Abstract
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) represent a spectrum of acute respiratory failure with diffuse, bilateral lung injury and severe hypoxemia caused by non-cardiogenic pulmonary edema. Failure may be initiated by pulmonary or extrapulmonary insults (e.g. pneumonia, sepsis, trauma, aspiration) that increase alveolar epithelial endothelial permeability, flood alveoli, and reduce lung compliance. The only treatment proven to improve survival is mechanical ventilation using a ‘lung protective strategy’ with tidal volume = 6 mL/kg predicted body weight. Although mortality can exceed 50%, survivors have a good prognosis for recovery of lung function.
Introduction: General Features of ALI/ARDS
Acute lung injury and the acute respiratory distress syndrome represent a spectrum of progressive respiratory failure affecting over 190,000 patients annually in the United States and causing 75,000 deaths.1,2 They are characterized by rapid-onset, diffuse, bilateral lung injury, severe hypoxemia, non-cardiogenic pulmonary edema, low alveolar ventilation/perfusion ratios (VA/Q), and abnormal physiological shunting of O2. Such acute respiratory failure usually occurs with neutrophil (PMN)-mediated lung inflammation. Pathological increases in permeability of alveolar epithelial and endothelial cells occur, leading to dyspnea, tachypnea, and arterial hypoxemia. These permeability defects increase efflux of proteins and fluids from blood into the alveolar interstitium and airspaces, while alveolar liquid clearance is impaired that normally resolves pulmonary edema.1 Consequently, alveolar O2 exchange deteriorates.
ALI/ARDS is a complex, multi-phase process. Initiating factors that cause early lung dysfunction may differ from those that perpetuate, complicate, or delay resolution. ALI/ARDS can be caused by pulmonary-specific mechanisms, notably severe pneumonia, aspirating gastric contents, embolism (fat, air, amniotic fluid), chest trauma, near-drowning, and inhaled noxious gases.1 ALI/ARDS can also occur due to systemic conditions, notably bacteremic sepsis, peritonitis or pancreatitis, hemorrhagic shock, burns, massive transfusions, and drug overdoses.1, 3 Such indirect factors reflect the pulmonary manifestation of acute systemic inflammation and generalized endothelial injury involving many organ systems. Approximately 15% of deaths from ALI/ARDS are due to progressive respiratory failure with intractable hypoxemia, increased deadspace ventilation, and hypercarbia. The main cause underlying death in ALI/ARDS (~75% patients) is the multiple organ dysfunction syndrome (MODS) involving lungs plus the cardiovascular system, kidneys, coagulation system, and/or liver.2, 4
Clinical Definitions of ALI/ARDS
In 1994, the American-European Consensus Conference on ARDS5 issued definitions that have been widely adopted by clinicians and researchers. These include:
1. Acute lung injury (ALI): A syndrome of acute and persistent lung inflammation with increased vascular permeability characterized by four clinical features:
Acute onset (within days of exposure to predisposing direct or indirect causes);
Diffuse bilateral infiltrates on x-ray consistent with non-cardiogenic pulmonary edema;
PaO2/FIO2 ≤ 300 mmHg, regardless of the level of positive end-expiratory pressure (PEEP) during mechanical ventilation. PaO2/FIO2 estimates lung oxygenation efficiency across the range of supplemental O2 provided to patients. PaO2 is measured in mmHg and FIO2 is a decimal between 0.21 and 1.00. For a subject with PaO2 = 100 mmHg on room air (FIO2 = 0.21), PaO2/FIO2 = 100/0.21 = 476 mmHg.
No clinical evidence of left-sided heart failure or elevated left atrial pressure, i.e. an elevated “wedge” pressure if a pulmonary artery catheter (PAC) had been placed, as in congestive heart failure (See Figures 1A & 1B). A relatively normal heart size by x-ray supports the clinical absence of left ventricular failure as the explanation for pulmonary edema. If a PAC is present, the wedge pressure is ≤ 18 mmHg. Clinical evidence of left heart failure includes systolic left ventricular (LV) dysfunction, plus appropriate physical findings, such as LV S3 gallop and peripheral edema, especially when supported by LV dilation or reduced ejection fraction by echocardiography or catheterization, or enlarged cardiac silhouette plus pleural effusions.
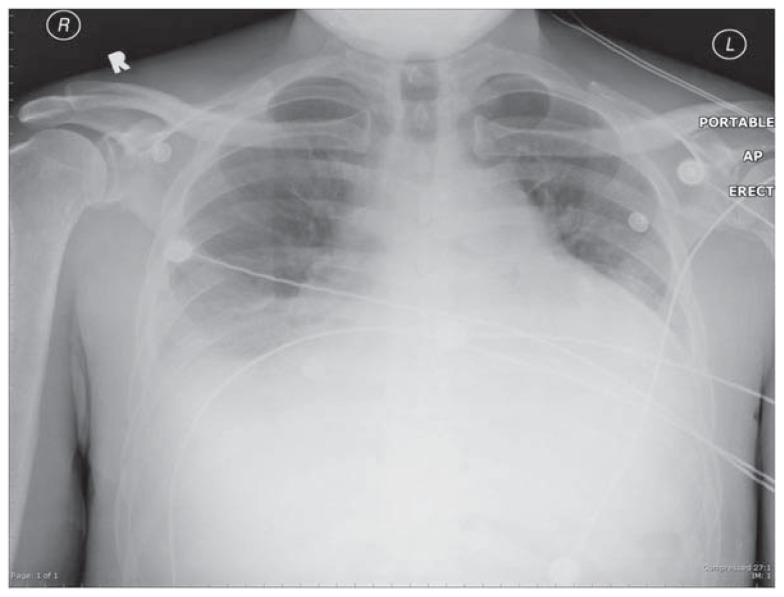
Figure 1A.
Chest radiograph showing cardiomegaly and bilateral pleural effusions. Such findings are compatible with elevated left atrial pressure, interstitial cardiogenic pulmonary edema, and LV systolic dysfunction as are seen in congestive heart failure.
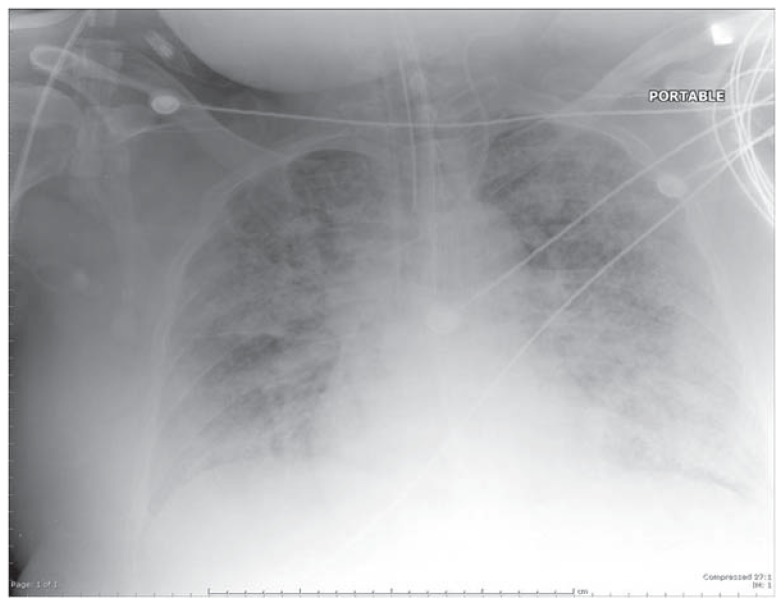
Figure 1B.
Chest radiograph showing diffuse, bilateral pulmonary infiltrates in all lung quadrants (acute air-space disease) typical of ALI/ARDS. The costophrenic angles are relatively clear, indicating absence of pleural effusions.
2. Acute respiratory distress syndrome (ARDS): A more severe physiological expression of ALI, at the further end of a spectrum of evolving lung injury characterized by:
Acute onset;
Diffuse bilateral infiltrates on x-ray consistent with pulmonary edema;
PaO2/FIO2 ≤ 200 mmHg;
No evidence of left-sided heart failure, including normal heart size on x-ray (See Figures 1A & 1 B). If a PAC is in place, the measured pulmonary wedge pressure is ≤ 18 mmHg.
Pathophysiology of ALI/ARDS
Normal lung function requires dry, open alveoli overlying perfused capillaries with intact gas exchange and a normal work of breathing. It requires coordinated interaction of several key physiological processes plus appropriate lung immune and inflammatory responses.
Three inter-related pathophysiologic abnormalities typify events that cause or precipitate ALI/ARDS.
1. Increased Microvascular Permeability (Non-Cardiogenic Pulmonary Edema)
Normal pulmonary capillary endothelium is selectively permeable, with serum proteins confined to the intravascular space, while low molecular weight molecules and water cross the membranes under hydrostatic and osmotic forces. The Starling Equation describes the forces directing fluid movement between the vessels and the interstitium:
Where:
Q=net transvascular fluid movement
K=filtration coefficient for permeability of the capillary endothelium
Pmv=hydrostatic pressure within microvessels
Ppmv=hydrostatic pressure in surrounding peri-microvascular space
σ=protein reflection coefficient, normally 1.0 (arbitrary units)
πmv=protein osmotic pressure in the circulation
πpmv=protein osmotic pressure in the peri-microvascular space
Balanced hydrostatic and osmotic forces normally allow only small quantities of fluid into the interstitium of alveolar septa. At least four mechanisms promote fluid retention in capillaries to prevent interstitial edema and alveolar flooding. First, most airspace liquid is cleared by apical Na+ transporters in alveolar epithelial cells. Second, larger plasma proteins like albumin (65kD) maintain osmotic gradients favoring water re-absorption. Third, tight junctions between pulmonary endothelial cells prevent leakage. Fourth, interstitial lymphatics return alveolar fluid to the circulation. Abnormalities in Starling forces during ALI/ARDS include:
Decreased σ during sepsis, causing larger proteins like albumin to enter the interstitium, so that protein-rich edema floods alveoli.
Reduced πmv from excessive i.v. infusions and/or reduced acute phase hepatic regulation of albumin, etc., favoring increased transvascular fluid flux into distal airspaces.
Increased Pmv from fluids infused to treat shock, hypovolemia, or decreased venous return secondary to increased intrathoracic pressure during positive-pressure ventilation.
Increased πpmv from plasma proteins entering alveolar interstitia, or from loss of alveolar epithelial Na+ transport, both retarding alveolar liquid clearance.
Collectively, these cause gravitationally-dependent edema and alveolar flooding, evident in inferior lung zones of supine patients (See Figure 2), worsening VA/Q mismatch and reducing PaO2.
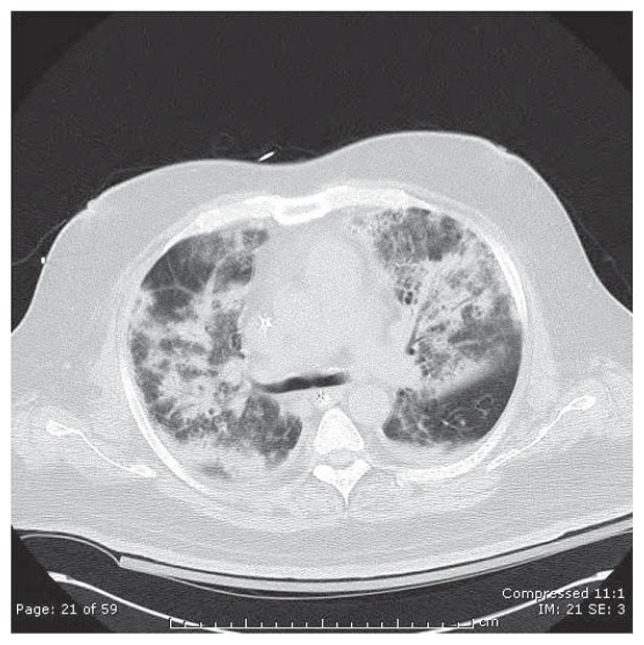
Figure 2.
Computed tomographic (CT) scan of the chest (lung attenuation window) in a patient with severe ARDS due to bilateral S. pneumoniae pneumonia. Note extensive opacification of alveolar units & prominent air bronchograms, compared with the small residual area of normal, radiolucent (black)-appearing lung in the right upper and left lower portions of this image.
2. Alveolar Instability and De-recruitment with Decreased Lung Compliance: Pathophysiology and Potential for Ventilator-Associated Lung Injury
Compliance quantifies changes in lung volumes (ΔV) caused by distending airway or intra-pleural pressures (ΔP) as during spontaneous respiration. Compliance (=ΔV/ΔP) is often reduced in ALI/ARDS, leading to alveolar instability and collapse when approaching end-expiratory pressures. This de-recruitment of formerly functioning alveoli results from several factors:
Alveolar flooding inactivates surfactant when plasma proteins leak into distal airspaces.
Such alveoli show increased surface tension, and can collapse (i.e. undergo atelectasis) during end-expiratory pauses in patients with ALI/ARDS. These distal airspaces fail to expand cyclically during inspiration due to excessively high alveolar opening pressures.
Functional residual capacity (FRC) characteristically falls in ALI/ARDS patients, who often breathe rapidly and shallowly to maximize minute ventilation (VE, L/min) while minimizing the work of breathing to expand non-compliant or “stiff ” lungs.
Superimposed mechanical ventilation-associated lung injury (VALI) may occur, especially at tidal volumes (VT) exceeding 10 mL/kg of predicted body weight (PBW) as conventionally used to ventilate adults with ALI/ARDS.
Since many alveoli may become atelectatic due to alveolar flooding and inflammatory exudates, mechanical ventilation with conventionally high VT to sustain VE is equivalent to forcing each positive-pressure inspiration into infant-sized lungs. Simultaneously, non-atelectatic alveoli receive excessive ventilation being redirected from atelectatic regions (See Figures 2, 3). This dilemma sets the stage for three forms of VALI that are not mutually exclusive:
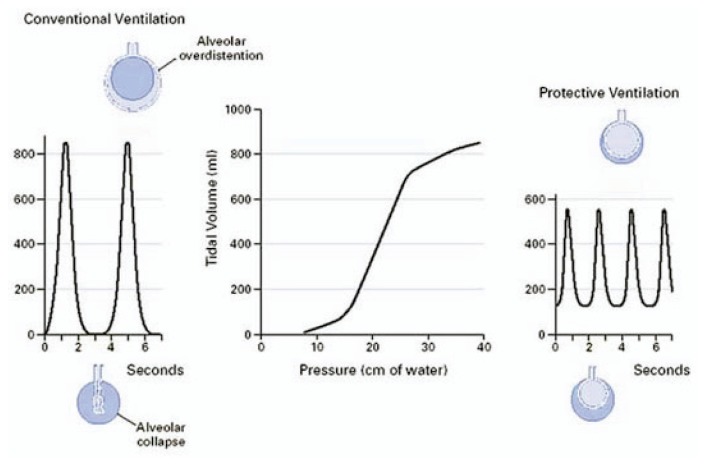
Figure 3.
Effects on achieved tidal volumes in sedated patients using conventional mechanical ventilation (left) or when using a protective lung ventilation strategy (right). From Tobin MJ7. © 2001, Massachusetts Medical Society. All rights reserved.
Barotrauma: pressure-related lung injury
Volutrauma: alveolar over-distension injury
Cyclical shear stress from excessive tidal swings in alveolar diameters
3. Dysregulated and Excessive Acute Lung Inflammatory Responses
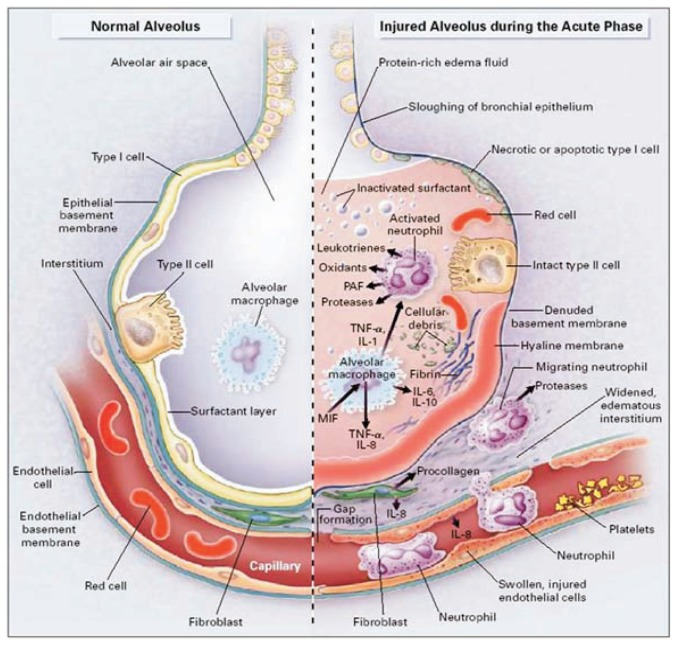
A hallmark of ALI/ARDS is excessive inflammation in the alveolar interstitium and airspaces (See Figure 4). This response involves massive neutrophil influx into alveolar structures initiated by upregulated adhesion molecules on PMN and vascular endothelia and sustained by additional chemotactic signals and host-derived mediators. Consequently PMNs in bronchoalveolar lavage fluid may approach 50% of all cells recovered, vs. ≤ 3% in healthy volunteers. Major inflammatory mediators in this acute phase include:
Figure 4.
The normal alveolus vs. the acutely injured alveolus in ALI/ARDS. From: Ware LB and Matthay M1. © 2000, Massachusetts Medical Society. All rights reserved.
Cytokines, notably tumor necrosis factor (TNF)-αɑ, interleukin (IL)-1ββ, IL-6, IL-8, IL-10, granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF);
Chemokines, including macrophage inhibitory factor and macrophage chemoattractant protein;
Arachidonic acid metabolites including prostanoids and leukotrienes;
Oxyradicals/oxidants, including superoxide anion and peroxynitrite;
Proteases that degrade alveolar structures and enhance inflammation;
Fibrin, following activation of tissue factor and the coagulation system.
Together, these processes cause acute lung dysfunction, manifested as tachypnea with distress, declining PaO2 or declining SaO2 by pulse oximetry, and chest radiographs or CT scans showing bilateral infiltrates. [Infiltrates may be asymmetric in patients with co-existing chronic obstructive pulmonary disease.] Thus progressively impaired gas exchange occurs, with VA/Q mismatching and physiological shunting causing hypoxemia. Additionally, physiological deadspace may increase despite a constant VE, retarding CO2 elimination. Ventilator-induced rises in intrathoracic pressure also inhibit venous return, with fewer alveoli perfused despite ongoing ventilation. In advanced cases of ARDS, CO2 excretion falls and PaCO2 rises.
Transfusion-related acute lung injury (TRALI) is an important form of ALI/ARDS with similar clinical features but temporally related to transfused blood products. TRALI is the leading cause of transfusion-related death in the U.S. Affected patients develop fever with cough, dyspnea, and hypoxemia within 6 h of receiving red cells, platelets, or fresh frozen plasma3.
Typical Presentation and Progression of ALI/ARDS
Despite many precipitating factors, most ALI/ARDS patients follow similar clinical courses characterized initially by severe hypoxemia requiring prolonged mechanical ventilatory support (days to weeks). Patients generally progress through three pathologic stages. An initial exudative stage with diffuse alveolar damage gives way over the first week to a proliferative stage with resolving pulmonary edema, proliferating type II cells, squamous metaplasia, interstitial infiltration by myofibroblasts, and deposition of collagen.1 For unclear reasons, some patients progress to a third fibrotic stage with accelerated collagen deposition, obliteration of normal lung architecture, diffuse fibrosis, and parenchymal cyst formation. This last subgroup accounts for most patients who die “of ” ALI/ARDS with intractable hypoxemia and increasing deadspace ventilation that require progressively higher FIO2’s and risk of lung O2 toxicity. [Prolonged ventilation with FIO2’s ≥ 0.60 is associated with increased inflammation and fibrosis.]
Physical exam usually reveals tachycardia, tachypnea, diffuse crackles over the chest, increased work of breathing, and use of accessory respiratory muscles (trapezius, scalene, sternocleidomastoid, and pectoralis). Laboratory findings are nonspecific and may include leukocytosis, evidence of disseminated intravascular coagulation (DIC), and lactic acidosis. Blood gases usually show acute respiratory alkalosis, reduced SaO2 (< 90%), decreased PaO2/FIO2, and severe hypoxemia reflecting right-to-left shunt physiology. Chest radiographic findings, while distinctive, are also seen in diffuse lung hemorrhage and acute interstitial edema. Importantly, ALI/ARDS is a clinical syndrome, not a specific disease. It is always caused by underlying conditions that must be diagnosed and treated, in addition to the respiratory failure.
As to the subsequent course in such patients, oxygenation usually improves over the first few days as edema resolves, but patients may remain ventilator-dependent due to continued hypoxemia, high VE requirements, and poor lung compliance. Radiographic densities become less opaque as edema resolves, but interstitial infiltrates may persist. In the exudative and proliferative phases, lung flooding may resolve but patients continue to exhibit increased dead-space ventilation seen as increased VE with normal or elevated PaCO2. Oxygenation may respond favorably to PEEP by reversing atelectasis (See Figure 3), or remain problematic because of surfactant dysfunction. Organizing fibrosis may occur in the proliferative phase, causing increased airway pressures, pulmonary hypertension, and honeycomb appearances on x-ray.
Complications in ALI/ARDS Patients
Complications occur daily in ICU patients, and those with ALI/ARDS and co-existing MODS are particularly prone to deep venous thrombosis, pulmonary thromboembolism, gastrointestinal bleeding, malnutrition, untoward effects from sedative and neuromuscular blocking medications, and superimposed catheter-related infections. In addition, several lung-specific complications may develop, most especially ventilation-related trauma, shear stress injury, and VALI already mentioned.1
In addition, nosocomial ventilator-associated pneumonia (VAP) in mechanically ventilated patients is a feared complication, with mortality rates of up to 50%. The “attributable mortality” for VAP is 33 – 50%, despite aggressive supportive care and antimicrobial therapy in affected patients. VAP develops in 9 – 27% of ventilated patients, and the risk of VAP increases 1 – 3% per day of intubation. Late-onset VAP, developing after ≥ five days of intubation and ventilation, is frequently caused by multiple drug resistant (MDR) organisms such as methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, or Acinetobacter baumanii. VAP is clinically diagnosed by the development of new fever, increased purulent secretions from the endotracheal tube, and new or worsening infiltrates on chest x-rays.
The manner in which ALI/ARDS patients are mechanically ventilated has immediate consequences on oxygenation, but also impacts acute inflammatory responses within alveoli. Thus, how such diffusely injured lungs are ventilated by positive-pressure ventilation may: 1) augment inflammatory injury by superimposing additive pressure-or volume-related trauma; 2) delay resolution of lung injury; or 3) enhance recovery of pulmonary gas exchange, lung cell function, and non-pulmonary organ homeostasis.
In many patients, their hypoxemia, hypotension, or rapid deterioration in lung compliance may not be immediately apparent, and a pneumothorax may be difficult to visualize on chest films obtained in the supine patient. Such pneumothoraces may enlarge in patients on positive pressure ventilation, compress central venous structures, and cause cardiogenic shock due to diminished ventricular filling. Urgent needle decompression and definitive chest-tube thoracostomy are required when pneumothorax is detected in a patient on mechanical ventilation.
Definitive Treatment of ALI/ARDS
In patients with acute respiratory failure, the first priority is to stabilize their acutely abnormal gas exchange and hemodynamics to prevent hypoxic organ damage, respiratory muscle fatigue and increased work of breathing, and cardiopulmonary arrest. Rapid clinical assessment followed by transfer to an ICU setting is important. Most patients will require endotracheal intubation and mechanical ventilation with a high initial FIO2 (commonly 1.00) to relieve hypoxemia, and application of PEEP to increase their FRC.
Diagnosing the underlying causes of ALI/ARDS is critically important. For patients with sepsis, aggressive determination of the cause (e.g. pneumonia, catheter-related bacteremic sepsis, intra-abdominal abscess following surgery) is indicated, since infections and purulence within closed spaces must be drained, and empiric broad-spectrum antimicrobial therapy begun quickly.
Given the increased vascular permeability in ALI/ARDS, the appropriate goals for fluid administration, volume status, and hemodynamic management will differ across patient categories. Thus, administering sufficient i.v. fluids to perfuse non-pulmonary organs may cause life-threatening hypoxemia. Similarly, aggressive diuresis may improve pulmonary gas exchange by lowering left atrial pressure and thus the gradient for alveolar flooding from leaky capillaries.. Early initiation of supplemental nutrition within 72 hours of ICU admission, preferably by enteral route, is indicated and has been associated with improved outcomes in limited trials.
“Lung Protective” Mechanical Ventilation Strategy
Historically, a tidal volume (VT) of 12 – 15 mL/kg predicted body weight (PBW) was recommended in ALI/ARDS. However, it is now clear that a low VT lung protective strategy reduces mortality and is the single most important evidence-based therapy that should be initiated in mechanically ventilated patients with ALI/ARDS. In 2000, the NIH ARDS Network published the findings of a randomized, controlled multi-center clinical trial in 861 patients6. The trial compared a low VT strategy (6 mL/kg and a plateau pressure < 30 cm H2O) with a higher VT cohort (12 mL/kg, plateau pressure < 50 cm H2O). Plateau pressure (Pplat) in vivo is that airway pressure during the brief pause after a mechanical inspiration ends and before the subsequent expiration begins. Measured Pplat in ventilated patients reflects the static distending pressure in the lungs when airflow (and thus, frictional resistance to airflow) is zero. As such, Pplat reflects lung compliance measured during a 0.5 sec inspiratory hold in a relaxed, sedated patient. The chief therapeutic goal of minimizing VALI is achieved when Pplat is < 30 cm H2O.
In this ARDS Net Trial, in-hospital mortality was 40% among the 12 mL/kg group vs. 31% in the 6 mL/kg group. This highly significant 22% reduction in mortality necessitated stopping the trial early for ethical reasons. With an absolute risk reduction of 9% using low VT ventilation, one life is saved for every 11 ALI/ARDS patients treated by this modality. There were also more ventilator-free days (thereby decreasing the likelihood of VAP) and more organ failure-free days in patients within the low VT group. The steps prescribed in the lung protective strategy6,7 to establish initial ventilator VT and frequency (f) adjustments are:
Calculate patient’s PBW, and set initial VT to 8 mL/kg PBW;
Set Ventilator Mode to “Volume Assist-Control”;
Decrease VT to 7 mL/kg after 1 – 2 h, then to 6 mL/kg PBW after another 1 – 2 h;
Set f to maintain adequate minute ventilation (but < 35 breaths/min);
Establish a Pplat goal of < 30 cm H2O;
Check Pplat with a 0.5 sec inspiratory pause every 4 h and after each change in PEEP or VT.
Other Evidence-Based Approaches for Treating ALI/ARDS
The pulmonary artery catheter (PAC) historically provided significant cardiopulmonary physiological data in ALI/ARDS patients, including estimation of wedge pressure, cardiac output, pulmonary artery and central venous (right atrial) pressures, mixed venous O2 saturation as a measure of tissue O2 utilization, and an estimate of RV preload based on ejection fraction analysis (RV end-diastolic volume index). However, PAC use to guide management and improve outcomes in ALI/ARDS was addressed by a recent NIH trial in 1,000 patients comparing hemodynamic management guided by a PAC vs. a central venous catheter8. Neither 60-day mortality nor days until ventilator weaning were improved by PACs, indicating no benefit for managing ALI/ARDS.
Although ALI/ARDS is an inflammatory process and steroid medications have powerful anti-inflammatory properties, no evidence-based report has shown significant benefit using methylprednisolone or other steroids to prevent or treat lung inflammation associated with ALI/ARDS. Indeed, high dose methylprednisolone therapy during the fibroproliferative stage of ARDS increased mortality at 60 and 180 days if begun > 2 weeks after diagnosis9.
Prognosis
Survival has improved for patients with ALI/ARDS, particularly since widespread institution of the lung protective strategy for mechanical ventilation. Overall mortality from ARDS uncomplicated by multiple organ dysfunction syndrome (MODS) remains ~ 25 – 35% despite improved ICU management, availability of new antimicrobial therapies, nutritional support, and other factors. However, mortality in patients with ALI/ARDS and MODS increases proportionately with the number of dysfunctional non-pulmonary organs, with highest mortality observed of 50 – 75% with concurrent septic shock plus MODS. Thus, severe sepsis, septic shock, and MODS are more powerful predictors of survival than respiratory parameters per se. Advanced age and pre-existing liver dysfunction also connote a poor outcome.
It is difficult to predict outcome in individual patients with ALI/ARDS based on initial severity of physiological impairments including oxygenation; such findings do not reliably distinguish survivors from non-survivors. Likewise, a patient’s initial respiratory compliance, radiographic findings, or required PEEP do not correlate with mortality. However, failure to improve clinically over the first several days, particularly regarding oxygenation, predicts a complicated course and greater mortality risk.
Long-term prognosis for recovery of lung function following ALI/ARDS is reasonably good with respect to lung function.4 Within six months after hospital discharge, spirometric lung volumes largely return to pre-morbid or predicted values, except in patients with pre-existing lung disease. At one year after discharge, the commonest pulmonary physiological finding is mild to moderate reduction in the single-breath diffusion capacity for carbon monoxide. Exertional O2 desaturation is seen in 6% of survivors. Both likely reflect persistent fibrotic remodeling that retards diffusive gas exchange in the distal airways and alveoli.
Biography
George M. Matuschak, MD, and Andrew J. Lechner, PhD, are both Professors in the Department of Pharmacological and Physiological Science and the Division of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine at the Saint Louis University School of Medicine.
Contact: matuscgm@slu.edu


Footnotes
Disclosure
None reported.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Looney MR, Gropper MA, Matthay MA. Transfusion-related lung injury. Chest. 2004;126:249–258. doi: 10.1378/chest.126.1.249. [DOI] [PubMed] [Google Scholar]
- 4.Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest. 2007;131:554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 5.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 6.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 7.Tobin MJ. Advances in mechanical ventilation. New Engl J Med. 2001;344:1986–1996. doi: 10.1056/NEJM200106283442606. [DOI] [PubMed] [Google Scholar]
- 8.The National Heart, Lung and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. New Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 9.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. New Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]