watermelon. Australian Journal of Experimental Agriculture. 2013;43:1269–1274. doi: 10.1071/EA02095. [DOI] [Google Scholar]
- Zhang et al. (2014).Zhang Z, Jhaveri DJ, Marshall VM, Bauer DC, Edson J, Narayanan RK, Robinson GJ, Lundberg AE, Bartlett PF, Wray NR, Zhao QY. A comparative study of techniques for differential expression analysis on RNA-Seq data. PLOS ONE. 2014;9:e103207. doi: 10.1371/journal.pone.0103207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al. (2015).Zheng H, Xiao C, Han K, Peng J, Lin L, Lu Y, Xie L, Wu X, Xu P, Li G, Chen J, Yan F. Development of an agroinoculation system for full-length and GFP-tagged cDNA clones of cucumber green mottle mosaic virus. Archives of Virology. 2015;160:2867–2872. doi: 10.1007/s0070. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2012).Zhu Y, Jin Y, Wei S, Li H, Zhang W. Functional analysis of the isopentenyltransferase gene MdIPT3a from apple (Malus pumila Mill.) Journal of Horticultural Science & Biotechnology. 2012;87:478–484. doi: 10.1080/14620316.2012.11512898. [DOI] [Google Scholar]
- Žižková et al. (2015).Žižková E, Dobrev PI, Muhovski Y, Hošek P, Hoyerová K, Haisel D, Procházková D, Lutts S, Motyka V, Hichri I. Tomato (Solanum lycopersicum L) SlIPT3 and SlIPT4 isopentenyltransferases mediate salt stress response in tomato. BMC Plant Biology. 2015;15:85. doi: 10.1186/s12870-015-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The typical green mottle mosaic symptom on CGMMV-infected leaves 14 days after inoculation (right panel), while not symptom on mock leaves (left panel). (B) No obvious symptoms of virus on the fruit of CGMMV-infected bottle gourd (the bottom panel) and the mock fruits (the upper panel). (C) Detection of CGMMV (the upper panel), ZYMV (the middle panel) and WMV (the bottom panel) on L. siceraria by RT-PCR. (D) Detection of CGMMV by western blot. Photos by Chenhua Zhang.
(A) Dissolution curves of candidate RGs on both leaf and fruit of bottle gourd. (B) Dissolution curves of candidate RGs on leaf of bottle gourd. (C) Dissolution curves of candidate RGs on fruit of bottle gourd.
(A) Dissolution curves of candidate RGs on both leaf and fruit of bottle gourd. (B) Dissolution curves of candidate RGs on leaf of bottle gourd. (C) Dissolution curves of candidate RGs on fruit of bottle gourd.
(A) Dissolution curves of candidate RGs on both leaf and fruit of bottle gourd. (B) Dissolution curves of candidate RGs on leaf of bottle gourd. (C) Dissolution curves of candidate RGs on fruit of bottle gourd.
The upper line “C”, indicates the cDNA template, the bottom line “G”, indicates the gDNA template. “M” indicates the DNA ladder marker. (A) PCR amplification products of all RGs in L. siceraria leaf. (B) PCR amplification products of all RGs in L. siceraria fruit.
The RPKM > 40, RPKMmax/min < 2.0, and CV < 0.3, p < 0.05. All the selected internal RGs have only one transcript.
The RPKM > 40, RPKMmax/min < 2.0, and CV < 0.2, p < 0.05. All the selected internal RGs have only one transcript, and are ranked from small to large according to their RPKMmax/min values.
The RPKM > 4 0, RPKMmax/ min < 2.0, and CV < 0.2, p < 0.05. All the selected internal RGs have only one transcript.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.