Abstract
We have devised a method of using intracellular combinatorial libraries to select antibodies that control cell fates. Many agonist antibodies have been selected with this method, and the process appears to be limited only by the availability of a phenotypic selection system. We demonstrate the utility of this approach to discover agonist antibodies that engage an unanticipated target and regulate macrophage polarization by selective induction of anti-inflammatory M2 macrophages. This antibody was used therapeutically to block autoimmunity in a classic mouse model of spontaneous systemic lupus erythematosus.—Han, K. H., Gonzalez-Quintial, R., Peng, Y., Baccala R., Theofilopoulos, A. N., Lerner, R. A. An agonist antibody that blocks autoimmunity by inducing anti-inflammatory macrophages.
Keywords: cathepsin G, CTSG, CD14, M2 macrophage
The fundamental problem of the immune system is that it must kill an offending organism or tumor with minimal damage to itself. This feat is difficult because the target and surrounding tissue are, chemically speaking, essentially the same. Thus, we know of many tissue injuries that are the result of the “shrapnel” from an immune attack—a kind of collateral damage. Nature has evolved at least 3 less-than-perfect solutions to the problem of protecting the integrity of the host during an immune attack. The first is containment, the second is high specificity of the T-cell– or antibody-mediated response, and the third is an elaborate system of checks and balances. Thus, macrophages engulf bacteria and contain them in vesicles into which highly toxic chemicals are pumped. On the cellular side, the system of checks and balances operates, for example, by balancing effector and regulatory T cells with proinflammatory and anti-inflammatory macrophages. However, in a therapeutic setting, one or the other arm of an otherwise balanced system may have to be favored. Such control has become one of the major goals in immunology for the treatment of autoimmunity and cancer. Given our increasing understanding of the importance of activating or suppressing immune cellular function, there is an urgent search for new cellular targets and molecules that will perturb cells that express them.
We have devised a method of using intracellular combinatorial libraries to select antibodies that control cell fates. Many agonist antibodies have been selected by this method, and the process appears to be limited only by the availability of a phenotypic selection system (1–6). As a discovery tool, the system is most effective when unbiased antibody libraries are used, because it allows the discovery of new targets and antibodies that activate the cells expressing them. The power of the system derives from its autocrine nature, as each cell expresses both 1 member of an antibody library containing ∼108 members and its potential targets. In this report, we show how the system can be used to discover agonist antibodies that engage unanticipated targets and regulate macrophage polarization by selective induction of anti-inflammatory M2 macrophages. These antibodies regulate the in vivo outcomes of an autoimmune disease.
MATERIALS AND METHODS
Mouse strains and cell lines
The following mouse strains were used: C57BL/6J, BALB/c, C3H/HeJ, C3H/HeOuJ, C57BL/10ScNJ, B6.129S4-CD14tm1frm/J, and MRL/MPJ-Fas (lpr)/J (The Jackson Laboratory, Sacramento, CA, USA). Cathepsin G (CTSG)-knockout mice were the generous gift of Dr. C. Pham (Washington University, St. Louis, MO, USA). Nuclear factor of activated T cells (NFAT)c1−/− and NFATc2flox/flox:CD4Cre mice were the generous gift of Dr. A. Rao (La Jolla Institute for Allergy and Immunology, La Jolla, CA, USA). The human embryonic kidney (HEK)293T cell line was maintained in DMEM containing 10% fetal calf serum (FCS), penicillin, and streptomycin (Thermo Fisher-Invitrogen, Carlsbad, CA, USA). The HEK293F cell line was maintained in Freestyle 293 Expression Medium with 4 mM Glutamax (Thermo Fisher-Invitrogen). Human total bone marrow cells (All-Cells, Alameda, CA, USA) were cultured in StemSpan serum-free medium with cytokine cocktail (Stemcell Technologies, Vancouver, BC, Canada). Mouse bone marrow cells were cultured in DMEM/F12 containing 10% FCS, penicillin, and streptomycin. The mice were housed and handled according to protocols approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute.
Combinatorial antibody library and lentivirus
Single-chain Fv (ScFv) genes were obtained from a naive human combinatorial antibody library (1 × 1011 library diversity) and subcloned into a lentiviral vector. Lentivirus was produced in HEK293T cells by cotransfection of lentiviral vectors with the pCMVD8.91 and pVSVg viral packaging vectors at a ratio of 1:1:1. Supernatants containing virus were collected at 48 h after transfection. Cell debris was removed by centrifugation and filtration through a 0.22 μm polyethersulfone membrane filter unit (EMS-Millipore, Billerica, MA, USA). The titer of the lentivirus preparation was determined with a Lenti-X p24 ELISA (Clontech, Mountain View, CA, USA). The virus preparations were divided into aliquots and frozen at −80°C.
Transduction and colony-forming cell assay
The bone marrow cells were incubated with lentivirus for 3 d at 37°C. Agonist antibodies were selected by a colony-forming cell assay with methylcellulose-based medium. Bone marrow cells were transduced with the lentiviral antibody library at a multiplicity of infection of 2 and added to the methylcellulose medium at final concentrations of 1.27% methylcellulose and ∼3 × 104 cells/ml. A total of 1.5 ml cell suspension was added to 35 mm diameter dishes and cultured on soft agar for 2 wk. The colonies were harvested with the aid of a micromanipulator (Sutter Instruments, Novato, CA, USA). The antibody genes from each colony were amplified by PCR with primer pairs customized for our lentiviral vector. The amplified antibody genes were analyzed by electrophoresis and recovered.
Purification of ScFv-Fc proteins
For single antibodies, the antibody expression vector was transfected into HEK293F cells. Antibodies from the pooled supernatants were purified using HiTrap Protein G high-performance (HP) columns with an ÄKTAxpress purifier (General Electric Healthcare, Marlborough, MA, USA). The buffer was exchanged to Dulbecco’s PBS (pH 7.4) and stored at 4°C. The vector encoding the ScFv-Fc tag fusion protein was transfected into HEK293F cells for transient expression.
Immunoprecipitation and mass spectrometry
For immunoprecipitation, mouse bone marrow cells were prepared and solubilized in lysis buffer. The lysates were incubated with selected antibody (named LKAb) for 2 h at 4°C, followed by incubation with 50 μl of protein G-Sepharose beads (Thermo Fisher-Pierce, Waltham, MA, USA). The eluent was introduced into the linear trap quadrupole mass spectrometer from a nano-ion source with a 2 kV electrospray voltage. The analysis method consisted of a full MS scan with a range of 400–2000 mass-to-charge ratio followed by data-dependent tandem mass spectrometry (MS/MS) on the 3 most intense ions from the full MS scan. The raw data from the linear trap quadrupole were searched by using the International Protein Index human FASTA database with the Mascot search engine (http://www.matrixscience.com/; Matrix Science, Boston, MA, USA).
Western blot analysis
Bone marrow cells were washed with PBS and then lysed in lysis buffer {50 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES; pH 7.2), 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 10% glycerol, and 1% Triton X-100}. The lysates were then centrifuged at 20,000 g for 15 min at 4°C. The proteins were denatured in Laemmli sample buffer (5 min at 95°C), separated by SDS-PAGE, and transferred to nitrocellulose membranes using a blot analysis system (iBlot; Thermo Fisher-Invitrogen). The membranes were blocked in PBS with Tween 20 (PBST) containing 5% BSA for 30 min before being incubated with antibodies for 3 h. A CTSG antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used for identification. After the membranes were washed several times with PBST, the blots were incubated with horseradish peroxidase–conjugated anti-goat or anti-rabbit antibody for 1 h. The membranes were then washed with PBST and developed by ECL. Phosphorylation was performed with phospho-AKT, ERK, and p38 (Cell Signaling Technology, Beverly, MA, USA).
Silencing CTSG mRNA
Mouse CTSG short hairpin (sh)RNA (Santa Cruz Biotechnology), used for producing lentiviral particles, contain 3 target-specific constructs designed to knock down gene expression. Mouse bone marrow cells were infected with lentivirus CTSG shRNA and cultured with LKAb or macrophage colony-stimulating factor (M-CSF) for 6 d.
Flow cytometry and cell sorting
Cells were stained with anti-mouse or human cluster of differentiation CD11b, CD11c, CD14, F4/80, CD16/32, CD36, CD86, MHCΙΙ, CD64, and CD200R (BD Biosciences, San Jose, CA, USA). Stained cells were analyzed with an LSRII flow cytometer (BD Biosciences). To obtain CD11b+ and CD11b− bone marrow cells, CD11b+ microbeads (15 μl/108 cells; Miltenyi Biotec, San Diego, CA, USA) were added to a suspension of 108 cells/ml of separation buffer and incubated for 15 min on ice, followed by washing. The cells were resuspended in separation buffer (500 μl/108 cells), run through a SuperMACS II Separation Unit equipped with an MS column (Miltenyi Biotec), and washed 3 times with buffer (500 μl), and flow-through cells were collected under pressure.
Real-time quantitative RT-PCR
Macrophages were obtained from bone marrow cells cultured with LKAb antibody or M-CSF, RNA was extracted (Qiagen, Valencia, CA, USA), and cDNA was synthesized (Thermo Fisher-Invitrogen). PCR was performed in triplicate using 400 ng cDNA, RT SYBR Green Supermix, primer sets specific for mouse arginase (ARG)-1 or indoleamine 2,3 dioxygenase (IDO)-1 gene sequences, and a C1000 thermal cycler (all from Bio-Rad Laboratories, Hercules, CA, USA).
CTSG enzymatic activity
To monitor enzyme activities, we used an assay based on CTSG-mediated cleavage of a specific substrate and release of the dye group pNA (4-nitroaniline). This assay was performed with mouse bone marrow lysates that were incubated with LKAb or CTSG inhibitor.
Cytokine assay
Cytokines were quantified in serum collected from individual mice using a mouse cytokine magnetic bead panel (EMS-Millipore, San Diego, CA, USA) according to manufacturer’s instructions.
Treatment with isolated antibody
MRL-Faslpr mice were treated with LKAb (75 µg/mouse, i.p.) 2 times per week. Treatments were initiated at 6 wk of age, and the experiment was terminated at 20 wk of age. Total and anti-chromatin serum IgG subclasses were assessed by ELISA with 96-well plates coated with goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and mouse chromatin, respectively. Bound antibodies were detected using alkaline phosphatase–conjugated goat antibodies (Caltag Laboratories, Carlsbad, CA, USA) to mouse IgG or IgG2a, the main autoantibody subclass in this model. Standard curves were generated with calibrated mouse serum (Accurate Chemical and Scientific Company, Westbury, NY, USA).
Statistical analysis
The data are expressed as means ± se. Statistical analysis was performed with Student’s t test or 1-way ANOVA and the post hoc test. The groups were analyzed by unpaired 2-tailed Student’s t test. Survival was analyzed by Kaplan-Meier plot and log-rank test. Results reaching P < 0.05 were significant.
RESULTS
Selection system
Intracellular combinatorial antibodies have been used recently to select a wide variety of potent agonists. Antibody libraries in lentiviruses are used to infect cells followed by cell selection based on phenotype. There are 2 entry points in such experiments. In the biased case, large antibody libraries (∼1011 members) are selected for binding to a given target, after which all binding antibodies are transferred to lentiviruses for infection of cells. The infected cells can be selected for those that have a particular phenotype using, for example, fluorescence-activated cell sorting (FACS) analysis or morphologic criteria. Alternatively, unbiased libraries can be used so that the targets are not preordained. This format allows simultaneous identification of new targets and antibodies that induce proliferation or differentiation of the cells that express them.
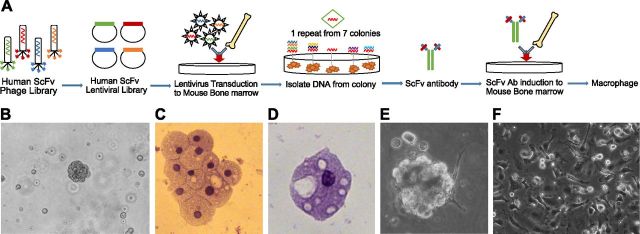
The general selection scheme is illustrated in Fig. 1A. An unbiased antibody library in lentiviruses containing ∼108 unique members was used to infect total mouse bone marrow cells. The infected cells were plated onto soft agar to observe the formation of colonies (Fig. 1B). Large colonies were harvested, and the morphology of the cells therein was determined. All colonies contained cells with the classic morphology of macrophages (Fig. 1C, D). The antibody gene integrated into the cells was recovered by PCR. One sequence was repeated in 7 colonies, and this antibody was chosen for further studies. This antibody gene was reinserted into lentivirus, and fresh cells were infected. The cells, now infected with a single lentivirus, also formed colonies (Fig. 1E) and contained cells with a morphology that was similar to that of the cells selected after the first round of infection. To characterize the selected antibody (LKAb), the encoding gene was transferred to a mammalian expression vector. When the purified antibody was incubated with bone marrow, a large percentage of the cells attached to the plate and developed a macrophage morphology (Fig. 1F).
Figure 1.
Selection of an agonist antibody that induces macrophage cell differentiation. A) Phenotype selection. The selection starts with a human ScFv phage library (109 members). ScFv genes were transferred to a lentiviral vector to make lentiviral intrabody libraries. Total mouse bone marrow cells were infected with the antibody library and plated on soft agar. B) After 2 wk of incubation, 7 colonies with compact morphologies had grown. C and D) These colonies were harvested, and the antibody genes were recovered by PCR. One sequence was present in all colonies and was used for further studies. Hematoxylin and eosin staining of cells from the colonies showed cells with the classic morphologies of macrophages. E) The experiment was repeated using the selected sequence incorporated into lentivirus. This antibody clone (identified as LKAb) again induced colonies with a similar morphology that contained cells with macrophage morphologies. F) After a 1 wk incubation with the purified LKAb antibody, bone marrow cells differentiated in culture into cells with morphology consistent with that of macrophages.
Identification of a novel target
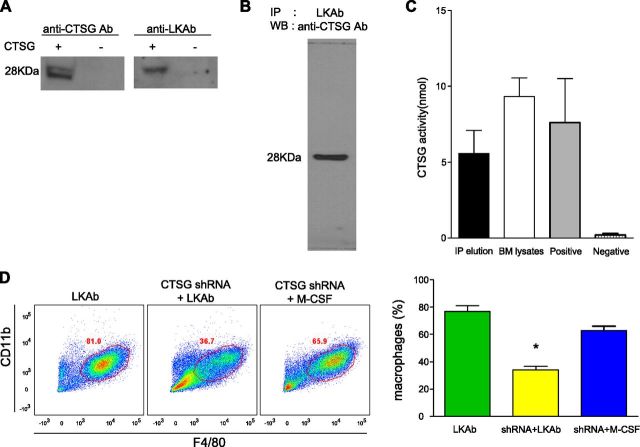
The purified antibody was incubated with an extract of total mouse bone marrow cells, and the immune complexes were captured on a protein A/G column. Proteins that reacted with the antibody were identified by silver staining of SDS gels, and their identity was determined by MS (Supplemental Fig. 1A, B). Four candidate proteins based on the number of peptide “hits” were tested for their ability to bind to antibody LKAb (Supplemental Fig. 1C). Of the candidate antigens, only CTSG bound to LKAb, as determined by Western blot analysis and ELISA (data not shown). CTSG is known to be secreted by activated leukocytes, but it has also been shown to be present on the surface of monocytic cells (7). CTSG was confirmed as the target antigen by comparing LKAb to a commercial anti-CTSG antibody using Western blot analysis of an extract from cells overexpressing CTSG (Fig. 2A). The commercial anti-CTSG antibody also reacted strongly with a protein from mouse bone marrow extracts that was first captured by our antibody (Fig. 2B). Moreover, the protein fraction captured by our antibody was shown to have CTSG activity (Fig. 2C). Finally, when CTSG expression was silenced with shRNA, the macrophage formation induced by our antibody was markedly reduced (Fig. 2D).
Figure 2.
Identification of a novel antigen recognized by LKAb. A) Both commercial anti-CTSG antibody and LKAb bound to a 28 kDa protein in Western blots of lysates from cells overexpressing CTSG. B) Lysates of mouse bone marrow cells were incubated with LKAb for immunopurification. Eluates from these immune complexes bound to the commercial anti-CTSG antibody in Western blots. C) A CTSG enzymatic assay showed that CTSG enzyme activity was present in the eluates and mouse bone marrow total lysates. D) Mouse bone marrow was incubated for 2 d with lentiviruses containing CTSG shRNA, followed by incubation with LKAb (10 μg/ml) or M-CSF (10 ng/ml) for 6 d. The cells were then stained with anti-CD11b and anti-F4/80. FACS analysis showed that the induced macrophage populations were dramatically reduced when CTSG mRNA was silenced. However, M-CSF still induced macrophages when CTSG mRNA was silenced. Right: percentage of macrophages. *P < 0.05; Student’s t test.
Selective induction of M2 macrophages
Mouse bone marrow cells were incubated with the selected antibody, and FACS analysis using CD11b and CD11c markers was performed to confirm that LKAb acted in a dose-dependent manner and that the induced cells were of macrophage, not dendritic, cell lineage (Fig. 3A). To determine which cell population underwent differentiation induced by our antibody, bone marrow cells were sorted according to the presence or absence of the CD11b marker. Only the CD11b− population differentiated into macrophages (Fig. 3B). By contrast, M-CSF induced macrophage differentiation in both populations. FACS analysis using the M1 markers CD16/32 and CD86 and the M2 markers CD36 (scavenger receptor), MHCΙΙ, and CD14 showed that the macrophages induced by the antibody were mostly of the M2 type (Fig. 3C). The high selectivity of our antibody for induction of M2 macrophages was confirmed by quantitative (q)RT-PCR analysis comparing the ability of the cells induced by either LKAb or M-CSF to express the M1 marker IDO1 or the M2 marker ARG-1. Cells induced by our antibody displayed high expression of ARG-1 and limited expression of IDO-1, whereas cells induced with M-CSF showed high expression of IDO1 and only low expression of ARG-1 (Fig. 3D).
Figure 3.
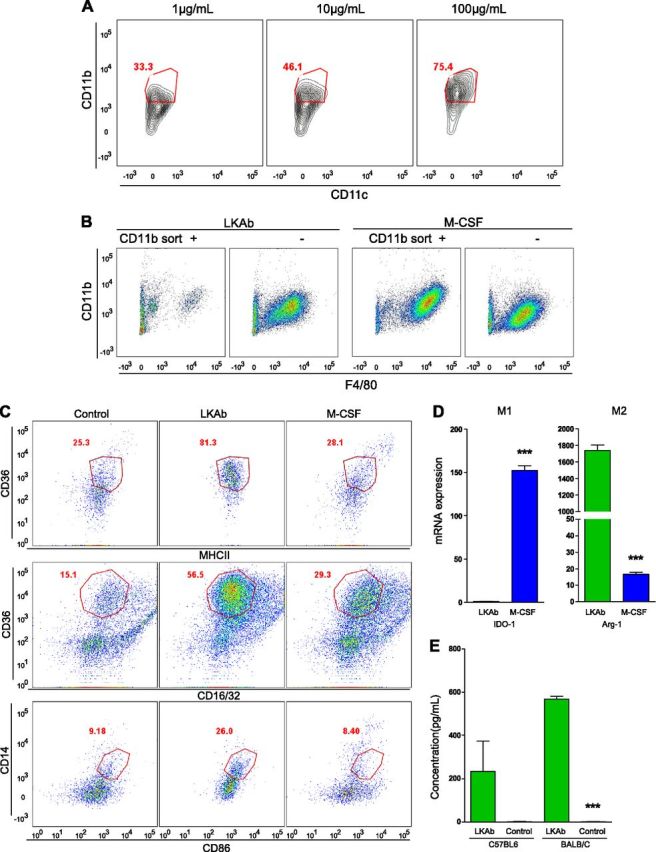
LKAb induced anti-inflammatory M2 macrophage differentiation. A) Mouse bone marrow cells were incubated with LKAb at the indicated concentrations (1–100 µg/ml) for 6 d. The cells were then stained with anti-CD11b and -CD11c and analyzed by FACS. Red outline: positive macrophage populations. LKAb induced differentiation of CD11b+ macrophages, but not CD11c+ dendritic cells, in a dose-dependent manner. B) Mouse total bone marrow cells were separated by using CD11b-specific magnetic beads, and the isolated CD11b+ or CD11b− populations were incubated with LKAb or M-CSF for 6 d. The cells were then stained with anti-CD11b and -F4/80 and analyzed by FACS. Macrophages differentiated mainly from the CD11b− population. C) Mouse bone marrow was incubated with medium, LKAb, or M-CSF for 6 d. Cells were then stained with anti-CD16/32 and -CD86 as M1 macrophage markers, and anti-CD36, -MHCΙΙ, and -CD14 as M2 macrophage markers. Red outline: M2-type-specific populations. The macrophages induced by LKAb selectively expressed M2 type markers. D) Mouse bone marrow was induced by LKAb antibody or M-CSF for 6 d. Cells were harvested, and total RNA was extracted for qRT-PCR analysis. IDO1 was used as an M1-specific gene and ARG-1 as an M2-specific gene. qRT-PCR showed high ARG-1 mRNA expression in macrophages induced by LKAb but only low levels of IDO1. E) Macrophages induced by the LKAb in vivo expressed M2 cytokines. LKAb and PBS were injected intraperitoneally into C57BL/6 or BALB/c mice 3 times/wk for 2 wk, and IL-10 levels in the serum were measured. Mice treated with LKAb showed dramatically increased levels of IL-10, one of the major anti-inflammatory cytokines. ***P < 0.0005; Student’s t test.
Expression of the cytokine IL-10 is another feature of M2 macrophages. To determine the in vivo effect of our antibody on IL-10 production, we injected LKAb intraperitoneally into C57BL/6 and BALB/c mice and found significantly higher levels of IL-10 in treated compared with control mice (Fig. 3E). To study the activation of signaling pathways, we treated fresh mouse bone marrow cells with the antibody, and the cell lysates were assessed by Western blot analysis with anti-phospho p38, ERK, and protein kinase B (AKT). ERK and AKT were activated to some extent, but p38 was strongly activated, consistent with its known role in M2 macrophage polarization (Supplemental Fig. 2) (8).
Mechanistic studies
Although CTSG is located on the surface of macrophages and polymorphonuclear leukocytes (PMNs), it lacks a signal transduction domain, and thus its role in cell activation is indirect. Nevertheless, there is an increasing awareness of the role that CTSG plays in the regulation of important cell surface receptors (9). In particular, CTSG has been reported to regulate monocyte activation by down-regulation of CD14, which is a critical coreceptor for TLR4 in the innate immune system and is involved in differentiation of cells in the macrophage lineage (10).
Given its role in the innate immune system and the high concentration of CD14 on the surface of monocytes, we assessed CD14 involvement in differentiation of macrophages by our antibody. Bone marrow cells from CTSG−/−, CD14−/− , and wild-type mice were incubated with LKAb, and FACS analysis with CD14 and F4/80 markers showed that this antibody increased CD14 expression in wild-type cells (Fig. 4A), presumably because the activity of CTSG was inhibited. However, no increase in CD14 was observed in the CTSG−/− or CD14−/− cells. In addition, antibody-mediated induction of the general macrophage marker F4/80 decreased significantly in bone marrow cells from CTSG−/− or CD14−/− mice. Consistent with these results, we found that LKAb inhibited CTSG activity by 50% (Fig. 4B).
Figure 4.
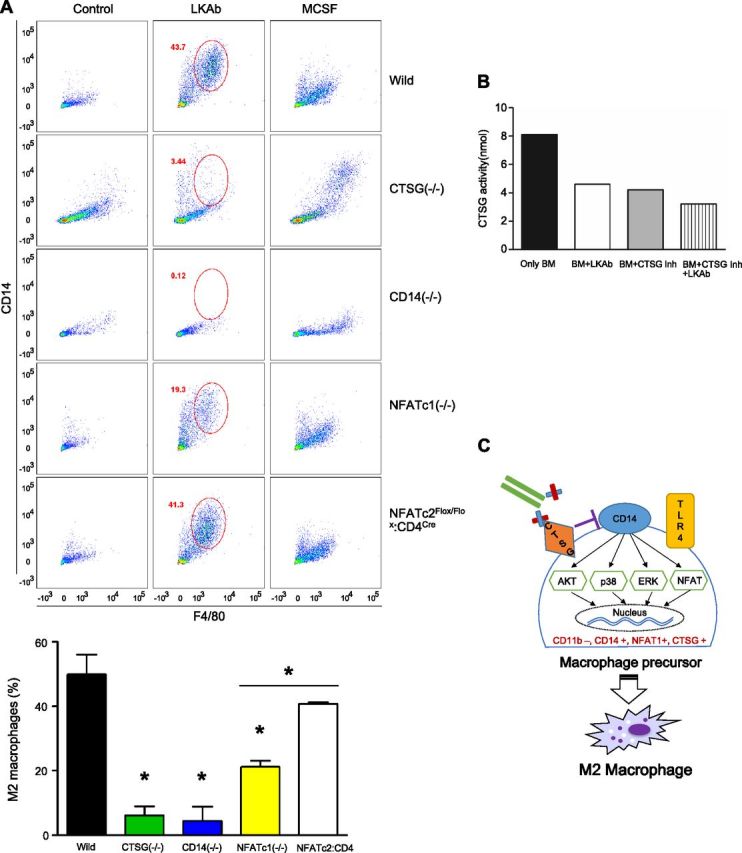
CTSG, CD14, and NAFAT play a key role in LKAb-dependent M2 macrophage differentiation. A) Bone marrow from wild-type, CTSG knockout, CD14 knockout mice, or NFAT-knockout mice were incubated with medium, LKAb, or M-CSF for 6 d. The cells were then stained with anti-CD14 and anti-F4/80. FACS analysis showed CD14 and F4/80 expression was increased by LKAb in normal mice. However, CD14 and F4/80 expression was significantly reduced in both the CTSG- and CD14-knockout mice. CD14 and F4/80 expression was reduced in NFATc1- but not in NFATc2:CD4Cre-knockout mice. Bottom: percentage of M2 macrophages. *P < 0.05; Student's t test. B) Wild-type mouse bone marrow cells were incubated with LKAb, a CTSG inhibitor, or both, and CTSG activity was determined. The antibody-induced reduction of CTSG activity was comparable to that of the inhibitor. C) Critical cell surface components and their downstream signaling pathways potentially involved in macrophage differentiation mediated by LKAb (depicted as bound to CTSG).
Because CD14 and TLR4 are coreceptors, we studied whether TLR4 is also essential for LKAb-mediated macrophage differentiation. Using bone marrow cells from wild-type and TLR4−/− mice, we showed that the cell-polarizing function of our antibody was TLR4 independent (Supplemental Fig. 3). The finding that LKAb needed CD14, but not TLR4, to induce macrophage differentiation left open the question of the mechanism of signal transduction. Unlike TLR4, CD14 does not have a signal-transduction domain. However, dendritic cells have been reported recently to be activated through an NFAT signaling pathway that is dependent on CD14 but independent of TLR4 (11). Therefore, we used NFAT-knockout mice to determine whether this pathway plays a role in macrophage differentiation induced by our antibody. Bone marrow cells from NFATc1−/− and NFATc2flox/flox:CD4Cre mice were incubated with LKAb and analyzed by FACS with the CD14 and F4/80 markers. We found that macrophage differentiation was impaired in NFATc1−/− mice (Fig. 4A). As expected, NFAT inactivation in nonmacrophage cells (such as CD4-expressing T cells and dendritic cells) had no consequence on macrophage differentiation by our antibody.
Thus, we have a picture of CTSG’s role in macrophage polarization, suggesting that this molecule operates through CD14 and NFAT to regulate macrophage activation and differentiation (Fig. 4C).
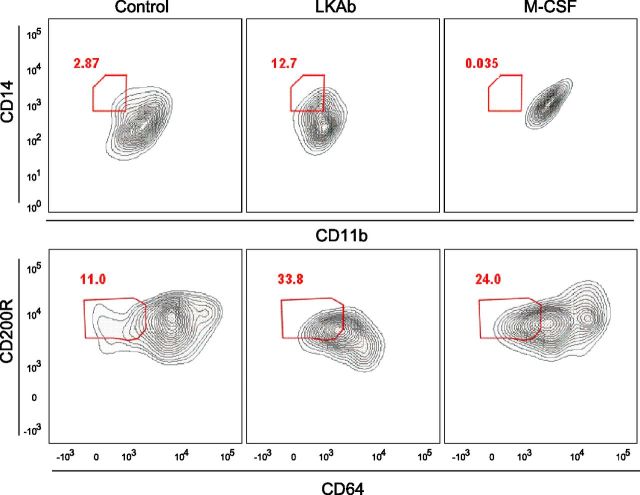
Inducing M2 macrophages from human bone marrow
Because the human and mouse CTSG proteins are highly homologous (Supplemental Fig. 4), we wished to test the ability of our antibody to induce macrophage formation from human total bone marrow. As in the mouse, LKAb selectively induced M2 macrophages, as evidenced by a large increase in cells bearing the CD14 and CD200R markers (Fig. 5)
Figure 5.
LKAb induces human bone marrow cells to differentiate into M2 macrophages. Human total bone marrow was incubated with medium, LKAb, or M-CSF for 6 d. Cells were then stained with anti-CD11b and -CD64 as M1 type markers and anti-CD14 and -CD200R as M2 markers. Red outline: M2-type specific populations. As with mice, LKAb also induced human bone marrow to differentiate into anti-inflammatory macrophages.
LKAb treatment reduces systemic autoimmunity in lupus-prone MRL-lpr mice
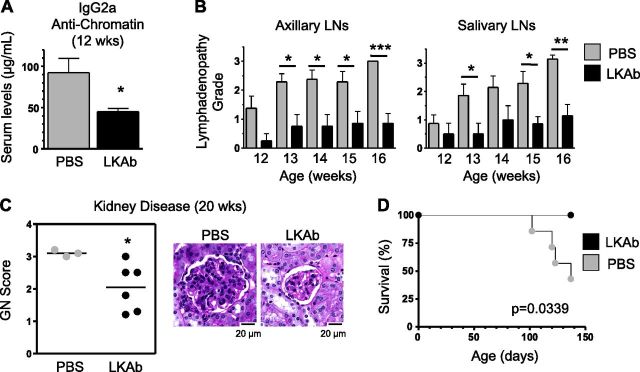
We next investigated whether this antibody could be used therapeutically in lupus, an autoimmune disease in which M1 macrophage infiltration appears to contribute significantly to end-organ pathology. For this experiment, we used MRL-Fas/lpr mice, which develop lupus-like manifestations with high titers of anti-nuclear autoantibodies, immune complex glomerulonephritis (GN), and early mortality, primarily because of defective Fas-mediated T- and B-cell apoptosis (12–14). Treatments with LKAb (75 μg/mouse, i.p. 2 times per week) were initiated at 6 wk of age, and the experiment was terminated at 20 wk of age, when >50% of the control (PBS-treated) mice were dead and the remaining exhibited signs of discomfort caused by advanced disease. The results showed significant reductions in disease parameters in LKAb-treated mice compared with controls. Thus, titers of anti-chromatin IgG2a autoantibodies, the main isotype induced in this model, were reduced in the serum of treated mice (Fig. 6A). The treatment also significantly inhibited progression of lymphadenopathy (Fig. 6B). Moreover, at 20 wk of age, treated mice had significantly reduced GN scores (Fig. 6C) and, accordingly, extended survival (Fig. 6D).
Figure 6.
Treatment with LKAb reduces lupus-like disease in MRL-lpr mice. Mice (6–7/group) were injected intraperitoneally with LKAb or PBS (from the age of 6 wk until termination of the experiment at 20 wk) and followed for manifestations of disease. A) IgG2a anti-chromatin autoantibody levels determined by ELISA at 12 wk of age. B) Progression of lymphadenopathy between 12 and 16 wk of age assessed by palpation of axillary and salivary lymph nodes (LNs) and scored on a 0–4 scale. C) Kidney disease determined by histologic examination for GN at 20 wk of age. Representative images for treated and control mice are shown. D) Kaplan-Meier plot representing survival rates of treated and control mice. *P < 0.05, **P < 0.005, ***P < 0.0005, LKAb-treated vs. PBS control-treated mice; Student’s t test.
DISCUSSION
In this article, we report on the selection of an antibody agonist that induces bone marrow cells to differentiate into M2 macrophages. The primary target of the antibody appears to be CTSG. The requirements for efficient macrophage polarization by this antibody include the presence of CTSG and CD14, which appear to operate via p38, a classic marker for macrophage differentiation (8, 15). The target is unusual, in that CTSG has been thought to be an effector molecule secreted by activated PMNs. There is, however, a growing body of evidence suggesting that the CTSG also plays a regulatory role in PMNs and macrophages. The first indication for an expanded functional role of CTSG was the finding that, in addition to the secreted form, CTSG is associated with the plasma membrane in PMNs and macrophages. Parallel studies showed that CTSG activates protease-activated receptor-4 in human platelets (16), regulates the urokinase receptor CD87 on monocytes (17), and promotes monocyte chemotaxis by activating protease-activated receptor-1 (18).
In general, the expression of CTSG on macrophages, together with CD14, is thought to play a role in the regulation of pro- and anti-inflammatory pathways by meditating M1/M2 polarization (8, 15, 19, 20). In a study particularly pertinent to our results, Le-Barillec et al. (10) showed that CTSG down-regulates LPS-mediated monocyte activation by proteolysis of CD14, leading to cleavage of its glycosylphosphatidylinositol (GPI) anchor. In this system, activation occurred through TLR4-mediated induction of NF-κB. The present study adds to these results by demonstrating that an agonist anti-CTSG antibody will not induce macrophage differentiation from bone marrow precursors in the absence of CD14. Thus, our data suggest a mechanistic model involving a set of interacting molecules on the macrophage plasma membrane that include CTSG, CD14, and TLR4. However, although the exact role of CTSG is not yet fully understood, it is tempting to speculate that CTSG functions via its catalytic activity, as is true of the class of protease-activated receptors such as Notch1 (21). This notion is especially intriguing relative to our studies in murine lupus, given that it has been reported that Notch1 signaling is essential for M2b macrophage polarization in lupus, operating through the PI3K and MAPK pathways (22). Thus, a proteolysis-based activation event has already been shown to impact an autoimmune disease via modification of macrophage polarization (22). In these studies as well as our current one, manipulation of proteolytic activity affected disease outcome. Blockage of Notch-1 through inhibition of γ-secretase ameliorated disease by reducing inflammatory M2b macrophage formation. In contrast, in our study, inhibition of another protease, CTSG, induced the formation of anti-inflammatory M2 macrophages and suppressed disease. In this scenario, CTSG may function as an autocrine regulator of macrophage polarization. Such autocrine-based regulation of cell differentiation involving cell surface proteases is now known to occur in many systems (17, 23–27).
In this model of autocrine regulation, the anti-CTSG antibody can influence cell fate by suppressing catalysis directly and perturbing the structural relationship between the enzyme and its substrate, presumably CD14 and NFAT. CD14-mediated regulation of dendritic cell fates starts with the activation of the Src family kinases, together with the involvement of phospholipase C (PLC)-γ2, which operate via Ca2+ mobilization and NFAT activation (11).
Relative to the therapeutic effect of the LKAb antibody in the MRL-Fas/lpr lupus model, our findings are compatible with the documented role of inflammatory macrophages in lupus kidney pathology, the primary cause of fatal lupus. Thus, the degree of macrophage infiltration in kidneys correlates with renal dysfunction in patients with systemic lupus erythematosus (28–30), and several markers typical of inflammatory M1 macrophages are elevated, including CD86, IFN-γ, IL-6, chemokine (C-C motif) ligand-2, and chemokine (C-X-C motif) ligand-10 (31). Preferential accumulation of M1 compared with M2 macrophages was also observed in kidneys of MRL-Fas/lpr mice, both during spontaneous nephritis and after ischemia/reperfusion renal injury (32). Moreover, reduction of kidney macrophage infiltrates by monocyte chemotactic protein-1 or colony stimulating factor-1 deficiency ameliorated disease in this model (33, 34).
In summary, using an intracellular combinatorial antibody library, we have uncovered an unanticipated function of CTSG in the differentiation of anti-inflammatory M2 macrophages. Although the mechanistic process by which this differentiation is promoted remains incompletely defined, our evidence suggests that this effect is dependent on inhibition of CTSG proteolytic activity, upregulation of CD14, and activation of NFAT-dependent signaling. The efficacy of this antibody in reducing nephritis in a mouse model of lupus suggests that a similar treatment would be applicable to several inflammatory syndromes in which inflammatory macrophages frequently predominate and mediate tissue damage in afflicted organs.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
Acknowledgments
The authors thank Dr. C. Pham (Washington University, St. Louis, MO, USA) and Dr. A. Rao (La Jolla Institute for Allergy and Immunology, La Jolla, CA, USA) for knockout mice; and Drs. T. Bartfai, B. Felding, and Y. J. Kang [The Scripps Research Institute (TSRI)] for scientific discussions. Funding was provided by TSRI. K.H. and R.A.L. are the inventors of the LKAb agonist antibody, which has been patented by TSRI. The patent has been licensed to Zebra Technologies. The remaining authors declare no conflicts of interest.
Glossary
- AKT
protein kinase B
- ARG
arginase
- CD
cluster of differentiation
- CTSG
cathepsin G
- FCS
fetal calf serum
- GN
glomerulonephritis
- GPI
glycosylphosphatidylinositol
- HEK
human embryonic kidney
- IDO
indoleamine 2,3 dioxygenase
- M-CSF
macrophage colony-stimulating factor
- MS
mass spectrometry
- NFAT
nuclear factor of activated T cells
- PBST
PBS-Tween
- PMN
polymorphonuclear leukocyte
- qRT-PCR
quantitative RT-PCR
- ScFv
single-chain Fv
- shRNA
short hairpin RNA
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Zhang H., Yea K., Xie J., Ruiz D., Wilson I. A., Lerner R. A. (2013) Selecting agonists from single cells infected with combinatorial antibody libraries. Chem. Biol. , 734–741 [DOI] [PubMed] [Google Scholar]
- 2.Xie J., Zhang H., Yea K., Lerner R. A. (2013) Autocrine signaling based selection of combinatorial antibodies that transdifferentiate human stem cells. Proc. Natl. Acad. Sci. USA , 8099–8104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H., Xie J., Lerner R. A. (2014) A proximity based general method for identification of ligand and receptor interactions in living cells. Biochem. Biophys. Res. Commun. , 251–255 [DOI] [PubMed] [Google Scholar]
- 4.Xie J., Yea K., Zhang H., Moldt B., He L., Zhu J., Lerner R. A. (2014) Prevention of cell death by antibodies selected from intracellular combinatorial libraries. Chem. Biol. , 274–283 [DOI] [PubMed] [Google Scholar]
- 5.Yea K., Xie J., Zhang H., Zhang W., Lerner R. A. (2015) Selection of multiple agonist antibodies from intracellular combinatorial libraries reveals that cellular receptors are functionally pleiotropic. Curr. Opin. Chem. Biol. , 1–7 [DOI] [PubMed] [Google Scholar]
- 6.Yea K., Zhang H., Xie J., Jones T. M., Yang G., Song B. D., Lerner R. A. (2013) Converting stem cells to dendritic cells by agonist antibodies from unbiased morphogenic selections. Proc. Natl. Acad. Sci. USA , 14966–14971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen C. A. (2008) Leukocyte cell surface proteinases: regulation of expression, functions, and mechanisms of surface localization. Int. J. Biochem. Cell Biol. , 1246–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perdiguero E., Sousa-Victor P., Ruiz-Bonilla V., Jardí M., Caelles C., Serrano A. L., Muñoz-Cánoves P. (2011) p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J. Cell Biol. , 307–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pham C. T. (2006) Neutrophil serine proteases: specific regulators of inflammation. Nat. Rev. Immunol. , 541–550 [DOI] [PubMed] [Google Scholar]
- 10.Le-Barillec K., Pidard D., Balloy V., Chignard M. (2000) Human neutrophil cathepsin G down-regulates LPS-mediated monocyte activation through CD14 proteolysis. J. Leukoc. Biol. , 209–215 [PubMed] [Google Scholar]
- 11.Zanoni I., Ostuni R., Capuano G., Collini M., Caccia M., Ronchi A. E., Rocchetti M., Mingozzi F., Foti M., Chirico G., Costa B., Zaza A., Ricciardi-Castagnoli P., Granucci F. (2009) CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature , 264–268 [DOI] [PubMed] [Google Scholar]
- 12.Theofilopoulos A. N., Dixon F. J. (1985) Murine models of systemic lupus erythematosus. Adv. Immunol. , 269–390 [DOI] [PubMed] [Google Scholar]
- 13.Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. (1992) Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature , 314–317 [DOI] [PubMed] [Google Scholar]
- 14.Watson M. L., Rao J. K., Gilkeson G. S., Ruiz P., Eicher E. M., Pisetsky D. S., Matsuzawa A., Rochelle J. M., Seldin M. F. (1992) Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J. Exp. Med. , 1645–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahay B., Patsey R. L., Eggers C. H., Salazar J. C., Radolf J. D., Sellati T. J. (2009) CD14 signaling restrains chronic inflammation through induction of p38-MAPK/SOCS-dependent tolerance. PLoS Pathog. , e1000687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrano G. R., Huang W., Faruqi T., Mahrus S., Craik C., Coughlin S. R. (2000) Cathepsin G activates protease-activated receptor-4 in human platelets. J. Biol. Chem. , 6819–6823 [DOI] [PubMed] [Google Scholar]
- 17.Beaufort N., Leduc D., Rousselle J. C., Magdolen V., Luther T., Namane A., Chignard M., Pidard D. (2004) Proteolytic regulation of the urokinase receptor/CD87 on monocytic cells by neutrophil elastase and cathepsin G. J. Immunol. , 540–549 [DOI] [PubMed] [Google Scholar]
- 18.Wilson T. J., Nannuru K. C., Singh R. K. (2009) Cathepsin G recruits osteoclast precursors via proteolytic activation of protease-activated receptor-1. Cancer Res. , 3188–3195 [DOI] [PubMed] [Google Scholar]
- 19.Pedron T., Girard R., Chaby R. (2000) Protein phosphorylation pathways involved during lipopolysaccharide-induced expression of CD14 in mouse bone marrow granulocytes. FEMS Immunol. Med. Microbiol. , 247–256 [DOI] [PubMed] [Google Scholar]
- 20.Tundup S., Srivastava L., Nagy T., Harn D. (2014) CD14 influences host immune responses and alternative activation of macrophages during Schistosoma mansoni infection. Infect. Immun. , 3240–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artavanis-Tsakonas S., Rand M. D., Lake R. J. (1999) Notch signaling: cell fate control and signal integration in development. Science , 770–776 [DOI] [PubMed] [Google Scholar]
- 22.Zhang W., Xu W., Xiong S. (2010) Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. J. Immunol. , 6465–6478 [DOI] [PubMed] [Google Scholar]
- 23.Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. (1991) Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell , 1057–1068 [DOI] [PubMed] [Google Scholar]
- 24.Zhou W., Slingerland J. M. (2014) Links between oestrogen receptor activation and proteolysis: relevance to hormone-regulated cancer therapy. Nat. Rev. Cancer , 26–38 [DOI] [PubMed] [Google Scholar]
- 25.Mackie E. J., Pagel C. N., Smith R., de Niese M. R., Song S. J., Pike R. N. (2002) Protease-activated receptors: a means of converting extracellular proteolysis into intracellular signals. IUBMB Life , 277–281 [DOI] [PubMed] [Google Scholar]
- 26.Driesbaugh K. H., Buzza M. S., Martin E. W., Conway G. D., Kao J. P., Antalis T. M. (2015) Proteolytic activation of the protease-activated receptor (PAR)-2 by the glycosylphosphatidylinositol-anchored serine protease testisin. J. Biol. Chem. , 3529–3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andréasson C., Heessen S., Ljungdahl P. O. (2006) Regulation of transcription factor latency by receptor-activated proteolysis. Genes Dev. , 1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuroiwa T., Lee E. G. (1998) Cellular interactions in the pathogenesis of lupus nephritis: the role of T cells and macrophages in the amplification of the inflammatory process in the kidney. Lupus , 597–603 [DOI] [PubMed] [Google Scholar]
- 29.Yang N., Isbel N. M., Nikolic-Paterson D. J., Li Y., Ye R., Atkins R. C., Lan H. Y. (1998) Local macrophage proliferation in human glomerulonephritis. Kidney Int. , 143–151 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Harris D. C. (2011) Macrophages in renal disease. J. Am. Soc. Nephrol. , 21–27 [DOI] [PubMed] [Google Scholar]
- 31.Orme J., Mohan C. (2012) Macrophage subpopulations in systemic lupus erythematosus. Discov. Med. , 151–158 [PubMed] [Google Scholar]
- 32.Iwata Y., Boström E. A., Menke J., Rabacal W. A., Morel L., Wada T., Kelley V. R. (2012) Aberrant macrophages mediate defective kidney repair that triggers nephritis in lupus-susceptible mice. J. Immunol. , 4568–4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesch G. H., Maifert S., Schwarting A., Rollins B. J., Kelley V. R. (1999) Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J. Exp. Med. , 1813–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lenda D. M., Stanley E. R., Kelley V. R. (2004) Negative role of colony-stimulating factor-1 in macrophage, T cell, and B cell mediated autoimmune disease in MRL-Fas(lpr) mice. J. Immunol. , 4744–4754 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.