Abstract
Angioedema is an increasing cause of hospitalizations in the United States. This syndrome presents with non-pitting, asymmetric swelling of the face, lips, tongue, larynx, genitalia, and extremities, although any part of the body can be involved. Common causes of angioedema include allergic reactions and ACE inhibitors. Hereditary angioedema is a rare form of angioedema that can be diagnosed by screening with a C4 level. In 2009, three new treatments for hereditary angioedema were approved for use in the United States, revolutionizing management of this rare disease.
Introduction
Angioedema is an increasing cause of hospitalizations in the United States and associated with significant medical expenditure1. Although this syndrome frequently occurs in the setting of an allergic reaction, other non-allergic forms of angioedema can present both a diagnostic and therapeutic challenge. Chronic, recurrent forms, including hereditary angioedema (HAE), acquired angioedema, medication associated angioedema, and idiopathic angioedema may present with recurrent, difficult to treat episodes leaving both the patient and practitioner feeling discouraged. This paper, therefore, will attempt to review the presentation, pathogenesis, diagnosis, and treatment options of chronic forms of angioedema with attention to newly released therapies for HAE.
Clinical Presentation
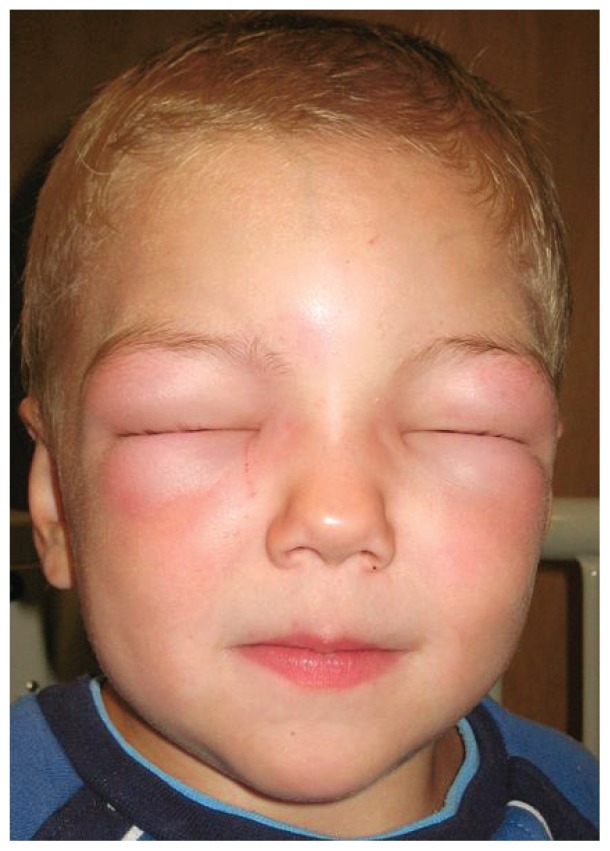
Angioedema is typically characterized by non-pitting, asymmetric swelling of the face, lips, tongue, larynx, genitalia, and extremities, although any part of the body can be involved. See Figure 1. In HAE, isolated swelling of the gastrointestinal tract frequently occurs and manifests as severe abdominal pain, nausea, vomiting, and diarrhea. This clinical presentation can be mistaken for infectious gastroenteritis, and patients can remain undiagnosed for several years after the onset of symptoms.2 Characteristically, neither ACE inhibitor induced angioedema nor HAE is associated with urticaria, but patients with HAE may have a prodromal serpiginous rash, known as erythema marginatum3. Finally, in HAE the triggering event may be trauma or stress; however, these episodes may also occur spontaneously.
Figure 1.
Facial Angioedema
(Image courtesy of Wikipedia; permission granted per the GNU Free Documentation License.)
Conversely, allergic angioedema typically occurs within several minutes of exposure to an identifiable allergic trigger, including food, medications, and insect stings. Depending on the severity of the reaction, urticaria, diarrhea, cough, wheeze, conjunctivitis, and rhinitis may also be present.
Pathogenesis
The pathogenesis of angioedema which results from an allergic reaction is a well defined consequence of direct mast cell activation. After binding and cross-linking of the high affinity IgE receptor by allergen associated IgE, the mast cell immediately degranulates, releasing inflammatory mediators, such as histamine into the dermis causing venodilation and extravasation of intravascular fluid. This is pathogenetically similar to urticaria; however, in urticaria syndromes, mast cell degranulation occurs more superficially in the epidermis, near pain receptors, leading to pruritus as a primary symptom with the characteristic wheal and flare. As the hallmark of an allergic reaction is diffuse mast cell degranulation, when angioedema is a result of an allergic trigger, urticaria often accompanies the swelling.
In non-allergic angioedema, the pathogenesis is distinctly different and is typically not accompanied by urticaria or pruritus. In these cases, an increase in bradykinin and complement derived mediators lead to the extravestation of fluid from the blood vessels again leading to localized skin swelling. This type is typical in both hereditary and acquired forms of angioedema, as well as angioedema associated with ACE inhibitors.
Angiotensin converting enzymes, in addition to mediating the body’s extracellular volume, degrades bradykinin; thus, the proposed mechanism by which ACE inhibitors lead to angioedema is by preventing breakdown of bradykinin4. It remains unclear why some individuals are susceptible to this bradykinin accumulation while others can tolerate ACE inhibitors for years without reaction.
HAE types I and II are autosomal dominant disorders due to heterogeneous mutations of the C1 INH gene (SERPING1). Mutations associated with HAE type I lead to no SERPING1 gene product (C1 INH); whereas, those associated with HAE type II lead to a dysfunctional gene product. Acquired angioedema is either a paraneoplastic disorder typically associated with lymphatic malignanices or due to an autoantibody to C1 INH.5
As C1 INH is a type of serine protease inhibitor or serpin, its absence or dysfunction leads to overactivation of proteases. It is the major inhibitor of at least three major proteases, including C1s and C1r, that along with C1q from the C1 complex of the classical complement pathway; the mannose binding lectin associated serine proteases (MASP); and factors XIa, XIIa, and kallikrein, proteases of the coagulation cascade. The unregulated degradation of C4 by uninhibited C1s/C1r and MASP in the classical and lectin complement pathways leads to the low C4 characteristic of HAE and acquired angioedema. The unregulated degradation of kininogen to bradykinin by uninhibited kallikrein leads to bradykinin accumulation. This bradykinin accumulation via the B2 receptor leads to venodilation and is the likely mechanism of angioedema in these syndromes; although, the exact mechanisms by which deficiency of this inhibitor leads to angioedema is not yet fully understood.
Diagnosis
In order to determine the etiology of angioedema, a detailed medical history must be taken with particular attention to identifying possible triggers, as well as the medication history and family history. See Table 1. Allergic angioedema has a rapid onset following contact with an allergic trigger, including food ingestion, hymenoptera stings, or medication administration. Allergic angioedema rapidly subsides after administration of anti-histamines and epinephrine and does not recur without repeat insult by the allergic trigger. Skin prick or blood allergy testing may be useful tools in diagnosis of allergic angioedema; while, an oral or intravenous challenge can confirm the diagnosis. Medications, in particular ACE inhibitors and non-steroidal anti-inflammatory agents, are associated with more refractory episodes of angioedema; however, prompt identification and withdrawal of the offending agent can prevent further episodes. Idiopathic angioedema is a diagnosis of exclusion and may or may not be associated with urticaria and pruritus.
Table 1.
Causes of Angioedema
Allergic Angioedema
|
Other chronic forms of angioedema are typically more difficult to diagnosis and patients may have numerous episodes before symptoms can be controlled. If a family history of angioedema is apparent, the triggering event is trauma, or if no obvious trigger for angioedema can be identified, lab testing for hereditary and acquired forms of angioedema is warranted.
Initial screening should include a C4 level which will be depressed both during as well as between attacks. If a C4 level is found to be low or clinical suspicion for hereditary or acquired angioedema is very high, follow-up testing of the C1 INH level and function is warranted. C4 levels are often low due to improbably handling; therefore, repeating a C4 level when further testing is sent is also advisable. In type 1 hereditary angioedema and acquired angioedema, the C1 INH level will be low, typically <30% of normal. A C1q level will differentiate between these two types of angioedema, as the C1q level will only be low in acquired angioedema but not type 1 HAE. Types 1 and 2 as well as acquired angioedema will all have a low C1 INH function, again typically <30% of normal. See Table 2. If acquired angioedema is diagnosed, a thorough workup looking for an underlying malignancy, in particular lymphoproliferative disorders is warranted. C3 levels are not helpful in classification of HAE or acquired angioedema.
Table 2.
Laboratory Findings in Hereditary and Acquired Angioedema
| C4 Level | C1-Esterase Level | C1-Esterase Function | C1q | |
|---|---|---|---|---|
|
|
||||
| Hereditary Angioedema Type 1 | ↓ | ↓ | ↓ | normal |
|
|
||||
| Hereditary Angioedema Type 2 | ↓ | normal | ↓ | normal |
|
|
||||
| Acquired Angioedema | ↓ | ↓ | ↓ | ↓ |
|
|
||||
| Other forms of angioedema (non-allergic, medications, idiopathic) |
normal | normal | normal | normal |
Treatment
Acute episodes of allergic angioedema are exquisitely responsive to therapy with anti-histamines, oral corticosteroids, and epinephrine; while other forms are refractory to this conventional therapy and may take hours to days to resolve. In all instances, particular attention must be paid to airway maintenance if the patient is experiencing tongue or laryngeal involvement. Supportive care should also include pain management and hydration if abdominal symptoms are predominant. Prompt removal of any suspected triggers is warranted.
Management of HAE may include both short term and long term prophylaxis as well as treatment of acute episodes. Prior to 2010, the only option for HAE prophylaxis was treatment with compounded androgens, including danazol and stanozolol or antifibrinolytic agents, such as aminocaprioic acid and tranexamic acid6–9. Both of these classes of medications, however, are associated with potentially prohibitive side effects. Androgens may cause virilization, weight gain, and hypertension10; while, antifibrinolytic agents have been reported to cause muscle necrosis, hypotension and menorrhagia.
In 2010, the FDA approved three products for treatment of HAE, revolutionizing the management of this disorder in the United States11. Two of these products are plasma derived C1 INH concentrate which have been used in Europe since 1973 with good efficacy and tolerability12–14. The third is a plasma kallikrein inhibitor and has emerged as an efficacious alternative to C1 INH therapy.15
One pasteurized plasma C1 INH concentrate, BerinertR, (CSL Behring, King of Prussia, PA) was approved in the United States and indicated for the treatment of acute attacks of angioedema associated with HAE. A second, nanofiltered plasma C1 INH concentrate, CinryzeTM, (ViroPharma, Exton PA) was also approved in the United States but received indication for the prophylaxis of acute angioedema associated with HAE. Both of these products are available as a lyophilized concentrate that is reconstituted with sterile water for intravenous administration. As such, patients or hospitals can store a single dose for 24–30 months at room temperature in preparation for an acute attack. Patients with HAE, therefore, can transport the medication to the emergency room in case of an attack and/or have their local emergency rooms prepared with the concentrate on formulary. Side effects of C1 INH concentrate are rare and include headache and fever. The primary limitation to utilization of these products is cost.
Take Home Points.
Angioedema is a increasing cause of hospitalizations in the United States.
Angioedema can result from both allergic triggers as well as non-allergic triggers.
Hereditary angioedema is a rare but treatable cause of recurrent angioedema and should be considered even when no family history is reported.
Two forms of C1 inhibitor concentrate and one kallikrein inhibitor are now available for treatment of hereditary angioedema. Trials are underway to expand indications for these new therapeutic agents.
The third product, ecallantide or KalbitorR (Dyax, Cambridge, MA) is a potent human plasma kallikrein inhibitor and has been introduced as an efficacious alternative to C1 INH concentrate. This product is not a blood product and is uniquely given subcutaneously. It is also indicated for treatment of acute attacks of angioedema associated with HAE. Side effects are typically mild and similar to C1 INH concentrate. Kalbitor did receive a black box warning because of the associated risk of anaphylaxis.
Future Progress
Much progress has been made in recent years in the treatment of HAE, while more innovation is expected in the coming years. Two additional therapies for treatment of HAE may be available in the United States soon. A recombinant form of C1 INH is in development and may emerge as a cheaper alternative to plasma derived C1 INH. In addition, a bradykinin B2-receptor antagonist, icatibant, is under investigation as an alternative therapy for acute attacks of HAE and ACE associated angioedema16–18.
Finally, with the new availability of C1 INH and ecallantide in the United States, studies are underway to expand the indications for their use. Clinicaltrials.gov lists several ongoing and completed studies investigating novel uses of these medications. Efficacy in ACE induced angioedema as well as kidney rejection is being investigated. Antecdotal evidence from Europe suggests that these products may be useful in management of acquired forms of angioedema and is a potential area of interest for future studies19.
Biography
Christina E. Ciaccio, MD, is an Assistant Professor, University of Missouri-Kansas City School of Medicine, and is in the Section of Allergy/Asthma/Immunology at Children’s Mercy Hospitals and Clinics and Truman Medical Center in Kansas City.
Contact: ceciaccio@cmh.edu

Footnotes
Disclosure
None reported.
References
- 1.Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol. 2008;101:185–92. doi: 10.1016/S1081-1206(10)60208-6. [DOI] [PubMed] [Google Scholar]
- 2.Frank MM, Gelfand JA, Atkinson JP. Hereditary angioedema: the clinical syndrome and its management. Ann Intern Med. 1976;84:580–93. doi: 10.7326/0003-4819-84-5-580. [DOI] [PubMed] [Google Scholar]
- 3.Farkas H, Harmat G, Fay A, et al. Erythema marginatum preceding an acute oedematous attack of hereditary angioneurotic oedema. Acta Derm Venereol. 2001;81:376–7. doi: 10.1080/000155501317140188. [DOI] [PubMed] [Google Scholar]
- 4.Nussberger J, Cugno M, Cicardi M. Bradykinin-mediated angioedema. N Engl J Med. 2002;347:621–2. doi: 10.1056/NEJM200208223470820. [DOI] [PubMed] [Google Scholar]
- 5.Agostoni A, Aygoren-Pursun E, Binkley KE, et al. Hereditary and acquired angioedema: problems and progress proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004;114:S51–131. doi: 10.1016/j.jaci.2004.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelfand JA, Sherins RJ, Alling DW, Frank MM. Treatment of hereditary angioedema with danazol. Reversal of clinical and biochemical abnormalities. N Engl J Med. 1976;295:1444–8. doi: 10.1056/NEJM197612232952602. [DOI] [PubMed] [Google Scholar]
- 7.Sheffer AL, Fearon DT, Austen KF. Clinical and biochemical effects of stanozolol therapy for hereditary angioedema. J Allergy Clin Immunol. 1981;68:181–7. doi: 10.1016/0091-6749(81)90181-0. [DOI] [PubMed] [Google Scholar]
- 8.Frank MM, Sergent JS, Kane MA, Alling DW. Epsilon aminocaproic acid therapy of hereditary angioneurotic edema. A double-blind study. N Engl J Med. 1972;286:808–12. doi: 10.1056/NEJM197204132861503. [DOI] [PubMed] [Google Scholar]
- 9.Sheffer AL, Austen KF, Rosen FS. Tranexamic acid therapy in hereditary angioneurotic edema. N Engl J Med. 1972;287:452–4. doi: 10.1056/NEJM197208312870907. [DOI] [PubMed] [Google Scholar]
- 10.Sloane DE, Lee CW, Sheffer AL. Hereditary angioedema: Safety of long-term stanozolol therapy. J Allergy Clin Immunol. 2007;120:654–8. doi: 10.1016/j.jaci.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Wasserman RL, Levy RJ, Bewtra AK, et al. Prospective study of C1 esterase inhibitor in the treatment of successive acute abdominal and facial hereditary angioedema attacks. Ann Allergy Asthma Immunol. 2011;106:62–8. doi: 10.1016/j.anai.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Kunschak M, Engl W, Maritsch F, et al. A randomized, controlled trial to study the efficacy and safety of C1 inhibitor concentrate in treating hereditary angioedema. Transfusion. 1998;38:540–9. doi: 10.1046/j.1537-2995.1998.38698326333.x. [DOI] [PubMed] [Google Scholar]
- 13.Farkas H, Jakab L, Temesszentandrasi G, et al. Hereditary angioedema: a decade of human C1-inhibitor concentrate therapy. J Allergy Clin Immunol. 2007;120:941–7. doi: 10.1016/j.jaci.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. 2010;363:513–22. doi: 10.1056/NEJMoa0805538. [DOI] [PubMed] [Google Scholar]
- 15.Cicardi M, Levy RJ, McNeil DL, et al. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med. 2010;363:523–31. doi: 10.1056/NEJMoa0905079. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt PW, Hirschl MM, Trautinger F. Treatment of angiotensin-converting enzyme inhibitor-related angioedema with the bradykinin B2 receptor antagonist icatibant. J Am Acad Dermatol. 2010;63:913–4. doi: 10.1016/j.jaad.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Bas M, Greve J, Stelter K, et al. Therapeutic efficacy of icatibant in angioedema induced by angiotensin-converting enzyme inhibitors: a case series. Ann Emerg Med. 2010;56:278–82. doi: 10.1016/j.annemergmed.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Cicardi M, Banerji A, Bracho F, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med. 2010;363:532–41. doi: 10.1056/NEJMoa0906393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicardi M, Zanichelli A. Acquired angioedema. Allergy Asthma Clin Immunol. 6:14. doi: 10.1186/1710-1492-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]