Abstract
Although uncommon, “brain cancer” is one of the most feared diseases that afflict human beings. While still regarded as one of the most deadly forms of primary brain neoplasm, recent advances in the treatment of glioblastoma (GBM) have offered new hope for patients, families, and clinicians. In the first part of this two-part review, we will focus on the multidisciplinary advances that have established the current treatment approach in the management of GBM. In the second part of this review, ongoing research will be presented including current clinical trials as well as some of the newer technologies that are forming the promise of the future.
Introduction
As a practicing neuro-oncologist, I have found that for patients and families facing a new diagnosis of GBM, the two thoughts that occur immediately are: “I have brain cancer,” and “I am going to die”. This bias is not limited to the lay community; even among closely allied medical professionals there exists a pessimism and fatalism in the approach to medical management of these patients. Furthermore, compared with the general oncology population, patients with brain cancer often have complicated constellations of physical and cognitive deficits and a challenging psychosocial dynamic, requiring the coordinated efforts of a multidisciplinary team for effective management. Despite these challenges, advances in treatment have not only lead to improved survival, but also, because of improved safety and side effect profiles, patients are continuing to lead full, active lives, even during ongoing cancer treatment.
The Central Brain Tumor Registry of the United States (CBTRUS) estimates that there will be approximately 63,000 new cases of primary malignant and non-malignant brain and central nervous system tumors (brain/CNS tumors) diagnosed in the United States in 20101.
Primary brain/CNS tumors are tumors which originate from within the brain or CNS as contrasted with metastatic tumors which originate elsewhere in the body. The burden of disease in the United States is high with an estimated 612,000 persons living with some type of primary brain/CNS tumor in 2004 (primary malignant = 124,000, primary non-malignant = 488,000)2. It is predicted 1.5% or 22,000 of all malignant cancer in the United States in 2009 will be primary malignant brain tumors, with GBM constituting over 50% or 12,000 3.
Glioblastoma is generally a cancer of older people, the incidence increases with advancing age until it peaks between the ages of 75–84 years and then declines.1 Regarding epidemiologic predictors, there appears to be a small increased risk for males compared with females and for Caucasians compared with non-Caucasians.1 Established risk factors for development of GBM include a prior history of ionizing radiation which includes atomic bomb exposure, prior radiotherapy for cancer and radiotherapy for tinea capitis4. While there are reported families with strong hereditary risk for development of GBM, these are rare, and the overwhelming majority of patients, over 95%, have no identified risk factor.4
There have been recent concerns over a potential link between mobile phone use and development of brain cancer in both the medical literature and the lay press; at present the data is conflicting, and a recent meta-analysis showed no overall positive correlation5. Challenges facing ongoing epidemiologic research efforts in this area stem from two factors: first, it can many years from exposure to the development of brain cancer; this hinders studies which rely on patient “recall” of past exposures. Secondly, the patterns of cell phone usage are changing.
Initial Presentation/Evaluation
A common reflection of patients facing a new diagnosis of GBM is “if I had gone to see the doctor sooner …,” or “if my primary care physician had only imaged me sooner …,” then we would have found the tumor earlier. While there are certain symptoms that occur commonly in patients with GBM, for example headaches or seizures, none of these are pathognomonic for brain tumor. A recent case-controlled study which sheds light on this issue examines the symptoms of patients presenting in the primary care setting and who ultimately were diagnosed with a primary brain tumor6. As Table 1 demonstrates, the predictive value of any particular symptom, or multiple symptoms, commonly associated with brain tumors is very low. For example, the symptom with the strongest predictive value, new-onset seizures, had a positive predictive value of 1.2%, meaning over 98% of patients with new-onset seizures did not have an underlying brain tumor. Headache complaints were even less helpful, with the likelihood of a brain tumor causing headaches was less than one in one-thousand. Regarding the concern of earlier diagnosis, there is no data showing that patients with “earlier” diagnosis have better outcomes; however, several analyses do suggest that physical debility (functional status) or cognitive dysfunction are independent predictors for a poorer outcome 7, 8.
Table 1.
Symptoms Associated with Brain Tumors
| Variable | Cases, n(%) n = 3,505 |
Controls, n(%) n = 24,021 |
Positive Predictive Value (95% CI) |
|---|---|---|---|
| Headache | 362 (10.2) | 261 (2.6) | 0.09% (0.08–0.10) |
| Motor Loss | 308 (8.7) | 731 (3.1) | 0.026% (0.024–0.030) |
| New-onset Seizure | 154 (4.4) | 8 (0.05) | 1.2% (1.0–1.4) |
| Confusion | 109 (3.1) | 47 (0.2) | 0.20% (0.16–0.24) |
| Weakness | 95 (2.7) | 42 (0.2) | 0.14% (0.11–0.18) |
| Memory Loss | 37 (1.1) | 64 (0.4) | 0.036% (0.026–0.052) |
| Visual disorder | 35 (1.0) | 62 (0.3) | 0.035% (0.025–0.051) |
Table 1 adopted from Reference #6 (Hamilton, Br J Gen Pract, 2007)
Radiology/Imaging Considerations
The initial diagnostic work-up for a patient with concerning symptoms not responding to conservative management, after the history and physical exam, typically involves some type of neuro-imaging. Computed Tomography (CT) scanning, which was developed almost 40 years ago, is fast, fairly inexpensive and widely available. Although CT imaging has largely been replaced by magnetic resonance imaging (MRI) in the management of patients with brain tumors, CT remains a good choice in initial imaging and in imaging the patient with acutely changing neurological symptoms due to its ability to rapidly demonstrate both blood product and gross abnormalities. Additionally, there is a group of patients with implanted metal or metallic foreign bodies, who cannot undergo MRI scan and are typically imaged only with CT imaging.
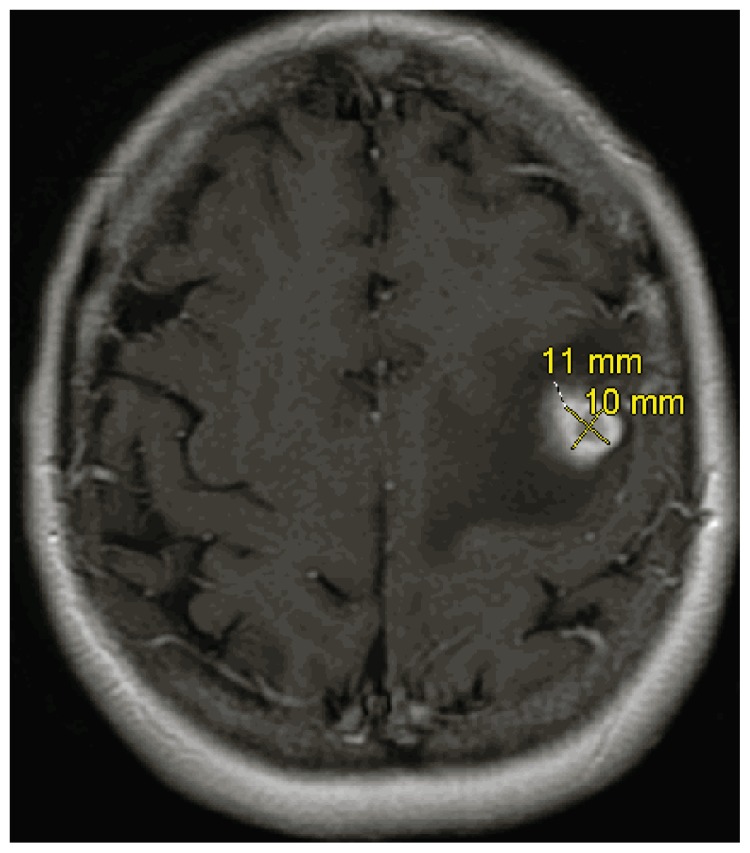
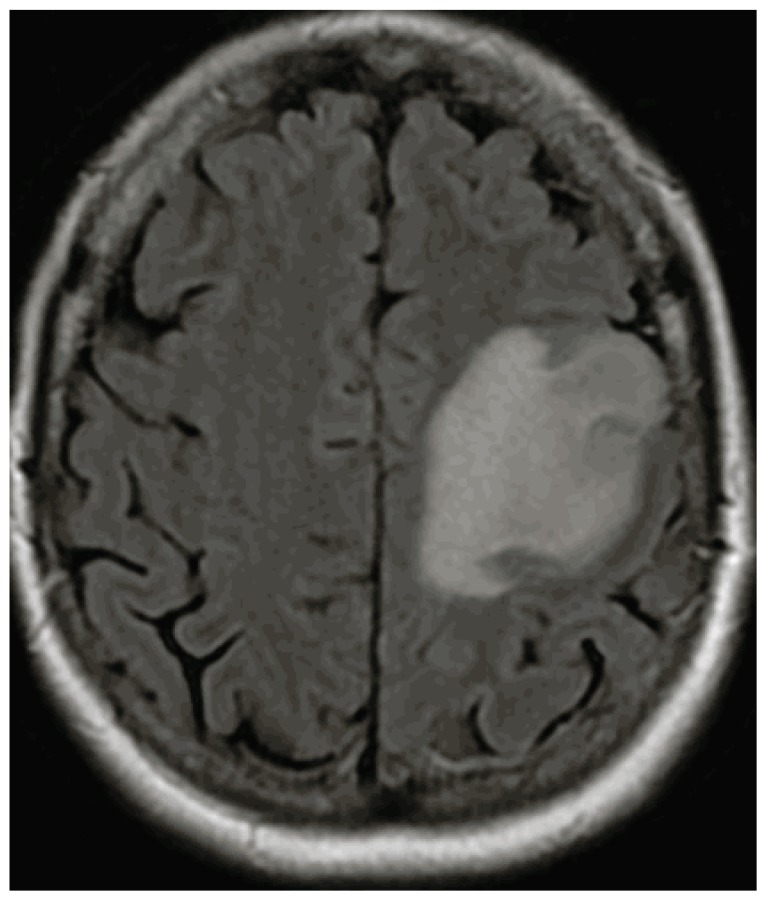
Since its initial clinical use about 30 years ago, the MRI, using high strength magnetic fields to generate multiple imaging sequences (pre- and post- contrast, T2 weighted and FLAIR sequences), is more specific and sensitive than CT, especially in evaluating non-enhancing lesions9, 10. Additionally, MRI can generate images in three planes (axial, coronal and saggital), whereas CT generates images only in the axial plane. The classic MRI image of GBM is a rim-enhancing lesion (See Image 1) with mass effect and surrounding FLAIR hyperintensity (See Image 2), usually solitary, but in about 10% of cases can be multifocal. The differential diagnosis can include other high grade gliomas, such as, anaplastic astrocytoma and anaplastic ependymoma, primary CNS lymphoma, metastatic tumors, brain abscess and other neurologic processes. In these circumstances, MRI imaging modalities including MR spectroscopy, perfusion imaging and diffusion scanning can be helpful. Finally, MRI imaging is important in developing both the surgical approach and anticipated extent of resection as well as for subsequent radiotherapy planning.
Image 1.
Axial MRI Image of GBM with Contrast.
Image 2.
Axial MRI, FLAIR Sequence.
In addition to the initial diagnosis, the other critical issue that faces the neuro-oncology team is the evaluation of growing enhancing lesions and/or increasing FLAIR hyperintensity (sometimes oversimplified as “swelling”) during treatment. Historically, growing lesions were very consistent with true tumor progression, but with the widespread use of Temozolomide chemotherapy as a radiosensitizer during radiotherapy (discussed later in this review), the issue of pseudo-progression, or radiation injury has been recognized. The incidence of progressive radiographic findings after radiation with concurrent Temozolomide has been reported between 40–50% of patients. Of these patients, 50% or higher can be pseudo-progression, the remainder being true tumor progression11,12. The challenge to the clinician is that pseudo-progression can have the same radiographic appearance as true tumor growth (enhancement/FLAIR progression) and can also cause neurologic symptoms.
Pseudo-progression typically appears within three months of completed radiotherapy, and most clinical trials currently open for recurrent GBM require at least a three-month interval after radiotherapy is complete before patients can become eligible. However, pseudoprogression or treatment-related change (necrosis/gliosis) can occur later in the clinical course as well; I have cared for patients with biopsy-proven necrosis/gliosis appearing many years after treatment completion. One critical difference is that although both true tumor progression and pseudo-progression can cause neurologic symptoms, pseudoprogression typically will peak and slowly resolve within a few weeks to months, whereas tumor progression typically continues unabated. MRI imaging modalities, including MR spectroscopy, which looks at chemical composition of a selected area of brain, MR perfusion and blood volume, looking at the vascularity of the brain can sometimes be helpful in differentiating these lesions. Interestingly, having a tumor with a methylated (silenced) resistance enzyme methylguanine methyltransferase (MGMT) appears to be a strong predictor of pseudo-progression, which may also predict better outcomes11.
Neuro-surgical Considerations
Despite the advances in neuro-imaging, ultimately for most patients, establishing a definitive diagnosis involves obtaining tissue, either through a core needle biopsy or an open resection/debulking of the tumor mass. Exceptions to this include tumors located entirely within eloquent tissue, such as the brain stem where a biopsy would be extremely risky. In general, while a stereotactic biopsy offers improved safety and more rapid post-operative recovery, several series suggest that the concordance rate between the specimen obtained at biopsy and the specimen obtained at subsequent resection can vary between 62–89%13, 14, 15. This modest concordance rate highlights the reality that gliomas in general and GBM, in specific, tend to be very heterogeneous tumors and the histological grading can vary even within different regions of the tumor specimen.
Another factor favoring an attempt at maximal tumor resection, as opposed to stereotactic biopsy, centers on the widely held belief supported by a number of retrospective reports, that greater extent of resection improves survival16, 17, 18, 19. Additionally, more extensive resections can improve quality of life through improved symptom control and greater ability to decrease/discontinue corticosteroids19. Accordingly, with the exception of tumors which are extensively infiltrated into eloquent brain tissue, a gross- or near-total resection is the goal of surgery. Interestingly, Hassaneen and colleagues found that for patients with multifocal or multicentric GBM (GBM presenting at multiple, discrete sites within the brain), performing multiple craniotomies during the initial operation with the goal of gross total removal of visible enhancing tumor also improved survival; outcomes were comparable to patients having only a single lesion which was gross totally resected20. Note that gross total resection refers to removal of visible enhancing tumor, and not the surrounding FLAIR hyperintensity, the latter typically contains microscopic deposits of tumor cells which require subsequent treatment – a concept that some patients do not fully understand.
Recent advances in neurosurgical technique have continued to improve neurosurgical safety while at the same time maximizing the extent of resection. Technologies such as intra-operative image guidance (real time 3-dimensional tumor visualization and localization intraoperatively), functional mapping (pre-operative mapping of motor or speech areas of the brain and the descending fiber tracts allowing the neurosurgeon to preserve these areas during surgery) and awake craniotomy21 are currently employed in appropriate patients.
Pathological Evaluation
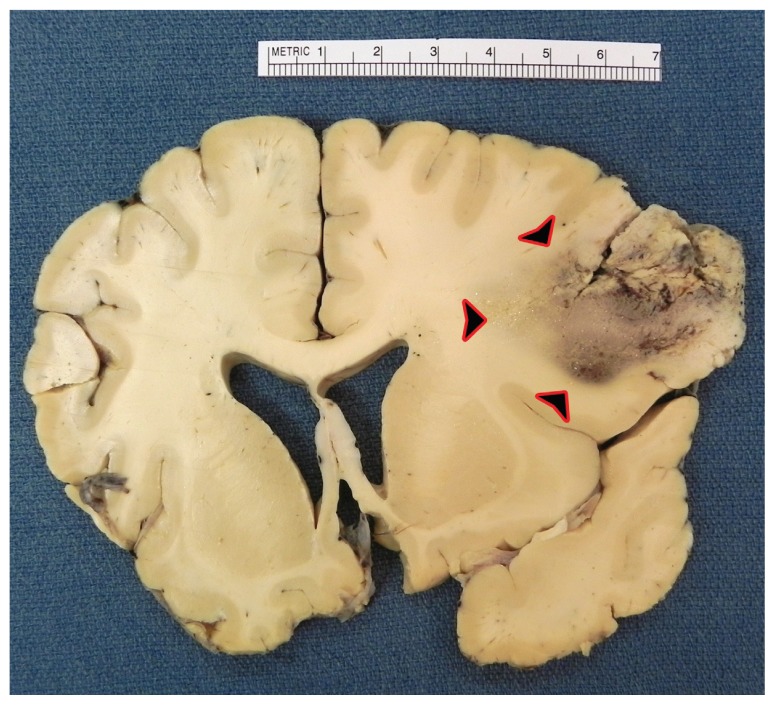
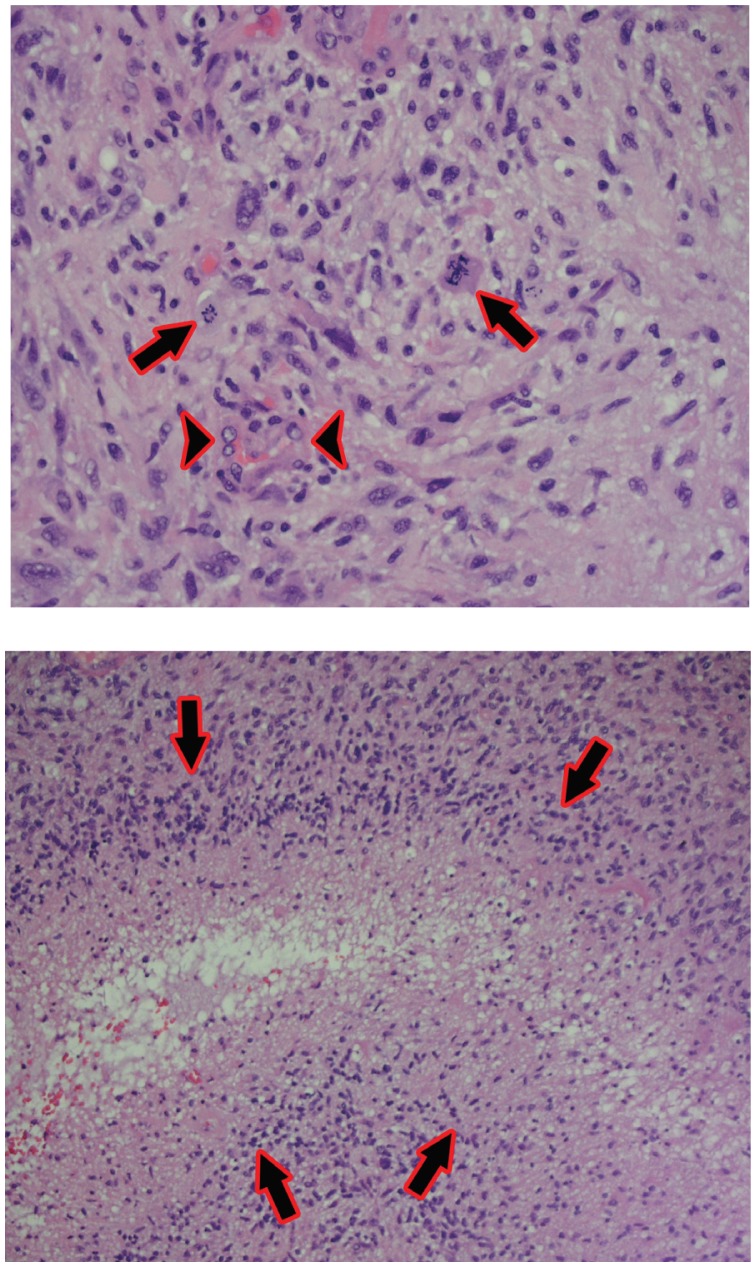
The original tumor name “Glioblastoma Multiforme” comes historically from the observation that both grossly and microscopically these tumors can take a variety of different forms and appearances (See Image 3). Glioblastoma are the most aggressive type of tumor known as “gliomas” or tumors arising from the glial cells. Glial cells, which compose the brain substance, include astrocytes, oligodendrocytes, and ependymal cells. There have been a number of different grading systems proposed over the years; at present, most neuro-pathologists utilize the World Health Organization (WHO) 22 system, which utilizes Grades 1 thru 4 to grade gliomas. Of note, the term “multiforme” (as in Glioblastoma Multiforme) has been dropped in the recent WHO classification system, instead referring to these tumors as Glioblastoma, but “GBM” continues to be a widely used acronym. Glioblastoma by definition, are WHO grade 4 tumors, the classical features include marked cellularity with nuclear atypia, mitotic activity, vascular/endothelial proliferation and pseudo-palisading necrosis (See Images 4 and 5). Interestingly, because of the low propensity of GBM to metastasize either within the central nervous system (CNS) or outside the CNS, there is not an associated staging system (staging describes extent of tumor spread) as there is for most other systemic cancers.
Image 3.
Coronal section of frontal lobe – Glioblastoma with variable appearance of tumor leading to use of term “multiforme.”
Images 4 and 5.
Top: 400x photomicrograph – Glioblastoma with marked cellularity, nuclear atypia, mitotic figures (arrows), and vascular/endothelial proliferation (arrowheads).
Bottom: 200x photomicrograph – Glioblastoma with pseudo-palisading of tumor cells around area of necrosis (arrows).
There are several findings in tumor specimens can be helpful in determining pathogenesis and/or prognosis. Approximately 10% of GBM’s appear to originate from an underlying lower grade glioma (a process called “transformation”); these tumors are called secondary GBM’s and may have regions of low grade tumor adjacent to highly malignant tumor. While most malignant gliomas originate from astrocytes (Astrocytoma), some appear to arise from oligodendrocytes. In a recent report, GBM’s with oligodendroglial features have been suggested to have better outcomes compared with those patients whose tumors do not have this finding23. Finally, multiple retrospective reports suggest that an enzyme thought to mediate resistance to alkylator (including BCNU (carmustine) and CCNU (lomustine)) and methylator chemotherapy (including Temozolomide – discussed later) known as methylguanine methyltransferase (MGMT) can predict treatment outcomes. Methylation of the promoter region for the encoding of MGMT appears to “silence” expression of this enzyme, resulting in low or absent quantities of MGMT within the tumor cell and a resulting reduced ability to repair chemotherapy damage24. More recently, it has been suggested that MGMT status predicts outcome to treatment independent of chemotherapy choice25. Although not “mandatory” we typically will offer patients MGMT testing at initial diagnosis, furthermore this is also typically offered a part of initial screening/enrollment into multiple clinical trials as well. While this does not dictate therapy per se, it can be helpful in management, especially in the face of pseudo- versus true- tumor progression after radiotherapy.
Radiation Oncology
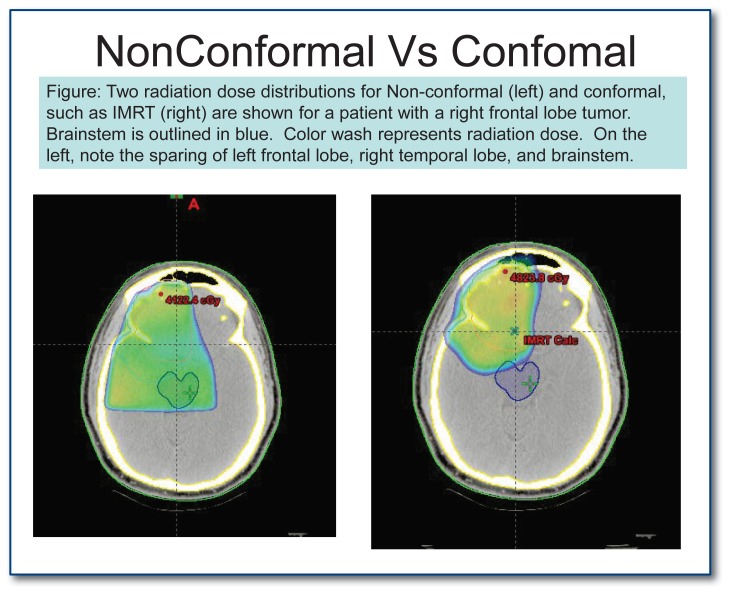
The use of radiotherapy in the management of cancer goes back almost a century. There have been a number of important improvements in the delivery of radiation in the treatment of patients with GBM, including targeted radiotherapy, known as “involved field radiation”, which results in reduced side effects compared with radiation delivered to the whole brain (utilized historically for GBM treatment). More modern technologies allow delivery of a fairly homogenous high dose of radiation to the target area with a rapid dose drop off outside of the target area. The use of post-operative radiotherapy in the treatment of GBM, when compared with surgery followed by supportive management (corticosteroids), has demonstrated to improve survival from two-three months to ten to twelve months. By comparison, chemotherapy improves survival by about two-four months in unselected populations. Current treatment strategies include the utilization of a technique called Intensity Modulated Radiation Therapy (IMRT) or Image Guided Radiation Therapy (IGRT) which has the benefit of more precise dose delivery to tumors near sensitive areas such as the optic apparatus or brainstem (See Image 6). A typical course of radiation treatment consists of approximately 30 treatments (called “fractions”) given weekdays for a total of six to six and a half weeks.
The current standard of care for patients with newly diagnosed GBM includes radiotherapy as detailed above, combined with daily dosing of oral Temozolomide chemotherapy26. Not all patients are appropriate candidates for the combination of radiation with Temozolomide, which is associated with a modest increase in toxicity compared with radiotherapy alone. In certain populations, such as the elderly and/or infirm patients with poor performance status, palliation with radiotherapy alone offers a reasonable choice with less toxicity. Recent reports have explored shorter courses of radiotherapy, for example over 3 weeks, which are more tolerable, show comparable rates of symptom control and do not appear to significantly lower survival, although these small reports make direct comparisons difficult27.
Neuro-Oncology/Medical Oncology
Historically, the benefit of chemotherapy in the management of GBM has been modest at best. A large meta-analysis finally confirmed a two-month median improvement in survival with the use of pre-Temozolomide chemotherapy era drugs (mainly BCNU (carmustine) and CCNU (lomustine)); this benefit was at the cost of significant toxicity28. In 2005, Stupp and colleagues published the results of a randomized trial comparing surgery followed by radiotherapy alone (the standard of care at the time) versus the same approach with the addition of an oral methylating chemotherapy drug called Temozolomide. Temozolomide (or Temodar) was given daily during radiotherapy, including weekends, and, after completion of radiation, was continued for six cycles (approximately six months) in a five-day on, 23-day off fashion. This addition of Temozolomide improved the median survival to 14.6 months with radiation/Temozolomide versus 12.1 months with radiation alone. Although the benefit appears modest, most patients tolerated Temozolomide well; only 8% of patients discontinued the drug due to toxicity26. Ultimately, this approach was approved by the FDA29.
Subsequent follow-up of patients on this trial showed improvements in survival out to five years: 9.8% of patients still alive who were treated with Radiotherapy/Temozolomide versus 1.9% of patients treated with Radiotherapy alone30. While this approach is extending survival in a minority of patients, the reality is that most patients will have progressive disease, on average within nine to ten months from the start of treatment26. Salvage options after progression include first and foremost clinical trial consideration. Off trial options include switching chemotherapy to one of the older nitrosureas such as BCNU (carmustine) or CCNU (lomustine) or continuing Temozolomide, but switching to a more prolonged dosing schedule (see below). Historically, salvage therapies at the time of GBM recurrence have demonstrated six-month progression-free survival (PFS-6) rates of up to 16%31. The use of CCNU at salvage after tumor progression on Temozolomide has modestly increased the PFS-6 to around 19%32. The observation that Temozolomide-based chemotherapy is generally well tolerated has led to the investigations of more prolonged dosing strategies. This is based in part on in-vitro observations that the resistance enzyme MGMT can be overcome by more frequent exposure to chemotherapy over time. Several recent papers looking at changing (or restarting) Temozolomide with a metronomic dosing schedule (dosed 50mg/m2/day, daily during a four-week cycle) after progression either during or after standard five-day on, 23-day off Temozolomide show PFS-6 rates 24% – 32% with acceptable toxicities33,34.
Over the past few years, interest in targeted anti-angiogenesis therapies has increased. Angiogenesis or (angio = blood vessel, genesis = creation/growth) is a critical process for tumor growth beyond a centimeter in size. Angiogenesis has been shown to be important in many diverse tumors, including colon, lung, breast and brain cancers. Bevacizumab is a targeted therapeutic agent which binds an extracellular protein known as VEGF (vascular endothelial growth factor), a protein which is necessary for angiogenesis. Recent trials have demonstrated a PFS-6 of 40–50% and overall survival of about nine months with the use of Bevacizumab at the time of progression on Temozolomide chemotherapy35. The results of this trial and others led to the accelerated approval of Bevacizumab by the FDA in May 2009. However, Bevacizumab comes with a number of common side effects, including fatigue and hypertension, as well as some rare, but potentially life-threatening side effects such as increased risk of intracranial hemorrhage, wound dehiscence, blood clot (DVT and PE), bowel perforation and progressive proteinuria. Additionally, with its long half-life (approaching 30 days), side effects may not resolve quickly with discontinuation. Still, it remains an important salvage therapy for some patients with recurrent GBM.
A major challenge in caring for patients with brain tumors in general and Glioblastoma in specific is the extremely common finding of neurocognitive impairment. One study found impairment on cognitive testing in at least one domain in 90% of patients and in three or more domains in 70% of patients; these tests were administered to patients prior to initiation of treatment36. Historically in clinical trials, if cognitive functioning was assessed at all, the Mini Mental Status Exam was utilized, a tool which was actually developed to screen for dementia. Many recent clinical protocols, however, are utilizing much more sensitive evaluations of cognitive function, repeated multiple times through the course of treatment. Ultimately, this will increase our understanding not only the degree of injury to cognitive functioning, but also at what point during treatment and in which specific areas these deficits occur.
Two additional comments for clinicians in regards to management of patients undergoing treatment for Glioblastoma. During the initial portion of treatment with radiotherapy with concurrent Temozolomide, and usually dexamethasone to control swelling, lymphopenia is often observed26. The original EORTC/NCIC trial26 recommended prophylaxis against PCP (Pneumocystis Pneumonia). This is also recommended in the FDA approval29. Although it is common in the United States to prophylax with Bactrim, our experience has been that the bone marrow suppressive effects, drug-drug interactions and sulfa allergies as well as patient compliance make this challenging at times. Our usual practice is to offer practice with pentamidine (Nebupent) 300mg via nebulizer monthly, which was one of the accepted prophylaxis strategies in the original EORTC/NCIC trial of Temozlomide26. The main side effect is mild respiratory irritation, typically prevented with albuterol inhalation (using a MDI) as pre-treatment. Our practice is to begin prophylaxis at the start of radiotherapy with concurrent Temozolomide and discontinue after recovery of lymphocyte counts, typically in the two to three months after completion of radiotherapy, but this can be delayed if corticosteroids are unable to be tapered off. The other question we are often is asked is in regards to whether brain cancer patients are able to receive the annual flu, pneumonia or other vaccines – these are safe, effective and recommended in this population37.
Conclusion
The current management of patients with Glioblastoma represents real improvements over many decades. Although not without toxicities, the safety and side effect profile of treatment for most patients including neurosurgery, radiotherapy and chemotherapy are much more acceptable than in years past. Improvements in these approaches, combined with advancements in supportive therapy have allowed most patients to tolerate ongoing treatment and still maintain a good quality of life during the process. Unfortunately, most patients still are not cured of their cancer with these modern approaches and that becomes the real challenge in moving forward. Future advances, discussed in Part 2 of this paper in a future edition of Missouri Medicine, will not only add to the efficacy of treatment, but will also reduce the toxicity further, allowing us to deliver helpful therapies to more patients suffering from this dreaded disease.
Biography
Michael E. Salacz, MD, practices Neuro-Oncology, Medical Oncology & Palliative Medicine, and is the Medical Director at Saint Luke’s Brain Tumor Center in Kansas City, Mo. Kenneth R. Watson, DO, practices Anatomic Pathology and David A. Schomas, MD, practices Radiation Oncology with St. Luke’s Health System. This is part one of a two-part series.
Contact: msalacz@saint-lukes.org

Footnotes
Disclosure
None reported.
References
- 1.CBTRUS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2006. Source Central Brain Tumor Registry of the United States; Hinsdale, IL: 2010. website: www.cbtrus.org. [Google Scholar]
- 2.Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the US by age, gender, behavior, and histology. Neuro–Oncology. doi: 10.1093/neuonc/nop066. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 4.Bondy ML, Scheurer ME, et al. Brain Tumor Epidemiology: Consensus from the Brain Tumor Epidemiology Consortium. Cancer Supplement. 2008 Oct 1;113(7):1953–68. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myung SK, Ju W, McDonnell DD, et al. Mobile Phone Use and the Risk of Tumors: A Meta-Analysis. Journal Clinical Oncology. 2009 Nov 20;27(33):5565–72. doi: 10.1200/JCO.2008.21.6366. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton W, Kernick D. Clinical Features of Primary Brain Tumors: A Case-control Study Using Electronic Primary Care Records. British Journal of General Practice. 2007 Sep;:695–99. [PMC free article] [PubMed] [Google Scholar]
- 7.Curran WJ, Scott CB, Horton J, et al. Recursive Partitioning Ananlysis of Prognostic Factors in Three Radiation Therapy Oncology Group Malignant Glioma Trials. J Nat Cancer Inst. 1993 May;85(9):704–10. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 8.Mirimanoff RO, Gorlia T, Mason W, et al. Radiotherapy and Temozlomide for Newly Diagnosed Glioblastoma: Recursive Partitioning Analysis of the EORTC 26981/22981-NCIC CE3 Phase III Randomized Trial. J Clin Oncol. 2006 Jun;24(16):2563–69. doi: 10.1200/JCO.2005.04.5963. [DOI] [PubMed] [Google Scholar]
- 9.Smith AS, Weinstein MA, Modic MT, et al. Magnetic Resonance with Marked T2-weighted Images: Improved Demonstration of Brain Lesions, Tumor and Edema. American Journal of Radiology. 1985 Nov;145:949–55. doi: 10.2214/ajr.145.5.949. [DOI] [PubMed] [Google Scholar]
- 10.Bradley WG, Waluch V, Yadley RA, Wycoff RR. Comparison of CT and MR in 400 Patients with Suspected Disease of the Brain and Cervical Spinal Cord. Radiology. 1984;152:695–702. doi: 10.1148/radiology.152.3.6463251. [DOI] [PubMed] [Google Scholar]
- 11.Brandes AA, Franceschi E, Tosoni A, et al. MGMT Promotor Methylation Status Can Predict the Incidence and Outcome of Pseudoprogression After Concomitant Radiochemotherapy in Newly Diagnosed Glioblastoma Patients. JCO. 2008 May 1;26(13):2192–7. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 12.Incidence of Early Pseudo-progression in a Cohort of Malignant Glioma Patients Treated with Chemoirradiation With Temzolomide. Cancer. 2008 Jul 15;113(2):405–10. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 13.Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro-oncology. 2001 Jul;3:193–200. doi: 10.1093/neuonc/3.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGirt MJ, Villavicencio AT, Bulsara KR, Friedman AH. MRI-Guided Stereotactic Biopsy in the Diagnosis of Glioma: Comparison of Biopsy and Surgical Resection Specimen. Surgical Neurology. 2003;59:277–82. doi: 10.1016/s0090-3019(03)00048-x. [DOI] [PubMed] [Google Scholar]
- 15.Feiden W, Steude U, Bise K, Gundisch O. Accuracy of Stereotactic Brain Tumor Biopsy: Comparison of the Histologic Findings in Biopsy Cylinders and Resected Tumor Tissue. Neurosurgery Review. 1991;14:51–6. doi: 10.1007/BF00338192. [DOI] [PubMed] [Google Scholar]
- 16.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent Association of Extent of Resection With Survival in Patients with Malignant Brain Astrocytoma. J Neurosurg. 2009 Jan;100:156–62. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 17.Laicroix M, Abi-Said D, Fourney DR, et al. A Multivariate Analysis of 416 Patients With Glioblastoma Multiforme: Prognosis, Extent of Resection and Survival. J Neurosurg. 2001 Aug;95:190–8. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 18.Tsitlakidis A, Foroglou N, Venetis CA, et al. Biopsy Versus Resection in the Management of Malignant Gliomas: a Systemic Review and Meta-analysis. J Neurosurg. 2009 Sep 11;:1–13. doi: 10.3171/2009.7.JNS09758. [DOI] [PubMed] [Google Scholar]
- 19.Brown PD, Maurer MJ, Rummans TA, et al. A Prospective Study of Quality of Life in Adults with Newly Diagnosed High-Grade Gliomas: The Impact of the Exent of Resection on Quality of Life and Survival. Neurosurgery. 2005 Sep;57(3):495–503. doi: 10.1227/01.neu.0000170562.25335.c7. [DOI] [PubMed] [Google Scholar]
- 20.Hassaneen W, Levine NB, Suki D, et al. Multiple Craniotomies in the Management of Multifocal and Multicentric Glioblastoma. J Neurosurg. 2010 Aug 6;:1–9. doi: 10.3171/2010.6.JNS091326. [DOI] [PubMed] [Google Scholar]
- 21.Bulsara KR, Johnson J, Villavicencio AT. Improvements in Brain Tumor Surgery: the Modern History of Awake Craniotomies. Neurosurg Focus. 2005 Apr;18:1–3. doi: 10.3171/foc.2005.18.4.6. [DOI] [PubMed] [Google Scholar]
- 22.Louis DN, Ohgaki H, Wiestler OD. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvati M, Formichella AI, D’Elia A. Cerebral glioblastoma with oligodendrogliomal component: Analysis of 36 Cases. J Neurooncol. 2009 Aug;94(1):129–34. doi: 10.1007/s11060-009-9815-6. [DOI] [PubMed] [Google Scholar]
- 24.Hegi ME, Liu L, Herman JG, et al. Correlation of O6-Methylguanine Methyltransferase (MGMT) Promoter Methylation With Clinical Outcomes in Glioblastoma and Clinical Strategies to Modulate MGMT Activity. JCO. 2008 Sep 1;26(25):4189–99. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 25.Rivera AL, Pelloski CE, Gilbert MR, et al. MGMT Promoter Methylation is Predictive of Response to Radiotherapy and Prognostic in the Absence of Adjuvant Alkylating Chemotherapy for Glioblastoma. Neuro-Oncology. 2010 Feb;12(2):116–21. doi: 10.1093/neuonc/nop020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. NEJM. 2005 Mar 10;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 27.Roa W, Xing JZ, Small C, et al. Current Developments in the Radiotherapy Approach to Elderly and Frail Patients With Glioblastoma Multiforme. Expert Rev Anticancer Ther. 2009;9(11):1643–50. doi: 10.1586/era.09.128. [DOI] [PubMed] [Google Scholar]
- 28.Glioma Meta-analysis Trialists (GMT) Group. Chemotherapy in Adult High-Grade Glioma: A Systematic Review and Meta-analysis of Individual Patient Data from 12 Randomised Trials. The Lancet. 2002 Mar 23;359:1011–18. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 29.Cohen MH, Johnson JR, Pazdur R. Food and Drug Administration Drug Approval Summary: Temozolomide Plus Radiation Therapy for the Treatment of Newly Diagnosed Glioblastoma Multiforme. Clin Cancer Res. 2005 Oct 1;11(19):6767–71. doi: 10.1158/1078-0432.CCR-05-0722. [DOI] [PubMed] [Google Scholar]
- 30.Stupp R, Hegi ME, Mason WP, et al. Effects of Radiotherapy With Concomitant and Adjuvant Temozlomide Versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009 May;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 31.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and Prognostic Factors in Recurrent Glioma Patients Enrolled Onto Phase II Clinical Trials. J Clin Oncol. 1999;17:2572–8. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 32.Wick W, Puduvalli VK, Chamberlain MC. Phase III Study of Enzastaurin Compared With Lomustine in the Treatment of Recurrent Intracranial Glioblastoma. J Clin Oncol. 2010 Mar 1;28(7):1168–74. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry JR, Belanger K, Mason WP, et al. Phase II Trial of Continuous Dose-Intense Temozolomide in Recurrent Malignant Glioma: RESCUE Study. J Clin Oncol. 2010 Apr 20;28(12):2051–7. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 34.Kong DS, Lee JI, Kim JH, et al. Phase II Trial of Low-Dose Continuous (Metronomic) Treatment of Temozolomide for Recurrent Glioblastoma. Neuro-Oncology. 2010 Mar;12(3):289–96. doi: 10.1093/neuonc/nop030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab Alone and in Combination With Irinotecan in Recurrent Glioblastoma. J Clin Oncol. 2009 Oct 1;27(28):4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 36.Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47:324–33. doi: 10.1097/00006123-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Gea-Banacloche JC. Influenza 2009: what you and your patients need to know. Commun Oncol. 2009;6:488–9. [Google Scholar]