Abstract
Fibroblast differentiation is an essential step during wound healing and fibrosis. Fibronectin (FN) is a major component of the extracellular matrix and occurs in two main forms: plasma and cellular FN. The latter includes the alternatively spliced domain A (EDA). Although EDA-containing cellular fibronectin (EDA-FN) is associated with fibroblast differentiation, how EDA-FN promotes differentiation is incompletely understood. In this study, we investigate the mechanism by which EDA-FN contributes to fibroblast differentiation with emphasis on the characterization of the EDA-FN receptor. We show that EDA-FN increases α-SMA expression (immunofluorescence), collagen deposition, cell contractility, and focal adhesion kinase (FAK) activation (immunoblotting); whereas plasma FN, a form lacking EDA, shows no effect. Primary lung fibroblasts constitutively express α4β7 integrin receptor (FACS and RT-PCR). Blocking of α4β7 reduces fibroblast adhesion to EDA-FN and inhibits α-SMA expression, collagen deposition, and FAK activation induced by EDA-FN. Using recombinant EDA-containing peptides, we demonstrate that the EDA segment is sufficient to induce fibroblast differentiation via binding to α4β7. EDA-FN induces MAPK-Erk1/2 activation and inhibition of MEK1/2 attenuates EDA-FN-induced α-SMA expression. Our findings demonstrate that EDA-FN induces fibroblast differentiation by a mechanism that involves binding of EDA to α4β7 integrin followed by activation of FAK and MAPK-associated signaling pathways.—Kohan, M., Muro, A. F., White, E. S., Berkman, N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to α4β7 integrin receptor and MAPK/Erk 1/2-dependent signaling.
Keywords: myofibroblasts, fibrosis, extracellular matrix
Fibroblasts are responsible for the preservation of extracellular matrix (ECM) homeostasis by maintaining a balance between synthesis and degradation of structural proteins in all the organs of the body. These cells have the ability to alter their phenotype from noncontractile, quiescent fibroblasts, and intermediate “protomyofibroblasts” to “myofibroblasts”, which are distinguished from their precursors by the expression of α-smooth muscle actin (α-SMA), their highly contractile capacity, and their ability to produce large amounts of ECM proteins (1, 2). The differentiation of fibroblasts into myofibroblasts requires the combined action of mechanical tension, TGF-β1 and EDA-containing fibronectin (FN) (1–3).
FN is a 440-kDa dimeric ECM glycoprotein that includes 3 different types of repeating segments, called type I, type II, and type III, the latter being the most abundant (4). The FN gene encodes 15 type III repeats, which are constitutively expressed, plus 2 that are alternatively spliced, the extra domain A (EDA) and B (EDB) (5). EDA and EDB are encoded by single exons, and their inclusion or exclusion from the FN mRNA is determined by a highly regulated alternative splicing mechanism (5). Two major forms of FN exist: plasma FN (pFN), a soluble dimeric form found in plasma secreted by hepatocytes, which lacks both EDA and EDB segments; and cellular FN (cFN), an insoluble cross-linked multimeric form found in ECM mainly secreted by activated fibroblasts as soluble dimers and contains variable proportions of EDA and/or EDB segments (5, 6).
Increased expression of the EDA-containing isoform of FN is found during embryogenesis, and once tissue development is completed its expression drops to negligible levels (7, 8). The FN isoform lacking both EDA and EDB domains is the most abundant form present in normal adult tissues (7–9). Under certain conditions such as wound healing or tissue fibrosis, where myofibroblasts are present in abundance, increased expression of EDA-containing cellular FN (EDA-FN) has been observed (10–12). Although EDA-FN is necessary for myofibroblast differentiation (11, 12), the mechanism by which it contributes to this effect still remains unknown. Serini et al. (12) have proposed that EDA-FN might interact with an uncharacterized cell surface receptor on fibroblasts and possibly facilitate TGF-β1-driven signals.
Integrins are a family of heterodimeric transmembrane receptors that bind ECM components (13). It is well established that both plasma and cellular FNs bind several different integrins triggering intracellular signals, including early activation of focal adhesion kinase (FAK), which has been associated with fibroblast differentiation (14, 15). α5β1 integrin, considered the “classic” FN receptor, binds to the RGD motif of FN and stimulates cell adhesion, migration, and proliferation (16, 17). Members of the α4/α9 subfamily of integrins include α4β1,α9β1, and α4β7 (16, 18). α4β1 and α9β1 are expressed by fibroblasts (19, 20) and are known to bind the EDA segment of FN (21). In contrast, expression of α4β7 integrin has not been reported in fibroblasts. In the present study, we show that lung fibroblasts constitutively express α4β7 integrin and provide evidence that EDA-FN stimulates fibroblast differentiation via binding of the EDA segment to α4β7 integrin followed by activation of downstream cell differentiation-linked signaling pathways.
MATERIALS AND METHODS
Antibodies and reagents
Anti-EDA-FN (3E2) and anti-α-smooth muscle actin (α-SMA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-α4β7 integrin (DATK32), FITC-conjugated anti-αE integrin (2E7), and anti-EDA-FN (IST-9) were purchased from Abcam (Cambridge, UK). Anti-α5β1 integrin (BMA5), anti-α9β1 integrin (Y9A2), and irrelevant isotype-matched IgG antibodies were purchased from Chemicon (Billerica, MA, USA). LEAF purified anti-β7 integrin (FIB27) was purchased from BioLegend (San Diego, CA, USA). Functional grade purified anti-β1 integrin (HMb1-1) was purchased from eBioscience (San Diego, CA, USA). Anti-α4β1 integrin (PS/2) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phospho-FAK (Tyr397), anti-FAK, anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (197G2), and anti-p44/42 MAPK (Erk1/2) (137F5) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Cy5-conjugated goat anti-mouse and HRP-conjugated goat anti-rabbit were purchased from Jackson Laboratories Inc (West Grove, PA, USA). EDA-FN was purchased from Sigma-Aldrich or isolated from media of cultured fibroblasts (11). pFN was purchased from Innovative Research (Novi, MI). MEK inhibitors U0126 and PD98059 were purchased from Cell Signaling Technology.
Fibroblast isolation and culture
C57Bl/6 mice were purchased from Harlan Laboratories Ltd (Jerusalem, Israel), and lung fibroblasts were extracted as described previously (22). The Hebrew University-Hadassah Medical School Animal Ethics Committee approved all animal protocols. Human lung fibroblasts were obtained from lung biopsies of patients undergoing thoracic surgery, and cells were maintained in DME with 10% FCS, glutamine, penicillin, and streptomycin. Informed consent was obtained. Cells between passages 2 and 4 were used for all experiments (n=3–4).
Production of EDA-containing and EDA-lacking recombinant peptides
FN sequences containing or lacking the EDA segment were generated from full-length cFN and full-length pFN cDNAs, respectively. PCR amplifications were performed using the following primers: forward, 5′-ACCATCACTGTGTATGCTGTCACT-3′; reverse, 5′-AAGTCCTGATACAACCACGGA-3′. These primers covered a sequence from base 4822 to base 5626 (numbers are as in the full-length cDNA sequence found at GeneBank) and generated two products: one of 804 bp containing the EDA segment flanked by the 11th and 12th type III repeats, and one of 534 bp lacking the EDA domain. PCR-amplified fragments were then cloned in the BamHI site of the pProEX Htb expression vector (Invitrogen Corp, San Diego, CA, USA). Competent BL21 cells were transfected, and expression of the peptide was induced by addition of isopropyl-β-d-thiogalactoside to a final concentration of 1 mM. Cells were then lysed and disrupted by freezing/thawing and sonication. Protein extracts were loaded on a 12% SDS-PAGE gel. Bands corresponding to the EDA-containing and EDA-lacking peptides were detected by staining the gel with 1 M KCl, cut out from the gel and electroeluted in 50 mM carbonate-bicarbonate buffer, pH 9.6. Only the purified EDA-containing recombinant peptide showed positive immunological reactivity to the anti-EDA monoclonal antibody 3E2.
Immunofluorescence
Fibroblasts (104 cells/well) were cultured on coverslips placed in 24-well plates and treated with EDA-FN, pFN, EDA-containing peptides, or peptides lacking the EDA segment (10 μg/ml, 48 h, 37°C). Cells were then fixed in 75% methanol, permeabilized in 0.2% Triton 100×, blocked in 1% BSA, and incubated with anti-α-SMA. Binding was detected using Cy5-conjugated goat anti-mouse antibody. Nuclei were counterstained with propidium iodide (Sigma). Slides were examined using a FluoView FV 300 confocal laser scanning biological microscope (Olympus Corporation, Center Valley, PA, USA). Ten fields were examined per slide. Quantification was assessed using Image-Pro Plus 6.0 (Media Cybernetics Inc., Silver Spring, MD, USA) on images acquired at ×600 and expressed as integrated optical density (IOD) as described previously (22). For blocking experiments, cells were preincubated (10–20 μg/ml, 45 min, 37°C) with anti-α4β7 integrin (DATK32), anti-α5β1 integrin (BMA5), anti-β7 integrin (FIB27), anti-β1 integrin (HMb1–1), irrelevant isotype-matched IgG, MEK1/2 inhibitor U0126 (10 μM, 1 h, 37°C), MEK1 inhibitor PD98059 (20 μM, 1 h, 37°C) or anti-EDA (IST-9; 25 μg/ml, 45 min, 37°C).
To determine whether the α4β7 integrin is expressed by fibroblasts in vivo, immunostaining was performed on 4–6 μm paraffined lung tissue sections from mice exposed to ovalbumin (22). After deparaffinization, slides were incubated with anti-α4β7 integrin together with anti-α-SMA as a marker for myofibroblasts. Binding was detected using Cy5 anti-mouse or FITC anti-rat antibodies. Tissue slides were examined using a FluoView FV 300 confocal laser scanning biological microscope.
Collagen deposition by fibroblast monolayers
Fibroblasts (5×104 cells/well) were seeded in 24-well plates, and collagen deposition was determined using the Sircol colorimetric assay (Biocolor, Belfast, UK), as described previously (22), in the presence or absence of EDA-containing cFN, pFN, EDA-containing peptides, or peptides lacking the EDA segment (10 μg/ml). Collagen deposition was expressed as fold increase relative to deposition by untreated cells. For blocking experiments, cells were preincubated with anti-α4β7 integrin (DATK32) (10 μg/ml, 45 min, 37°C) or anti-EDA (IST-9) (25 μg/ml, 45 min, 37°C).
Collagen gel contraction assay
Three-dimensional gel contraction was performed in collagen matrices as described previously (23). After 24 h at 37°C, gels were photographed and their size was determined using ImageJ (National Institutes of Health, Bethesda, MD, USA). Results were expressed as percentage of initial gel area.
ELISA for TGF-β1
ELISA for TGF-β1 was performed on cell culture medium using a commercial kit (R&D Systems, Minneapolis, MN, USA).
Fibroblast adhesion assay
Flat-bottom 96-well plates (BD Biosciences, San Jose, CA, USA) were coated with EDA-FN (20 μg/ml, 1 h, 37°C). Plates were then blocked with 1% BSA (30 min, 37°C). Fibroblasts (5×103 cells/well) were seeded into individual wells. Plated cells were centrifuged to ensure equal dispersion and were incubated for 20 min (37°C, 5% CO2). Nonadherent cells were removed by centrifuging the plate upside-down for 5 min at 500 RPM. Adherent cells were fixed and stained with 0.5% crystal violet, 1% formaldehyde, 20% methanol followed by destaining with PBS. Relative adherence was determined by reading the plate at 550 nm in an EL808 Ultra Microplate Reader (Bio-Tek Instruments, Winooski, VT, USA). For the indicated conditions, fibroblasts in suspension were preincubated with function-blocking antibodies (20 μg/ml, 30 min) on ice prior to plating.
RT-PCR for β7 integrin
Cellular RNA was isolated using TriReagent (Sigma). RT-PCR was performed using standard conditions (30 cycles) (Promega, Madison, WI, USA). cDNAs from murine lung fibroblasts and spleen-derived lymphoid cells were amplified using the following primers: forward, 5′-CCTACGACTCTGGGCTCTTG-3′; reverse, 5′-ACAGGTCAGCCTCAGAGCAT-3′.
cDNAs from human lung fibroblasts and blood-derived T lymphocytes were amplified using the following primers: forward, 5′-ACCGTGACCCTTGAACACTC-3′; reverse, 5′-TGCAACTCCACAATCAGCTC-3′.
PCR products (194 bp for murine cells and 239 bp for human cells) were visualized by ethidium bromide-stained agarose gel electrophoresis. A glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA was used as control. Some cells were exposed to 5 ng/ml of TGF-β1.
Flow cytometry
Cells were incubated with phycoerythrin-conjugated anti-α4β7 integrin (DATK32); FITC-conjugated anti-αE integrin (2E7) or irrelevant isotype-matched IgG for 30 min at room temperature. Fluorescence was analyzed using a FACStar analyzer (Becton Dickinson, Franklin Lakes, NJ, USA). Data were processed with the CellQuest software (BD Biosciences, San Jose, CA, USA).
Western blotting
Samples were prepared from cells grown in 6-well plates by addition of Nonidet P-40 lysis buffer supplemented with protease inhibitor cocktail (Pierce, Rockford, IL, USA), sodium vanadate (Sigma), and PMSF (Sigma). Protein concentrations were determined using the Bradford assay (Bio-Rad, Hercules, CA, USA). Lysates were then separated by 9% SDS-PAGE and samples were transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked with 5% dry milk in TBS/Tween-20 (TBS-T) and incubated with anti-phospho-FAK, anti-FAK, anti-phospho-Erk1/2, and anti-Erk1/2 antibodies. Membranes were incubated with HRP-conjugated anti-rabbit IgG, and blots were developed with the SuperSignal West Pico Chemiluminiscent Substrate kit (Thermo Scientific, Rockford, IL, USA) and exposed to Fuji Medical X-Ray films (FujiFilm Corp., Tokyo, Japan). Image analysis was performed using ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA).
Data analysis
The mean ± se values are given for each group. One-way analysis of variance (ANOVA) followed by Bonferroni posttest was performed to compare groups. Values of P < 0.05 were considered statistically significant.
RESULTS
EDA-FN induces fibroblast differentiation
We first compared the effects of EDA-FN and the non-EDA-containing form pFN on fibroblasts isolated from lungs of C57Bl/6 mice and evaluated different aspect of fibroblast differentiation, namely α-SMA expression, collagen production, collagen gel contractility, and TGF-β1 release.
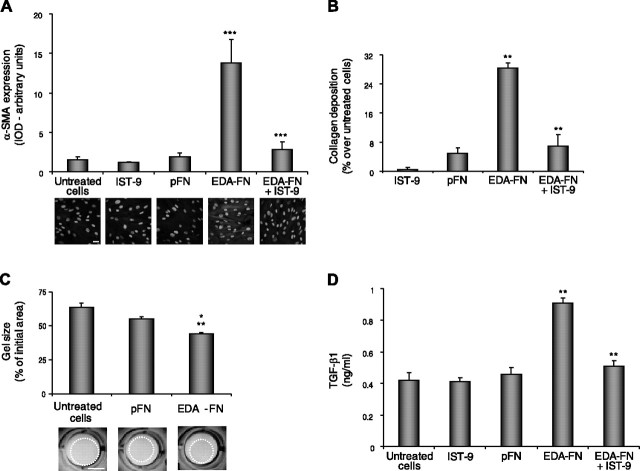
Expression of α-SMA was assessed by quantitative immunofluorescence (22). EDA-FN (10 μg/ml, 48 h) increased α-SMA expression in comparison with untreated cells and pFN (Fig. 1A, P<0.001). pFN (10 μg/ml, 48 h) did not induce changes in α-SMA expression levels in comparison with untreated cells (Fig. 1A). Preincubation with anti-EDA antibody (IST-9) inhibited EDA-FN-induced α-SMA expression (Fig. 1A; P<0.001).
Figure 1.
EDA-FN induces markers of fibroblast differentiation. Primary lung fibroblasts were exposed to 10 μg/ml EDA-FN or non-EDA-containing pFN), and markers of fibroblast differentiation were evaluated as described in Materials and Methods. EDA-FN induced expression of α-SMA (A), ***P< 0.001 vs. untreated cells and pFN; collagen deposition (B), **P < 0.01 vs. pFN; cell contractility (C), *P < 0.05 vs. pFN, **P < 0.01 vs. untreated cells; and TGF-β1 release (D), **P < 0.01 vs. untreated cells and pFN. Preincubation with anti-EDA monoclonal antibody IST-9 inhibited the effects induced by EDA-FN. Experiments were performed 3 times; means ± se are shown. Scale bars = 10 μm (A); 1 cm (C).
Fibroblast collagen deposition was measured by the Sircol colorimetric assay (22). EDA-FN (10 μg/ml, 48 h) increased fibroblast collagen deposition, whereas pFN (10 μg/ml, 48 h) showed minimal effect on collagen deposition (Fig. 1B; 28±2% for EDA-FN vs. 5±3% for pFN over untreated cells, P<0.01). Preincubation with anti-EDA antibody (IST-9) inhibited EDA-FN-induced collagen deposition (Fig. 1B; P<0.01).
Fibroblast contractility was determined by the collagen gel contraction assay (23). After 24 h, untreated cells reduced the gel to 64% of its initial size at time 0 (Fig. 1C). EDA-FN (10 μg/ml, 24 h) reduced the size of the gel by an additional 20% in comparison with untreated cells (Fig. 1C, P<0.01), whereas pFN (10 μg/ml, 24 h) reduced the gel by only an additional 8% as compared to untreated cells.
TGF-β1 secretion into culture medium by lung fibroblasts was determined by ELISA. While EDA-FN increased TGF-β1 release by 2-fold in comparison with untreated cells (Fig. 1D, P<0.01), exposure to pFN did not affect TGF-β1 release. Pretreatment with anti-EDA antibody (IST-9) inhibited EDA-FN-induced TGF-β1 release (Fig. 1D, P<0.01).
α4β7 integrin is constitutively expressed in lung fibroblasts and mediates fibroblast adhesion to EDA-FN
The effect exhibited by EDA-FN on fibroblast differentiation suggests the existence of a cell surface receptor that mediates this process. In an attempt to identify such a receptor, we first determined fibroblast adhesion to EDA-FN in the presence of blocking antibodies (20 μg/ml) for integrins α4β1, α9β1, and α4β7. Blocking of integrin α4β7 inhibited the adhesion of fibroblast to EDA-FN (Fig. 2A; P<0.05). In addition, blocking of α4β1, an integrin receptor expressed by fibroblasts (19), which is known to bind the EDA segment (21), also abrogated cell adhesion to EDA-FN, whereas blocking of integrin α9β1 showed no effect (Fig. 2A; P<0.01).
Figure 2.
α4β7 integrin is constitutively expressed in lung fibroblasts and mediates adhesion to EDA-FN. A) Lung fibroblasts were preincubated with blocking antibodies and seeded in 96-well plates previously coated with EDA-FN to determine cell adhesion. Blocking of α4β7 and α4β1 integrins inhibited fibroblast adhesion to EDA-FN. *P < 0.05, **P<0.01 vs. untreated cells). Experiments were performed 3 times; means ± se are shown. B) mRNA expression of β7 was evaluated by RT-PCR in murine primary lung fibroblasts (lane 1). Addition of 5 ng/ml TGF-β1 to lung fibroblasts did not alter β7 mRNA expression (lane 2). Murine lymphoid cells (lane 3) were used as a positive control. C) mRNA expression of β7 in human primary lung fibroblasts (lane 1) and in human T lymphocytes (lane 3, positive control). No band was observed in the absence of cDNA (lane 2, negative control). D) Staining for α4β7 integrin (black histograms) and irrelevant IgG isotype control (gray histograms) was evaluated by flow cytometry on murine primary lung fibroblasts and lymphoid cells (positive control). E) Expression of α4β7 integrin (green, left panel), α-SMA (red, middle panel), and colocalization (yellow, right panel) was evaluated in murine lung tissue by immunostaining (white arrows). Scale bar = 10 μm. Experiments were repeated 3 times; representative images are shown.
The α4 subunit is known to be expressed on fibroblasts as part of the α4β1 complex (15, 19). In contrast, the β7 subunit has not previously been identified on fibroblasts. We then evaluated the expression of both the β7 subunit and the α4β7 integrin complex in lung fibroblasts by RT-PCR and flow cytometry. Primary murine lung fibroblasts constitutively expressed β7 integrin mRNA (Fig. 2B, lane 1). Addition of TGF-β1 (5 ng/ml, 48 h) did not increase β7 integrin mRNA levels (Fig. 2B, lane 2). Primary human lung fibroblasts also expressed the β7 integrin subunit (Fig. 2C). FACS analysis demonstrated expression of the α4β7 complex in murine lung fibroblasts (mean fluorescence intensity − fold over control = 12.8±0.7) (Fig. 2D). We did not observe positive staining for the αE subunit, which also forms a complex with the β7 subunit (13) (data not shown). We also observed positive immunostaining for α4β7 integrin in vivo in myofibroblasts from lung tissue of OVA-treated mice (Fig. 2E).
Blocking of α4β7 integrin inhibits EDA-FN-induced myofibroblast differentiation
To investigate whether α4β7 mediates EDA-FN-induced fibroblast differentiation, cells were preincubated with anti-α4β7 blocking antibodies in the presence or absence of EDA-FN and α-SMA expression, collagen production and TGF-β1 release were determined. Blocking of α4β7 inhibited EDA-FN-induced α-SMA expression (P<0.001; Fig. 3A). Preincubation with anti-α5β1 blocking antibodies did not exert inhibitory effects on fibroblast differentiation. Fibroblasts were also preincubated with blocking antibodies against the β7 and β1 subunits. While pretreatment with anti-β7 integrin antibodies completely inhibited the induction of α-SMA expression observed after exposure to 10 μg/ml EDA-FN (Fig. 3A; P<0.001); anti-β1 integrin antibodies did not alter EDA-FN-induced α-SMA expression. The absence of effect with anti-β1 antibodies rules out a role for β1-containing integrins such as α4β1 and α9β1 on fibroblast differentiation. Pretreatment with anti α4β7 integrin blocking antibodies also reduced EDA-FN-induced collagen production (Fig. 3B) and EDA-FN-induced TGF-β1 release (Fig. 3C) to levels similar to those observed for untreated cells (internal control) (31±5% over control without anti-α4β7 vs. 7±4% over control with anti-α4β7 for collagen production, P<0.001; 1.04±0.06 ng/ml without anti-α4β7 vs. 0.63±0.05% ng/ml with anti-α4β7 for TGF-β1 release, P<0.001).
Figure 3.
EDA-FN induces myofibroblast differentiation via α4β7 integrin. Fibroblasts were exposed to 10 μg/ml EDA-FN in the presence of blocking antibodies for α4β7, α5β1, β7, or β1 integrins and expression of α-SMA was assessed by immunofluorescence, quantified and expressed as integrated optical density (IOD). Experiments were performed 4 times; representative images are shown. A) Preincubation with anti-α4β7 or anti-β7 blocking antibodies (45 min, 37°C) inhibited EDA-FN-induced α-SMA expression in fibroblasts. Scale bar = 20 μm. B, C) EDA-FN-induced collagen deposition, assessed by the Sircol assay (B) and EDA-FN-induced TGF-β1 release, determined by ELISA (C), were abrogated in fibroblasts preincubated with anti-α4β7 blocking antibodies. ***P < 0.001.
The EDA segment is sufficient to induce a myofibroblast phenotype through binding to α4β7 integrin
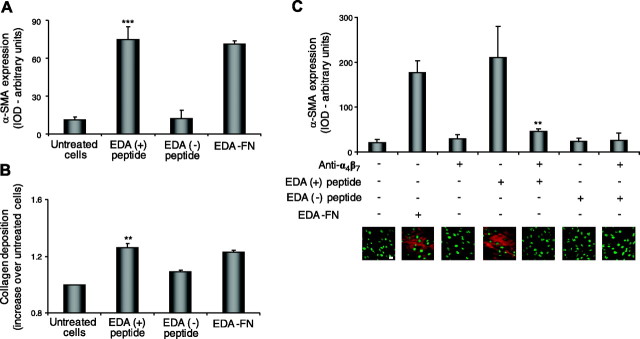
To determine whether the ability of EDA-FN to induce fibroblast differentiation is attributable to the EDA segment and—if so—due to direct binding of the EDA segment to α4β7, we generated recombinant fragments of FN containing or lacking the EDA segment. Recombinant peptides (10 μg/ml) containing the EDA segment (EDA+ peptides) increased α-SMA expression (Fig. 4A; P<0.001 vs. untreated cells) and collagen production by 26 ± 2% (Fig. 4B; P<0.01 vs. untreated cells). The effect observed for the EDA+ peptides was similar to that observed for EDA-FN. Recombinant peptides lacking the EDA segment (EDA− peptides) were used as control and showed no effect.
Figure 4.
The EDA segment of EDA-FN is sufficient to induce myofibroblast differentiation via α4β7 integrin. To test the contribution of the EDA segment to fibroblast differentiation, cells were exposed to 10 μg/ml of recombinant peptides containing (EDA+) or lacking (EDA−) the EDA segment (see Materials and Methods). A, B) EDA+ peptides increased expression of α-SMA (A) and collagen deposition (B) in a manner similar to EDA-FN. No effect was observed after treatment with EDA− peptides used as control. C) Exposure to anti-α4β7 integrin blocking antibodies abrogated the effect observed for EDA+ peptides on α-SMA expression. Scale bar = 10 μm. **P < 0.01, ***P < 0.001. Experiments were performed 3 times; representative images are shown.
To determine whether the effect of the EDA segment is via binding to α4β7 integrin, we evaluated the expression of α-SMA after exposure to EDA+ peptides in the presence or absence of anti α4β7 blocking antibodies. We observed that preincubation with anti-α4β7 antibodies completely abrogated the increased expression of α-SMA that was induced by 10 μg/ml EDA+ peptides (Fig. 4C; P<0.01).
EDA-FN enhances FAK activation via integrin α4β7
Binding of different sequences of FN to integrin receptors is known to activate FAK via phosphorylation of tyrosine residues (24, 25). The ratio between phosphorylated FAK and total FAK was determined by quantitative immunoblotting. EDA-FN (10 μg/ml) induced phosphorylation of the tyrosine 397 of FAK on lung fibroblasts in a time-dependent manner, with a maximal effect observed after 6 h of incubation (3.1-fold over untreated cells) (Fig. 5A). We also compared FAK activation between EDA-FN and pFN and observed that cells exposed to 10 μg/ml EDA-FN significantly increased the phosphorylated FAK:FAK ratio in comparison to 10 μg/ml pFN (Fig. 5B; 1.04±0.08 for EDA-FN vs. 0.59±0.07 for pFN; P<0.01). To determine whether the EDA segment can induce FAK activation, the phosphorylated FAK:FAK ratio was assessed in fibroblasts exposed to EDA+ peptides or EDA− peptides. Exposure of cells to 10 μg/ml EDA+ peptides for 3 h increased phosphorylated FAK:FAK ratio in comparison with cells treated with 10 μg/ml EDA− peptides (Fig. 5C). No differences were detected between cells treated with EDA− peptides and untreated cells. To evaluate whether EDA-FN activates FAK via α4β7 integrin, cells were exposed to 10 μg/ml EDA-FN in the presence or absence of anti-α4β7 integrin antibodies (preincubation for 45 min at 37°C). As shown in Fig. 5D, blocking of α4β7 integrin decreased phosphorylated FAK:FAK ratio induced by EDA-FN to levels similar to untreated cells (0.75±0.09 in the presence of anti-α4β7 integrin vs. 1.24±0.11 in the absence of anti-α4β7 integrin; P<0.01).
Figure 5.
EDA-FN activates FAK via α4β7 integrin receptor. Cell lysates obtained from fibroblasts exposed to EDA-FN, non EDA-containing pFN, EDA-containing peptides (EDA+) or EDA-lacking peptides (EDA−) were separated by SDS-PAGE and subjected to immunoblotting with anti-phospho-FAK and anti-FAK antibodies. Densitometric analysis calculated as ratio of phospho-FAK to total FAK was then assessed. A) Time course of FAK activation by EDA-FN showed a maximum effect after 3–6 h of incubation. B) Exposure to EDA-FN increased FAK activation in comparison with pFN. **P < 0.01 vs. pFN; ***P < 0.001 vs. untreated cells. C) Lysates from cells treated with EDA+ peptides showed greater FAK activation than lysates treated with EDA− peptides. D) Blocking of α4β7 integrin reduced EDA-FN-induced FAK activation. **P < 0.01. Blots shown are representative of 2 or 3 independent experiments from different lysates.
EDA-FN-mediated fibroblast differentiation is MAP kinase Erk1/2 dependent
The intracellular signals involved in EDA-FN-mediated fibroblast differentiation are virtually unknown. We explored whether the p44/42 MAP kinase (ERK 1/2) signaling pathway, which is key in cell differentiation, proliferation and motility (26), contributes to fibroblast differentiation. Exposure to 10 μg/ml EDA-FN and EDA-containing peptides enhanced phosphorylation of p44/42 MAPK (Erk1/2) in lung fibroblasts in a time-dependent manner with maximal effect observed after 3 h of incubation (3.7-fold over untreated cells) (Fig. 6A). Pretreatment (1 h, 37°C) with 10 μg/ml of MEK1/2 inhibitor U0126 or 20 μg/ml of MEK1 inhibitor PD98059 inhibited EDA-FN-induced α-SMA expression in fibroblasts (Fig. 6B; P<0.001 for U0126 and P<0.01 for PD98059 vs. EDA-FN). U0126 showed higher inhibitory capacity (96% inhibition) in comparison with PD98059 (62% inhibition).
Figure 6.
MAP kinase Erk1/2 activation by EDA-FN is required for fibroblast differentiation. Cell lysates obtained from fibroblasts exposed to 10 μg/ml EDA-FN or EDA-containing peptides were separated by SDS-PAGE and subjected to immunoblotting with anti-phospho-p44/42 MAPK (Erk1/2) and anti-p44/42 MAPK (Erk1/2) antibodies. A) EDA-FN activates Erk1/2, showing maximum effect after 3 h of incubation. B) Lysates from cells treated with EDA+ peptides showed greater Erk1/2 activation than lysates treated with EDA− peptides. Blots shown are representative of 2 experiments from different lysates. C) Fibroblasts were preincubated (1 h, 37°C) with MEK inhibitors U0126 (10 μg/ml) and PD98059 (20 μg/ml) and then exposed to 10 μg/ml EDA-FN for 48 h. Cell expression of α-SMA in response to EDA-FN was totally inhibited by U0126 and partially inhibited by PD98059, as assessed by immunofluorescence. **P < 0.01; ***P < 0.001. Experiments were performed 3 times; data are presented as means ± se.
DISCUSSION
Fibroblast differentiation to a myofibroblast phenotype is an essential step in the process of fibrogenesis (1, 2). Although a link between EDA-FN and fibroblast differentiation has been known for more than 15 yr (10), the mechanism by which this interaction occurs has not been fully elucidated. In the present study, we show that α4β7 integrin is constitutively expressed on lung fibroblasts and provide evidence that EDA-FN induces fibroblast differentiation through binding of the EDA segment to α4β7 integrin receptor and activation of FAK and MAP kinase Erk1/2.
Previous studies have reported that EDA-FN is more potent than the EDA-lacking form pFN in promoting fibroblast adhesion, migration, and proliferation (27, 28). EDA-FN also induces the expression of α-SMA in lipocytes more efficiently than pFN, resulting in the differentiation of these cells into myofibroblast-like cells (10). In this study we show that EDA-FN, but not pFN, induces α-SMA expression, collagen production, gel contractility, and TGF-β1 release on primary lung fibroblasts. These effects are blocked by the anti-EDA monoclonal antibody (IST-9), thus adding further evidence for a role of the EDA segment of cFN on fibroblast differentiation.
Although ours and several other studies suggest the presence of a specific cell surface receptor that binds EDA to mediate the effect of EDA-FN on fibroblast differentiation (2, 10, 12, 29), no such receptor has been identified to date. In this study we demonstrate that α4β7 integrin is constitutively expressed in murine and human lung fibroblasts and that cell adhesion to EDA-FN is partially mediated by this receptor. Expression of the α4β7 integrin has previously been reported in human and murine eosinophils, basophils, NK cells, CD4+ T, B lymphocytes and endothelial cells (30–32). This integrin plays a role in cell adhesion, rolling, differentiation, and survival of eosinophils and lymphocytes (30–34). β7-deficient mice have impaired recruitment of lymphocytes, mast cell progenitors and eosinophils to the gastrointestinal tract (35). We did not observe the expression of the αE subunit, which has also been shown to form complexes with β7 (13, 30). Cytokines and growth factors are likely to regulate the expression of α4β7. While tumor necrosis factor α and interleukin (IL)-1 β increase β7 expression in endothelial cells (32), IL-5 and platelet-activating factor (PAF) do not affect α4β7 expression on eosinophils (34, 35). Addition of TGF-β1 increases the expression of α4 subunit on lung fibroblasts (15); however, we found that it does not alter the expression of the β7 subunit in lung fibroblasts.
We found that EDA-FN induces lung fibroblast differentiation via α4β7 integrin, since blocking of this integrin attenuates this effect. Furthermore, using β7 blocking antibodies, we demonstrated that the β7 subunit is essential for the stimulatory effect of EDA-FN. α4β7 integrin binding sites have been previously identified in minimal sequences that share the dipeptide DV present on the CS-1 segment of the IIICS region (36, 37) and in the KLDAPT sequence present on the III5 repeat (38) of all forms of FN. A previous study has reported that EDA-FN increases eosinophil survival through α4β7 integrin and these authors hypothesize that this effect is mediated by the α4β7 integrin binding site on the CS-1 peptide (33). Our findings clearly demonstrate that fibroblast differentiation is not mediated via the α4β7 binding site present on the IIICS or the III5 regions as pFN does not induce differentiation. In the present study, we provide evidence for the existence of another binding site for α4β7 located on the EDA-segment; however, the amino acid sequence that binds α4β7 integrin has not yet been identified. It has been shown that the EDGIHEL sequence included in the EDA segment binds α4β1 and α9β1 integrins (21). The EDA domain includes DV segments (ref. 39, GenBank accession number AF 095690) and it is possible that α4β7 binds to EDA via DV-containing sequences. Further studies are necessary to address this issue.
It has been proposed that the EDA segment might not induce fibroblast differentiation directly, but rather its insertion into the FN molecule brings about a conformational change thereby increasing the accessibility of the RGD binding site to α5β1 (27) or exposing other integrin binding sites present on FN. Our study demonstrates that this is unlikely to be the case for fibroblast differentiation. This is supported by three lines of evidence: EDA-FN-induced α-SMA expression is not affected by the addition of blocking antibodies against α5β1 and β1 integrins; recombinant EDA-containing peptides are sufficient to induce fibroblast differentiation; and the addition of anti-α4β7 blocking antibodies inhibits recombinant EDA-containing peptides-induced α-SMA expression. Thus, taken together, these findings strongly suggest that the EDA segment itself binds to α4β7 integrin and thereby induces a myofibroblast phenotype. Our results are consistent with previous studies showing that antibodies against EDA (clone IST-9) inhibit fibroblast differentiation and that addition of a recombinant EDA segment promotes 3T3 cell adhesion (10, 12, 29). Jarnagin et al. (10) found that recombinant EDA-containing peptides increased α-SMA expression in lipocytes; while Serini et al. (12) showed inhibitory effects for recombinant EDA fragments on α-SMA expression in skin fibroblasts. These discrepancies may be due to the different structure of the peptides used.
The intracellular signal transduction pathways triggered following the binding of EDA-FN to integrin receptors have yet not been elucidated. Binding of different isoforms of FN to integrins activates focal adhesion kinase (FAK) (24), an early mediator of integrin-linked intracellular signals (25). FAK activation has been previously associated with fibroblast differentiation (14, 15). We show in this study that the EDA segment triggers FAK activation following binding to α4β7 integrin. Interestingly, Thannickal et al. (15) found that induction of myofibroblast differentiation via TGF-β1 requires integrin-dependent FAK activation and they suggest that this effect may be through induction of integrin receptors and/or enhanced FN expression in response to TGF-β1. Mitogen-activated protein kinase (MAPK) Erk1/2 is involved in cell differentiation (26). We found that EDA-FN and EDA-containing peptides activate MAPK Erk1/2 in primary fibroblasts. Blocking of MAPK Erk1/2 by biochemical inhibitors of MAPK kinase MEK1 and MEK2 abrogated EDA-FN-induced α-SMA expression. Activation of Erk2 by EDA-FN has also been observed in CHO cells but, in contrast to our findings, in this study, Erk2 activation was observed in the absence of FAK (28). Our results, therefore, demonstrate that activation of MAPK Erk1/2 is essential for fibroblast differentiation and is mediated by the EDA segment of FN.
In summary, we demonstrate that EDA-FN contributes to fibroblast differentiation through binding of the EDA segment to α4β7 integrin and activation of the intracellular signal transduction proteins FAK and MAP kinase Erk1/2. Our study advances present understanding of the mechanism by which EDA-FN mediates fibroblast differentiation and fibrogenesis. How EDA-FN interacts with TGF-β1 and/or mechanical tension to induce fibroblast differentiation requires further investigation.
Acknowledgments
This research was supported by grants from the Israel Science Foundation (1408/08) and the Chief Scientist Office of the Ministry of Health, Israel (4921).
The authors are grateful to Ashley M. Cornett, M.Sc., for her technical assistance in performing cell adhesion assays. The authors declare no conflict of interest.
REFERENCES
- 1..Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C., Brown R. A. (2002) Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat. Rev. Mol. Cell. Biol. , 349–363 [DOI] [PubMed] [Google Scholar]
- 2..Gabbiani G. (2003) The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. , 500–503 [DOI] [PubMed] [Google Scholar]
- 3..Hinz B., Phan S. H., Thannickal V. J., Galli A., Bochaton-Piallat M. L., Gabbiani G. (2007) The myofibroblast: one function, multiple origins. Am. J. Pathol. , 1807–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4..Hynes R. O. (1990) In Fibronectins (1st Ed.) (, Rich A. Editor), Springer-Verlag, New York, New York, USA [Google Scholar]
- 5..Kornblihtt A. R., Pesce C. G., Alonso C. R., Cramer P., Srebrow A., Werbajh S., Muro A. F. (1996) The fibronectin gene as a model for splicing and transcription studies. FASEB J. , 248–257 [DOI] [PubMed] [Google Scholar]
- 6..Muro A. F., Chauhan A. K., Gajovic S., Iaconcig A., Porro F., Stanta G., Baralle F. E. (2003) Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J. Cell Biol. , 149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7..Chauhan A. K., Iaconcig A., Baralle F. E., Muro A. F. (2004) Alternative splicing of fibronectin: a mouse model demonstrates the identity of in vitro and in vivo systems and the processing autonomy of regulated exons in adult mice. Gene , 55–63 [DOI] [PubMed] [Google Scholar]
- 8..Oyama F., Murata Y., Suganuma N., Kimura T., Titani K., Sekiguchi K. (1989) Patterns of alternative splicing of fibronectin pre-mRNA in human adult and fetal tissues. Biochemistry , 1428–1434 [DOI] [PubMed] [Google Scholar]
- 9..Pagani F., Zagato L., Vergani C., Casari G., Sidoli A., Baralle F. E. (1991) Tissue-specific splicing pattern of fibronectin messenger RNA precursor during development and aging in rat. J. Cell Biol. , 1223–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10..Jarnagin W. R., Rockey D. C., Koteliansky V. E., Wang S. S., Bissell D. M. (1994) Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J. Cell Biol. , 2037–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11..Muro A. F., Moretti F. A., Moore B. B., Yan M., Atrasz R. G., Wilke C. A., Flaherty K. R., Martinez F. J., Tsui J. L., Sheppard D., Baralle F. E., Toews G. B., White E. S. (2008) An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am. J. Respir. Crit. Care. Med. , 638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12..Serini G., Bochaton-Piallat M. L., Ropraz P., Geinoz A., Borsi L., Zardi L., Gabbiani G. (1998) The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by TGF-β1. J. Cell Biol. , 872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13..Takada Y, Ye X, Simon S. (2007) The integrins. Genome Biol. , 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14..Fernandes D. J., Bonacci J. V., Stewart A. G. (2006) Extracellular matrix, integrins and mesenchymal cell function in the airways. Curr. Drug Targets , 567–577 [DOI] [PubMed] [Google Scholar]
- 15..Thannickal V. J., Lee D. Y., White E. S., Cui Z., Larios J. M., Chacon R., Horowitz J. C., Day R. M., Thomas P. E. (2003) Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. , 12384–12389 [DOI] [PubMed] [Google Scholar]
- 16..Leiss M., Beckmann K., Giros A., Costell M., Fassler R. (2008) The role of integrin binding sites in FN matrix assembly in vivo. Curr. Opin. Cell Biol. , 502–507 [DOI] [PubMed] [Google Scholar]
- 17..Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M. (1989) Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J. Cell Biol. , 863–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18..White E. S., Baralle F. E., Muro A. F. (2008) New insights into form and function of fibronectin splice variants. J. Pathol. , 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19..White E. S., Thannickal V. J., Carskadon S. L., Dickie E. G., Livant D. L., Markwart S., Toews G. B., Arenberg D. A. (2003) Integrin α4β1 regulates migration across basement membranes by lung fibroblasts. Am. J. Respir. Crit. Care Med. , 436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20..Kanayama M., Kurotaki D., Morimoto J., Asano T., Matsui Y., Nakayama Y., Saito Y., Ito K., Kimura C., Iwasaki N., Suzuki K., Harada T., Li H. M., Uehara J., Miyazaki T., Minami A., Kon S., Uede T. (2009) Alpha9 integrin and its ligands constitute critical joint microenvironments for development of autoimmune arthritis. J. Immunol. , 8015–8025 [DOI] [PubMed] [Google Scholar]
- 21..Liao Y. F., Gotwals P. J., Koteliansky V. E., Sheppard D., Van De Water L. (2002) The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J. Biol. Chem. , 14467–14474 [DOI] [PubMed] [Google Scholar]
- 22..Kohan M., Breuer R., Berkman N. (2009) Osteopontin induces airway remodeling and lung fibroblast activation in a murine model of asthma. Am. J. Respir. Cell Mol. Biol. , 290–296 [DOI] [PubMed] [Google Scholar]
- 23..Puxeddu I., Bader R., Piliponsky A. M., Reich R., Levi-Schaffer F., Berkman N. (2006) The CC chemokine eotaxin/CCL11 has a selective profibrogenic effect on human lung fibroblasts. J. Allergy Clin. Immunol. , 103–110 [DOI] [PubMed] [Google Scholar]
- 24..Garcia A. J., Boettiger D. (1999) Integrin-fibronectin interactions at the cell-material interface: initial integrin binding and signaling. Biomaterials , 2427–2433 [DOI] [PubMed] [Google Scholar]
- 25..Guan J. L., Shalloway D. (1992) Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature , 690–692 [DOI] [PubMed] [Google Scholar]
- 26..Zhang Y., Dong C. (2007) Regulatory mechanisms of mitogen-activated kinase signaling. Cell. Mol. Life Sci. , 2771–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27..Manabe R., Ohe N., Maeda T., Fukuda T., Sekiguchi K. (1997) Modulation of cell-adhesive activity of fibronectin by the alternatively spliced EDA segment. J. Cell Biol. , 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28..Manabe R., Oh-e N., Sekiguchi K. (1999) Alternatively spliced EDA segment regulates fibronectin-dependent cell cycle progression and mitogenic signal transduction. J. Biol. Chem. , 5919–5924 [DOI] [PubMed] [Google Scholar]
- 29..Xia P., Culp L. A. (1995) Adhesion activity in fibronectin's alternatively spliced domain EDa (EIIIA): complementary to plasma fibronectin functions. Exp. Cell Res. , 517–527 [DOI] [PubMed] [Google Scholar]
- 30..Shaw S., Brenner M. (1995) The β7 integrin in mucosal homing and retention. Semin. Immunol. , 335–342 [DOI] [PubMed] [Google Scholar]
- 31..Lundahl J., Sehmi R., Hayes L., Howie K., Denburg J. A. (2000) Selective upregulation of a functional beta7 integrin on differentiating eosinophils. Allergy , 865–872 [DOI] [PubMed] [Google Scholar]
- 32..Brezinschek R. I., Brezinschek H. P., Lazarovits A. I., Lipsky P. E., Oppenheimer-Marks N. (1996) Expression of the beta 7 integrin by human endothelial cells. Am. J. Pathol. , 1651–1660 [PMC free article] [PubMed] [Google Scholar]
- 33..Meerschaert J., Vrtis R. F., Shikama Y., Sedgwick J. B., Busse W. W., Mosher D. F. (1999) Engagement of alpha4beta7 integrins by monoclonal antibodies or ligands enhances survival of human eosinophils in vitro. J. Immunol. , 6217–6227 [PubMed] [Google Scholar]
- 34..Walsh G. M., Symon F. A., Lazarovils A. L., Wardlaw A. J. (1996) Integrin alpha 4 beta 7 mediates human eosinophil interaction with MAdCAM-1, VCAM-1 and fibronectin. Immunology , 112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35..Brandt E. B., Zimmermann N., Muntel E. E., Yamada Y., Pope S. M., Mishra A., Hogan S. P., Rothenberg M. E. (2006) The alpha4beta7-integrin is dynamically expressed on murine eosinophils and involved in eosinophil trafficking to the intestine. Clin. Exp. Allergy , 543–553 [DOI] [PubMed] [Google Scholar]
- 36..Rüegg C., Postigo A. A., Sikorski E. E., Butcher E. C., Pytela R., Erle D. J. (1992) Role of integrin alpha 4 beta 7/alpha 4 beta P in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J. Cell Biol. , 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37..Hynes R. O. (1987) Integrins: a family of cell surface receptors. Cell , 549–554 [DOI] [PubMed] [Google Scholar]
- 38..Moyano J. V., Carnemolla B., Domínguez-Jiménez C., García-Gila M., Albar J. P., Sánchez-Aparicio P., Leprini A., Querzé G, Zardi L., Garcia-Pardo A. (1997) Fibronectin type III5 repeat contains a novel cell adhesion sequence, KLDAPT,. which. binds. activated. alpha4beta1. and. alpha4beta7. integrins. J. Biol. Chem. , 24832–24836 [DOI] [PubMed] [Google Scholar]
- 39..Muro A. F., Iaconcig A., Baralle F. E. (1998) Regulation of the fibronectin EDA exon alternative splicing. Cooperative role of the exonic enhancer element and the 5′ splicing site. FEBS Lett. , 137–141 [DOI] [PubMed] [Google Scholar]