Abstract
An estimated 170 million people in the world are infected with hepatitis C virus (HCV). These individuals are at risk for developing complications like cirrhosis and/or hepatocellular carcinoma. Occurrence of HCV has been recorded to be high in certain parts of the world like Africa and Southeast Asia. The prevalence is considerably lower in the United States, with an estimated number of people with positive HCV antibodies around 1.8% of the population and an estimated 3.1 million individuals having active HCV infection1. Treatment of hepatitis C has undergone a complete overhaul several times over the past decade and continues to evolve striving for constant improvement. We now are at the cusp of yet another such overhaul with the protease inhibitors about to be introduced into the market.
Introduction
With the approval of peginterferon and ribavirin in 2001 by the FDA, this combination has become the standard of care for patients, infected with HCV. Historically SVR rates for patients infected with HCV genotype-1 receiving treatment with pegylated interferon and ribavirin is around 40% to 50%. SVR rates are even lower in “difficult to treat” patients, such as African-Americans, high baseline viral load or those with HIV co-infection. More than one-third of patients classified as peginterferon/ribavirin non-responders are becoming a major public health concern. These patients are at higher risk for developing complications of chronic liver disease, including decompensated cirrhosis and hepatocellular cancer. With the progression of liver disease and the development of HCC, the need for liver transplantation is growing along with a substantial economic burden2.
The likelihood of achieving an SVR can be predicted by viral or host factors. Viral factors include genotype followed by the viral level. The genotype does not predict the natural history of infection; it does however predict the likelihood of treatment response, and, in many cases, determines the duration of treatment. In most prospective studies, genotype has been proposed to be the strongest predictor of response, however recently it has been shown that RVR is a stronger predictor of SVR than genotype3. SVR rates were higher in patients who had genotypes 2 or 3 and lower pre-treatment HCV RNA levels. Host factors of poor response include male sex, age at infection, duration of infection, Hispanics or African-Americans, presence of hepatic steatosis, genetic factors, and the patient’s immune response4,5.
The recent discovery of certain polymorphisms in the IL-28B gene has given new insight into on-treatment response rates. Variations in the gene have been linked to better response rates amongst people with chronic HCV infection. The IL-28B gene encodes interleukin 28, also known as interferon lambda, a cytokine with antiviral activity. Thompson et al. evaluated the clinical relevance of on-treatment virologic response and SVR in genotype 1 patients with respect to IL-28B polymorphism. Patients were genotyped as CC, CT, or TT. The CC genotype was observed more frequently in Caucasians (37%), followed by Hispanics (29%), and African-Americans (14%). The TT genotype was more common in African-Americans (37%) as compared to Hispanics (22%) or Caucasians (12%). The CC genotype was associated with improved early viral kinetics and viral suppression such that by week two of treatment the median reduction in viral load was 2-log or greater. The effect was similar amongst all races.
A recent abstract presented at EASL 2011 by Poordad et al. evaluated SVR rates in patients treated with pegylated interferon, ribavirin and boceprevir. Patients were tested for the IL-28B polymorphism. Amongst the treatment naïve patients, SVR rates were higher by 50% in the CC type patients over CT or TT, while in the boceprevir arms they were 27% higher in the CC genotype as compared to the CT and TT genotypes. For treatment failure patients there was a clear advantage for boceprevir in all categories. A multivariate analysis showed that IL-28B genotype was a stronger predictor than other baseline variables. Therefore IL-28B is being considered as one of the strongest pre-treatment predictors of SVR. However studies are concluding that RVR at week 4 is still the best predictor of SVR and treatment success regardless of IL-28B status. 6,7.
Treatment for Genotype 1
The current recommended treatment for HCV genotype 1 patients is 48 weeks with pegylated interferon and ribavirin. This treatment duration may be tailored by viral response using viral kinetics. A recent prospective trial demonstrated that patients with genotype 1 baseline HCV RNA levels ≤600,000IU/ml who undergo RVR achieve an SVR of up to 90% with either 48 or 24 weeks8. These studies have shown that a shorter duration of treatment with 24 weeks may be sufficient in HCV genotype 1 patients who demonstrate RVR and have low baseline HCV RNA levels, similar to those with genotype 2 and 3 patients exhibiting RVR.
Patients who may need to be considered for longer treatment duration than 24 weeks include those who have a baseline viral load of >600,000 IU/ml, cirrhosis, coinfection with HIV, or are immunosuppressed. Several recent studies have suggested that extending treatment beyond 48 weeks may lead to improved SVR rates in some genotype 1 patients9,10. Some studies have demonstrated that the probability of attaining SVR has been shown to be greater with a longer period of undetectable serum HCV RNA during treatment11. Two recent studies have helped provide insight into extending treatment. Berg et al. compared therapy extension for 72 weeks with standard duration of 48 weeks in genotype 1 HCV patients who received interferon-based therapy and found no overall difference in SVR (54% versus 53%) or relapse rates (21% versus 29%) between the two groups.
However, in a subgroup analysis in patients who were late responders (virus level HCV RNA < 6000IU/ml at 12 weeks but undetectable virus at 24 weeks), extending the duration of treatment to 72 weeks from 48 weeks decreased the relapse rate significantly. Patients who did not have a serum HCV RNA <6000IU/ml at 12 weeks had a high rate of relapse regardless of treatment duration. Of note, there was a higher incidence of dropouts in the 72-week treatment arm than the 48-week treatment arm, particularly between weeks 48 and 72, even with the lower ribavirin dose of 800mg, which possibly explains the lack of difference between treatment groups. Aggressive adherence measures should be undertaken to maximize response rates when considering extending therapy. Similar results were noted in a study by Sanchez-Tapias et al. that examined the role of 72 weeks of therapy compared with 48 weeks in HCV patients of all genotypes who did not achieve an RVR (TeraViC-4 trial) with pegylated interferon and ribavirin 9,10. Extension of therapy duration to 72 weeks within these genotype 1 patients improved the SVR rate (44 versus 28%) significantly by decreasing the relapse rate (53 versus 17%).
Pearlman et al. compared SVR rates among slow responder genotype-1 patients with 48 weeks’ treatment versus extension of treatment to 72 weeks. They noted that SVR rates were superior in those patients who were treated for 72 weeks versus 48 weeks (38% versus 18%, respectively)11. Even though there were increased dropouts in the 72-week group, extension of therapy appears to be an option in those who fail to clear virus by week 12, and may be an option in those who do not undergo week-four RVR. While some of these studies used flat-dose ribavirin, weight-based dosing of ribavirin should be used in an effort to minimize relapse. A recent study by Fried et al. studied the efficacy of high-dose pegylated interferon alfa-2a and ribavirin compared with conventional dose treatment in genotype-1 patients with features predicting a poor response to treatment. Patients were randomized to double-blind treatment with peginterfron alfa-2a at 180 or 270μg/week plus ribavirin at 1200 or 1600mg/day for 48 weeks. Patients randomized to higher doses of peginterferon and ribavirin experienced the highest rates of SVR and the lowest relapse rates (47 and 19%, respectively)12.
A New Era of Treatment for HCV Genotype-1 Patients
Thus far treatment for HCV has consisted of therapies to stimulate the immune system and interfere in a non-specific manner with viral replication. With the increasing understanding of the HCV life cycle and of the structural features of the HCV proteins there has been a shift in investigational focus towards direct acting anti-viral therapy (DAA) for HCV. This treatment inhibits HCV proteins that are essential for intracellular replication. Newer data have demonstrated promise for 2 protease inhibitors Boceprevir and Telaprevir, both of which improved SVR while decreasing the duration of treatment. These drugs are referred to as direct acting antiviral agents13,14.
NS3/4A Protease Inhibitors
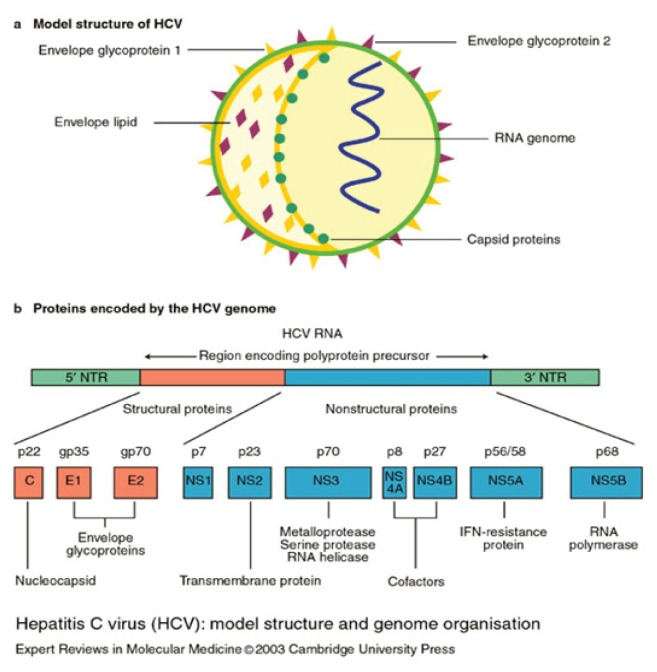
HCV is a single-stranded RNA molecule approximately 9600 nucleotides in length. Viral protein synthesis is mediated by an internal ribosome-entry site that binds directly to ribosomes, and RNA is translated into a polyprotein of 3000 amino-acids that is proteolytically cleaved into 4 structural and 6 non-structural (NS) proteins. The structural proteins are used to assemble new viral particles and the NS proteins support viral RNA replication 15 (See Figure 1).
Figure 1.
Hepatitis C virus: model structure and genome organization.
NS2 metalloprotease and NS3 serine protease are two viral proteolytic enzymes that allow the production of nonstructural proteins from the HCV polyprotein. The NS2 enzyme cleaves itself at its C-terminus, activating the second protease, the NS3 serine protease which is contained within the N-terminal of the multifunctional NS3 protein. The NS3 protease is responsible for all subsequent downstream cleavages of the polyprotein. Much attention has been paid to the NS3 site for anti-viral therapeutics. A key feature of the NS3 protease is that one strand of its seven stranded N-terminal beta-barrel structure is supplied in trans by the NS4A cofactor protein or peptide. Kinetic and structural studies have shown that the HCV NS3 serine protease requires intercalation of a strand of NS4A cofactor for proper alignment of the catalytic triad and full proteolytic activity. Without the intercalation of the NS4A strand, the N-terminus remains partially disordered resulting in imperfect alignment of the catalytic triad and a corresponding roughly 950-fold drop in catalytic efficiency.16,17
Telaprevir is an orally bioavailable NS-3 protease inhibitor which binds to the enzyme covalently but reversibly. Two landmark studies, PROVE-1 (conducted in the United States) and PROVE-2 (conducted in Europe) showed excellent response to therapy in treatment naïve genotype 1 patients. Results showed SVR rates of up to 70% when telaprevir was added to the regimen consisting of peginterferon and ribavirin18,19. Subsequent (ADVANCE and ILLUMINATE) studies have been conducted evaluating SVR rates with telaprevir based triple therapy in treatment naïve genotype 1 patients. Both the ADVANCE and ILLUMINATE trials evaluated the possibility of tailoring treatment based on achieving RVR, whereby those patients succeeding to exhibit such a response would be entitled to have a shorter duration of treatment thus helping minimize unnecessary exposure to protease inhibitors and chances of mutation in the HCV virus. Thus all such patients who achieve RVR can be safely given 12 or 24 weeks of telaprevir based therapy with an expected SVR rate of 70%, whereas those patients who still exhibit viral response and become negative by week 12 should be considered for 48 weeks of treatment with a similar expected SVR rate20,21.
With the success rates noted in treatment naïve patients, focus was then shifted to evaluate response rates in the difficult to treat genotype-1 patient population. The PROVE-3 study was conducted with patient enrollment in 53 international sites. SVR rates were quite similar in patients receiving 12 or 24 weeks of telaprevir based triple therapy (51% and 53%). When the data was evaluated by prior response to treatment those patients who were considered non-responders exhibited SVR rates of about 39% in patients receiving telaprevir. Patients with prior relapse to treatment had a much more successful response to telaprevir based therapy with SVR rates of about 75%. Patients with cirrhosis also did considerably well with treatment with similar results when compared to those without advanced fibrosis. Results show benefit for all those patients who completed telaprevir based therapy, since SVR rates were maintained at 48 weeks after end of treatment. These data are very encouraging for patients who have failed prior standard of care treatment.22.
Boceprevir is the other protease inhibitor that has been specifically designed to inhibit the HCV NS3 protease, thus enabling it to inhibit viral replication in HCV infected host cells. The mechanism of action involves the formation of stable but reversible covalent bonds between the ketoamides of boceprevir and the NS3 protease active site serine.
Phase I studies showed excellent response rates and HCV virus reduction within two weeks of combined therapy with peginterferon and ribavirin. Boceprevir was seen to be tolerated well both alone and in combination with peginterferon/ribavirin. Subsequent phase II studies that were conducted in the Unites States and Europe (SPRINT-1) showed promising data for treatment naïve genotype 1 patients. They used a lead in strategy with a hypothesis that both Peg IFN and RBV reach a steady state concentration in four weeks and with the lead in strategy, patients have a protease inhibitor added when the backbone drug levels have been optimized and the patient’s immune system has been optimally activated. This approach may minimize the period of time when there is a period of “functional monotherapy” with a DAA, possibly reducing the likelihood for the development of resistance. This strategy may also have the potential to reduce the likelihood of development of resistance by identifying patients who are responders to IFN/RBV before giving them a Protease Inhibitor or other DAA drugs13,14.
The phase II study (SPRINT-1) was conducted in treatment naïve HCV genotype -1 patients. Patients who received boceprevir had higher SVR rates (75%)13,14. A phase-III study (SPRINT-2) evaluated the effects of boceprevir in treatment naïve genotype-1 patients with two different cohorts black and non-black23. A response guided arm was initiated in this study which allowed roughly half of the patients to be treated with a 4 week lead-in followed by 24 weeks of triple therapy with boceprevir24. Response rates were again noted to be higher in patients who received boceprevir compared to the control group. SVR rates amongst non-blacks were 40% to 70%. SVR rates amongst blacks were 23% to 53%. These data confirmed that addition of boceprevir increased SVR rates amongst all patients, irrespective of race. In addition this trial showed that the treatment duration could be tailored to individual patients who exhibit RVR in an attempt to minimize exposure to medication beyond necessity13,14,23.
Additionally a non-responder phase 3 study RESPOND-2 was conducted to look at the effects of boceprevir on re-treatment of genotype 1 patients who had partial response or relapse on previous treatment with peginterferon and ribavirin. SVR rates were higher amongst patients receiving boceprevir (59% to 66%) compared to those being treated with peg-interferon and ribavirin alone25.
Conclusions
These are exciting times for patients who have previously failed therapy with standard of care peginterferon and ribavirin. Treatment with both boceprevir and telaprevir has proven to increase SVR rates by at least 20% over the standard therapy of 48 weeks of peginterferon and ribavirin. The emergence of resistance will need to be monitored very carefully as these newer and more potent drugs are added to the interferon and ribavirin backbone drugs.
Biography
Bruce R. Bacon, MD, is the James F. King, MD, Endowed Chair in Gastroenterology, Professor of Internal Medicine. Omer Khalid, MD, is a Subspecialty Resident (Fellow). Both are in the Division of Gastroenterology and Hepatology at the Saint Louis University Liver Center at Saint Louis University School of Medicine.
Contact: baconbr@slu.edu


Footnotes
Disclosures
None reported.
References
- 1.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999 Aug 19;341(8):556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 2.Saracco G, Rizzetto M. Predictors of response to interferon therapy. Dig Dis Sci. 1996;41:115S–120S. doi: 10.1007/BF02087886. [DOI] [PubMed] [Google Scholar]
- 3.Fried MW, Hadziyannis SJ, Shiffman ML, Messinger D, Zeuzem S. Rapid Virological Response Is the Most Important Predictor of Sustained Virological Response Across Genotypes in Patients with Chronic Hepatitis C Virus Infection. J Hepatol. 2011 doi: 10.1016/j.jhep.2010.10.032. Manuscript in print. [DOI] [PubMed] [Google Scholar]
- 4.Conjeevaram HS, Fried MW, Jeffers LJ, et al. Peg interferon and ribavirin treatment in African American and Caucasian American patients with Hepatitis C genotype 1. Gastroenterology. 2006;131:470–477. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Torres M, Jeffers LJ, Sheikh MY, et al. Peg interferon alfa-2a and ribavirin in Latino and non-latino whites with Hepatitis C. N Engl J Med. 2009;360:257–267. doi: 10.1056/NEJMoa0805062. [DOI] [PubMed] [Google Scholar]
- 6.Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010 Jul;139(1):120–129 e118. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Poordad F, Bronowicki JP, Gordon SC, et al. IL28B polymorphism predicts virologic response in patients with hepatitis C genotype 1 treated with boceprevir(BOC) combination therapy. 2010 [Google Scholar]
- 8.Jensen DM, Morgan TR, Marcellin P, et al. Early identification of HCV genotype 1 patients responding to 24 weeks peg interferon alfa-2a (40KD)/ribavirin therapy. Hepatology. 2006;43:954–960. doi: 10.1002/hep.21159. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Tapias JM, Diago M, Escartín P, et al. Peg interferon alfa-2a plus ribavirin for 48 vs 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology. 2006;131:451–460. doi: 10.1053/j.gastro.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Berg T, von Wagner M, Nasser S, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 vs 72 weeks of peg interferon alfa-2a plus ribavirin. Gastroenterology. 2006;130:1086–1097. doi: 10.1053/j.gastro.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Pearlman BL, Ehleben C, Saifee S. Treatment extension to 72 weeks of peg interferon and ribavirin in hepatitis C genotype 1 infected slow responders. Hepatology. 2007;46:1688–1694. doi: 10.1002/hep.21919. [DOI] [PubMed] [Google Scholar]
- 12.Fried MW, Jensen DM, Rodriguez-Torres M, et al. Improved outcomes in patients with heptitis C with difficult to treat characteristics: randomized study of higher doses of peg interferon alfa-2a and ribavirin. Hepatology. 2008;48:1033–1043. doi: 10.1002/hep.22448. [DOI] [PubMed] [Google Scholar]
- 13.Kwo PY, Lawitz EJ, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376(9742):705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 14.Berman K, Kwo PY. Boceprevir, an NS3 protease inhibitor of HCV. Clin Liver Dis. 2009;13(3):429–439. doi: 10.1016/j.cld.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Bartenschlager R. Hepatitis C virus replicons: potential role for drug development. Nat Rev Drug Discov. 2002;1:911–916. doi: 10.1038/nrd942. [DOI] [PubMed] [Google Scholar]
- 16.Taremi SS, Beyer B, Maher M, et al. Construction, expression, and characterization of a novel fully activated recombinant single-chain hepatitis C virus protease. Protein Science. 1998;7:2143–2149. doi: 10.1002/pro.5560071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R, Beyer BM, Durkin J, et al. A Continuous Spectrophotometric Assay for the Hepatitis C Virus Serine Protease. Analytical Biochemistry. 1999;270:268–275. doi: 10.1006/abio.1999.4109. [DOI] [PubMed] [Google Scholar]
- 18.McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827–1838. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 19.Hézode C, Forestier N, Dusheiko G, et al. Telaprevir and peginteron with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839–1850. doi: 10.1056/NEJMoa0807650. [DOI] [PubMed] [Google Scholar]
- 20.Sherman KE, Flamm SL, Afdhal NH. Telaprevir in combination with peginterferon alfa2a and ribavirin for 24 or 48 weeks in treatment-naive genotype 1 HCV patients who achieved an extended rapid viral response: Final results of phase 3 ILLUMINATE study. 61st AASLD; Boston, MA. October 29–November 2010; 2010. [Google Scholar]
- 21.Jacobson IM, McHutchison GJ, Dusheiko MG. Telaprevir in combination with peginterferon and ribavirin in genotype 1 HCV treatment-Naive patients: Final results of phase 3 ADVANCE study. 61st AASLD; Boston, MA. October 29–Novermber 2010; 2010. [Google Scholar]
- 22.McHutchison JG, Manns MP, Muir AJ, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292–1303. doi: 10.1056/NEJMoa0908014. [DOI] [PubMed] [Google Scholar]
- 23.Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir with peginterferon/ribavirin for untreated chronic hepatitis C. 2011 currently in print. [Google Scholar]
- 24.Poordad F. Big changes are coming in hepatitis C. 2011 doi: 10.1007/s11894-010-0153-9. manuscript in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for treatment of chronic hepatitis C genotype 1 nonresponders. 2011 currently in print. [Google Scholar]