Abstract
Background
MicroRNA-647 (miR-647) has been reported to repress cell tumorigenic phenotype, while the function of miR-647 in non-small cell lung cancer was obscure.
Methods
The effect of miR-647 and TRAF2 on A549 and H1299 cells was explored through Methyl thiazolyl tetrazolium (MTT) assay, colony formation and cell cycle assays. Luciferase reporter assays, reverse transcription quantitative PCR (RT-qPCR) and Western blot assay were carried out to determine that TRAF2 is directly regulated by miR-647. The effect of miR-647/TRAF2 axis on p65 protein level in nucleus or total was detected by Western blot assay.
Results
Here, we found that miR-647 was high expression in tumor that under argon-helium cryoablation treatment in contrast to the tumor under non argon-helium cryoablation treatment and inhibited cell proliferation of A549 and H1299 cells by inducing G1-S transition. TRAF2 was confirmed as a target of miR-647. TRAF2 overexpression partially rescued the suppressive function of miR-647 in A549 and H1299 cells. Moreover, we found that miR-647 repressed lung carcinogenesis by attenuating NF-κB pathway.
Conclusion
In all, our study demonstrates that miR-647 functions as tumor suppressor via targeting and down-regulating the expression of TRAF2 and NF-κB signaling pathway in non-small cell lung cancer.
Keywords: miRNA, miR-647, TRAF2, NF-κB, NSCLC, argon–helium cryoablation
Introduction
Lung cancer is the most commonly occurring cancer worldwide, with non-small cell lung cancer (NSCLC) representing the majority of cases. The prognosis of NSCLC remains poor, with only 5-year overall survival rate.1 There is thus an urgent need to elucidate the molecular pathogenesis of NSCLC and determine the optimal strategies for diagnosis and therapy. Argon–helium cryoablation is a very important, minimally invasive cryogenic technique, which produces better results than heat and freeze treatments alone,2,3 although the underlying mechanism by which it works is unclear.
MicroRNAs (miRNAs) are a class of small noncoding RNAs ranging from 21 to 24 nt in length. They regulate gene expression by degrading their target mRNAs or suppressing protein translation4 and have vital roles in cell proliferation, metastasis, apoptosis, and the cell cycle.5–7 Recent reports showed that miR-647 regulates cell tumorigenic phenotypes in gastric cancer, as well as inhibits cell invasion and metastasis,8 and suppresses cell proliferation by inducing cell cycle arrest and apoptosis.9 However, the role of miR-647 in NSCLC has been unclear.
Herein, we show that miR-647 is highly expressed in NSCLC tumors that have been subjected to argon–helium cryoablation treatment, in contrast to those that underwent other treatments. Moreover, miR-647 suppresses cell viability and proliferation of NSCLC cells by delaying G1/S phase transition. We also present evidence that TNF receptor-associated factor 2 (TRAF2) is the target of miR-647 and demonstrate that miR-647 regulates cell proliferation and the cell cycle of NSCLC cells partly through altering TRAF2 expression. Importantly, miR-647 represses the NF-κB signaling pathway that was promoted by TRAF2 in NSCLC. This study may provide important insights into the carcinogenesis of NSCLC under altered regulation of miRNA pathways.
Materials and methods
Cell culture and transfection
Lung cancer cell lines, A549 and H1299, were purchased from the American Type Culture Collection and were maintained in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum. The cells were cultured at 37°C in the presence of 5% CO2. All the transfection was performed with Lipofectamine™ 2000 transfection reagent (Thermo Fisher Scientific) following the manufacturer’s protocol.
Vector construction
The miR647 mimics and miR control were purchased from Thermo Fisher Scientific. The overexpression plasmid for TRAF2, and gene expression plasmids for the 3′ untranslated region (UTR) and mutant 3′ UTR of TRAF2 were purchased from Thermo Fisher Scientific.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR) assay
RNA was isolated using the mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA) following the manufacturer’s protocol. RNA was reverse-transcribed into cDNA with the following primers: miR-647-RT: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACGAA GGA-3′; U6-RT: 5′-GTCGTATCCAGTGCAGGGTC CGAGGTGCACTGGATACGACA AAATATGG-3′. The following primers were used for the PCR: miR-647-forward: 5′-TGCGGGTGGCTGCACTCACT-3′; miR-647-reverse: 5′-CCAGTGCAGGGTCCGAGGT-3′; U6-forward: 5′-TGCGGGTGCTCGCTTCGGCAGC-3′; U6-reverse: 5′-CCAGTGCAGGGTCCGAGGT-3′; TRAF2-forward: 5′-CTGCCCTTGCTGTCCTGT-3′; TRAF2-reverse: 5′-CCCTCGTTTCTGCCTGTC-3′; β-actin-forward: 5′-CTACGTCGCCCTGGACTTCGAGC-3′; β-actin-reverse: 5′-GATGGAGCCGCCGATCCACACGG-3′. The detailed experimental protocol, including the Western blotting protocol used to detect proteins, was previously described by Zhang et al.10 Anti-TRAF2, anti-p65, and anti-GAPDH were from Abcam (Cambridge, UK).
Luciferase reporter assay
A549 and H1299 cells were seeded in a 48-well plate and transiently cotransfected with Renilla luciferase (pRL-TK) and miR-647 mimics, miR-control, pLuc-TRAF2-3′ UTR1, pLuc-TRAF2-3′ UTR1-mutant, pLuc-TRAF2-3′ UTR2, and pLuc-TRAF2-3′ UTR2-mutant. Then, 48 h posttransfection, fluorescence detection was carried out using a luciferase assay system (Promega Corporation, Fitchburg, WI, USA) according to the manufacturer’s protocols.
MTT and colony formation assays
The MTT and colony formation assays were performed as described by Zhao et al.11
Cell cycle analysis by flow cytometry
A549 and H1299 cells were seeded in six-well plates and incubated for 72 h in complete RPMI-1640 medium. In this study, cell cycle analysis was performed as described by Zhang et al.10
Clinical NSCLC specimens
Clinical specimens consisted of 20 tumors that had undergone argon–helium cryoablation treatment and were less than 5 cm in diameter (the median diameter before treatment was 3.5 cm), and 20 tumors that had undergone non-argon–helium cryoablation treatment and were also less than 5 cm in diameter (the median diameter before treatment was 3.3 cm); they were collected from Tianjin University of Traditional Chinese Medicine (TCM) Affiliated Wuqing Hospital of TCM. The patients in the argon–helium cryosurgery group had either declined to undergo surgery or surgery had not been clinically indicated. The non-argon–helium cryoablation treatment involved surgery. This research was approved by the ethics committee of Tianjin University of TCM Affiliated Wuqing Hospital of TCM, and all patients provided written informed consent for the use of their tissue in medical research.
Statistical analyses
All statistical analyses were performed at least three times in independent experiments and analyzed using two-tailed unpaired Student’s t-tests. P≤0.05 was considered to be statistically significant.
Results
miR-647 was upregulated in lung cancer after argon–helium cryoablation treatment
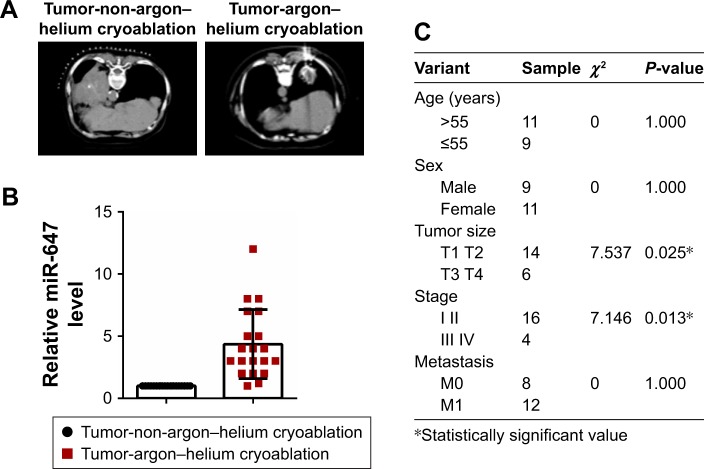
To determine the expression of miR-647, we examined 20 tumors that had undergone argon–helium cryoablation treatment and 20 tumors that had undergone non-argon–helium cryoablation treatment using qRT-PCR. The results showed that tumors in the argon–helium cryoablation treatment group were smaller than those in the non-argon–helium cryoablation group (Figure 1A). As shown in Figure 1B, the expression of miR-647 was higher in the argon–helium cryoablation treatment group than in the non-argon–helium cryoablation group. We also analyzed the correlation between miR-647 levels and clinicopathological characteristics of patients in the argon–helium cryoablation treatment group and found that high expression of miR-647 was associated with lower tumor stage (Figure 1C). Overall, these data showed that argon–helium cryoablation treatment decreased the size of tumors and that miR-647 expression was related to the clinicopathological characteristic of patients undergoing argon–helium cryoablation treatment.
Figure 1.
miR-647 was upregulated in lung cancer after argon–helium cryoablation treatment.
Notes: (A) Computed tomography scans of tumor that had undergone argon–helium cryoablation treatment (left) and tumor that had undergone non-argon–helium cryoablation treatment (right). (B) The expression of miR-647 was determined by qRT-PCR in 20 lung cancer specimens after argon–helium cryoablation treatment or non-argon-helium cryoablation treatment. (C) Correlation between miR-647 and clinicopathological characteristics of patients undergoing argon–helium cryoablation treatment.
Abbreviations: miR-647, microRNA-647; qRT-PCR, quantitative real-time polymerase chain reaction.
miR-647 inhibited cell proliferation and the cell cycle in NSCLC cells
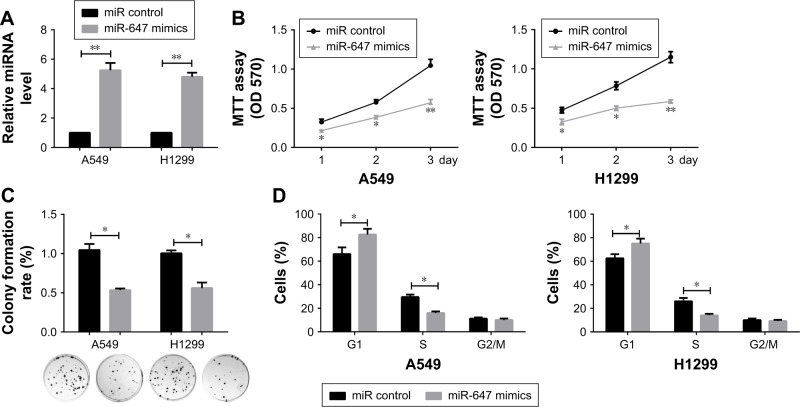
To investigate the effects of miR-647 on NSCLC cells, an RT-PCR assay was carried out to verify the confidence of the miR-647 mimics in A549 and H1299 cells (Figure 2A). MTT and colony formation assays were also performed to determine the role of miR-647 in the proliferation of A549 and H1299 cells. The results indicated that miR-647 mimics could inhibit cell viability (Figure 2B) and colony formation activity, in comparison with the control vector (Figure 2C). In order to investigate whether miR-647 inhibited NSCLC cell proliferation via altering cell cycle progression, cell cycle assays were performed using flow cytometry analysis. The results indicated that miR-647 mimics led to an increase in the number of cells in G1 phase and a decrease in those in S phase (Figure 2D). Overall, these data demonstrated that miR-647 inhibited proliferation of NSCLC cells through inducing G1-S transition.
Figure 2.
miR-647 inhibited cell proliferation and cell cycle in NSCLC cells.
Notes: (A) The confidence of miR-647 mimics in A549 and H1299 cells was confirmed by qRT-PCR. Proliferation of A549 and H1299 cells was determined by MTT assay (B) and colony formation assay (C), Representative graphs of the colony formation are presented. (D) Cell cycle assays were carried out in A549 and H1299 cells using flow cytometry. *P<0.05; **P<0.01.
Abbreviations: miR-647, microRNA-647; NSCLC, non-small cell lung cancer; qRT-PCR, quantitative real-time polymerase chain reaction.
TRAF2 was targeted by miR-647
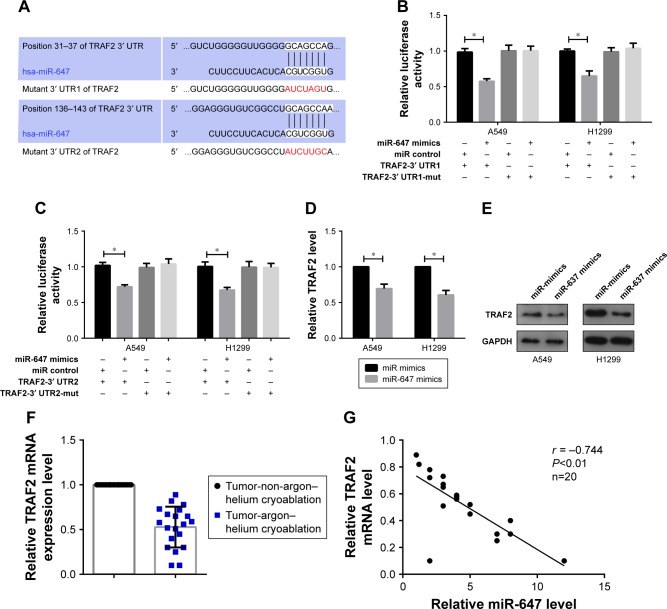
To explore which target genes mediate the effect of miR-647 on NSCLC cells, we used bioinformatics analyses to predict candidate targets. Based on the functions of candidate targets, TRAF2 was chosen for further study. Bioinformatics analysis revealed two binding sites for miR-647 in the 3′ UTR of TRAF2; therefore, we constructed two luciferase reporter plasmids, one containing the wild-type 3′ UTR of TRAF2 and the other containing a 3′ UTR mutant (Figure 3A). The luciferase reporter assay showed that miR-647 mimics decreased the luciferase fluorescence intensity of the wild-type 3′ UTR-TRAF2, but not that of the mutant 3′ UTRs-TRAF2, compared with the control group that was transfected with miR control (Figure 3B and C). Moreover, we observed using qRT-PCR and Western blotting that TRAF2 mRNA and protein levels were markedly decreased by miR-647 in NSCLC cells (Figure 3D and E). Having demonstrated that miR-647 was highly expressed in the argon–helium cryoablation treatment group compared with the non-argon–helium cryoablation treatment group, we also detected the expression of TRAF2 in order to analyze the relationship between miR-647 and TRAF2. The results indicated that TRAF2 was downregulated in the argon–helium cryoablation treatment group in comparison with the non-argon–helium cryoablation treatment group (Figure 3F). Furthermore, as shown in Figure 3G, there was a negative correlation between miR-647 and TRAF2 mRNA in the argon–helium cryoablation treatment group. These results indicated that TRAF2 was a direct target of miR-647.
Figure 3.
TRAF2 was targeted by miR-647.
Notes: (A) Two potential miR-647-binding sites in the TRAF2 3′ UTR were predicted using TargetScan 7.1; the mutant nucleotides are shown. A549 and H1299 cells were cotransfected with miR-647 mimics or the control vector with TRAF2-3′ UTR1, TRAF2-3′ UTR1-mut (B) or TRAF2-3′ UTR2, TRAF2-3′ UTR2-mut (C) for the Luciferase reporter assay. (D and E) A549 and H1299 cells were transfected with miR-647 mimics or the control vector to determine the mRNA and protein expression levels of TRAF2 by qRT-PCR and Western blotting. (F) The expression of TRAF2 was determined by qRT-PCR in 20 lung cancer tissue specimens that had undergone argon–helium cryoablation treatment or non-argon–helium cryoablation treatment. (G) Pearson’s correlation analysis indicated a negative correlation between the expression of miR-647 and TRAF2 (r = −0.744, P<0.01) *P<0.05.
Abbreviations: miR-647, microRNA-647; RT-PCR, quantitative real-time polymerase chain reaction; TRAF2, TNF receptor-associated factor 2; UTR, untranslated region.
The miR-647/TRAF2 axis regulated the tumorigenic phenotype of NSCLC cells
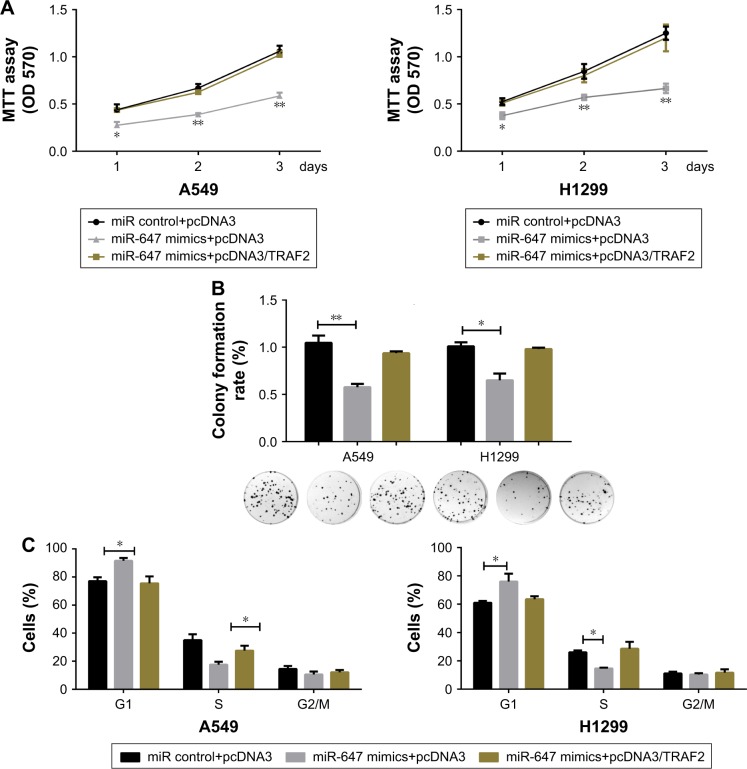
To further confirm that the effects of miR-647 on cell proliferation and cell cycle progression in NSCLC cells were mediated by TRAF2, several rescue experiments were performed. Functional rescue experiments showed that miR-647-mediated repression of cell viability and colony formation ability in NSCLC cells was counteracted by ectopic expression of TRAF2 (Figure 4A and B). Moreover, as shown in Figure 4C, TRAF2 overexpression reestablished G1-S transition repressed by miR-647. These findings implicated TRAF2 in the effects of miR-647 on cell tumorigenic characteristics in NSCLC cells.
Figure 4.
miR-647/TRAF2 axis regulated the tumorigenic phenotype of NSCLC cells.
Notes: A549 and H1299 cells were cotransfected with miR-647 mimics and pcDNA3/TRAF2 or the control vector. The transfected cells were examined using CCK8 assays to determine the cell tumorigenic phenotype (A and B), colony formation assays (C), and cell cycle assays. *P<0.05; **P<0.01.
Abbreviations: miR-647, microRNA-647; NSCLC, non-small cell lung cancer; TRAF2, TNF receptor-associated factor 2.
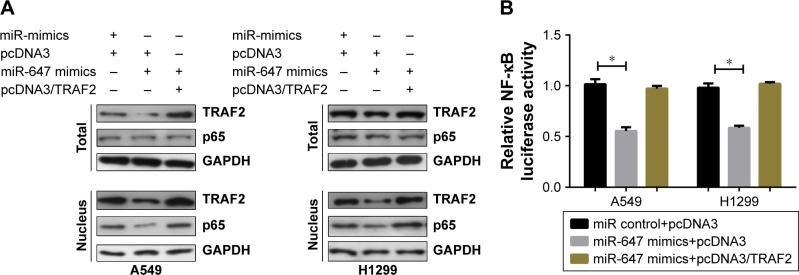
miR-647 inhibited the TRAF2-mediated NF-κB signaling pathway
Previous reports have suggested that TRAF2 plays a carcinogenic part in lung tumorigenesis through activating the NF-κB pathway.12,13 In order to determine whether miR-647 could activate the NF-κB signaling pathway, we detected the localization of p65 protein in A549 and H1299 cells using Western blotting. As shown in Figure 5A, miR-647 mimics decreased the level of the activated nuclear form p-p65 in comparison with the control vector; furthermore, the ectopic expression of TRAF2 restored the inhibitory function p65. Moreover, luciferase reporter assays demonstrated that TRAF2 partly neutralized the inhibitory effect of miR-647 on NF-κB transcriptional activity in A549 and H1299 cells (Figure 5B). These data demonstrated that miR-647 inhibited the TRAF2-mediated NF-κB signaling pathway.
Figure 5.
miR-647 inhibited the TRAF2-mediated NF-κB signaling pathway.
Notes: A549 and H1299 cells were cotransfected with miR-647 mimics and pcDNA3/TRAF2 or the control vector. Western blot analysis of TRAF2 and p65 protein levels in whole cell lysate and nucleus (A). Luciferase reporter assays were performed to analyze NF-κB activity (B). *P<0.05.
Abbreviations: miR-647, microRNA-647; NSCLC, non-small cell lung cancer; TRAF2, TNF receptor-associated factor 2.
Discussion
Aberrant expression of miRNAs influences tumorigenic phenotypes, including cell proliferation, the cell cycle, migration, and invasion, thereby accelerating the development of cancer. In NSCLC, aberrant expression of miRNAs is a key regulatory factor underlying carcinogenicity, with miR-204,14 miR-129b,15 miR-1298,16 miR-107,17 miR-342-3p,18 and miR-37319 all having been implicated in previous reports. Here, we demonstrated that miR-647 was highly expressed in tumors that had undergone argon–helium cryoablation treatment, compared with those that had undergone non-argon–helium cryoablation. We speculated that argon–helium cryoablation might induce a decrease or increase of transcription factors, thus resulting in the upregulation of miR-647. However, this requires further validation. miR-647 suppressed proliferation of NSCLC cells by delaying G1/S phase transition. Based on these findings, we investigated the molecular mechanism underlying the role of miR-647 in NSCLC cells.
Generally, miRNAs perform their cellular functions through downregulating the expression of their target genes.20 In this study, we used TargetScan (Prediction of icroRNA targets; http://www.targetscan.org/vert_72/) to predict and identify TRAF2 as a new target gene of miR-647. Furthermore, we showed that the luciferase fluorescence intensity of the 3′ UTR1 and 3′ UTR2 of the TRAF2 reporter vector were decreased by miR-647. In addition, we showed that miR-647 reduced endogenous mRNA and protein levels of TRAF2. These findings demonstrate that TRAF2 is a direct target of miR-647.
TRAF2 belongs to the family of the TRAF proteins. It was first identified as a component of TNF receptor-2 (TNFR2) and then TNFR1 signaling complexes.21,22 Recent studies have shown that TRAF2 is associated with several important signaling pathways in cancer. The TNF/TRAF2/ASK1/p38 kinase pathway may contain useful biomarkers to identify lung cancer patients at high risk.23 TRAF2 regulates mTORC2 signaling via interaction with OTUD7B.24 It is also able to modulate two kinase cascades that lead to the activation of JNK and NF-κB to regulate cell biological processes. We found that TRAF2 mediated the inhibition of cell proliferation and cell cycle arrest by miR-647 in A549 and H1299 cells. TRAF2 mediates multiple downstream signaling pathways of TNFR1 and TNFR2, including the activation of NF-κB pathways.25,26 Moreover, we showed using Western blotting that miR-647 decreased the protein levels of endonuclear p65 in A549 and H1299 cells, indicating that miR-647 inhibits the TRAF2-mediated NF-κB signaling pathway.
Conclusion
Our findings indicated that argon–helium cryoablation treatment can decrease the size of tumors and that miR-647 is highly expressed in tumors after argon–helium cryoablation treatment. Moreover, miR-647 targets the two 3′ UTRs of TRAF2 and downregulates its expression. TRAF2 mediates the inhibition of miR-647 in cell proliferation and the cell cycle in NSCLC cells. These findings may provide insight into the progression of NSCLC and help to drive the development of new techniques for diagnosis and therapy.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Hu KW, Li QW, Zuo MH, Sun T, Jiang M. Clinical observation on the combined treatment of 57 cases of non-small cell lung cancer using argon-helium cryosurgery and Chinese herbal medicine. Chin J Integr Med. 2007;13(3):224–227. doi: 10.1007/s11655-007-0224-4. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Guo Z, Wang HT, Liu F, Ni H. Clinical study of cryoablation in the salvage treatment of stage III non-small cell lung cancer. Zhonghua Yi Xue Za Zhi. 2011;91(31):2205–2207. Chinese. [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Shirafkan N, Mansoori B, Mohammadi A, Shomali N, Ghasbi M, Baradaran B. MicroRNAs as novel biomarkers for colorectal cancer: new outlooks. Biomed Pharmacother. 2018;97:1319–1330. doi: 10.1016/j.biopha.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Castro D, Moreira M, Gouveia AM, Pozza DH, De Mello RA. MicroRNAs in lung cancer. Oncotarget. 2017;8(46):81679–81685. doi: 10.18632/oncotarget.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynam-Lennon N, Maher SG, Reynolds JV. The roles of microRNA in cancer and apoptosis. Biol Rev Camb Philos Soc. 2009;84(1):55–71. doi: 10.1111/j.1469-185X.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 8.Ye G, Huang K, Yu J, et al. MicroRNA-647 targets SRF-MYH9 axis to suppress invasion and metastasis of gastric cancer. Theranostics. 2017;7(13):3338–3353. doi: 10.7150/thno.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao W, Wei W, Zhan Z, Xie D, Xie Y, Xiao Q. Role of miR-647 in human gastric cancer suppression. Oncol Rep. 2017;37(3):1401–1411. doi: 10.3892/or.2017.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Dai J, Deng H, et al. miR-1228 promotes the proliferation and metastasis of hepatoma cells through a p53 forward feedback loop. Br J Cancer. 2015;112(2):365–374. doi: 10.1038/bjc.2014.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao JL, Zhang L, Guo X, et al. miR-212/132 downregulates SMAD2 expression to suppress the G1/S phase transition of the cell cycle and the epithelial to mesenchymal transition in cervical cancer cells. IUBMB Life. 2015;67(5):380–394. doi: 10.1002/iub.1381. [DOI] [PubMed] [Google Scholar]
- 12.Zheng M, Morgan-Lappe SE, Yang J, et al. Growth inhibition and radio-sensitization of glioblastoma and lung cancer cells by small interfering RNA silencing of tumor necrosis factor receptor-associated factor 2. Cancer Res. 2008;68(18):7570–7578. doi: 10.1158/0008-5472.CAN-08-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borghi A, Verstrepen L, Beyaert R. TRAF2 multitasking in TNF receptor-induced signaling to NF-kappaB, MAP kinases and cell death. Biochem Pharmacol. 2016;116:1–10. doi: 10.1016/j.bcp.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Lv HY, Zhou DM, Zhang EN. miR-204 suppresses non-small-cell lung carcinoma (NSCLC) invasion and migration by targeting JAK2. Genet Mol Res. 2016;15(2) doi: 10.4238/gmr.15026415. [DOI] [PubMed] [Google Scholar]
- 15.Zheng L, Qi YX, Liu S, Shi ML, Yang WP. miR-129b suppresses cell proliferation in the human lung cancer cell lines A549 and H1299. Genet Mol Res. 2016;15(4) doi: 10.4238/gmr15048367. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Dang J, Chang KY, et al. miR-1298 inhibits mutant KRAS-driven tumor growth by repressing FAK and LAMB3. Cancer Res. 2016;76(19):5777–5787. doi: 10.1158/0008-5472.CAN-15-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia H, Li Y, Lv X. MicroRNA-107 inhibits tumor growth and metastasis by targeting the BDNF-mediated PI3K/AKT pathway in human non-small lung cancer. Int J Oncol. 2016;49(4):1325–1333. doi: 10.3892/ijo.2016.3628. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Xue X, Fei X, Hou W, Zhang Y, Liu L, Hu R. miR-342-3p suppresses cell proliferation and migration by targeting AGR2 in non-small cell lung cancer. Cancer Lett. 2018;412:170–178. doi: 10.1016/j.canlet.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Qu J, Zhou L, Liao F, Wang J. MicroRNA-373 inhibits cell proliferation and invasion via targeting BRF2 in human non-small cell lung cancer A549 cell line. Cancer Res Treat. 2017 Oct 12; doi: 10.4143/crt.2017.302. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothe M, Wong SC, Henzel WJ, Goeddel DV. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78(4):681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 22.Shu HB, Takeuchi M, Goeddel DV. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc Natl Acad Sci U S A. 1996;93(24):13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildebrandt MA, Roth JA, Vaporciyan AA, et al. Genetic variation in the TNF/TRAF2/ASK1/p38 kinase signaling pathway as markers for postoperative pulmonary complications in lung cancer patients. Sci Rep. 2015;5:12068. doi: 10.1038/srep12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B, Jie Z, Joo D, et al. TRAF2 and OTUD7B govern a ubiquitin-dependent switch that regulates mTORC2 signalling. Nature. 2017;545(7654):365–369. doi: 10.1038/nature22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 26.Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269(5229):1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]