Abstract
Expanding gaps between the number of patients awaiting transplantation and the number who receive organs in the United States has been associated with heightened disease severity among transplant candidates and more common use of organs from non-standard donors. We summarize data on the economic consequences of liver and renal allograft quality in contemporary practice. Policy makers and providers must work together to ensure that financial disincentives do not lead to wastage of life-saving organs.
Introduction
Over the past several decades, technical advances in liver and of kidney transplantation procedures, increased prevalence of major causes of end-stage failure of the kidney (e.g., diabetes, hypertension) and liver (e.g., hepatitis C), and a relatively static organ supply have fueled expanding gaps between the number of patients awaiting transplantation and the number who receive organs in the United States. For example, from 1997 to 2010 the number of patients on the waiting list for a kidney transplant increased more than two-fold, to >80,000 patients 1.
In contrast, the total number of kidney transplants per year increased from 11,700 in 1997 to a peak of 17,000 in 2006, and has remained at approximately 16,000 annual transplants through 2010 1. In 2010, the number of patients awaiting a kidney transplant was approximately five-times the number transplants performed 1. Similarly, while 16,000 patients are now awaiting liver transplantation, the annual transplant rate has grown only modestly over the past decade from approximately 4,000 to 6,000 total transplants each year 1. As the 11 geographic regions directing organ distribution within the United Network for Organ Sharing (UNOS) are heterogeneous in size and population, organ demand-to-supply imbalances vary by location across the country. Transplant centers, particularly in areas with the most severe organ shortages, are faced with heightened illness burdens among patients needing transplants, and many turn increasingly to non-standard organs to serve their patients. Simultaneously, efforts to contain healthcare spending without adequate recognition of the cost implications of recipient illness severity and donor quality are posing unprecedented clinical and economic challenges to the provision of transplant care.
The Interplay of Recipient Illness Severity and Allograft Quality on Liver Transplant Costs
The costs and profitability of liver transplantation are directly linked to interactions of allograft supply, recipient disease severity, and donor quality. Adoption of severity-of-illness based liver allocation in 2002 based on the Model for End-Stage Liver Disease (MELD) score to promote more equitable distribution of donated livers according to medical necessity has reduced overall waiting list mortality but also increased the average illness severity and medical complexity of patients reaching transplantation 2. As the MELD system does not resolve geographic differences in organ supply, significant variation in transplant access persists in the MELD-era as a function of region and DSA 3. In regions with the most limited organ supply, average MELD score is >28 at the time of transplant, compared with only 20 in the least competitive regions.
Recent studies have quantified associations of recipient illness severity with the costs of liver transplant care. Single center studies demonstrated that higher MELD at transplant is associated with longer lengths of transplant stay, including more use of pharmaceuticals, laboratory tests, radiographs, and dialysis services 4. Recently, integration of national registry and administrative data have enabled some large-scale studies of cost drivers in liver transplantation. Based on a linkage of Organ Procurement and Transplantation Network (OPTN) registry data with private payer billing claims, Buchanan et al. estimated that transplant admission charges were $152,819 (p<0.05) higher in patients with MELD 28–40 compared to 15–20 5. Integration of OPTN registry data with cost accounting data from the University HealthSystem Consortium identified dramatic associations of illness severity with hospital costs defined by an activity-based costing metric according to a non-linear functional form, such that MELD scores above 25 were associated with rapidly escalating costs. For example, incremental transplant hospital costs for patients with MELD >30 exceed those for patients with MELD <10 by approximately $80,000. Thus, centers in high demand areas that are able to provide allografts to substantial proportions of their candidates only at advanced levels of disease severity bear additional financial strains.
To address the ongoing liver allograft shortage, transplant centers increasingly use organs from donors with characteristics that increase the risk of allograft failure. The Donor Risk Index (DRI) developed by Feng et al. is a continuous, objective measure of organ quality based upon donor characteristics available at the time of the organ offer that predicts the likelihood of graft failure at three months post transplant 6. Retrospective study of Scientific Registry of Transplant Research (SRTR) registry data by Schaubel et al. found that transplantation with high-DRI livers improves the relative risk of mortality compared to continued waiting with possible later transplantation using a lower-DRI organ for patients with MELD >=20, but notably worsened survival for low-MELD patients 7.
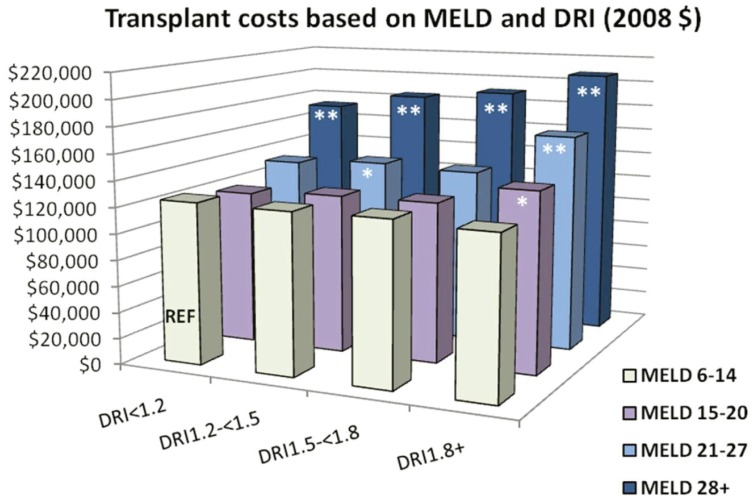
Another recent study of integrated OPTN registry and University HealthSystem consortium data sought to quantify the impact of donor quality, as assessed through the DRI, on total inpatient costs (transplant and all readmissions) for one year following liver transplantation8. DRI was ≥ 1.8 in 24.6% of transplants and 1.5–1.8 in 22.0%. After adjustment for recipient characteristics, the costs of both transplant and first year inpatient care increased in a graded manner with DRI levels. Donors in the highest DRI quartile (≥ 1.8) added nearly $12,000 to the cost of transplantation and nearly $22,000 to post-transplant costs in comparison to the lowest risk donors (DRI <1.2). Among the individual components of the DRI, donation after cardiac death (increase of $20,769 vs. brain dead donors) had the greatest impact on transplant costs. Importantly, the impact of donor quality on cost varied across severity of illness. While high DRI increased the cost of transplant at all MELD strata, the magnitude of the cost differential according to organ quality was greatest in the high MELD patients. Patients in the lowest MELD quartile (6–14) had a modest rise in cost when receiving donors from the highest DRI group. In the highest MELD quartile (>28), the total cost of transplant care as a function of DRI was more pronounced, rising nearly $35,000 across DRI levels (See Figure 1).
Figure 1.
Synergistic effect of MELD and DRI on liver transplant costs.
Interaction of DRI, MELD score and total transplant cost (in thousands of US dollars), n= 7,575, adjusted for: sex, Age, race, diagnosis (Hepatocellular carcinoma, Hepatitis B Virus, Hepatitis C Virus, other), recipient blood type, prior liver transplant, pre-transplant dialysis, MELD score, simultaneous liver-kidney transplantation and region * P<0.05; ** P<0.0001. Reproduced with permission from “The Interaction Among Donor Characteristics, Severity of Liver Disease, and the Cost of Liver Transplantation” 8. (Permission granted from Wiley 2/24/11)
The observation of a synergistic impact of lower allograft quality and high recipient illness severity on overall transplant costs is particularly important given evidence that the survival benefit of high DRI organs is generally confined to the high MELD patients 7. Thus, transplant centers that choose to use higher risk allografts for high MELD patients to improve patient survival may be substantially economically disadvantaged. Given the geographic variation in the average MELD score at transplant due to current organ supply disparities, the economic implication of these two factors may be greatest for transplant centers serving areas with the most severe organ shortages.
Revised organ sharing proposals to achieve better equity in transplant access have been considered by the UNOS Liver and Intestine Transplant Committee. These proposals are intended to increase the aggregate clinical benefit of available liver allografts by raising the MELD thresholds for local use and thereby increasingly sharing within a geographic region. Data prepared for the UNOS Liver and Intestine Transplant Oversight committee by the SRTR was recently used to evaluate the cost-effectiveness of alternative organ sharing proposals. In March 2010, the SRTR presented a series of analyses of the clinical impact of revising the current liver allocation system with a two-tiered system of liver allocation that varies the MELD thresholds for local and regional utilization of available allografts 9. The SRTR applied the Liver Simulated Allocation System model (LSAM) to predict the clinical impact of these proposed allocation systems on transplants performed and mortality. Compared to the current allocation system, simulation analysis suggested that adoption of a MELD 15–25 two-tiered regional sharing approach would result in 88 fewer deaths annually. Using the estimated cost impacts of illness severity from integrated OPTN registry and University HealthSystem Consortium data, Axelrod et al. estimated that the cost per quality-adjusted life year (QALY) saved would be $17,000, comparing favorably to incremental cost effectiveness ratios of $50,000 to $100,000 per QALY generally considered cost-effective in the United States 10.
Cost Implications of the Renal Transplant Shortage and Allograft Quality
Under the current allocation system, waiting times for deceased donor kidney transplantation vary dramatically across UNOS regions. Median waiting time for a blood group O kidney among patients listed in 2003–2004 ranged from 2.8 years to 7.8 years across regions 1. Patients who require extended durations of pretransplant dialysis accumulate a high prevalence of comorbid conditions such as congestive heart failure and coronary artery disease11, increasing the complexity and cost of their transplant care.
To expedite the placement of non-standard renal allografts from brain-dead deceased donors, UNOS implemented a separate policy for the allocation of kidneys from Expanded Criteria Donors (ECD), defined by older donor age and risk factors including donor hypertension, death due to stroke, and elevated terminal serum creatinine. By definition, ECD kidneys are associated with lower expected allograft survival compared to kidneys from standard criteria brain dead donors 12. Use of non-standard renal allografts has become more common in response to the renal organ shortage. From 1993 to 2008, the relative frequency of ECD allograft use rose from 7.4% to 22% among U.S. kidney transplants performed. Use of kidneys donated after cardiac death (DCD transplants) also increased from <1% to 12.4% in this period 13.
ECD kidneys can offer a survival advantage compared to continued waiting on dialysis, although significant benefit appears limited to sub-groups such as older patients, patients with diabetes, and those who live in areas with very long waiting times 14. The incidence of delayed graft function (DGF), the need for dialysis in the first week after transplant, is approximately 30% for ECD transplants, significantly higher than the incidence of 20% among transplants from standard criteria deceased donors 13. Because of the ischemia inherent in procurement of DCD organs, DGF is also common after DCD kidney transplantation, affecting 40% of such allografts in recent US practice 13.
Increased risk of early dialysis requirements after transplantation drives higher early expenditures after transplantation of non-standard renal allografts 15. In an analysis of financial data from one center, Englesbe et al. found that transplant of non-standard allografts is associated with a financial loss for the transplant center 16. Based on data for Medicare-insured adults transplanted at one center between 1999 and 2005, statistically significant decreases in medical center margins were identified for ECD recipients (−$5887) and in cases of DGF (−$4937). An annual decline in medical center margin of -$5278 per year was also observed, related to both increasing costs and decreasing Medicare reimbursements.
Effective organ preservation may reduce the discard rate of marginal organs and also attenuate the excess transplant costs associated with use of these organs. OPTN registry data from 1999 to 2005 indicates a lower discard rate nationally when ECD kidneys are preserved with pulsatile machine perfusion (pumping) compared to cold storage: 29.7%, compared to 43.6% for unpumped ECD kidneys (adjusted odds ratio 0.51, P<0.0001) 17. In a retrospective analysis of data for Medicare-insured ECD kidney transplant recipients in 1995–2004 captured in the United States Renal Data System (USRDS), the cost of the transplant hospitalization was significantly lower for ECD transplants that received pulsatile machine perfusion compared to cold storage alone 18. After adjusting for other recipient, donor and transplant factors, pulsatile perfusion was associated with a $2,131 reduction (P = 0.007) in hospitalization costs. Use of pumping for preservation was also associated with lower DGF risk (27% of pumped and 38% of non-pumped, P< 0.0001). The impact of pumping on the costs of ECD transplantation should be evaluated in prospective studies.
A possible reimbursement-based mechanism to minimize financial disincentives to use non-standard organs that we believe warrants consideration is modification of the Diagnosis Related Group (DRG) for kidney and liver transplantation according to organ quality. Allografts associated with better outcomes and thus a lower cost (standard criteria organs) could have a DRG associated with a lower reimbursement by Medicare compared to DRGs for organs shown to be associated with poorer outcomes, such as ECD kidneys, some DCD kidneys, and high DRI livers. In the future, kidney transplant reimbursement could be graded based on a donor profile index or another continuous scale. The financial implications of potential revisions of allocation policy targeted at resolving disparities in the supply of standard criteria organs across geographic regions also warrants increased attention to better inform debates on allocation policy reform.
Conclusions
While significant clinical benefits may be achieved through the appropriate use of liver and renal allografts from non-standard donors 7, 14, financial disincentives to use of these organs pose the risk of systematic reduction to the maximal use of available donated organs. As the availability of high-quality deceased donor organs declines with the aging of the American population and other measures that reduce the number of brain-dead donors (e.g., seatbelt laws and improved care by trauma centers), and economic pressures mount in the healthcare industry, the financial stability of transplant centers will be increasingly challenged. Viable organs are undoubtedly wasted due in part to concerns for associated financial risks. Appropriate policy decisions at the national level, sound contracting strategies, and continuous improvement in clinical practices are vital to ensure optimal use of available organs for as many patients as possible without prohibitive expenses to providers or payers 19, 20. We believe that tighter linkage of transplant reimbursement to the severity of recipient illness and to metrics of donor quality is a potential strategy for compensating centers for the risks of appropriate clinical care. Efforts to address geographic variation in organ supply within possible revisions to the liver and kidney allocation systems should be considered to improve equity in organ access, and also warrant evaluation as a possible means to reduce economic barriers to life-saving transplant procedures.
Acknowledgments
Drs. Lentine and Schnitzler receive support from an American Recovery and Reinvestment Act grant from the National Institute of Diabetes Digestive and Kidney Diseases, RC1 1RC1DK086450-01. Dr. Lentine also received career development support from an NIDDK grant, K08DK073036. The authors thank Drs. David Axelrod and Paolo Salvalaggio for their research collaboration, valuable insights and critical review of the manuscript.
Biography
Krista L. Lentine, MD, MS, is an Associate Professor of Medicine in the Nephrology Division, Saint Louis University Department of Internal Medicine, and in the Saint Louis University Center for Outcomes Research. Mark A. Schnitzler, PhD, is also at the Saint Louis University Center for Outcomes Research.
Contact: lentinek@slu.edu

Footnotes
Disclosure
None reported.
References
- 1.Organ Procurement and Transplant Network. [Access date February 23, 2011]. http://optn.transplant.hrsa.gov/latestData/viewDataReports.asp.
- 2.Olthoff KM, Brown RS, Jr, Delmonico FL, Freeman RB, McDiarmid SV, Merion RM, Millis JM, Roberts JP, Shaked A, Wiesner RH, Lucey MR. Liver Transpl; Summary report of a national conference: Evolving concepts in liver allocation in the MELD and PELD era; December 8, 2003; Washington, DC, USA. 2004. pp. A6–22. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JP, Dykstra DM, Goodrich NP, Rush SH, Merion RM, Port FK. Geographic differences in event rates by model for end-stage liver disease score. Am J Transplant. 2006;6:2470–5. doi: 10.1111/j.1600-6143.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 4.Washburn WK, Pollock BH, Nichols L, Speeg KV, Halff G. Impact of recipient MELD score on resource utilization. Am J Transplant. 2006;6:2449–54. doi: 10.1111/j.1600-6143.2006.01490.x. [DOI] [PubMed] [Google Scholar]
- 5.Buchanan P, Dzebisashvili N, Lentine KL, Axelrod DA, Schnitzler MA, Salvalaggio PR. Liver transplantation cost in the model for end-stage liver disease era: looking beyond the transplant admission. Liver Transpl. 2009;15:1270–7. doi: 10.1002/lt.21802. [DOI] [PubMed] [Google Scholar]
- 6.Feng S, Goodrich N, Bragg-Gresham J, Dyskstra D, Punch J, DebRoy M, Greenstein S, Merion R. Characteristics associated with liver graft failure: The concept of a Donor Risk Index. Am J Transplant. 2006;6:783–90. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 7.Schaubel DE, Sima CS, Goodrich NP, Feng S, Merion RM. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8:419–25. doi: 10.1111/j.1600-6143.2007.02086.x. [DOI] [PubMed] [Google Scholar]
- 8.Salvalaggio PR, Dzebisashvili N, MacLeod KE, Lentine KL, Gheorghian A, Schnitzler MA, Hohmann S, Segev DL, Gentry SE, Axelrod DA. The interaction among donor characteristics, severity of liver disease and the cost of liver transplantation. Liver Transpl. 2011 doi: 10.1002/lt.22230. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [Access date November 24, 2010]. http://optn.transplant.hrsa.gov/liver.asp.
- 10.Axelrod D, Gheorghian A, Schnitzler MA, Dzebisashvili N, Salvalaggio PR, Tuttle-Newhall J, Segev DL, Gentry S, Hohmann S, Pomfret L, Lentine KL. The Economic Implications of Broader Sharing of Liver Allografs. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03443.x. [in press] [DOI] [PubMed] [Google Scholar]
- 11.Lentine KL, Hurst FP, Jindal RM, Villines TC, Kunz JS, Yuan CM, Hauptman PJ, Abbott KC. Cardiovascular risk assessment among potential kidney transplant candidates: approaches and controversies. Am J Kidney Dis. 2010;55:152–67. doi: 10.1053/j.ajkd.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, Delmonico FL, Wynn JJ, Merion RM, Wolfe RA, Held PJ. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002;74:1281–6. doi: 10.1097/00007890-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Renal Data System. USRDS 2008 Annual Data Report. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. Altas of ESRD, Transplantation http://www.usrds.org/2008/view/esrd_07.asp. [Google Scholar]
- 14.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK. Deceased-donor characteristics and the survival benefit of kidney transplantation. Journal of the American Medical Association. 2005;294:2726–33. doi: 10.1001/jama.294.21.2726. [DOI] [PubMed] [Google Scholar]
- 15.Whiting JF, Woodward RS, Zavala EY, Cohen DS, Martin JE, Singer GG, Lowell JA, First MR, Brennan DC, Schnitzler MA. Economic cost of expanded criteria donors in cadaveric renal transplantation: analysis of Medicare payments. Transplantation. 2000;70:755–60. doi: 10.1097/00007890-200009150-00007. [DOI] [PubMed] [Google Scholar]
- 16.Englesbe MJ, Ads Y, Cohn JA, Sonnenday CJ, Lynch R, Sung RS, Pelletier SJ, Birkmeyer JD, Punch JD. The effects of donor and recipient practices on transplant center finances. Am J Transplant. 2008;8:586–92. doi: 10.1111/j.1600-6143.2007.02098.x. [DOI] [PubMed] [Google Scholar]
- 17.Sung RS, Christensen LL, Leichtman AB, Greenstein SM, Distant DA, Wynn JJ, Stegall MD, Delmonico FL, Port FK. Determinants of discarded expanded criteria donor kidneys: impact of biopsy and machine perfusion. American Journal of Transplantation. 2008;8:783–92. doi: 10.1111/j.1600-6143.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 18.Buchanan PM, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Association of lower costs of pulsatile machine perfusion in renal transplantation from expanded criteria donors. Am J Transplant. 2008;8:2391–401. doi: 10.1111/j.1600-6143.2008.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Englesbe M, Dimick J, Mathur A, Ads Y, Welling T, Pelletier S, Heidt D, Magee J, Sung R, Punch J, Hanto D, DC Who pays for biliary complications following liver transplant? A business case for quality improvement. Am J Transplant. 2006;6:2978–82. doi: 10.1111/j.1600-6143.2006.01575.x. [DOI] [PubMed] [Google Scholar]
- 20.Abecassis M. Financial outcomes in transplantation--a provider’s perspective. Am J Transplant. 2006;6:1257–63. doi: 10.1111/j.1600-6143.2006.01329.x. [DOI] [PubMed] [Google Scholar]