Abstract
Intracerebral hemorrhage (ICH) is a devastating event, carrying a very high morbidity and mortality rate. Hypertension and age-related amyloid angiopathy are the strongest risk factors for ICH, but smoking, anticoagulation with warfarin, excessive alcohol intake and cocaine also increase risk. This, the fourth in a Missouri Medicine series on stroke summarizes the clinical and imaging aspects of making the diagnosis of ICH. Current medical and surgical therapies are discussed as well as predictors of outcome and recommendations for secondary prevention.
Introduction
Intracranial hemorrhage includes epidural hematoma, subdural hematoma, subarachnoid hemorrhage (SAH), intraventricular hemorrhage (IVH), hemorrhagic transformation of ischemic stroke (HT), venous hemorrhage from cortical vein or sinus thrombosis and intracerebral hemorrhage. The primary focus of this, the fourth in a Missouri Medicine six part series on Stroke, is intracerebral hemorrhage (ICH) which, for the purposes of this discussion, will include intraventricular hemorrhage (IVH). Part 5 will deal with subarachnoid hemorrhage (SAH).
Intracerebral hemorrhage accounts for 10–15% of all strokes and carries very high morbidity and mortality rates that have not changed over the last 30 years. At one year, mortality ranges from 51% to 65% depending on the location of the hemorrhage.1 Half of the deaths occur in the first two days. At six months, only 20% of patients are expected to be independent.2 The incidence of hemorrhage increases exponentially with age and is higher in men than in women.
Clinical Presentation and Pathogenesis
Sudden onset of focal neurological deficit which progresses over minutes to hours is the major presenting feature of ICH. The nature of the deficits reflects the location of the initial bleeding and subsequent edema. Seizures, vomiting, headache, and diminished level of consciousness are common associated symptoms. Both headache and diminished level of conscious are uncommon in acute ischemic strokes.
The majority of patients with primary ICH develop measureable lesion expansion over the initial few hours. The degree of hematoma growth is an independent determinant of mortality and functional outcome. The mass effect of the primary bleeding may cause lesions to migrate and dissect through less-dense white matter and into the ventricles resulting in increased intracranial pressure. A hematoma incites local edema and neuronal damage in the adjacent brain parenchyma which typically lasts from 5 days to 2 weeks, with the largest increase in edema occurring in the first 72 hours.3 Thrombin within the hematoma plays a central role in promoting perihematomal edema. Hemoglobin and its products, heme and iron, are potent mitochondrial toxins leading to cell death.
Diagnosis and Assessment
The early risk of neurological deterioration and cardiopulmonary instability in ICH is high, making urgent diagnosis and management critical. The history must be taken quickly. It is important to know whether there is any history of trauma, hypertension, excessive use of alcohol, any use of drugs either by prescription or recreation that could play a role such as cocaine, warfarin, aspirin, clopidogrel, or any hematologic disorder.
The first priority in the physical examination is to assess vital signs and determine if intubation is required for safety during imaging. It is important to determine if acute myocardial injury is a risk in patients with severely elevated blood pressure (BP).After the patient is medically stabilized, the next step is to obtain stat labs to include protime/INR, PTT, CBC with platelet count, D-dimer, fibrinogen, electrolytes, BUN/creatinine, glucose, liver functions and type and screen to blood bank and then get the patient to an imaging study as fast as possible.
Imaging
CT head scanning has clarified the natural history of ICH and is the major test in use to differentiate between acute ICH, SAH, and ischemic stroke. It is an extremely sensitive test to detect both ICH and SAH and to identify the size and location of the hemorrhage. Hematoma expansion, highly associated with clinical deterioration and poor outcomes, is evident in nearly 40% of cases within the first 3 hours after onset of symptoms is also well-documented with CT scanning.4,5 An approximate volume of the hemorrhage can be determined by multiplying the maximum length (cm) times the maximum width (cm) times the number of transverse CT scan cuts (height in cm) and dividing by 2.
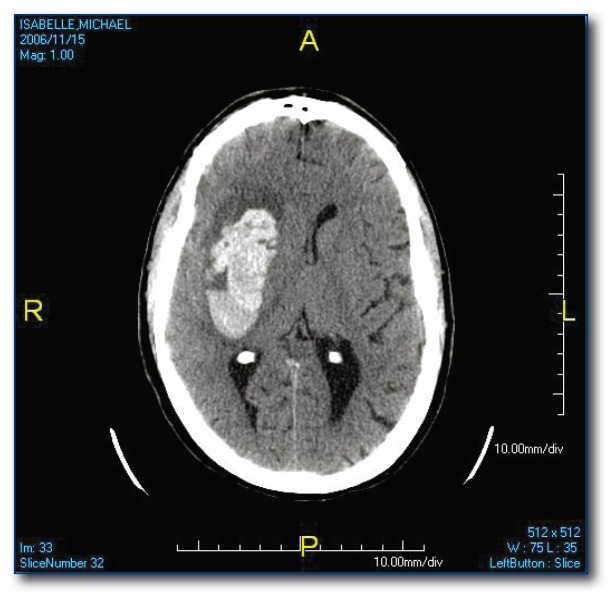
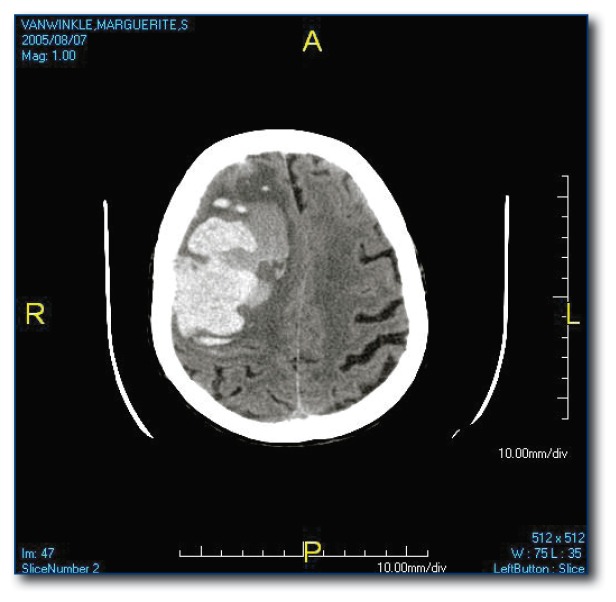
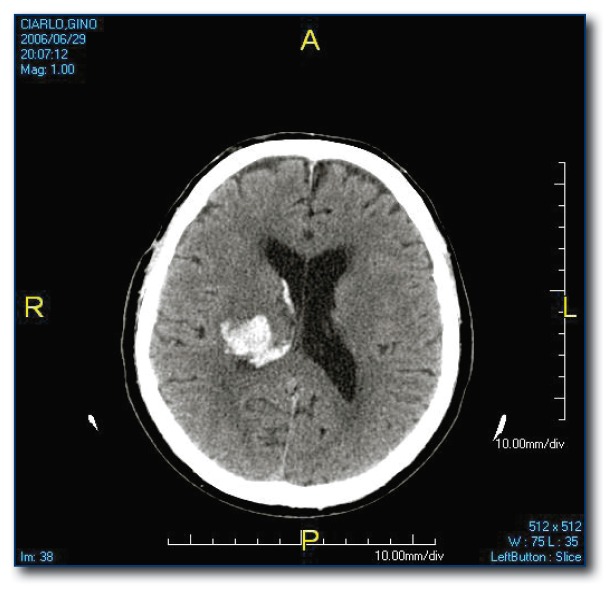
The most common site for hypertensive ICH is the putamen (see Figure 1) but it may occur in other subcortical locations. Lesions in the peripheral brain parenchyma (lobar hemorrhages; see Figure 2) are usually attributed to amyloid angiopathy in the elderly, but may also be due to hypertension. Hemorrhages may dissect from the brain parenchyma into the adjacent ventricular space or they may be isolated to the intraventricular space (see Figure 3), both carrying a poor prognosis.6
Figure 1.
Putamenal hypertensive hemorrhage. Typical location for hemorrhage secondary to hypertension.
Figure 2.
Lobar hemorrhage likely related to amyloid angiopathyv.
Figure 3.
Intraventricular hemorrhage, usually related to hypertension.
CT with contrast and CT angiography (CTA) may identify associated aneurysms, tumors and underlying AVMs, although MR scanning is more sensitive for AVMs, especially cavernomas. MRI is a reasonable alternative to CT scanning but is usually not as practical in most hospitals.
Digital subtraction angiography (DSA) is the gold standard for identification of aneurysms and AVMs. Most cases compatible with either hypertensive or amyloid angiopathy etiologies will not require angiography. A search for a secondary cause is recommended when:
Age <45 years
No history or presence of hypertension
Unusual location i.e. temporal lobe
Increased edema on initial CT scan
Multiple hemorrhages present
Irregular shape of the hemorrhage
Treatment of ICH
Primary Therapy
There are no evidence-based primary therapies that improve outcomes for acute ICH. Clinical trials have shown that early treatment with recombinant Factor VIIa prevents early ICH expansion, but clinical outcomes were not changed.7
Medical Management
Blood Pressure
General medical management includes attention to airway, oxygenation, hydration, glucose <180, temperature at 37.5 degrees C, early nutrition and mobilization, and prophylaxis for deep vein thrombosis. The head of the bed should be up 30 degrees and the patient should be monitored in an ICU setting.
The target blood pressure should be individualized depending on factors including history of hypertension, intracranial pressure, age, presumed cause of the hemorrhage and interval since onset. In several retrospective studies, elevated systolic blood pressure greater than 160 mm HG on admission was associated with growth of the hematoma but this has not been demonstrated in prospective studies of ICH growth. The ASA Guidelines suggest the following approach for elevated blood pressure in spontaneous ICH:
If SBP >200, aggressive reduction of blood pressure using continuous intravenous infusion with frequent BP monitoring every 5 minutes
If SBP >180 and evidence for elevated ICP, then monitor ICP and lower blood pressure using intermittent or continuous intravenous medications to keep cerebral perfusion pressure >60–80 mm Hg
If SBP >160 and no evidence for elevated ICP then consider a modest reduction of blood pressure to 150/90 using intermittent or continuous intravenous medications.
Check BP every 15 minutes.
Preferred agents are beta blockers (labetalol, esmolol), ACE inhibitor (enalapril), calcium channel blocker (nicardipine) or hydralazine.8
Whether more aggressive control of blood pressure could decrease hematoma expansion without compromising perfusion of the surrounding brain is unknown. Ongoing studies (ATACH and INTERACT) may provide more guidance on acute BP management.
Intracranial Pressure (ICP)
Cerebral perfusion pressure should be >70mm Hg. Initial treatment should use the simple measures of elevating the head of bed to 30 degrees combined with pain management and sedation. Mass effect causing significant elevation of ICP with a risk of herniation may be managed emergently with osmotic agents (mannitol, hypertonic saline) or hyperventilation, but none of these therapies has been formally studied in clinical trials.
Seizures
In the first two weeks after ICH 5–10% of patients will have a clinically manifest seizure, reflecting cortical involvement. In addition, with continuous EEG monitoring, 30% of patients will demonstrate electrical seizure activity without clinically evident seizures. The clinical significance of this is unclear. Anticonvulsant therapy is indicated for clinical seizures and could be considered in those cases where there is electrical seizure activity and the patient is not waking up as expected. The appropriate duration of anticonvulsant treatment is not established.
Warfarin Related ICH
Warfarin accounts for a substantial proportion of cases of ICH presenting to general hospitals. Among patients with supratentorial ICH admitted to Massachusetts General Hospital over a seven year period, 23.4% were taking warfarin.9
The risk factors are age, hypertension, intensity of anticoagulation,and poor balance.
The goal of treatment is to rapidly reverse the coagulation defect to minimize hematoma growth. The following options are available:
Vitamin K1 can be administered intravenously at 10–25 mg to correct the INR, but it will take several hours to reach a normalized INR..
Fresh frozen plasma (FFP) at 15–20 mL/kg can be used to correct the INR faster. It has the disadvantage of requiring the infusion of large volumes that can lead to volume overload. There is also the potential for allergic reaction and infection. Often Vitamin K will be started immediately and FFP added when it is available.
Prothrombin complex concentrate is gaining popularity because it requires smaller volumes and corrects the coagulopathy quickly. There is the risk of inducing thromboembolic events. There is no risk of infection.
Recombinant Factor VIIa has the potential to normalize the INR very quickly, but it has not been tested in this setting. The short half-life of 2.6 hours might require repeated injections, and there is a risk of thromboembolic complications.
Surgical Management
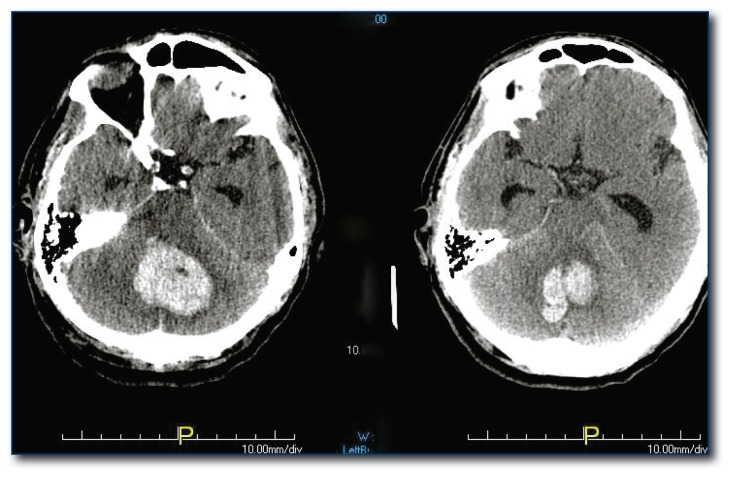
The single mandated indication for neurosurgical decompression is cerebellar hemorrhage (see Figure 4) causing decreased level of consciousness, hydrocephalus or brainstem compression. Early craniotomy prior to significant brainstem compression is critical. The best surgical candidates are patients with an initial Glasgow Coma Scale (GCS) <14 and hematomas >40 mL while those with higher GCS and smaller lesions are likely to have a good outcome with conservative, non-surgical management.10
Figure 4.
Cerebellar hemorrhage. This is the one potential neurosurgical emergency.
The largest trial looking at surgical intervention for supratentorial ICH is the STITCH Trial.11 Patients were randomized to surgery or medical therapy. Mortality and favorable outcomes were equal in the two groups. Surgery does not appear to be helpful in most cases and is probably harmful in those presenting in coma. The subset of patients who may benefit from early evacuation of the clot are those who have lobar hemorrhages within 1cm of the surface of the brain and milder clinical deficits (GCS>9). Craniotomy in this group was associated with 29% relative benefit in functional outcome when compared to medical management. The STITCH II Trial is currently randomizing patients with lobar hemorrhages to early open surgery versus conservative management.
Minimally invasive surgery involves stereotactic placement of a cannula into the center of the clot with subsequent dosing of tPA through the cannula to dissolve the clot from the inside out. The ongoing MISTIE Trial is testing this technique in supratentorial ICH and the CLEAR Trial is testing the same procedure for intraventricular hemorrhage. Early results indicate significant decrease in clot size. Whether this will translate to improved clinical outcomes remains to be seen.
Predictors of Outcomes
The volume of the ICH and the clinical grade on the Glasgow Coma Scale on admission are the most powerful predictors of 30-day mortality.12 Hemispheric lesions >30 cc have a high mortality rate. Patients with GCS <9 and hematoma >60 cc have a 90% mortality rate.8 Intraventricular involvement with associated hydrocephalus predicts a mortality rate of 43% at 30 days.13,14 Brainstem hemorrhages, even when small, carry a poor prognosis. Age over 80 years also carries a higher risk of mortality.
Recovery and Secondary Prevention
Early mobilization and involvement of the neuro-rehabilitation team is essential to maximizing recovery. People with severe ICH have the potential to do better than those with severe ischemic stroke.
There is a 2.1–3% risk of recurrent ICH per patient year. Lobar hemorrhages have a higher risk of recurrence that is likely related to underlying amyloid angiopathy. Other factors increasing the risk of recurrence are older age, anticoagulation, and the APOE genotype which is associated with amyloid deposition.
The major factor in preventing recurrence is blood pressure control. The odds ratio for recurrent ICH with untreated hypertension in one study was 3.5 but only 1.4 for treated hypertension suggesting that treatment of hypertension can prevent ICH.15 Smoking, heavy alcohol use and cocaine are also associated with increased rates of ICH so these should be addressed with cessation strategies.
The issue of anticoagulation in patients who have had an ICH and also have atrial fibrillation or another condition where warfarin is indicated is a difficult one. Anticoagulated patients have a higher likelihood of recurrent ICH when the prior location of the hemorrhage was lobar rather than subcortical. Overall, there is a relatively strong contraindication to using warfarin in people who have had an ICH. There is weaker evidence against the use of antiplatelet therapy. This issue should be addressed on a case by case basis.
Biography
Marilyn M. Rymer, MD, is a Professor of Medicine at the University of Missouri - Kansas City School of Medicine and practices at the Saint Luke’s Brain and Stroke Institute. MSMA member since 1981, she is the lead author and collaborator of Missouri Medicine’s Micro-Series on Stroke. This article is fourth in a series of six.
Contact: mrymer@saint-lukes.org

Footnotes
Disclosure
None reported.
References
- 1.Flaherty ML, Woo D, Haverbusch M, et al. Racial Variations in Location and Risk of Intracerebral Hemorrhage. Stroke. 2005;36(5):934–937. doi: 10.1161/01.STR.0000160756.72109.95. [DOI] [PubMed] [Google Scholar]
- 2.Counsell C, Boonyakamkul S, Dennis M, et al. Primary Intracerebral Hemorrhage in the Oxfordshire Community Stroke Project, 2: Prognosis. Cerebrovascular Disease. 1995;5:26–34. [Google Scholar]
- 3.Juvela S, Kase C. Advances in Intracerebral Hemorrhage Management. Stroke. 2006;37:301–4. doi: 10.1161/01.STR.0000200445.68303.25. [DOI] [PubMed] [Google Scholar]
- 4.Brott T, Broderick J, Kothari R, et al. Early Hemorrhage Growth in Patients with Intracerebral Hemorrhage. Stroke. 1997;28(1):1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Hill MD, Silver FL, Austin PC, et al. Rate of Stroke Recurrence in Patients with Primary Intracerebral Hemorrhage. Stroke. 2003;31(1):123–127. doi: 10.1161/01.str.31.1.123. [DOI] [PubMed] [Google Scholar]
- 6.Arakawa S, Saku Y, Ibayashi S, et al. Blood Pressure Control and Recurrence of Hypertensive Brain Hemorrhage. Stroke. 1998;29:1806–9. doi: 10.1161/01.str.29.9.1806. [DOI] [PubMed] [Google Scholar]
- 7.Mayer S, Brun NC, Begtrup K, et al. Recombinant Activated Factor VII for Acute Intracerebral Hemorrahge. New England JMed. 2005 Feb 24;352(8):777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 8.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage in Adults: 2007 Update: A Guideline from the American Stroke Association Stroke Council. Stroke. 2007;38:2001–23. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 9.Rosand J, Eckman MH, Knudsen KA, et al. The Effect of Warfarin and Intensity of Anticoagulation on Outcome of Intracerebral Hemorrhage. Archives of Int Med. 2004 Apr 26;164(8):880–884. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi S, Sato A, Kageyama Y, et al. Treatment of Hypertensive Cerebellar Hemorrhage—Surgical or Conservative Management. Neurosurgery. 1994;32:246–50. [PubMed] [Google Scholar]
- 11.Mendelow AD, Gregson BA, Fernandes HM, et al. Early Surgery versus Initial Conservative Treatment in Patients with Spontaneous Supratentorial Intracerebral Hemorrahge in the International Surgical Trial in Intracerebral Hemorrhage (STICH): A Randomized Trial. Lancet. 2005 Jan 29;365(9457):387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 12.Broderick J, Brott T, Duldner JE, et al. Volume of Intracerebral Hemorrhage: A Powerful and Easy to Use Predictor of 30-day Mortality. Stroke. 1993;24(7):987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 13.Tuhrim S, Horowitz D, Sacher M, et al. Validation and Comparison of Models Predicting Survival Following Intracerebral Hemorrhage. Critical Care Med. 1995;23:950–4. doi: 10.1097/00003246-199505000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: A Previously Unrecognized Predictor of Poor Outcomes from Supratentorial Intracerebral Hemorrhage. Stroke. 1998;29(7):1352–1357. doi: 10.1161/01.str.29.7.1352. [DOI] [PubMed] [Google Scholar]
- 15.Woo D, Haverbusch M, Sekar P, et al. Effect of Untreated Hypertension on Hemorrhagic Stroke. Stroke. 2004;35(7):1703–1708. doi: 10.1161/01.STR.0000130855.70683.c8. [DOI] [PubMed] [Google Scholar]