Abstract
Background
In nanomedicine, gold nanoparticles (AuNPs) have demonstrated versatile therapeutic efficiencies and, in particular, have been developed for the treatment of various cancers due to their high selectivity in killing cancer, not healthy, cells.
Methods
In this study, AuNPs were conjugated with the cell-penetrating peptide Cys-(Arg)8-Asp-Ser (CRRRRRRRRGDS) by direct cross-linking of the cysteine’s thiol group to the gold surface and a fibronectin-derived RGD group was also used due to its efficacy toward cancer cell targeting and possible promotion of healthy fibroblast functions.
Results
Ultraviolet–visible absorbance spectrum and transmission electron microscope images of the synthesized peptide-capped AuNPs (PEP-AuNPs) validated the formation of AuNP aggregates. The presence of peptides on AuNPs was confirmed by Fourier transform infrared spectroscopy and quantified by a bicinchoninic acid assay. After being modified with the arginine-rich peptide, the AuNPs possessed a positive charge, as their zeta potential increased from −23.81±8.43 mV to 8 mV on average. In this manner, an easy method to conjugate AuNPs was shown here. Further, MTS assays were performed using healthy human dermal fibroblasts. After 24 hours of treatment with PEP-AuNPs, the cell density increased dramatically to around 25,000 cells/cm2. Results further showed a very high half-maximal inhibitory concentration of 69.2 µM for the PEP-AuNPs (indicating low toxicity).
Conclusion
The results showed for the first time the ability of PEP-AuNPs to promote human dermal fibroblast cell viability, which after further investigation, may show an ability to replace cancerous tissue with healthy soft tissue.
Keywords: gold nanoparticles, cell-penetrating peptide, cysteine, fibroblast
Introduction
In nanomedicine, gold nanoparticles (AuNPs) have provided many solutions for the treatment of a variety of diseases, particularly cancer.1 Currently, chemotherapy is the most common treatment for cancer. However, delivering sufficient concentrations of therapeutics to cancer cells without producing off-target side effects against healthy cells remains a problem. One of the options to resolve this problem is to engineer new therapies that are more cytocompatible with noncancerous cells and are able to directly target cancer cells. In this way, less of the therapeutic is needed and the side effects toward off-target cells are minimized. Due to their unique physicochemical properties and their ability to bind amine or thiol groups as well as their plasmon properties, AuNP functionalization has been the object of intense research for cancer diagnostics and therapies. AuNPs have previously been used in thermal therapies or as radiosensitizers for anticancer treatments,2 and, with the appropriate design, conjugated AuNPs are also able to target specific cells for use in drug delivery.3 Such technologies are solving crucial issues in cancer treatments by increasing antitumor efficiency with fewer side effects. Other studies, like those by Li et al,4 have already demonstrated the capacity of AuNPs to enhance the anticancer effects of drugs, when they are conjugated to AuNPs, compared to ordinary methods, when the therapeutics are not cross-linked to AuNPs. However, while AuNPs have been widely studied for anticancer effects, little attention has been paid to modifying them to regenerate healthy tissue after the tumor is destroyed. Faced with those encouraging results and future challenges, various combinations of functionalized AuNPs must be tested to find new potential treatments.
The beneficial properties of AuNPs largely rely on their conjugation. This study focused on the conjugation of a specific peptide, the CRRRRRRRRGDS peptide, whose physical properties make it an ideal peptide for cancer therapeutics. First, this peptide is a rich arginine peptide, which makes it a cell-penetrating peptide (CPP), and has the capacity to penetrate cells through endocytosis.5 To allow for endocytosis, CPPs are very hydrophilic, which allow them to bind the surface receptors of the cell membrane, leading to internalization. Second, the terminal Arg–Gly–Asp–Ser (RGDS) sequence is derived from fibronectin,6 a widely distributed protein in the extracellular matrix of cells. Of its many functions, fibronectin supports cellular growth. Because of this, cells can recognize the RGD groups and form a localized docking adhesion point on the extracellular matrix. Cancer cells, which possess overexpressed RGD receptors,7 can therefore be highly targeted by this CPP. Several previous studies have already used this RGD peptide and observed this phenomenon of discrimination between normal and cancer cells.8 But the beneficial effects, or not, of using the RGD peptide linked to a CPP is something that this study wanted to explore. Further, nanoparticles functionalized with such groups may promote the functions of cells (such as fibroblasts) to regenerate tissue after killing cancer cells.
In the present report, the aim was to study the assembly of these two promising technologies, AuNPs and the CRRRRRRRRGDS peptide as a first step for treating cancer cells. For the first time, we successfully synthesized AuNPs conjugated with peptides which not only demonstrated limited toxicity toward human dermal fibroblasts (HDFs), it actually promoted their function, indicating that they should be further studied for the regeneration of healthy tissue in areas where cancer cells once were. For that purpose, synthesis was performed using a cysteine amino acid in the peptide sequence, a well-known method used to obtain peptide-capped AuNPs (PEP-AuNPs).9 The cysteine’s thiol group can therefore directly cross-link the gold surface. The different results of the study have highlighted the successful synthesis of conjugated AuNPs with cell-penetrating peptides, and the cytocompatible nature of those PEP-AuNPs on HDF cells.
Materials and methods
Materials
CRRRRRRRRGDS peptides were purchased from Biomatik (Cambridge, ON, Canada). All other reagents were purchased from Sigma-Aldrich (St Louis, MO, USA) and were used as received.
Synthesis of PEP-AuNPs
AuNPs with a 20 nm diameter from Sigma-Aldrich were used in this work. Nanoparticles were modified using the thiolterminated peptide CRRRRRRRRGDS. In a typical reaction, 4 mL of AuNPs in a citrate buffer (pH =6) or in phosphate-buffered saline (pH =7.5) (7.2×1011 particles/mL) were degassed for 30 minutes. Then, 4 mL of a solution of peptides was prepared at the desired concentration in deionized water, before being degassed. The AuNPs were added to the peptide solution. This solution was then stirred for 16 hours at room temperature. To remove the unreacted peptides from the solution, AuNPs were centrifuged at 11,000 rpm for 30 minutes in glass tubes, the supernatant was decanted, and the particles were resuspended in distilled water. This process was repeated three times. PEP-AuNPs collected from this process were suspended in 4 mL of deionized water, sterilized by passing through a 0.22 µm syringe filter (Corning Inc., Corning, NY, USA), and stored in the dark at 4°C. Different pH values and peptide concentrations were tested to find the optimal conditions to obtain stable PEP-AuNPs.
Determination of the peptide concentration by the BCA assay
To determine the concentration of peptides attached to the gold surface, the Thermo Scientific™ Pierce™ BCA Protein Assay (Waltham, MA, USA) was used. A standard curve between 5 and 2,000 µg/mL was created following the manufacturer’s instructions in a 96-well plate with three replicates per sample. The plate was incubated at 37°C for 30 minutes and absorbance was read at 562 nm.
Physicochemical characterization of PEP-AuNPs
Transmission Electron Microscopy (TEM)
TEM images were acquired using a JEM 1010, JEOL electron microscope (JEOL, Tokyo, Japan) with an 80 keV acceleration voltage to confirm particle size and microstructure. Samples were prepared for TEM imaging by adding 5 µL of the nanoparticle suspension on a copper-coated TEM grid and then were dried at room temperature.
Dynamic lighting scattering (DLS) and zeta potential
The mean hydrodynamic diameter and zeta potential were estimated using a DLS analyzer (90Plus Zeta, Brookhaven Instruments Corporation, Holtsville, NY, USA) and the software provided by the manufacturer. For this purpose, 2 mL of each sample was measured in a disposable cuvette. The samples were analyzed three times at 25°C.
Fourier transform infrared (FTIR) spectroscopy
Infrared spectra analysis of AuNPs and PEP-AuNPs was performed using a Perkin Elmer Spectrum 100 FT-IR Spectrometer (Perkin Elmer, Waltham, MA, USA). Samples were deposited onto PTFE IR sample cards (International Crystal Laboratories, Garfield, NJ, USA) and dried under vacuum overnight. All spectra were recorded at a resolution of 1 cm−1 over a wavenumber range of 400–4,000 cm−1.
Ultraviolet-visible spectroscopy
Ultraviolet–visible absorbance spectra of AuNPs and PEP-AuNPs were acquired using a Molecular Devices SpectraMax M3 UV spectrometer (Molecular Devices, San Jose, CA, USA). Samples were deposited in a 96-well plate at a volume of 100 µL. All spectra were recorded at a resolution of 1 nm over a wavelength range of 400–750 nm.
Fibroblast growth
Cell Culture
HDFs (Detroit 551 – CCL-110) from the American Type Culture Collection (Manassas, VA, USA) were used to determine mammalian cell response to treatments with PEP-AuNP via an MTS assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI, USA). Cells were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin; all these reagents were also purchased from the American Type Culture Collection. The cultures were maintained in an incubator at 37°C with a humidified atmosphere of 5% CO2 until 80% confluency was achieved. Then, cells were enzymatically detached from the surface of the T-75 flask using 0.5% trypsin and collected by centrifugation at 1,400 rpm for 5 minutes.
Cell viability assay
For testing, cells were first seeded on a 96-well plate at a density of 5×104 cells/mL and allowed to adhere in an incubator at 37°C and 5% CO2 for 24 hours. After incubation, the culture medium was replaced with 100 µL of the PEP-AuNPs suspension at different concentrations in fresh media, 10 µL of PEP-AuNP samples at the desired concentration, and 90 µL of media. Control wells without nanoparticles received only 100 µL of medium. The cells were then incubated for 24 hours. Viability was determined by replacing media and nanoparticle suspensions with an MTS reagent (cell culture media: MTS reagent at 5:1 v/v ratio) followed by incubation for 3 hours at 37°C and 5% CO2. The OD was measured at 490 nm and the cell density of each sample was calculated with a calibration curve plotted by OD against known cell numbers.
Statistical analysis
The results were statistically analyzed using Student’s t-tests. Differences in the results were considered statistically significant when P<0.05 and highly significant when P<0.01.
Results and discussion
Characterization of the AuNPs
The shape and size of the AuNPs was confirmed by TEM analysis as spheres 19.0±3.0 nm in diameter. A zeta potential of −23.81±8.43 mV for the AuNPs stabilized in citrate buffer was validated by DLS measurements.
Direct cross-linking of PEP peptides to the AuNPs
Conjugation of PEP peptides to AuNPs was carried out at different pH values and cysteine-peptide concentrations to optimize the PEP-AuNP formation. To determine the PEP-AuNP formation, ultraviolet–visible absorbance was used. At a pH of 7.5, AuNPs were stabilized in phosphate-buffered saline, which demonstrated a decrease in absorbance intensity with increasing concentrations of peptide solution (Figure 1), leading to a completely colorless solution of AuNPs. This phenomenon could be related with the formation of PEP-AuNPs aggregates, affecting the plasmon band. Those aggregates prevent all medical uses of the AuNPs as it is impossible to purify them by centrifugation.
Figure 1.
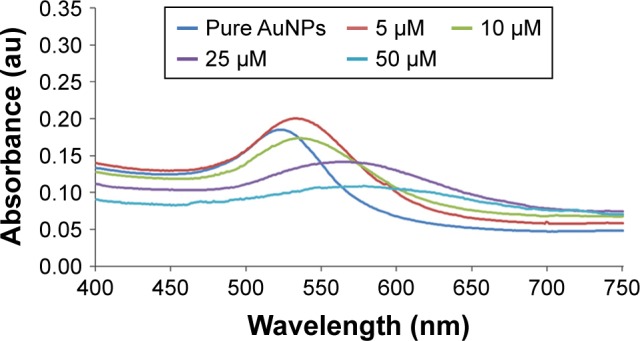
Ultraviolet spectra of PEP-AuNPs at different concentrations of initial peptide solutions compared to pure AuNPs in PBS.
Abbreviations: AuNP, gold nanoparticle; PEP-AuNPs, peptide-capped AuNPs.
At a pH of 6, AuNPs were stabilized in a citrate buffer, which increased the stability of the reaction. Initially, pure AuNPs in citrate presented only one plasmon resonance band with a λmax of 523 nm (Figure 2). However, the presence of the cysteine-peptide enlarged the peak and offset it to the left. These observations suggest the formation of aggregates of AuNPs, which increased in number and size when the peptide concentration was increased. This can be explained by the intermolecular association of peptide chains. Majzik et al10 observed the same phenomenon and justified this observation by electrostatic interactions of the negatively charged carboxylate acids and positively charged amino groups. Nevertheless, the aggregates were less large than those observed at a pH of 7.5 and purification of the AuNPs was possible. Based on these facts, the AuNPs stabilized in citrate buffer demonstrated a better capacity to be conjugated by cysteine-peptides. No stable PEP-AuNPs were obtained from the AuNPs in PBS.
Figure 2.
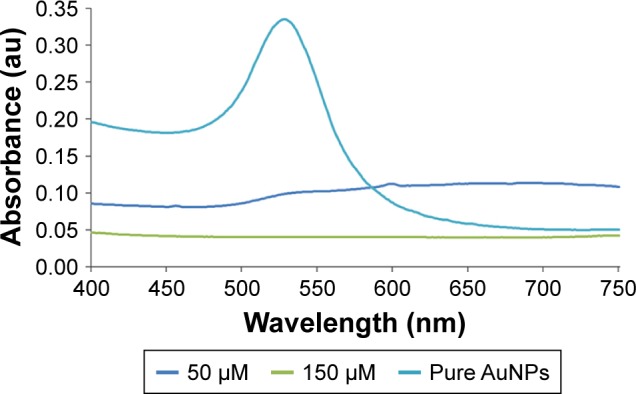
Ultraviolet spectra of PEP-AuNPs at different concentrations of initial peptide solutions compared to pure AuNPs in citrate buffer.
Abbreviations: AuNP, gold nanoparticle; PEP-AuNP, peptide-capped AuNP.
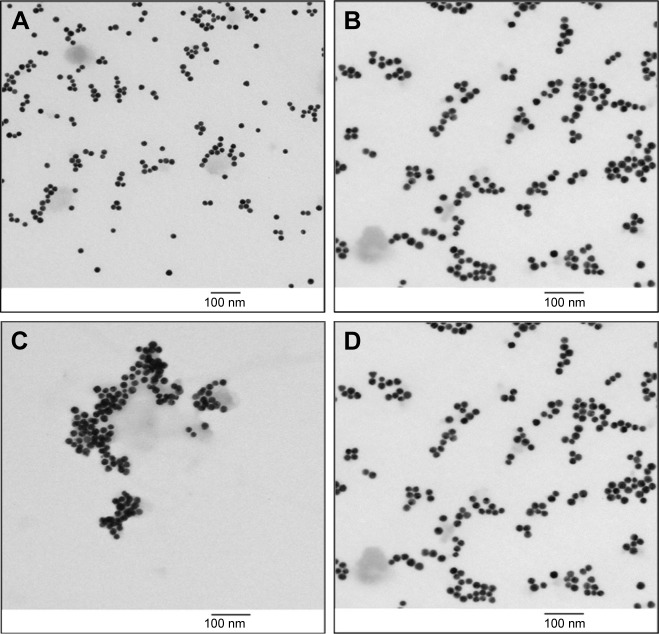
TEM images of the PEP-AuNPs confirmed the formation of aggregates (Figure 3A and B) and showed the impact of the cysteine-peptide on an initially stable suspension of AuNPs in citrate buffer. To overcome AuNP aggregation, which clearly can be an obstacle for cell penetration, sonication was used to reduce the number and size of aggregates. This method showed a significant result (Figure 3C and D). Data collected with TEM and ultraviolet-spectrometry demonstrated that if the initial concentration of the peptide is higher than 25 µM, aggregation occurs at too high of a degree, progressively leading to a complete degradation of the solution after several days.
Figure 3.
TEM images of AuNPs: (A) pure AuNPs in citrate buffer, (B) PEP-AuNPs immediately after the synthesis, (C) PEP-AuNPs before centrifugation, (D) and PEP-AuNPs after centrifugation.
Note: Scale bars =100 µm.
Abbreviations: AuNP, gold nanoparticle; PEP-AuNP, peptide-capped AuNP; TEM, transmission electron microscopy.
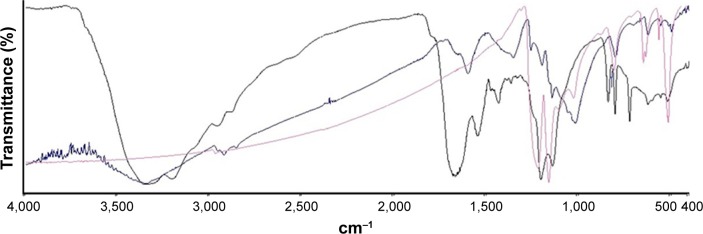
The presence of the bound peptides on the gold surface was validated using two different methods. Through infrared spectroscopy, a typical FTIR spectra of dried PEP-AuNPs was found (Figure 4). The different peaks in the spectra are associated with different vibrations: 3,330 cm−1 for N-H stretching primary amine; 1,598 cm−1 for C=O stretching; and 1,350 cm−1 for C-H bending. The other peaks are difficult to assign because of the superposition of the peaks from characteristic vibrations of the peptides and of the AuNPs. Nevertheless, the broad peak centered at 3,330 cm−1 is the most interesting peak, as it indicates the presence of amine primary groups. The pure peptide FTIR spectra also showed a distinctive peak around 3,300 cm−1, whereas the pure AuNPs spectrum did not contain peaks in this area. This result is a strong indication of the successful conjugation of peptides onto the AuNPs.
Figure 4.
FTIR spectra: (blue) PEP-AuNPs; (black) pure peptide; and (pink) naked AuNPs.
Abbreviations: AuNP, gold nanoparticle; FTIR, Fourier transform infrared spectroscopy; PEP-AuNP, peptide-capped AuNP.
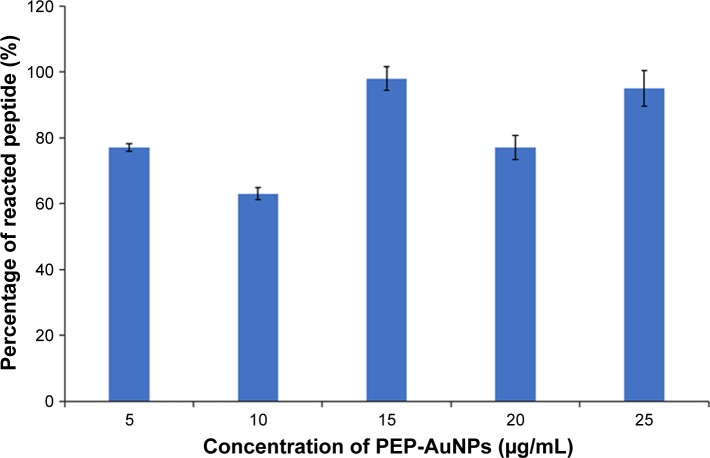
The results of the BCA assay on PEP-AuNPs are shown in Figure 5. A quadratic regression of the data from the BCA standard curve instead of a classic linear regression was utilized to acquire more accurate results. Figure 5 shows the different concentrations measured after the reaction and purification. First, the process revealed the presence of peptides in all cases, which further validates the formation of a covalent bond between the thiol groups of the cysteine and the gold surface. Second, the percentage of reacting peptides displayed a certain variability. Even at low concentrations of peptides, the percentage of the reacting peptide fell sufficiently below 100%. It seems that the reaction was not total but at an equilibrium. This can be explained by aggregations of AuNPs, leading to nonhomogenous distributions.
Figure 5.
Results from the BCA assay.
Notes: Data = mean ± SEM; N=3.
Abbreviations: AuNP, gold nanoparticle; PEP-AuNP, peptide-capped AuNP; SEM, standard error of the mean.
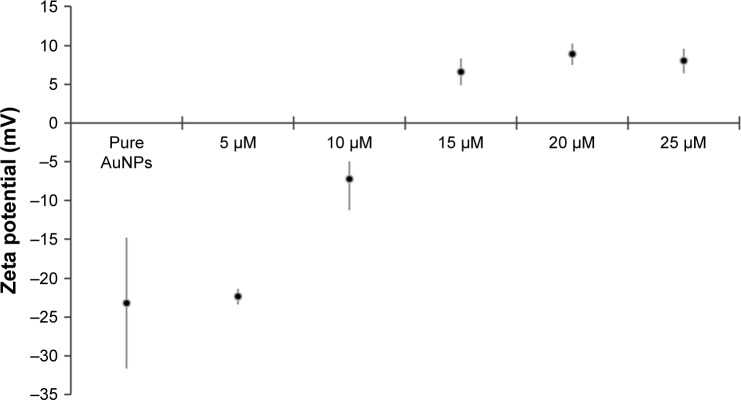
Zeta potential results for the PEP-AuNPs prepared with various initial peptides concentrations are shown in Figure 6. AuNPs in citrate buffer are negatively charged and have good stability with a zeta potential of −23.81±8.43 mV. The zeta potential of the PEP-AuNPs increased with increases in initial concentration and became positively charged at 15 µM, which signifies that there was enough peptide to change the charge of the conjugated particles. Indeed, these results are in agreement with the design of the CRRRRRRRRGDS peptide, where the arginine-rich CPPs engender a positive charge layer around the AuNPs. Consequently, the penetration of AuNPs through the cell membranes became possible with a positive zeta potential. Therefore, PEP-AuNPs with an initial peptide concentration lower than 15 µM may not be ideal for cancer therapeutics. Nevertheless, for peptide concentrations from 15 to 25 µM, the zeta potential values are low, between 5 and 10 mV, indicating weak stability of the PEP-AuNP suspension, which will rapidly generate aggregation and sedimentation. For the four last concentrations there was a plateau, which indicated that the maximal value of zeta potential for the PEP-AuNPs was potentially reached. These results are in accordance with the previous observations made with the TEM images and the ultraviolet spectra.
Figure 6.
Zeta potential measurements for the different PEP-AuNPs at different concentrations of initial peptide solutions compared to pure AuNPs in citrate buffer. Notes: Data = mean ± SEM; N=3.
Abbreviations: AuNP, gold nanoparticle; PEP-AuNP, peptide-capped AuNP; SEM, standard error of the mean.
Fibroblasts
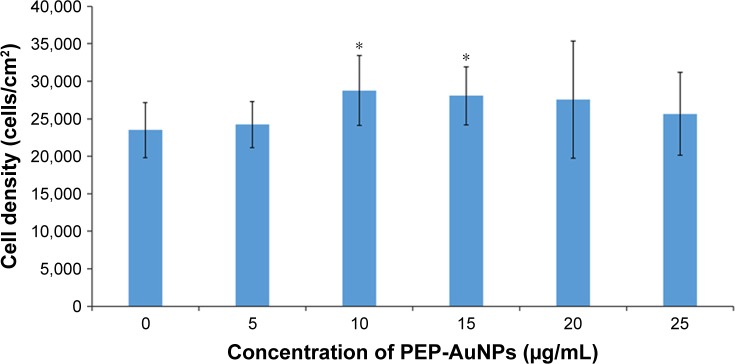
To evaluate fibroblast functions in the presence of the PEP-AuNPs on HDF cells and to ensure that the cells incubated with nanoparticles did not have any negative effects, cytotoxicity was assessed with an MTS assay (Figure 7). The PEP-AuNPs did not exhibit any cytotoxicity toward HDF cells at any peptide concentration tested. For 10 and 15 µM, cell density was higher than control samples (P<0.05), indicating a high cytocompatibility of the nanoparticles. The MTS assay also showed that the PEP-AuNPs actually enhanced the growth of HDF cells, which suggests (although clearly requiring more experiments) their potentially positive role in increasing healthy tissue growth after killing cancer cells. However, the mechanism behind this phenomenon is unknown. These results are encouraging for the future investigation of the presently prepared AuNPs.
Figure 7.
HDF density exposed to PEP-AuNPs for 24 hours in serum-free medium.
Notes: Data = mean ± SEM; N=3 (five samples per group); and *P<0.01 represents significant differences compared to the controls.
Abbreviations: AuNP, gold nanoparticle; HDF, human dermal fibroblast; PEP-AuNP, peptide-capped AuNP; SEM, standard error of the mean.
Conclusion
This study demonstrated that the rich arginine peptide CRRRRRRRRGDS can be conjugated to AuNPs by direct cross-linking using a simple method. It is necessary to control peptide concentration to avoid aggregations of PEP-AuNPs engendered by peptide interactions, which can potentially block the cell penetration and reduce the lifetime of the AuNPs. The PEP-AuNPs also not only had low cytotoxicity toward HDF cells, but it actually promoted their growth. Those results highlight the capacity to fabricate cytocompatible conjugated AuNPs with CPP, which can be beneficial for the development of new treatments such as anticancer treatments and even for regenerating healthy tissue after the killing of cancer cells. Further studies to better understand the effect of such conjugated AuNPs should validate their efficiency as a cancer therapeutic and their capacity to specifically target cancer cells. Other methods of synthesis to cross-link the peptide and avoid aggregation may increase efficacy, such as the use of polyethylene glycol as an intermediary.
Acknowledgments
The authors would like to thank Northeastern University for the funding, William Fowle for TEM training, and all the members of the Nanomedicine Lab for their help and advice concerning the biological experiments.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cai W, Gao T, Hong H, Sun J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl. 2008;1:17–32. doi: 10.2147/NSA.S3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Br J Radiol. 2012;85(1010):101–113. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu X, Trase I, Ren M, Duval K, Guo X, Chen Z. Design of nanoparticle-based carriers for targeted drug delivery. J Nanomater. 2016;2016:1–15. doi: 10.1155/2016/1087250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li CM, Zhang L, Hou YH, Li N, Che MH. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using anti-EGFR antibody as a targeting agent. World Chinese J Dig. 2015;23(12):1890–1896. [Google Scholar]
- 5.Tung CH, Weissleder R. Arginine containing peptides as delivery vectors. Adv Drug Deliv Rev. 2003;55(2):281–294. doi: 10.1016/s0169-409x(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 6.Humphries MJ, Olden K, Yamada KM. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986;233(4762):467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Li Y, Shen Y, Wang A, Wang S, Xie T. The functions and applications of RGD in tumor therapy and tissue engineering. Int J Mol Sci. 2013;14(7):13447–13462. doi: 10.3390/ijms140713447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang R, Sun L, Webster TJ. Selective inhibition of MG-63 osteosarcoma cell proliferation induced by curcumin-loaded self-assembled arginine-rich-RGD nanospheres. Int J Nanomedicine. 2015;10:3351–3365. doi: 10.2147/IJN.S78756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lévy R, Thanh NT, Doty RC, Christopher Doty R, et al. Rational and combinatorial design of peptide capping ligands for gold nanoparticles. J Am Chem Soc. 2004;126(32):10076–10084. doi: 10.1021/ja0487269. [DOI] [PubMed] [Google Scholar]
- 10.Majzik A, Fülöp L, Csapó E, et al. Functionalization of gold nanoparticles with amino acid, beta-amyloid peptides and fragment. Colloids Surf B Biointerfaces. 2010;81(1):235–241. doi: 10.1016/j.colsurfb.2010.07.011. [DOI] [PubMed] [Google Scholar]