Abstract
Hepatocellular carcinoma (HCC) is increasing in incidence in the United States and is strongly associated with chronic liver disease and cirrhosis. Surgical therapy with liver transplantation or resection remains the mainstay of curative therapy for patients with HCC. Therapeutic decisions in patients with HCC are complex and are best approached via a multidisciplinary group of liver transplant and hepatobiliary surgeons, oncologists, and hepatologists. In this manuscript, we review the current surgical management of HCC.
Introduction
Hepatocellular carcinoma (HCC) is an aggressive neoplasm associated with chronic liver disease. It is one of the most common malignancies in the world and it is the third leading cause of cancer mortality worldwide.1 The incidence of HCC has doubled in the United States over the last two decades.2 Chronic viral hepatitis is the most common risk factor, but any setting in which there is chronic inflammation of the liver places a patient at higher for the development of cirrhosis and subsequently HCC. Chronic inflammation is the backdrop for genetic mutations to amass and drive cells toward malignancy.1 Because symptomatology is non-specific, patients are often diagnosed with advanced disease at their initial presentation. For those with localized disease, surgery represents the only hope for cure. The optimal treatment of a patient with HCC depends on the anatomic extent of the tumor, the patient’s underlying liver function, the efficacy of the treatment and the potential additive effects of treatment options.
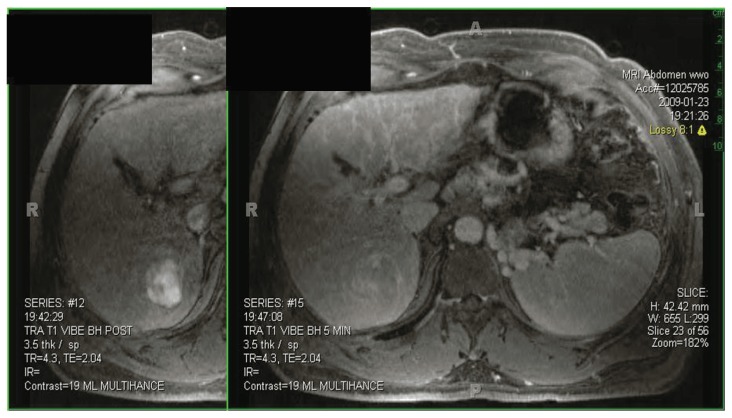
The surgical evaluation of patients with hepatocellular carcinoma begins with the diagnosis and assessment of the primary tumor burden. In the vast majority of patients, noninvasive methods can reliably establish the diagnosis and staging of HCC and biopsy of the tumor is rarely necessary. Either dynamic multiphase computed tomography (CT) or magnetic resonance imaging (MRI) should be used to detect HCC. If CT is utilized, contrasted multiphase imaging is imperative. Currently contrasted MRI is considered to be the most noninvasive method for HCC detection.3,4 The MRI appearance of HCC is classically characterized as early arterial contrast enhancement followed by early washout on the delayed phase imaging (See Figure 1). Completion of the metastatic work-up includes CT of the chest and a bone scan, which assesses the two most common sites of distant metastasis of HCC. Currently, positron emission scanning (PET) scanning has a highly selective role in the detection of occult HCC metastasis.
Figure 1. HCC Diagnosis.
Early arterial contrast enhancement followed by early washout on delayed phase imaging characterizes the appearance of HCC on MRI.
There are numerous staging methods for hepatocellular carcinoma. The American Joint Committee on Cancer (AJCC) Tumor Node Metastasis (TNM) system was revised in 2010, but continues to recognize the most important predictors of prognosis: number/size of tumors and the presence of vascular invasion.5 These prognostic factors were emphasized by the landmark paper by Mazzaferro et al. which established liver transplantation as the optimal therapy for early stage HCC on the background of cirrhosis.6 By limiting transplantation to early HCC (single lesion < 5cm or one to three lesions each < 3 cm, absence of vascular invasion, and no regional or distant metastasis) the Milan group demonstrated that four year outcomes following orthotopic liver transplantation (OLT) for HCC were comparable with OLT in patients with benign indications. The Milan criteria which have been validated by further studies by other groups are now the basis for selecting patients with HCC for OLT. Currently in the United States, livers are allocated for transplantation using the Model for End Stage Liver Disease (MELD) score which accurately predicts the three-month mortality of patients awaiting liver transplantation (See Table 1). The MELD system is of little value to patients with relatively compensated cirrhosis who have early stage HCC. Thus in 2002, the United Network for Organ Sharing (UNOS) adopted the Milan criteria for allocating exception points to those listed for OLT with HCC. Currently patients listed for OLT with Stage II HCC (T2, N0, M0; 1 nodule ≤ 5 cm or up to three tumors all ≤ 3 cm) can receive 22 MELD exception points which results in liver transplantation within six to twelve months at most centers.
Table 1. Model for End Stage Liver Disease.
Serum levels of Creatinine, INR, and total bilirubin are the parameters used to predict survival among patients with cirrhosis.
| Model for End Stage Liver Disease |
|---|
| = [0.957ln(Cr)+0.378ln(bili)+1.12ln(INR)+0.643]×10 |
unos.org
As a result of the favorable results following OLT, an increase in surveillance and early detection as well as an increasing incidence of HCC, liver transplantation for HCC has quadrupled in last ten years7. The current overall five- and ten-year survival following transplant for HCC is 67% and 50% respectively.7 This compares favorably with the overall five- and ten-year survival rates of transplant for all causes, 73% and 59%, respectively. 7 However, excitement for the use of OLT as the preferred treatment modality for HCC is also driven by recent reports of 64–95% disease free survival following OLT at five years and 56–95% at ten years (See Table 2).
Table 2. Liver Transplantation for HCC.
These results compare favorably to patients treated with surgical resection for HCC
| Author | Journal | Date | n | MELD | Overall Survival (%) | Disease Free Survival (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-yr | 3-yr | 5-yr | 10-yr | 1-yr | 3-yr | 5-yr | 10-yr | |||||
| Lee et al | J Surg Oncol | 2009 | 48 | 12 | 85 | 78 | 78 | 92 | 89 | 89 | ||
| Margarit et al | Liv Transplant | 2005 | 36 | N/A | 78 | 65 | 60 | 77 | 64 | 56 | ||
| Mazzaferro et al | Lancet One | 2009 | 444 | N/A | 73 | 69 | 95 | 95 | ||||
| Sugawara et al | Dig Dis | 2007 | 68 | N/A | 91 | 82 | 75 | 93 | 90 | 90 | ||
| Moonka et al | Transplant Proc | 2009 | 117 | N/A | 90 | 81 | 81 | 92 | 83 | 78 | ||
| Bellavance et al | JOGS | 2008 | 134 | 11 | 91 | 79 | 66 | 96 | 89 | 82 | ||
To continue to improve these results, many centers routinely use locoregional (i.e. transarterial chemoembolization, radiofrequency ablation, or percutaneous ethanol injection) therapies as a neoadjuvant strategy to complement OLT. These strategies are often refered to as “bridge to transplantation” as they are designed to prevent HCC progression while a patient is awaiting transplant. The use of neoadjuvant therapy is associated with low rates of progression beyond the Milan criteria (0–15%) during the first six months awaiting transplant.8, 9, 10, 11 This strategy is reported to reduce the drop-out rate of those listed for OLT and to potentially select appropriate candidates for downstaging. In addition, the use of TACE has been associated with improved survival following OLT for HCC2.
Many authors feel that the Milan criteria are too restrictive and that expanding the maximum tumor size would allow OLT in more patients without compromising results, but others feel that the expanding criteria for transplanting HCC will tax an already scarce resource. There is evidence that the outcomes following transplantation for stage II and stage III patients are similar12. Further, researchers at the University of San Francisco have shown that modest expansion of the Milan criteria do not adversely affect outcome in a retrospective analysis13. One- and five-year survival were similar following transplant for Stage IIIA HCC (“UCSF” criteria: single lesion </= 6.5 cm, in diameter or two lesions </= 4.5 cm with total diameter </= 8 cm)13. These findings have been prospectively validated based on preoperative imaging with one- and five-year recurrence-free probabilities of 96.9% and 93.6%, respectively14. In this study the median follow-up was 26.1 months. There is little enthusiasm for extending liver transplantation beyond the UCSF criteria as there is an approximately 30% decrease in five-year recurrence free probability in patients whose explant pathology demonstrates histology beyond UCSF criteria14. In addition, radiographic evaluation understages TIII tumors in approximately 30% of cases, which can result in the inadvertent transplantation of patients beyond UCSF criteria14. Thus, many groups advocate the used of a neoadjuvant “downstaging” strategy to select patients with advanced HCC for transplantation.
While the utility of locoregional therapy as a bridge to transplantation has been well substantiated, the use of these therapies as a strategy to “downstage” patients is being investigated. The biological aggressiveness of particular tumors is difficult to assess preoperatively. In patients who present beyond Milan criteria, many investigators feel that there exists a subgroup with favorable tumor biology that would benefit from OLT. Successful downstaging using pre-operative locoregional therapy would be the criteria to demonstrate a group with favorable biology. Our group has demonstrated that roughly 25% of patients presenting with advanced HCC (stage III/IV) can be downstaged to meet Milan criteria15. Further, downstaged patients have equivalent or better early and medium term outcomes following OLT. Among the patients who presented beyond Milan criteria and were successfully downstaged using TACE, 94% were alive at a median of 19.6 months after OLT.15 Setting criteria that demonstrates favorable biology through successful downstaging is a rational approach in the evaluation of patients for OLT with advanced HCC.
Surgical resection of HCC is considered the standard therapy for patients who do not have underlying liver disease. The results for surgical resection in this setting are variable and depend on tumor size, presence of vascular invasion, and status of the surgical margin. A tumor over 5cm significantly decreases the five-year survival (63 vs. 37%).16 An independent, but equally poor predictor is the presence of vascular invasion. Resection of tumors without vascular invasion in non-cirrhotics with HCC resulted in one-, three-, and five-year survival rates of 93%, 75%, and 53%, versus 57%, 16%, and 6%, with invasion, respectively.17 Finally, one series reported no five-year survivors among those with positive surgical margins versus 39% with an R0 resection.17
In patients with cirrhosis, resection for HCC is much more controversial. The decision to resect HCC is dependent on many factors including tumor burden and location, underlying liver function and overall fitness for a major operative procedure. Further, given the excellent outcome following OLT, the patient’s suitability to become a transplant candidate should be assessed by a multidisciplinary team before proceeding with resection.
Characteristics that preclude resection include bilobar tumors, tumors that invade adjacent organs or major vasculature, and extrahepatic disease. Anatomic resection with negative margins is the goal in any cancer operation, but without adequate hepatic reserve, there are significant risks. Operative morbidity and mortality are greatly elevated in the setting of cirrhosis. Peri-operative mortality in cirrhotic patients is best estimated using the MELD score.18, 19, 20 A score less than nine is generally considered safe for limited liver resection19, 20. Recent papers examining liver resection for HCC have been limited to patients with MELD scores </= 1220 (See Table 3). As shown in Table 3, the overall survival at one- and five-years following resection of HCC in highly selected patients with cirrhosis is similar to that of OLT. However, the one-, five-, and ten-year disease free survival rates are much worse following resection.
Table 3. Surgical Resection for HCC.
While overall survival is similar among patients treated with either OLT or surgical resection for HCC, disease-free survival is increased in the OLT group.
| Author | Journal | Date | n | MELD | Overall Survival (%) | Disease Free Survival (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-yr | 3-yr | 5-yr | 10-yr | 1-yr | 3-yr | 5-yr | 10-yr | |||||
| Cherqui et al | Ann Surg | 2009 | 67 | 8 | 73 | 42 | ||||||
| Lee et al | J Surg Oncol | 2009 | 82 | 12 | 87 | 75 | 58 | 74 | 59 | 57 | ||
| Margarit et al | Liv Transplant | 2005 | 37 | N/A | 92 | 70 | 50 | 84 | 34 | 18 | ||
| Shah, et al | Surgery | 2007 | 193 | CPA | 85 | 68 | 53 | 72 | 48 | 39 | ||
| Bellavance et al | JOGS | 2008 | 245 | 9 | 93 | 71 | 46 | 88 | 62 | 40 | ||
| Torzilla | Arch Surg | 2008 | 61 | N/A | 93 | 81 | 77 | 30 | ||||
| Park | Trans Proced | 2009 | 213 | CPA | 92 | 78 | 69 | 52 | 79 | 57 | 44 | 19 |
| Kamiyama | J Surg Oncol | 2010 | 287 | CPA | 78 | 56 | 42 | 25 | ||||
The idea of using surgical resection as a bridge to transplant has been examined by several groups. These studies come from outside the US where the transplant allocation schemes are different. The results of these studies are variable with the largest concern being that 25% to 41% of patients who underwent resection and then recurred were no longer within Milan criteria.21, 22, 23, 24, 25 One of the larger and best designed of these studies used an intention-to-treat analysis and showed a decreased overall survival in patients who were resected prior to transplantation24. The use of resection for HCC in a cirrhotic patient who is within Milan criteria remains a controversial decision at most centers in the US.
Summary
The surgical management of HCC is complex and is dependent on multiple factors. The standard of care therapy for patients with underlying liver disease, remains liver transplantation with or without neoadjuvant locoregional therapy. In general, surgical resection should be reserved for patients without underlying liver disease and only in unusual instances should primary resection be considered in a cirrhotic patient who meets the Milan criteria and is otherwise an appropriate transplant candidate. Downstaging strategies should be considered for patients who present outside the Milan criteria. Cirrhotic patients with HCC are challenging and an incorrect initial decision can damage future therapeutic options worsening a patient’s overall prognosis. The optimal therapeutic strategy for an individual patient should result from a multidisciplinary approach that incorporates the efforts of hepatobiliary and liver transplant surgeons, hepatologists, and oncologists. The patient’s suitability for transplantation, current institutional expertise, and current organ allocation schemes are all important considerations.
Biography
Christopher D. Anderson, MD, MSMA member since 2010, is Assistant Professor of Surgery, Section of Abdominal Transplant Surgery, and Associate Residency Program Director. Bernard J. DuBray, Jr., MD, MSMA member since 2011, is a Resident in General Surgery, Research Fellow, Section of Abdominal Transplantation. William C. Chapman, MD, is Professor and Chief, Section of Transplantation, and Chief, Division of General Surgery. All are at Washington University School of Medicine in St. Louis.
Contact: andersonc@wudosis.wustl.edu

Footnotes
Disclosure
None reported.
References
- 1.Trevisani F, et al. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010;42(5):341–7. doi: 10.1016/j.dld.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Bharat A, et al. Pre-liver transplantation locoregional adjuvant therapy for hepatocellular carcinoma as a strategy to improve longterm survival. J Am Coll Surg. 2006;203(4):411–20. doi: 10.1016/j.jamcollsurg.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Burrel M, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38(4):1034–42. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 4.Elsayes KM, et al. MRI characterization of 124 CT-indeterminate focal hepatic lesions: evaluation of clinical utility. HPB (Oxford) 2007;9(3):208–15. doi: 10.1080/13651820701216950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edge SB, Byrd DR, Compton CC, et al. AJCC (American Joint Committee on Cancer) Cancer Staging Manual. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferro V, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 7.OPTN/SRTR Annual Report 1998–2007. 2008. HHS/HRSA/HSB/DOT. [Google Scholar]
- 8.Andorno E, et al. Preliminary results of liver transplantation for hepatocellular carcinoma among allocation organ policy strategies, neoadjuvant treatments, and intention-to-treat analysis. Transplant Proc. 2008;40(6):1972–3. doi: 10.1016/j.transproceed.2008.05.061. [DOI] [PubMed] [Google Scholar]
- 9.Jarnagin W, et al. Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford) 2010;12(5):302–10. doi: 10.1111/j.1477-2574.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddala YK, et al. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: outcome with chemoembolization. Liver Transpl. 2004;10(3):449–55. doi: 10.1002/lt.20099. [DOI] [PubMed] [Google Scholar]
- 11.Graziadei IW, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9(6):557–63. doi: 10.1053/jlts.2003.50106. [DOI] [PubMed] [Google Scholar]
- 12.Goodman J, et al. Liver transplantation for hepatocellular carcinoma: expanding special priority to include stage III disease. Arch Surg. 2005;140(5):459–64. discussion 464. [Google Scholar]
- 13.Yao FY, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 14.Yao FY, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587–96. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 15.Chapman WC, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248(4):617–25. doi: 10.1097/SLA.0b013e31818a07d4. [DOI] [PubMed] [Google Scholar]
- 16.Zhou XD, et al. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91(8):1479–86. doi: 10.1002/1097-0142(20010415)91:8<1479::aid-cncr1155>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Lang H, et al. Survival and recurrence rates after resection for hepatocellular carcinoma in noncirrhotic livers. J Am Coll Surg. 2007;205(1):27–36. doi: 10.1016/j.jamcollsurg.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Teh SH, et al. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 2007;132(4):1261–9. doi: 10.1053/j.gastro.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Teh SH, et al. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9(9):1207–15. doi: 10.1016/j.gassur.2005.09.008. discussion 1215. [DOI] [PubMed] [Google Scholar]
- 20.Cucchetti A, et al. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12(6):966–71. doi: 10.1002/lt.20761. [DOI] [PubMed] [Google Scholar]
- 21.Del Gaudio M, et al. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: University of Bologna experience. Am J Transplant. 2008;8(6):1177–85. doi: 10.1111/j.1600-6143.2008.02229.x. [DOI] [PubMed] [Google Scholar]
- 22.Ikegami T, et al. The timing of liver transplantation after primary hepatectomy for hepatocellular carcinoma: a special reference to recurrence pattern and Milan criteria. Transplantation. 2008;86(5):641–6. doi: 10.1097/TP.0b013e3181814de2. [DOI] [PubMed] [Google Scholar]
- 23.Margarit C, et al. Resection for hepatocellular carcinoma is a good option in Child-Turcotte-Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl. 2005;11(10):1242–51. doi: 10.1002/lt.20398. [DOI] [PubMed] [Google Scholar]
- 24.Adam R, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238(4):508–18. doi: 10.1097/01.sla.0000090449.87109.44. discussion 518–9. [DOI] [PMC free article] [PubMed] [Google Scholar]