Abstract
Ion channels play important roles in numerous physiological processes, including the control of heart rate, propagation of the nerve impulse, and insulin secretion. Abnormal functioning of ion channels can cause disease. This article presents an overview of the diseases in humans that are a result of mutations in ion channels.
Introduction
Movement of charged molecules in and out of the cell is often energetically unfavorable due to the presence of the cell membrane. Ion channels are membrane-intrinsic proteins that form water-filled pores through the membrane, providing a pathway for ions to cross. Many channels are able to control the flow of ions in response to a variety of signals, and act as critical control points in a vast array of physiological phenomena, e.g. the electrical activity of neurons and muscles that allow us to think and move, and the secretion of insulin necessary for glucose homeostasis. It is not surprising that mutation of genes coding for ion channel proteins result in disease. This article presents a brief (and therefore necessarily incomplete) overview of human diseases that are associated with mutations of ion channels, and begins with three paradigms of ion channel function and associated diseases: (1) generation of the action potential and Long QT syndrome, (2) salt reabsorption in the kidney and Bartter’s syndrome, and (3) the regulation of insulin secretion and diabetes/congenital hyperinsulinism. Diseases grouped by channel type will also be presented. Diseases that are the result of acquired altered ion channel function are not discussed.
Long QT Syndrome
The cardiac action potential is the result of a complex interplay of currents through ion channels. Some of the major currents that underlie the cardiac action potential are shown in Figure 1A. Initially, influx of sodium ions (INa) through activated sodium channels depolarizes the membrane, forming the rapid upward phase. Sodium channels then rapidly inactivate, but the depolarized cell membrane activates voltage-gated potassium and calcium channels. Calcium influx through calcium channels tends to depolarize the membrane, while potassium efflux through potassium channels tends to repolarize the membrane. These two opposing effects create the plateau of the cardiac action potential. Repolarization occurs with closure of the calcium channels. In an ECG, ventricular depolarization is represented by the QRS complex, while the T wave is due to repolarization (See Figure 1B).
Figure 1.

The cardiac action potential and surface ECG. Some of the major ionic currents involved are indicated.
Potassium currents also assist in repolarization of the cardiac cell membrane, and include the slowly activating potassium current (IKs) due to the potassium channel complex KV7.1(KCNQ1) + minK(KCNE1), and the rapidly activating potassium current (IKr) through KV11.1 (KCNH2) + MiRP1 (KCNE2)1. Decreased K currents as a result of mutations in these K channel complexes can cause a prolongation of the action potential and lengthening of the QRS to T wave interval on an ECG (Fig 1B), manifesting as Long QT (LQT) syndrome. Patients with LQT are predisposed to sudden death due to cardiac arrhythmias and Torsades de Pointes, particularly under stressful situations that increase adrenergic tone. Profound deafness can be seen in patients with LQT due to mutations in KV7.1, as it is necessary for hair cell development in the inner ear.
The potassium channel Kir2(KCNJ2), also plays an important role in repolarization (See Figure 1A, IK1). Mutations in this channel are associated with Andersen-Tawil syndrome1, characterized by a milder QT prolongation, periodic paralysis, cardiac dysrhythmias and dysmorphic features.
Mutations that delay closure of calcium and sodium channels, prolonging depolarization, are also associated with LQT syndrome1, and include activating mutations in the SCN5A gene (sodium channel NaV1.5) that cause a persistent sodium current in the plateau phase. Timothy syndrome is due to mutations in CACNA1C (calcium channel CaV1.2, ICa in Fig 1A) that cause delayed channel closure, manifested as LQT along with syndactyly, autism, and facial dysmorphisms.
In contrast, loss-of-function mutations in NaV1.5, or gain-of-function mutations in KV7.1 or KV11.1 lead to early repolarization associated with arrhythmias, and can be seen in Brugada’s and short QT syndrome1.
Bartter’s Syndrome
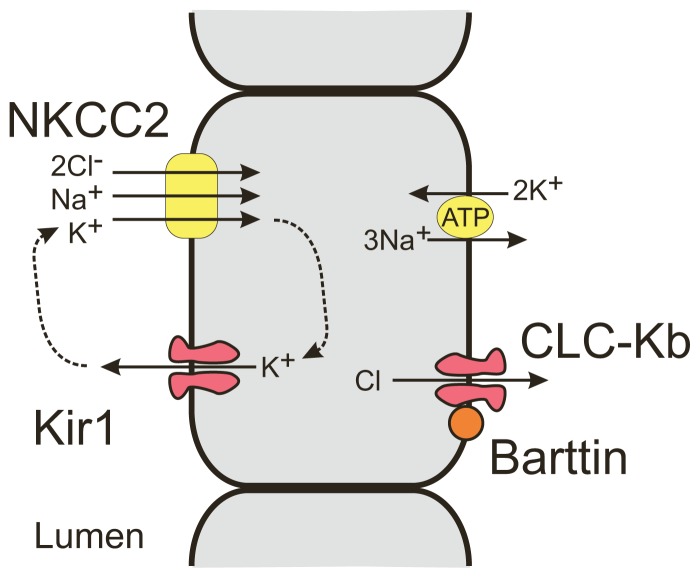
Sodium reabosorption in the thick ascending loop of Henle occurs in cotransport with K+ and Cl+ ions through the NaK2Cl cotransporter (See Figure 2). The luminal concentration of K is many fold lower than Na, and continued reabsorption requires recycling of K back into the lumen through Kir1 channels. Na and Cl ions then exit the cell through the 3Na/2K ATPase and CLC-Kb channels, both found in the basolateral membrane. Mutations in genes that code for Kir1 resulting in a loss of K recycling is associated with the severe antenatal form of Bartter’s syndrome2, characterized by polyhydramnios, premature delivery, and a failure to thrive. Neonates develop severe salt wasting, hypokalemia, metabolic acidosis, hyperprostaglandinemia, and hypercalciuria that can lead to osteopenia and nephrocalcinosis. Mutations in the CLC-Kb channels that reduce Cl− efflux result in classic Bartter’s syndrome2, a milder form that is typically diagnosed in school-age children. Bartter’s may also be the result of a mutation in Barttin, a chaperone protein that is required for proper trafficking of the CLC-Kb channel to the membrane2. In addition, Barttin is required for trafficking of ClC-Ka and ClC-Kb channels in the inner ear, and loss of Barttin function results in deafness.
Figure 2.
Schematic of ion channels and transporters involved in sodium reabsorption in the distal loop of Henle.
Regulation of Insulin Secretion
The mechanism of insulin secretion in response to blood glucose levels is well characterized3 (See Figure 3). Elevations of blood glucose (such as that occurring after eating) increases ATP and decreases ADP in the pancreatic β-cell due to glucose metabolism. KATP channels, which are inhibited by ATP and activated by ADP, close in response to the increase in ATP to ADP ratio. KATP channels are responsible for maintaining the resting membrane potential, and closure of these channels leads to membrane depolarization and activation of voltage-dependent calcium channels, followed by a rise of intracellular calcium, which then stimulates insulin secretion. Conversely, when glucose concentrations decrease, the ATP/ADP ratio decreases, KATP channels open, and insulin secretion is inhibited.
Figure 3.
Paradigm of insulin secretion from the pancreatic β-cell.
In this paradigm, the KATP channel plays a key role in the regulation of insulin secretion. Loss of KATP channel activity would be expected to cause persistant membrane depolarization, and hypersecretion of insulin. Congenital hyperinsulinism is a rare disorder characterized by inappropriate insulin secretion and hypoglycemia that can be very severe. Loss-of-function mutations in the subunits that form the KATP channel (Kir6.2 KCNJ11 and SUR1 ABCC8) have been found in patients with congenital hyperinsulinism3, 4. In contrast, patients with mutations that increase KATP channel activity display an undersecreting phenotype and hypoinsulinemia called neonatal diabetes. These patients develop diabetes before 6 months of age that may be transient or permanent. A polymorphism of the Kir6.2 gene (E23K) that results in a mild decrease in sensitivity to inhibition by ATP may be associated with an increased risk in developing type II diabetes3.
Other Channelopathies, Grouped by Ion Channel Type
Voltage-Gated Potassium (KV) Channels
In addition to cardiac arrhythmias (discussed above), KV channels are associated with syndromes that affect the neuron. Mutations in KV1.1(KCNA1)2 are associated with episodic ataxia (EA), and reduce K conductance, leading to prolonged action potentials and increased cell excitability. EA patients have bouts of ataxia and loss of balance, provoked by stress, startle, or heavy exertion such as exercise.
KCNQ2 and KCNQ3 encode for proteins that heteromultimerize into a K channel that underlies the M-current2. This K current is important in controlling excitability of many neurons. Benign familial neonatal convulsions is associated with mutations resulting in increased neuronal excitability due to decreased M-currents. Patients have tonic-clonic seizures within the first seven days of life. Seizures spontaneously remit after about three months, apparently due to compensation by other neuronal currents, but patients are more susceptible to developing seizures later in life.
The BK (KCNMA) channel, a calcium activated K channel widely expressed in the central nervous system, is associated with a syndrome of generalized epilepsy and paroxysmal dyskinesia. Mutations increase the activity of this K channel5.
Voltage-Gated Sodium (NaV) Channels
In muscle, mutations that cause persistant activity of the voltage-gated sodium channel NaV1.4 (SCN4A) have been associated with cell hyperexcitability, manifested as repetitive action potential generation and impaired muscle relaxation (myotonia), as seen in paramyotonia congenita and potassium-aggravated myotonias6. Depending on the size of the Na currents, excessive depolarization and paralysis can also occur, as seen in hyperkalemic periodic paralysis7. Symptoms often can be triggered by high potassium diets, presumably through raising serum potassium levels which leads to a mild depolarization of muscle membrane and further enhancing sodium channel activity. In contrast, mutations that decrease NaV1.4 activity have been associated with hypokalemic periodic paralysis (HypoPP)7. Episodes of paralysis can be triggered by events that lower serum potassium, which can occur after high carbohydrate meals with elevated insulin levels, or under stress with release of adrenaline, resulting in hyperpolarization of the membrane and making activation of these mutant channels even more difficult.
In neurons, mutations of voltage gated sodium channels have complex effects, and the same mutation can lead to both increased and decreased cell excitability8. Mutations in SCN1A(NaV1.1), SCN2A(NaV1.2), and SCN1B (sodium-channel β-subunit), have been associated with a variety of seizure disorders including generalized epilepsy with febrile seizures plus, severe myoclonic epilepsy of infancy, and the syndrome of intractable childhood epilepsy with generalized tonic–clonic seizures. Mutations in SCN9A(NaV1.7) are associated with familial primary erythermalgia, a rare autosomal dominant disorder characterized episodes of redness, swelling, and painful burning in the extremities.
Voltage-Gated Calcium (CaV) Channels
In response to depolarization of the sarcomeric membrane, calcium channels of the T-tubules open allowing entry of calcium and muscle contraction. Mutations that decrease the activity of CaV1.1(CACNA1S), expressed in the transverse tubules of skeletal muscle cells, can cause HypoPP7. CaV1.1 mutations associated with HypoPP reduce activation of the channel. Similar to the Na channel associated HypoPP, hypokalemia worsens the condition by hyperpolarizing the cell membrane and making it more difficult to activate the calcium channel.
Mutations in the CACNA1F (CaV1.4, highly expressed in the retina) is associated with incomplete congenital stationary night blindness. Patients with this disorder have night blindness and decreased visual acuity, thought due to defects in photoreceptor transduction. Mutations result in either non-functional channels or shift the voltage-dependence of opening to more hyperpolarized potentials.
Familial hemiplegic migraine with aura associated with hemiparesis, episodic Ataxia type-2, and spinocerebellar ataxia type-6 are all associated with mutations in the calcium channel gene, CACNA1A (CaV2.1, expressed heavily in the cerebellar Purkinje cells)9–11. They are all associated with cerebellar degeneration but differ in rate and extent of disease progression.
Epithelial Sodium Channels (ENaC)
ENaC is located in the apical membrane of epithelial cells of the lung, kidney, and colon, and are important in the transcellular transport of sodium ions. Liddles syndrome is a rare form of hypertension associated with hyperactive ENaC channels in the distal nephron. Patients have severe hypertension at an early age, hypokalemia, metabolic acidosis, and low serum aldosterone levels. Mutations found in Liddles block ubiquitination of ENaC12 so that it is no longer properly degraded, leading to increased sodium reabsorption and hypertension. Treatment by blockade of the ENaC with amiloride or triamterene is effective. Conversely, loss-of-function mutations in ENaC subunits (genes SCNN1A, SCNN1B, and SCNN1G) are associated with autosomal recessive pseudohypoaldosteronism Type-1. Most of the mutations are predicted to result in non-functional channels, as a result of frameshift or early stop codons12. Patients present with salt-wasting, hyperkalemia, metabolic acidosis, and high serum aldosterone levels.
Chloride Channels (ClC)
Mutations in ClC-1 have been associated with congenital myotonias7, 13, characterized by stiffness due to slow relaxation of the muscles after contraction. ClC-1 is almost exclusively expressed in skeletal muscle and is important in repolarization and maintanence of the resting membrane potential. Mutations that lead to truncated proteins are seen in the recessive form (Becker’s), while mutant subunits that are able to interact with normal subunits and interfere with their function are seen in the autosomal dominant form (Thomsen’s). These mutations result in hyperexcitable muscle cells. Findings in Thomsen’s are noticeable at birth or in early childhood, and are progressive. Muscle hypertrophy is a common sign.
Cyclic-Nucleotide Gated (CNG) Channels
CNG channels of the retina are critical in visual transduction. In the dark, high levels of cGMP activate CNG channels, allowing calcium influx and stimulating neurotransmitter release. Light activates cGMP phosphodiesterase, leading to closure of CNG channels and shutting off neurotransmitter release. Mutations of the rod cell CNG channel (CNGA1) are associated with retinitis pigmentosa (RP)14. Patients with RP suffer from night blindness, progressive reduction of the visual field, and abnormal pigmentation of the retina. Mutations in CNGA3 and CNGB3, both encoding for CNG channels in cone cells, are associated with achromatopsia14, characterized by the inability to perceive color and poor visual acuity.
Nicotinic Cetylcholine Receptor (nAChR) Channels
nAChR are cation selective channels that are important in neuromuscular signaling. Binding of acetylcholine activates these channels causing membrane depolarization of the postsynaptic neuromuscular junction and muscle contraction. Mutations in the nAChR are associated with two syndromes, the Slow Channel Syndrome (SCS) and the Fast Channel Syndrome(FCS)7. Patients usually present at birth with muscle weakness, rapid fatigue, and progressive muscle atrophy. In SCS, mutant AChR channels have increased activity and longer channel openings, producing a prolonged depolarization of the muscle cell membrane. Excessive depolarization results in inactivation of sodium channels, accounting for the muscle weakness. In contrast to SCS, nAChR channels either fail to open or close too quickly in FCS. Mutations in FCS appear to decrease binding affinity of acetylcholine and the ability of the channel to open.
Glycine Receptor
Mutations in this channel are associated with a very rare disorder called Startle Disease13 This autosomal dominant disease causes stiffening of the muscles in response to startling. Severe responses can occur, along with injuries as the rigidity prevents patients from reaching out when falling. Similar symptoms can be seen with strychinine poisoning, a glycine channel inhibitor2. Mutations found in this disease decrease the activity of the glycine receptor and its inhibitory actions, important for normal muscle tone and motor neuron firing patterns. Treatment includes agonists of GABA chloride channels (e.g. clonazepam), presumably by compensating for the decreased chloride current through the glycine receptor.
Ionotropic Gamma-Aminobutyric Acid (GABAA) Receptors
Like glycine receptors, GABAA receptors are chloride selective channels, and have an inhibitory action in the brain. Rare mutations of the GABRA1, GABRG2 or GABRD gene, encoding the α1, γ2 or δ subunit of the GABA receptor have been associated with seizure disorders13, 15.
Aquaporins
The autosomal recessive and dominant forms of nephrogenic diabetes insipidus (NDI) are associated with loss of function mutations in the aquaporin 2 channel (AQP2)16. Multiple disease-causing AQP2 mutations have been identified. In autosomal recessive NDI, the mutations result in misfolded aquaporin proteins that are retained in the endoplasmic reticulum. In contrast, autosomal dominant mutations result in subunits that are able to associate with normal subunits, resulting in mistrafficking or loss of function of the heteromeric channel. A small number of people have been identified with severe or total deficiency in aquaporin-1 (AQP1)16, but they are generally healthy with a mild defect in the urinary concentrating ability.
Connexins (Cx)
Connexin subunits assemble into gap junctions providing electrical and metabolic coupling between cells. Numerous human disorders associated with mutations in connexins are known, and are grouped into seven major categories: neuropathic or myelin disorders, nonsyndromic and syndromic deafness, skin diseases, cataracts, oculodentodigital dysplasia, and idiopathic atrial fibrillation17. Charcot Marie Tooth is an X-linked disorder associated with mutations in Cx32 channels, which are important in delivery of metabolites between the layers of the myelin sheath formed by Schwann cells. Mutations in Cx26, Cx30, and Cx31 are associated with deafness and skin disorders that can occur separately or together. Cx46 and Cx50 are found in the lens and mutations may result in congenital cataracts. Mutations of Cx40 have been linked to atrial fibrillation.
TRP Channels
The TRP channels are a family of relatively non-selective cation channels. TRP channels are expressed throughout the body and respond to a wide variety of stimuli. TRPC6 is expressed in the podocytes of the nephron, mutations in this channel is associated with focal segmental glomerulosclerosis18, a disease characterized by nephrotic range proteinuria and often progressive renal failure. The mechanism by which mutations cause disease is unknown.
Hypomagnesemia with secondary hypocalcemia is an autosomal recessive genetic disorder due to mutations in TRPM618. TRPM6 is expressed in the distal convoluted tubule of the nephron and in the gut, and is important for transcellular magnesium absorption. It is often associated with severe hypocalcemia that can present as convulsions and spasms in early infancy, because decreased serum magnesium levels inhibit the secretion of parathyroid hormone. Treatment includes high doses of magnesium salts.
The PKD2 gene encodes for the TRPP2 channel, critical in calcium signal transduction and regulated by polycystin-1 (PKD1). Loss-of-function mutations in either of these proteins, give rise to autosomal dominant polycystic kidney disease. Dysregulation of calcium signaling is likely a major cause19.
Summary
Ion channels play critical roles in a wide variety of physiological processes, not limited to excitable cells. Mutations of genes that encode for ion channels underlie many human diseases. A better understanding of how these channels function, both on a molecular and cellular level, will undoubtedly provide a rational framework to help develop targeted treatments for many of these diseases.
Biography
Decha Enkvetchakul, MD, is an Assistant Professor in the Department of Pharmacological and Physiological Science at the Saint Louis University School of Medicine.
Contact: denkvetc@slu.edu

Footnotes
Disclosures
None reported.
References
- 1.Morita H, Wu J, Zipes DP. The QT syndromes: long and short. Lancet. 2008;372(9640):750–63. doi: 10.1016/S0140-6736(08)61307-0. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft FM. Ion Channels and Diseases. Academic Press; 2000. [Google Scholar]
- 3.Koster JC, Permutt MA, Nichols CG. Diabetes and insulin secretion: the ATP-sensitive K+ channel (K ATP) connection. Diabetes. 2005;54(11):3065–72. doi: 10.2337/diabetes.54.11.3065. [DOI] [PubMed] [Google Scholar]
- 4.Huopio H, et al. K(ATP) channels and insulin secretion disorders. Am J Physiol Endocrinol Metab. 2002;283(2):E207–16. doi: 10.1152/ajpendo.00047.2002. [DOI] [PubMed] [Google Scholar]
- 5.Du W, et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37(7):733–8. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- 6.George AL., Jr Inherited disorders of voltage-gated sodium channels. J Clin Invest. 2005;115(8):1990–9. doi: 10.1172/JCI25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurkat-Rott K, Lehmann-Horn F. Muscle channelopathies and critical points in functional and genetic studies. J Clin Invest. 2005;115(8):2000–9. doi: 10.1172/JCI25525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waxman SG. Channel, neuronal and clinical function in sodium channelopathies: from genotype to phenotype. Nat Neurosci. 2007;10(4):405–9. doi: 10.1038/nn1857. [DOI] [PubMed] [Google Scholar]
- 9.Jen JC, et al. Primary episodic ataxias: diagnosis, pathogenesis and treatment. Brain. 2007;130(Pt 10):2484–93. doi: 10.1093/brain/awm126. [DOI] [PubMed] [Google Scholar]
- 10.Kordasiewicz HB, Gomez CM. Molecular pathogenesis of spinocerebellar ataxia type 6. Neurotherapeutics. 2007;4(2):285–94. doi: 10.1016/j.nurt.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 11.de Vries B, et al. Systematic analysis of three FHM genes in 39 sporadic patients with hemiplegic migraine. Neurology. 2007;69(23):2170–6. doi: 10.1212/01.wnl.0000295670.01629.5a. [DOI] [PubMed] [Google Scholar]
- 12.Riepe FG. Clinical and molecular features of type 1 pseudohypoaldosteronism. Horm Res. 2009;72(1):1–9. doi: 10.1159/000224334. [DOI] [PubMed] [Google Scholar]
- 13.Planells-Cases R, Jentsch TJ. Chloride channelopathies. Biochim Biophys Acta. 2009;1792(3):173–89. doi: 10.1016/j.bbadis.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Biel M, Michalakis S. Function and dysfunction of CNG channels: insights from channelopathies and mouse models. Mol Neurobiol. 2007;35(3):266–77. doi: 10.1007/s12035-007-0025-y. [DOI] [PubMed] [Google Scholar]
- 15.Gardiner M. Molecular genetics of infantile nervous system channelopathies. Early Hum Dev. 2006;82(12):775–9. doi: 10.1016/j.earlhumdev.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Noda Y, et al. Aquaporins in kidney pathophysiology. Nat Rev Nephrol. doi: 10.1038/nrneph.2009.231. [DOI] [PubMed] [Google Scholar]
- 17.Dobrowolski R, Willecke K. Connexin-caused genetic diseases and corresponding mouse models. Antioxid Redox Signal. 2009;11(2):283–95. doi: 10.1089/ars.2008.2128. [DOI] [PubMed] [Google Scholar]
- 18.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005(272):re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 19.Delmas P, et al. Polycystins, calcium signaling, and human diseases. Biochem Biophys Res Commun. 2004;322(4):1374–83. doi: 10.1016/j.bbrc.2004.08.044. [DOI] [PubMed] [Google Scholar]