Abstract
Genes under the control of the cyclooxygenase-2 (Cox-2), human epidermal growth factor receptor 2 (Her-2), and survivin promoters were constructed and delivered to murine and human carcinoma cells. It was found that PCox-2-driven reporter expression was strong and correlated well with endogenous Cox-2 levels, while PHer-2 and Psurvivin yielded poor results, consistent with the three distinct expression mechanisms used by cancer cells to overexpress the endogenous versions of the selected genes. The PCox-2 was then used to drive the expression of caspase genes both in vitro and in vivo to bring about targeted apoptosis of carcinoma cells successfully. The results led to the following conclusions. 1) When selecting a promoter/enhancer for expression-targeted gene delivery, it is not enough to perform a microarray on some tumor tissue and select the control element associated with the greatest amount of gene up-regulation vs. normal controls. The mechanism of expression for the particular gene should be taken into account to prevent lengthy and costly research trials. 2) When overexpression is due to activator binding, a predictive model based on endogenous gene expression levels, overall cell transfectability, and cell doubling rates can be used to predict expression-targeted gene delivery outcomes with significant accuracy.—Dobek, G. L., Zhang, X., Balazs, D. A., Godbey, W. T. Analysis of promoters and expression-targeted gene therapy optimization based on doubling time and transfectability.
Keywords: Cox-2, Her-2, survivin
One of the major problems of cancer therapy is the lack of tumor specificity of modern drugs (1). Dividing cells are often targeted, including untransformed cells undergoing mitosis. This lack of specificity yields serious side effects for the patient as normal tissues face destructive attack by the chemotherapeutic agent. Although cancer gene therapy has attracted great attention since the first gene therapy protocol for cancer treatment was approved in 1989 (2, 3), it still faces the specificity problem in that transgene expression ideally should be targeted to tumor cells only. Using tumor-selective promoters to target gene expression is one method under investigation. Genes have been identified that show little or no activity in normal tissue but are up-regulated in certain types of tumors (1).
Because the present investigations utilize the cyclooxygenase type 2 (Cox-2), survivin, and human epidermal growth factor receptor 2 (Her-2) promoters, a brief introduction to the genes follows.
Cox catalyzes the conversion of arachidonic acid to prostaglandin H2 in the biosynthesis of prostanoids (4). Typically, Cox-2 is not expressed unless the cell is stimulated by growth factors, tumor promoters, cytokines, or hormones (5, 6). However, Cox-2 also mediates the production of prostaglandin-E2, which is associated with neoplasia through the promotion of cell survival, cell growth, migration, invasion, and angiogenesis (7). An up-regulation of Cox-2 expression has been demonstrated in various cancer types, including the esophagus (8), stomach (9, 10), colon (11–13), and bladder (14–17), but not in normal cells derived from the same tissues, making it a focus for cancer chemotherapy and targeted gene delivery (8–18).
Survivin, a member of the inhibitor of apoptosis (IAP) gene family, inhibits natural cell death and promotes cellular proliferation (19–21). Survivin is strongly expressed during embryonic and fetal development, but undetectable in terminally differentiated normal tissues (22, 23). Moreover, survivin mRNA or protein expression has been found in various cancers, including bladder (24), colon (25), esophageal (26), breast (27), and melanoma (28), as well as cell lines such as NIH-3T3 (29). In addition to gene therapy, other therapeutic trials targeting survivin include antisense oligonucleotides, siRNA, ribozymes, immunotherapy, and small-molecular-weight molecules (30–34). The investigation of survivin as a cancer biomarker has shown encouraging results, with the urinary level of survivin associated with bladder cancer presence, advanced stage, higher tumor grade, metastases, and mortality (35, 36).
Her-2 is a transmembrane receptor protein found to be overexpressed in approximately one-third of primary breast carcinomas (37). Her-2 overexpression has been shown to enhance proliferative, prosurvival, and metastatic signals in breast cancer cell lines (38–40). The overexpression of Her-2 in breast tumors makes it a potential target for cancer chemotherapy and gene delivery. Approximately half of Her-2-positive breast tumors respond to trastuzumab, a monoclonal antibody with Her-2 targeting properties (41). Several studies have focused on adeno-associated virus-mediated gene delivery of trastuzumab and other anti-Her2 antibodies to suppress the growth of Her-2 positive tumors (42–44). A phase I gene therapy clinical trial utilized a Her-2 promoter fragment/cytosine deaminase chimera combined with systemic administration of the prodrug fluorocytosine (5-FC), resulting in reduced tumor volumes (45). These results support the potential use of a Her-2 promoter for the targeted gene therapy of certain breast cancers.
This study aimed to establish the effects of endogenous gene expression on the expression of nonvirally delivered genes. We sought to determine whether a correlation between transcription levels of native genes and transfection success of expression-targeted plasmids using the same promoter elements existed. We used the endogenous expression levels and expression-targeted transfection efficiency data to predict the efficacy of promoter-driven anticancer treatments in vitro and in a mouse orthotopic model of bladder cancer.
MATERIALS AND METHODS
Cells
The murine transitional cell carcinoma cell lines MB49 (Anthony Atala, Wake Forest University Baptist Medical Center, Winston Salem, NC, USA) and MBT2 (Yi Luo, University of Iowa, IA, USA), the BT474 and SkBr3 human breast tumor cell lines [American Type Culture Collection (ATCC), Manassas, VA, USA], the NIH/3T3 murine fibroblast cell line (ATCC), and HFF-1 cell line (ATCC) were used for the current investigations. MB49 and 3T3 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA, USA), 100 U/ml penicillin, and 100 U/ml streptomycin (Invitrogen). MBT2, SkBr3, and HFF-1 cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 U/ml streptomycin. BT474 cells were also cultured in RPMI 1640 but with additional supplementation with 0.02 mg/ml bovine insulin. Cultures were maintained at 37°C and 5% CO2 with medium changes every 2–3 d.
Plasmids
Survivin-GFP and survivin-iCasp9
The plasmid pEGFP-N1 (Clontech/Takara Bio Co., Mountain View, CA, USA) was used as the vector for construction of a survivin-driven plasmid coding for an enhanced green fluorescent protein (EGFP) or inducible form of caspase 9 (iCasp9), with the original CMV promoter being replaced by the murine survivin promoter. The inserted promoter sequence (Roswell Park Cancer Institute, Buffalo, NY, USA) was amplified via PCR using the following set of primers: forward, 5′-AAGattaatCATGCCCTGCGCCCGCC-3′ (AseI site in lowercase); reverse, 5′-AAGaagcttCCTCCGCCAAGACGAC-3′ (HindIII site in lowercase).
Following digestion with AseI and HindIII, the PCR-amplified survivin promoter was ligated between the AseI and HindIII sites of the pEGFP-N1 vector. The survivin-iCasp9 plasmid was constructed through modification of the survivin-GFP plasmid, by replacing the EGFP exon with an exon coding for iCasp9 (exon supplied as a generous gift from David Spencer, Baylor College of Medicine, Houston, TX, USA) using SacII and BamHI restriction sites. Transformed colonies of E. coli DH5α (New England Biolabs, Ipswich, MA, USA) were selected on kanamycin-containing Luria-Bertani agar plates, and plasmid identities were verified by restriction analysis and sequencing.
Cox-2-GFP and Cox-2-iCasp9
The plasmid pEGFP-N1 was also used as the vector for construction of the murine Cox-2-promoter-driven plasmids, which code for EGFP or iCasp9. The murine Cox-2 promoter, also known as the murine 12-O-tetradecanoylphorbol-13-acetate (TPA)-inducible-sequence clone 10 (TIS10) promoter (46), was provided by Carol Pilbeam (University of Connecticut Health Center, Farmington, CT, USA). Plasmids were constructed as described previously (47).
Her2-GFP
Genomic DNA was isolated from human foreskin fibroblasts via chloroform-phenol extraction. Based on a published promoter sequence (48), the Her2 (c-erbB-2) promoter was obtained through PCR amplification using the following set of primers: forward, 5′-AAGattaatCCTGGAAGCCACAAGGTAAACACAA-3′ (AseI site in lowercase); reverse, 5′-AGgaattcGGGCTCCCCTGGTTTCTCCGGT-3′ (EcoRI site in lowercase).
The Her2/Neu-promoter-driven-GFP plasmid (Her2-GFP) was then constructed in a fashion similar to that described above for the survivin-GFP plasmid, again using the plasmid pEGFP-N1 as the vector. Plasmid identities were verified by restriction analysis and sequencing.
Real-time quantitative PCR
Cells were trypsinized and pelleted via centrifugation at 500 g for 5 min. Total RNA extraction was performed using the RNeasy mini kit (Qiagen Inc., Valencia, CA, USA), and first-strand cDNA synthesis was performed using an iScript Select cDNA synthesis kit with random primers (Bio-Rad, Hercules, CA, USA). Real-time PCR analyses were performed using an iCycler (Bio-Rad) in a total volume of 25 μl that contained 12.5 μl SYBR green supermix (Bio-Rad), 200 nM forward and reverse primers (Integrated DNA Technologies, Coralville, IA, USA), and 1 μl template. Reaction mixtures were incubated at 95°C for 3 min, and reactions were allowed to proceed via 40 cycles of melting at 95°C for 10 s, annealing at 58°C for 30 s, and extension at 72°C for 45 s. The housekeeping gene GAPDH (GenBank; locus: BC020407, accession: BC020407) or 18s rRNA were used as internal references.
The primer sequences used for the detection of murine Cox-2 mRNA (cDNA) (locus: NM_011198, accession: NM_011198) were as follows: forward, 5′-TGCTCACGAAGGAACTCAGC-3′; reverse, 5′-AAATCCTGTGCTCATACATTCCC-3′.
The primer sequences used for the detection of murine survivin mRNA (cDNA) (locus: NM_009689, accession: NM_009689) were as follows: forward: 5′-TGTACCTCAAGAACTACCGCATCG-3′; reverse; 5′-CTATGCTCCTCTATCGGGTTGTCATC-3′.
The primer sequences used for the detection of Her2 mRNA (cDNA), also known as ERBB2 (locus: NM_001005862, accession: NM_001005862), were as follows: forward, 5′-ACCTAGCGGAGCGATGCCCAACCAG-3′; reverse, 5′-AGATTTCTTTGTTGGCTTTGGGGGA-3′.
Quantification was performed using the efficiency-corrected relative quantification method (49):
| (1) |
where “ratio” represents the relative mRNA level of the sample as compared to that of the control, “target” refers to the cDNA for the gene of interest for that particular experiment (such as Cox-2 or survivin), and “reference” represents GAPDH cDNA. E is the amplification efficiency of each primer set. As negative controls, we used the 3T3 and HFF-1 cell lines, and “sample” refers to the particular cancer cell line used to generate the given total-RNA sample.
Inducible caspases and their activation
iCasp9 is a caspase protein that remains inactive until dimerized via an exogenously delivered activator, AP21087 (Ariad, Cambridge, MA, USA). The activator allowed for post-translational control of the delivered gene (50). The use of post-translational control allowed for a distinction between cell death that occurred as a result of the iCasp9 gene being delivered vs. cell death that may have resulted from the general act of nonviral transfection. The AP20187 was diluted in ice-cold ethanol before addition to the cells with a final concentration of 100 nM. Cells undergoing delivery of iCasp9 received the activator solution every 12 h for 60 h following each individual transfection. The activator was administered in vitro by the addition of 2.86 μl of 100 μg/ml AP20187 (in ethanol) to 2 ml of fresh growth medium.
In vitro transfections
Cells were plated in 6-well tissue culture dishes at a concentration of 105 cells/well and allowed to incubate for 12–16 h prior to transfection. The polycation polyethylenimine (PEI), with a reported weight-average molecular weight of 25 kDa (Sigma-Aldrich, St. Louis, MO, USA), was used as the gene delivery vehicle (51). Because cell lines have different sensitivities to PEI dosages, each cell line was transfected with an experimentally determined optimum of PEI/DNA complexes (52). The ratio of polymer to plasmid was held constant (7.5:1.0), but the number of complexes delivered was customized for each cell type.
PEI and DNA solutions were prepared separately in 50-μl volumes using normal saline as the diluent; then the polymer was added to the plasmid to produce 100 μl (per dose) of transfection complexes, which were allowed to incubate for 15 min prior to addition to cell wells that contained 2 ml of serum-free medium (51). Transfections were allowed to proceed for 2 or 4 h at 37°C depending on the experiment, after which the transfection medium was replaced with growth medium.
Assessments of transfection efficiencies were determined via a FACSCantoII flow cytometer (BD Biosciences, San Jose, CA, USA). The relative activities of expression-targeted plasmids under the control of the Cox-2 promoter were determined by normalizing results from positive control CMV-GFP transfections: the number of cells expressing Cox-2-driven GFP was divided by the number of cells expressing CMV-driven GFP.
For cell survival experiments involving the Cox-2-iCasp9, survivin-iCasp9, or DsRed plasmids, plates were washed twice with PBS to remove nonadherant cells, and the remaining cells were trypsinized and counted using flow cytometry. Experiments were performed in triplicate, with 104 cells counted per experimental run per cell type, at a flow rate of 1 μl/s. At least 3 experimental runs were performed for each cell type.
Cell doubling rates
Cells were plated in 6-well tissue culture dishes at a concentration of 105cells/well. Cell counts were conducted via hemacytometer every 12 h, beginning 48 h after seeding. To determine the times needed for population doubling, the average numbers of cells at each time point were plotted vs. time, and exponential curves were fit to the data. The exponential curves, by definition, took the form y = aebx, which yielded doubling times equal to ln (2)/b.
Animal orthotopic tumor model
All experiments were performed with the approval of the Tulane University Institutional Animal Care and Use Committee. Female, 3- to 5-wk-old specific pathogen-free C57Bl/6J mice (Mus musculus) were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and allowed 7 d to acclimate before the start of the study. Mice were randomly assigned to treatment or control groups. The model was established as in our previously published protocol (53). Following draining of the bladder, burn lesions were created via transurethral catheterization and delivery of 2.5 W delivered by a Bovie electrocautery unit (Bovie Medical Corp., St. Petersburg, FL, USA) set to coagulation. The wire was removed, and 100 μl MB49 cell suspension with a concentration of 1 × 106 cells/ml was delivered into the bladder. The syringe was left attached to the catheter to prevent efflux of the cell suspension, and the catheterized mice were maintained under anesthesia for 90 min to allow for cell attachment to the burn sites.
Ultrasound
Tumors were allowed to grow for 3–5 d, and then mice underwent ultrasound examination of the urinary bladder to monitor tumor growth. For ultrasound examination, animals were anesthetized as described above. Mice were placed in dorsal recumbancy, and abdominal skin was prepared by clipping the fur. A 24-gauge i.v. catheter was inserted transurethrally into the urinary bladder. Sterile normal saline (100 μl) was instilled into the bladder through the catheter to improve bladder visibility on ultrasound. Ultrasound conduction gel was applied to the abdomen, and ultrasonography was performed to assess the bladder wall and lumen. When a majority of the mice showed evidence of bladder tumors on ultrasound, treatments began the following day.
In vivo treatments
Mice were anesthetized and catheterized to empty the bladder of urine as described previously (53). Transfection complexes were delivered intravesically via transurethral catheterization with a total volume of 100 μl. Transfection solution remained in the bladder for 2 h, after which the catheters were removed and animals were allowed to recover. The treatment was repeated every other day for a total of 6 transfections/mouse. Treatment groups included mice receiving saline only as a negative control, mice receiving survivin-iCasp9 or Cox-2-iCasp9 DNA only, and mice receiving both survivin-iCasp9 and Cox-2-iCasp9 in the same dose or separately on alternate days of treatment.
AP20187 solutions were administered daily starting the day following the first treatment, with 100 μl being delivered per dose by i.p. injection. Each dose consisted of 4 μl (2 mg/kg) of AP20187 suspended in 10 μl polyethylene glycol (PEG 400) and 86 μl 2% Tween 20. Mice were euthanized 2 d after the last treatment, and bladders were harvested to obtain tumor weights.
Statistics
For analysis of the cell survival forecast model, predicted values were plotted against the means of observed values, and 95% confidence intervals were plotted based on Student's t test (using 2 degrees of freedom; ref. 54). Pair-wise comparisons were made using heteroscedastic t tests. Groups of data were compared for differences in means via single-factor ANOVA.
RESULTS
Correlations between expression-targeted transgene expression and transcription of the corresponding endogenous gene
Real-time PCR was used to measure the relative mRNA levels of endogenous murine Cox-2 and survivin of 6 cell types: HFF-1 (normal human foreskin fibroblast), NIH-3T3 (normal murine fibroblast), MC38 (murine colon cancer), MBT2 (murine bladder cancer), MB49 (murine bladder cancer), and CT26.CL25 (murine colon cancer). The same method was used to analyze human Her-2 mRNA levels in NIH-3T3, BT474 (human breast cancer), and SkBr3 (human breast cancer) cell lines. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was selected as the internal reference to correct for possible PCR loading errors in all cases except for Her-2 experiments, which used 18s rRNA. Associated data are shown in Table 1.
Table 1.
Relative expression levels of Cox-2, Her-2, and survivin genes
| Gene | Cell type | Expression level |
|---|---|---|
| Endogenous (murine) Cox-2 | HFF-1 | ND |
| 3T3a | 1.00 ± 0.30 | |
| MC38 | 2.19 ± 0.30 | |
| MBT2 | 4.00 ± 1.16 | |
| MB49 | 10.91 ± 4.68 | |
| CT26.CL25 | 15.80 ± 1.10 | |
| Endogenous (human) Her2 | HFF-1a | 1.00 ± 0.40 |
| 3T3 | 0.01 ± 0.00 | |
| BT474 | 23.38 ± 4.13 | |
| SkBr3 | 43.33 ± 6.11 | |
| Endogenous (murine) Survivin | HFF-1a | 1.00 ± 1.67 |
| 3T3 | 24.03 ± 5.33 | |
| MC38 | 19.14 ± 2.59 | |
| MBT2 | 26.61 ± 3.15 | |
| MB49 | 16.38 ± 4.71 | |
| CT26.CL25 | 28.21 ± 4.15 |
Expression levels were determined by real-time PCR. Values are averages ± sd. ND, not detected.
All data for each gene are normalized to the cell type indicated, with that average value defined as 1.00.
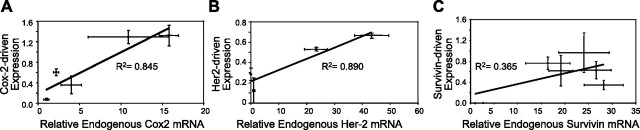
Transfection efficiencies achievable through the use of the Cox-2, Her-2, and survivin promoters were assessed by transfecting the cell lines with Cox-2-GFP (the GFP gene under the control of the Cox-2 promoter), Her-2-GFP, or survivin-GFP plasmids, with CMV-GFP transfections serving as positive controls for each cell line. The CMV promoter was chosen as the positive control because it is a strong promoter that has yielded robust transfection success in a multitude of cell types. Transfection efficiencies were measured via a FACSCantoII flow cytometer (BD Biosciences), and normalized to CMV-driven transfections to correct for variations in transfectability due to cell type. It was found that the expression of delivered genes coding for GFP and driven by the Cox-2 promoter positively correlated with the level of transcription of the endogenous Cox-2 gene (R2=0.849; Fig. 1A). A positive correlation also existed between Her-2-driven reporter gene expression and the endogenous level of Her-2 mRNA (R2=0.890; (Fig. 1B). However, the expression level of GFP reporter driven by the survivin promoter did not correlate well with endogenous survivin mRNA levels (R2=0.365; Fig. 1C).
Figure 1.
Relations between expression-targeted transfection efficiencies (normalized to CMV-driven control transfections) and relative transcription rates of endogenous genes utilizing the same promoter (as determined by real-time PCR on whole-cell RNA). Each panel addresses a different promoter. A) Cox-2. B) Her-2. C) Survivin. Error bars = 1 sd, solid lines represent linear regressions, goodness of fit is shown by R2 values.
Apoptotic activity of Cox-2-iCasp9 and survivin-iCasp9 in vitro
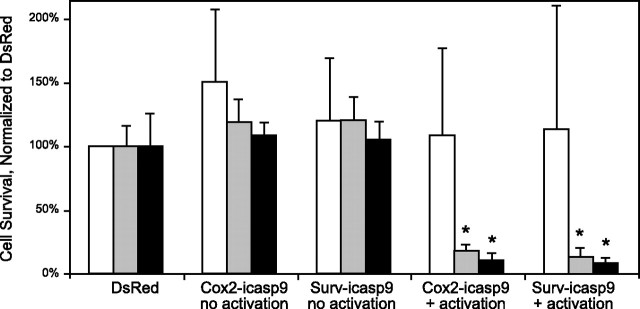
MB49, MBT2, and HFF-1 cells were transfected with plasmids coding for iCasp9 that were driven by the Cox-2 or survivin promoters. Transfections with the reporter plasmid DsRed (driven by the CMV promoter) and Cox-2-iCasp-9-transfected cells not receiving subsequent activation of the delivered-gene product were used as controls. HFF-1 cells were used as a negative control cell type to confirm specificity of the expression-targeted plasmids because neither murine Cox-2 nor murine survivin was detected in HFF-1 cells (Table 1). It was observed that MB49 and MBT2 cells transfected with survivin-directed genes coding for iCasp9, with post-translational product activation, had significantly fewer surviving cells as compared to the same transfections without subsequent iCasp9 activation (t test, n≥4, P<0.001; Fig. 2). No significant differences were found between the unactivated iCasp9 controls and cultures transfected with the DsRed reporter for the three cell types examined (ANOVA; n≥5/group). No significant differences were found in cell survival among any of the HFF-1 groups (ANOVA; n=3/group, P=0.879).
Fig. 2.
Delivery of survivin-driven or Cox-2-driven genes coding for an exogenously inducible form of caspase 9 results in cell death after post-translational product activation in survivin-expressing cells in vitro. All observed data were normalized to control transfections of CMV-driven DsRed plasmids. Open bars, HFF1 cells; shaded bars, MB49 cells; solid bars, MBT2 cells. Error bars = 1 sd. *P < 0.001 vs. corresponding no-activation group; t test (n≥4).
Tumoricidal activity of Cox-2-iCasp9 and survivin-iCasp9 in vivo
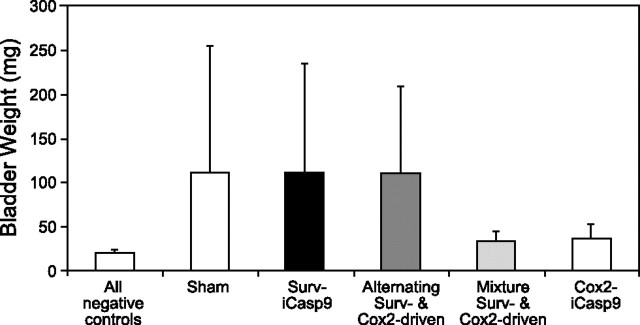
These experiments utilized treatment every other day, with a total of 6 treatments. Survivin-iCasp9 transfection had no noticeable effect on the size of MB49 tumors in the mouse orthotopic model of transitional cell carcinoma (Fig. 3). Likewise, survivin-iCasp9 alternated every other treatment with Cox-2-iCasp9 had no effect on tumor size. However, mice treated with either Cox-2-iCasp9 or a mixture of survivin-iCasp9 and Cox-2-iCasp9 (using the same total amount of DNA per treatment in each case) had noticeably smaller tumor burdens when compared with mice treated with saline only. The negative controls for these experiments all utilized animals that received no cells but were treated with Cox-2-iCasp9, alternating Surv/Cox-2-driven iCasp9, or a mixture of Surv/Cox-2-driven iCasp9 plasmids, with subsequent administration of the AP20187 activator solution as described in Materials and Methods.
Figure 3.
Survivin- vs. Cox-2-directed apoptosis of MB49 cells in vivo. Sham-treated control mice, mice treated with survivin-directed iCasp9 plasmids, and mice treated with alternating survivin- and Cox-2-directed plasmids had large, red bladders containing solid tumors. Bladders from negative control group mice and mice treated with gene therapy complexes containing mixtures of survivin- and Cox-2-directed plasmids or Cox-2-iCasp9 plasmids only were clear and generally devoid of large tumors. There was not a significant difference between these latter groups (P>0.05; ANOVA; n≥4). Negative control treatments involved the delivery of Cox-2-iCasp9, alternating Surv/Cox-2-driven iCasp9, or a mixture of Surv/Cox-2-driven iCasp9 plasmids, with subsequent AP20187 activation, in animals without tumor cells.
Figure 3 lumps all negative controls together for readability, but ANOVA analysis was performed using each negative control as an independent group. ANOVA indicated no significant difference in final bladder weights between the 3 negative control groups, mice receiving a mixture of Surv- and Cox-2-driven iCasp9 plasmids, and mice receiving straight Cox-2-iCasp9 plasmids (all with activation; P>0.05; n≥4). Comparisons by t test between each of these two treatment groups and the negative controls lumped together also indicated a lack of significant difference between groups (P>0.05; n≥4). Due to the low level of survivin-driven reporter expression in MBT2 cells, as well as the lack of survivin-driven efficacy against the MB49 tumors, in vivo experiments were not performed using the MBT2 bladder tumor cells in order to prevent unnecessary use of animals.
Determination of cell doubling rates
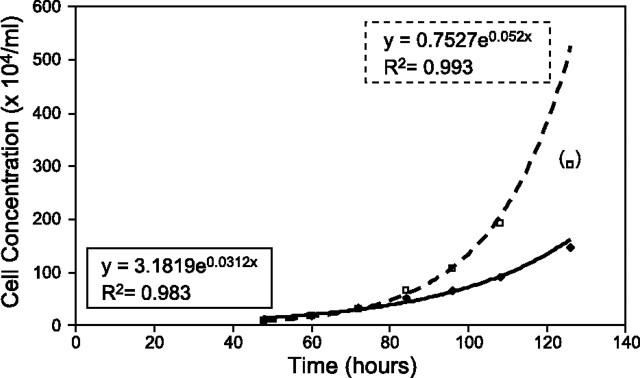
The Cox-2-promoter was selected for further modeling work because of the strength of correlation shown by Fig. 1 and the slope of the regression line in the same figure. The cell lines CT26.CL25 and MB49 were chosen for modeling because they showed the highest endogenous Cox-2 transcription levels of the cell types examined, which was synonymous with using the cell types tested that produced the greatest amount of Cox-2-driven GFP expression. Proliferation rates were determined empirically through cell counts taken during the exponential growth phase for several cultures, with exponential curves being fit to the data after the fact. The preciseness of fit was reflected through the R2 values for the two curves: 0.983 for the CT26.CL25 cells and 0.993 for the MB49 (Fig. 4). Using the formula presented in Materials and Methods, it was found that the doubling times were 22.2 h for the CT26.CL25 cells and 13.3 h for the MB49 cells.
Figure 4.
Observed cell concentrations for CT26.CL25 (solid diamonds, solid trace) and MB49 cells (open squares, dashed trace) at various times after initially plating 105 cells. Exponential curves were fit to the two data sets; curve equations and corresponding R2 values are shown beside each curve. Data point shown in parentheses was not used to generate an exponential curve because it was deemed to belong to the deceleration phase of the growth curve. Curves yielded doubling times of 22.2 h for CT26.CL25 cells and 13.3 h for MB49 cells.
Combination of cell cycle and endogenous expression data to predict outcomes
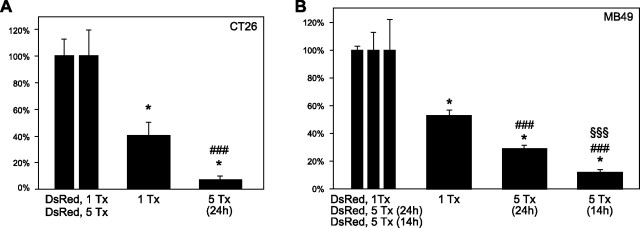
Although the combination of caspases 3 and 9 could effectively induce apoptosis in 48–60% of CT26.CL25 and MB49 cells after one transfection, the treatment regimen was further optimized to include repeated gene deliveries. Transfections with caspases 3 plus 9, a combination determined to be effective in our previous work (52), were administered to the two cell lines every 24 h for a total of 5 treatments. For the CT26.CL25 cell line, results revealed a dramatic reduction in cell viability from 40.51% to 6.98% as measured by FACS (Fig. 5A). For the MB49 cell line, cell viability was reduced from 52.56% to 28.87% (Fig. 5B). The difference in survival rates can be explained partially by the difference in doubling times between the two cell lines. With a doubling time of 22.2 h, the CT26.CL25 cells were being treated roughly once per cell doubling. However, having a doubling time of 13.3 h implies the MB49 cells were doubling almost twice between successive treatments, which would increase the number of cells that survived the treatment regimen. Holding the treatment frequency of 24 h for the CT26.CL25 cells as a standard, transfections occurred roughly once every 1.08 cell cycles. For a better comparison, the time between treatments for the MB49 cells would have to be adjusted to (1.08 cell cycles) × (13.3 h/cell cycle) = 14.36 h for a better comparison. A treatment regimen of 5 treatments, occurring every 14 h, was therefore adopted for the MB49 cells, which yielded a survival rate of 11.88% (Fig. 5B).
Figure 5.
Cell survival following 1 or 5 treatments with Cox-2-driven genes encoding inducible forms of caspases 3 and 9 in MB49 cells (A) and CT26.CL25 cells (B). All cell survival rates were normalized to control transfections of CMV-driven DsRed reporter plasmids utilizing the same transfection schedule. Number of treatments (Tx) is shown below each bar. When multiple treatments were administered, time (h) between treatments is shown in parentheses. Control transfections are shown together at left as a reference regarding sd. Error bars = 1 sd. *P < 0.025 vs. control transfections (n≥3); ###P<0.001 vs. Tx (n≥3); §§§P < 0.001 vs. 5 Tx (24 h; n≥3).
A predictive model for cell numbers, taking into account the cell cycle and expression-targeted transfection efficiency (which takes into account endogenous gene expression levels) was next derived as follows: Assuming exponential growth, the number of cells in culture at time t can be represented by y = AeBt. Given values for A and B, to predict the number of cells in culture at time t one needs only to plug a value for t into this equation. However, if certain discrete events that kill a percentage of the cells occur during the culture process, the model must be changed accordingly.
Suppose we are going to use expression-targeted gene therapy to deliver genes that code for proteins that will bring about cell death through apoptosis. Apoptotic cell death is important here because a specific molecule signaling death in one cell will not affect neighboring cells. Because the premise of expression targeting is that cells must express a related endogenous product, the promoter for which serves as the driving force behind transcription of the delivered gene, the percentage of cells in a culture that express a sufficient amount of the endogenous gene using the same promoter is important. For example, consider expression targeting that utilizes the Cox-2-promoter to direct the expression of delivered genes to Cox-2-overexpressing cells (such as carcinoma cells).
Before the first treatment, there will be y cells in the culture, (αy) expressing Cox-2, and (1 − α)y not expressing Cox-2.
If we let ε = the transfection efficiency of a transfection system that utilizes a given gene delivery vehicle with a specific promoter-exon combination to transfect a specific cell type, then after the first treatment we can predict that there will be [(1 − ε) · (αy)] + [(1 − α) · y] surviving cells. After the first treatment, the total number of cells can be treated as having 2 terms: one represents cells not expressing the gene of interest, which will grow exponentially according to negy = negAeBt; and one represents cells expressing the gene of interest, which will also grow exponentially according to posyn = posy(n–1) · eBt, then be reset by a factor of ε to give posyn = (1 − ε)posy(n–1) · eBt. This procedure can be performed for repeated transfections to yield a predicted number of surviving cells in culture following the treatment regimen (assuming exponential growth for each subset of cells over the entire treatment course).
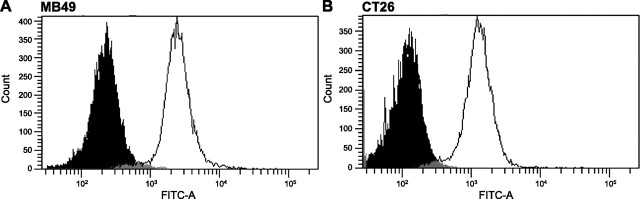
The above forecast method was used to predict the number of cells that would be in culture following repeated transfections with Cox-2-driven caspases 3 and 9, and compared to cells counted following such treatments. The value for α was found to be equal to 1 based on the fixation and labeling of untransfected cells with anti-Cox-2 antibodies followed by FACS analysis (Fig. 6). The values of A and B were determined via empirical cell count data shown in Fig. 4. Finally, because transfection efficiencies are rather difficult to determine when delivering plasmids that code for apoptosis-inducing genes, the expression-targeted transfection efficiencies for each cell type were determined using Cox-2-driven reporter genes.
Figure 6.
FACS data to detect the presence of anti-Cox-2 fluorescent antibody in MB49 cells (A) and CT26 cells (B). Solid curves, unlabeled controls; open curves, labeled cells.
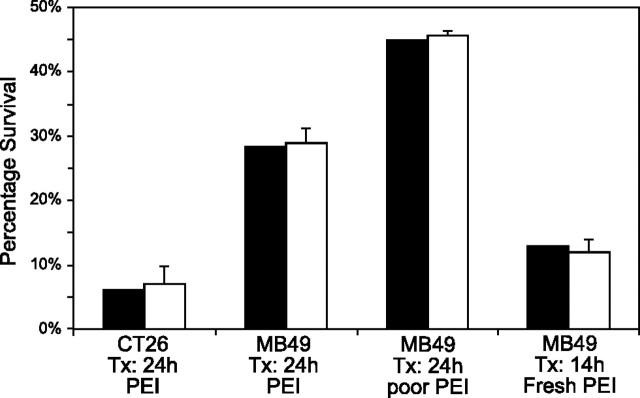
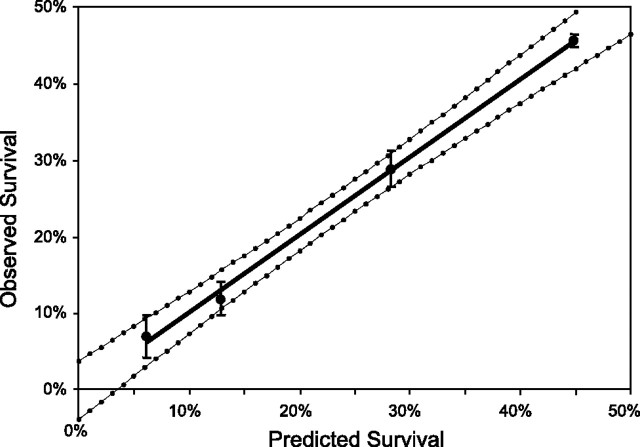
Comparisons between the predicted and observed numbers of surviving cells are shown in Fig. 7. Treatment groups were selected to be similar to those used for Fig. 5, with the addition of a group that used a suboptimal preparation of PEI (by design) to test the predictive nature of the described formula. Statistical significance was determined by plotting the observed vs. the predicted numbers of surviving cells and applying the t statistic for 95% confidence and 2 degrees of freedom to generate a function for the confidence interval (Fig. 8). From the figure, it can be seen that the regression line for the data points falls within the 95% confidence envelope, as does the origin.
Figure 7.
Predicted (solid bars) and observed (open bars) survival percentages following various treatments with Cox-2-driven genes encoding inducible forms of caspases 3 and 9. All observed data were normalized to control transfections of CMV-driven DsRed plasmids. Error bars = 1 sd (n≥3).
Figure 8.
Statistical analysis of observed vs. predicted survival data shown in Fig. 7. Dotted curves show the 95% confidence interval around the linear regression to the data (based on the t statistic, n=4, 2 degrees of freedom). Error bars = 1 sd.
DISCUSSION
Cox-2 is one of the isoforms of the cyclooxygenase enzyme, which acts as the catalyst in the conversion of arachidonic acid to prostaglandin H2 (4). Cox-2 overexpression has been demonstrated in a wide variety of carcinomas but not in unstressed, untransformed cells derived from the same tissues, which makes it a focus for cancer chemotherapy and targeted gene delivery (8–17). In the past, we have shown that the Cox-2 promoter can be used to target transgene expression to Cox-2-overexpressing cancer cells (51). The present study demonstrates a useful correlation between the expression of reporter genes under the control of the Cox-2 promoter and transcriptional activity associated with the expression of the endogenous Cox-2 gene. Consistent with our previous findings (51, 52), using the Cox-2 promoter for the delivery of caspase genes resulted in increased MB49 and MBT2 cell apoptosis in vitro and reduced MB49 tumor volumes in vivo. Even at a dose consisting of half the amount of Cox-2-iCasp9 DNA, tumor sizes in vivo were reduced significantly. These data showed that one can take advantage of the high Cox-2 promoter transcriptional activity to produce apoptosis in Cox-2 overexpressing tumor cells.
It should be noted that Cox-2 is overexpressed during the inflammatory process. This finding could imply that the delivery of transgenes directed by the Cox-2 promoter is to be discouraged for systemic gene therapies. However, low levels of endogenous Cox-2 expression are not enough to drive significant levels of Cox-2-directed transgene expression, as shown previously in human prostate epithelial cells and primary fibroblasts (51). More research is needed regarding the amount of inflammation that can be present in a tissue during Cox-2-directed gene therapy applications. Localized therapies present no difficulties, though, as no problems were observed during in vivo bladder transfections.
Her-2 is a transmembrane receptor protein that is overexpressed in several types of cancers, including one-third of primary breast carcinomas (37). The Her-2 data presented in Fig. 1B revealed that the transcriptional activity of transgenes directed by the Her-2 promoter correlate with the transcriptional activity of the endogenous Her-2 promoter. On the surface, this finding may seem to indicate that delivery of Her-2-directed anticancer genes could be an effective treatment for Her-2/Neu-positive breast cancers, but this is not the case. Transfectability of the BT474 and SkBr3 breast cancer types tested was low, even with CMV-driven plasmids. In addition, the slope of the regression line related to the Her-2 data (1×10−2, Fig. 1B) was much less than that for the Cox-2 data (0.0812, Fig. 1A).
The low slope is not surprising in light of the mechanism of Her-2 up-regulation: rather than increased Her-2 production resulting from increased expression of transcriptional proteins specific to Her-2 promoters/enhancers, the up-regulation is due to an excess of copies of the Her-2 promoter (gene amplification) (55). One might therefore predict that the expression of Her-2-driven transgenes (containing one copy of the Her-2 promoter per plasmid) would be approximately equal for all Her-2-positive cell types, regardless of the amount of endogenous Her2 being expressed. In consideration of the low transfectability of the Her-2-positive cells (as tested) and the low slope of the Her-2-directed transgene expression vs. endogenous Her-2 mRNA regression line, Her-2-directed gene delivery was not tested in vivo, and is not indicated for treatment of Her-2/Neu-positive breast cancers.
Survivin is the smallest member of the mammalian IAP gene family and may play important roles in the survival of cancer cells and malignance progression (19–21). Transcriptional activities of cell-specific promoters are typically expected to correlate with the level of expression of the corresponding endogenous genes, as in the case of the Cox-2 promoter. However, the survivin data presented herein revealed a lack of correlation between survivin-promoter-driven GFP expression and endogenous survivin mRNA levels. This difference may be due to epigenetic regulation of the promoter as has been suggested by a number of studies (56–59). One such investigation reported that methylation of the survivin promoter in endometrial tissue will prevent binding of p53, a tumor suppressor protein. This lack of binding to the promoter leads to a higher expression of survivin and is correlated positively with carcinogenesis (56). Another investigation found that methylation of the promoter and interactions with p53 played a role in survivin gene repression in human colorectal carcinomas (58). The dichotomy of effects regarding promoter methylation indicates that methylation patterns of the survivin promoter (or the effects of such methylation) may be tissue specific. Until more information is gathered regarding the methylation patterns of the survivin promoter for specific cancers, survivin-directed gene delivery is not indicated for treatment of survivin-positive cancers.
In summary, we have demonstrated that a correlation exists between the expression of genes under the control of the Cox-2 promoter and transcriptional activity associated with the endogenous Cox-2 gene. We have also developed a model that, when such a correlation exists, can be used to predict the efficacy of expression-targeted delivery of proapoptotic genes to cancer cells. The predictive power of the model demonstrates that cell cycle should be taken into account when determining a treatment regimen, and that the transfection potential of the gene carrier is also important. Finally, in terms of selecting a promoter/enhancer for expression-targeted gene delivery, it is not enough to perform a microarray on some tumor tissue and select the control element associated with the greatest amount of gene up-regulation vs. normal controls. The mechanism of expression for the particular gene should be taken into account to prevent lengthy and costly research trials.
Acknowledgments
The authors thank Dr. John C. Prindle, Jr. (Tulane University), for assistance with statistical analyses. The authors thank Ariad (Cambridge, MA, USA; http://www.ariad.com/regulationkits) for providing the AP20187.
This work was funded by a National Science Foundation CAREER award (CBET-0846395) and the Department of Comparative Medicine, Tulane University. The authors declare no conflicts of interest associated with these investigations.
REFERENCES
- 1.Nettelbeck D. M., Jerome V., Muller R. (2000) Gene therapy: designer promoters for tumour targeting. Trends Genet. , 174–181 [DOI] [PubMed] [Google Scholar]
- 2.Dachs G. U., Dougherty G. J., Stratford I. J., Chaplin D. J. (1997) Targeting gene therapy to cancer: a review. Oncol. Res. , 313–325 [PubMed] [Google Scholar]
- 3.Rosenberg S. A., Aebersold P., Cornetta K., Kasid A., Morgan R. A., Moen R., Karson E. M., Lotze M. T., Yang J. C., Topalian S. L., Merino M. J., Culver K., Miller A. D., Blaese R. M., Anderson W. F. (1990) Gene transfer into humans–immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N. Engl. J. Med. , 570–578 [DOI] [PubMed] [Google Scholar]
- 4.Simmons D. L., Botting R. M., Hla T. (2004) Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. , 387–437 [DOI] [PubMed] [Google Scholar]
- 5.Howe L. R., Subbaramaiah K., Brown A. M., Dannenberg A. J. (2001) Cyclooxygenase-2: a target for the prevention and treatment of breast cancer Endocr. Relat. Cancer , 97–114 [DOI] [PubMed] [Google Scholar]
- 6.Trifan O. C., Hla T. (2003) Cyclooxygenase-2 modulates cellular growth and promotes tumorigenesis. J. Cell. Mol. Med. , 207–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chell S., Kaidi A., Williams A. C., Paraskeva C. (2006) Mediators of PGE2 synthesis and signaling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochim. Biophys. Acta , 104–119 [DOI] [PubMed] [Google Scholar]
- 8.Shamma A., Yamamoto H., Doki Y., Okami J., Kondo M., Fujiwara Y, Yano M., Inoue M., Matsuura N., Shiozaki H., Monden M. (2000) Up-regulation of cyclooxygenase-2 in squamous carcinogenesis of the esophagus. Clin. Cancer Res. , 1229–1238 [PubMed] [Google Scholar]
- 9.Rajnakova A., Moochhala S., Goh P. M., Ngoi S. (2001) Expression of nitric oxide synthase, cyclooxygenase, and p53 in different stages of human gastric cancer. Cancer Lett. , 177–185 [DOI] [PubMed] [Google Scholar]
- 10.Ristimaki A., Honkanen N., Jankala H., Sipponen P., Harkonen M. (1997) Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. , 1276–1280 [PubMed] [Google Scholar]
- 11.Ferrandez A., Prescott S., Burt R. W. (2003) COX-2 and colorectal cancer. Curr. Pharm. Des. , 2229–2251 [DOI] [PubMed] [Google Scholar]
- 12.Kargman S. L., O'Neill G. P., Vickers P. J., Evans J. F., Mancini J. A., Jothy S. (1995) Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res. , 2556–2559 [PubMed] [Google Scholar]
- 13.Kutchera W., Jones D. A., Matsunami N., Groden J., McIntyre T. M., Zimmerman G.A., White R. L., Prescott S. M. (1996) Prostaglandin H synthase 2 is expressed abnormally in human colon cancer: evidence for a transcriptional effect. Proc. Natl. Acad. Sci. U. S. A. , 4816–4820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bostrom P. J., Aaltonen V., Soderstrom K. O., Uotila P., Laato M. (2001) Expression of cyclooxygenase-1 and -2 in urinary bladder carcinomas in vivo and in vitro and prostaglandin E2 synthesis in cultured bladder cancer cells. Pathology , 469–474 [DOI] [PubMed] [Google Scholar]
- 15.Mohammed S. I., Knapp D. W., Bostwick D. G., Foster R. S., Khan K. N., Masferrer J. L., Woerner B. M., Snyder P. W., Koki A. T. (1999) Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res. , 5647–5650 [PubMed] [Google Scholar]
- 16.Shirahama T., Sakakura C. (2001) Overexpression of cyclooxygenase-2 in squamous cell carcinoma of the urinary bladder. Clin. Cancer Res. , 558–561 [PubMed] [Google Scholar]
- 17.Yoshimura R., Sano H., Mitsuhashi M., Kohno M., Chargui J., Wada S. (2001) Expression of cyclooxygenase-2 in patients with bladder carcinoma. J. Urol. , 1468–1472 [PubMed] [Google Scholar]
- 18.Ghosh N., Chaki R., Mandal V., Mandal S. (2010) COX-2 as a target for cancer chemotherapy. Pharmacol. Rep. , 233–244 [DOI] [PubMed] [Google Scholar]
- 19.Ambrosini G., Adida C., Altieri D. (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. , 917–921 [DOI] [PubMed] [Google Scholar]
- 20.Altieri D. (2003) Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene , 8581–8589 [DOI] [PubMed] [Google Scholar]
- 21.Ambrosini G., Adida C., Sirugo G., Altieri D. (1998) Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J. Biol. Chem. , 11177–11182 [DOI] [PubMed] [Google Scholar]
- 22.Altieri D. C. (2003) Validating survivin as a cancer therapeutic target. Nat. Rev. Cancer , 46–54 [DOI] [PubMed] [Google Scholar]
- 23.Adida C., Crotty P. L., McGrath J., Berrebi D., Diebold J., Altieri D. C. (1998) Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am. J. Pathol. , 43–49 [PMC free article] [PubMed] [Google Scholar]
- 24.Swana H. S., Grossman D., Anthony J. N., Weiss R. M., Altieri D. C. (1999) Tumor content of the antiapoptosis molecule survivin and recurrence of bladder cancer. N. Engl. J. Med. , 452–453 [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki H., Altieri D. C., Lu C. D., Toyoda M., Tenjo T., Tanigawa N. (1998) Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. , 5071–5074 [PubMed] [Google Scholar]
- 26.Kato J., Kuwabara Y., Mitani M., Shinoda N., Sato A., Toyama T., Mitsui A., Nishiwaki T., Moriyama S., Kudo J., Fujii Y. (2001) Expression of survivin in esophageal cancer: correlation with the prognosis and response to chemotherapy. Int. J. Cancer , 92–95 [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K., Iwamoto S., Gon G., Nohara T., Iwamoto M., Tanigawa N. (2000) Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin. Cancer Res. , 127–134 [PubMed] [Google Scholar]
- 28.Grossman D., McNiff J. M., Li F., Altieri D. C. (1999) Expression and targeting of the apoptosis inhibitor, survivin, in human melanoma. J. Invest. Dermatol. , 1076–1081 [DOI] [PubMed] [Google Scholar]
- 29.McCrann D. J., Ravid K. (2010) Survivin localization during endomitosis of high ploidy mouse megakaryocytes. Blood , 2192–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan B., O'Donovan N., Duffy M. (2009) Survivin: A new target for anti-cancer therapy. Cancer Treat. Rev. , 553–562 [DOI] [PubMed] [Google Scholar]
- 31.Honma I., Kitamura H., Torigoe T., Takahashi A., Tanaka T., Sato N. (2009) Phase I clinical study of anti-apoptosis protein survivin-derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer Immunol. Immunother. , 1801–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikkawa K., Fujii R., Kuramoto T., Mori T., Inagaki T., Kohjimoto Y., Iwahashi M., Yamaue H., Hara I. (2009) Dendritic cells with transduced survivin gene induce specific cytotoxic T lymphocytes in human urologic cancer cell lines. J. Urol. , 222–228 [DOI] [PubMed] [Google Scholar]
- 33.Ma A., Huang W., Wu Z., Hu J., Li T., Zhou X., Wang Y. (2010) Induced epigenetic modifications of the promoter chromatin silence survivin and inhibit tumor growth. Biochem. Biophys. Res. Commun. , 592–597 [DOI] [PubMed] [Google Scholar]
- 34.Ku J. H., Seo S. Y., Kwak C., Kim H. H. (2010) Cytotoxicity and apoptosis by survivin small interfering RNA in bladder cancer cells. BJU Int. , 1812–1816 [DOI] [PubMed] [Google Scholar]
- 35.Shariat S. F., Ashfaq R., Karakiewicz P. I., Saeedi O., Sagalowsky A. I., Lotan Y. (2007) Survivin expression is associated with bladder cancer presence, stage, progression, and mortality. Cancer , 1106–1113 [DOI] [PubMed] [Google Scholar]
- 36.Margulis V., Lotan Y., Shariat S. F. (2008) Survivin: a promising biomarker for detection and prognosis of bladder cancer. World. J. Urol. , 59–65 [DOI] [PubMed] [Google Scholar]
- 37.Press M. F., Pike M. C., Chazin V. R., Hung G., Udove J. A., Markowicz M., Danyluk J., Godolphin W., Sliwkowski M., Akita R., Patterson M. C., Slamon D. J. (1993) Her-2/neu expression in node-negative breast cancer: direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res. , 4960–4970 [PubMed] [Google Scholar]
- 38.Benoit V., Relic B., Leval X., Chariot A., Merville M. P., Bours V. (2004) Regulation of HER-2 oncogene expression by cyclooxygenase-2 and prostaglandin E2. Oncogene , 1631–1635 [DOI] [PubMed] [Google Scholar]
- 39.Hung M. C., Zhang X., Yan D. H., Zhang H. Z., He G. P., Zhang T. Q., Shi D. R. (1992) Aberrant expression of the c-erbB-2/neu protooncogene in ovarian cancer. Cancer Lett. , 95–103 [DOI] [PubMed] [Google Scholar]
- 40.Ignatoski K. M., Maehama T., Markwart S. M., Dixon J. E., Livant D. L., Ethier S. P. (2000) ERBB-2 overexpression confers PI 3′ kinase-dependent invasion capacity on human mammary epithelial cells. Br. J. Cancer , 666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macrinici V., Romond E. (2010) Clinical updates on EGFR/HER-targeted agents in early-stage breast cancer. Clin. Breast Cancer (Suppl. 1), E38–E46 [DOI] [PubMed] [Google Scholar]
- 42.Wang G., Qiu J., Wang R., Krause A., Boyer J. L., Hackett N. R., Crystal R. G. (2010) Persistent expression of biologically active anti-HER2 antibody by AAVrh. 10-mediated gene transfer. Cancer Gene Ther. , 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang M., Shi W., Zhang Q., Wang X., Guo M., Cui Z., Su C., Yang Q., Li Y., Sham J., Liu X., Wu M., Qian Q. (2006) Gene therapy using adenovirus-mediated full-length anti-HER-2 antibody for HER-2 overexpression cancers. Clin. Cancer Res. , 6179–6185 [DOI] [PubMed] [Google Scholar]
- 44.Deshane J., Siegal G. P., Alvarez R. D., Wang M. H., Feng M., Cabrera G., Liu T., Kay M., Curiel D. T. (1995) Targeted tumor killing via an intracellular antibody against erbB-2. J. Clin. Invest. , 2980–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandha H. S., Martin L., Rigg A., Hurst H. C., Stamp G. W. H., Sikora K., Lemoine N. R. (1999) Genetic prodrug activation therapy for breast cancer: a phase I clinical trial of erbB-2-directed suicide gene expression. J. Clin. Oncol. , 2180–2189 [DOI] [PubMed] [Google Scholar]
- 46.Fletcher B. S., Kujubu D. A., Perrin D. M., Herschman H. R. (1992) Structure of the mitogen-inducible TIS10 gene and demonstration that the TIS10-encoded protein is a functional prostaglandin G/H synthase. J. Biol. Chem. , 4338–4344 [PubMed] [Google Scholar]
- 47.Zhang X., Atala A., Godbey W. T. (2008) Expression-targeted gene therapy for the treatment of transitional cell carcinoma. Cancer Gene Ther. , 543–552 [DOI] [PubMed] [Google Scholar]
- 48.Ishii S., Imamoto F., Yamanashi Y., Toyoshima K., Yamamoto T. (1987) Characterization of the promoter region of the human c-erbB-2 protooncogene. Proc. Natl. Acad. Sci. U. S. A. , 4374–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. , e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin L., Zeng H., Chien S., Otto K. G., Richard R. E., Emery D. W., Blau C. A. (2000) In vivo selection using a cell-growth switch. Nat. Genet. , 64–66 [DOI] [PubMed] [Google Scholar]
- 51.Godbey W. T., Atala A. (2003) Directed apoptosis in Cox-2-overexpressing cancer cells through expression-targeted gene delivery. Gene Ther. , 1519–1527 [DOI] [PubMed] [Google Scholar]
- 52.Zhang X., Turner C., Godbey W. T. (2009) Comparison of caspase genes for the induction of apoptosis following gene delivery. Mol. Biotechnol. , 236–246 [DOI] [PubMed] [Google Scholar]
- 53.Dobek G. L., Godbey W. T. (2011) An orthotopic model of murine bladder cancer. J. Vis. Exp. , 10.3791/2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Box G. E. P., Hunter W. G., Hunter J. S. (1978) Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building. John Wiley & Sons, New York [Google Scholar]
- 55.Hurst H. C. (2001) Update on HER-2 as a target for cancer therapy: the ERBB2 promoter and its exploitation for cancer treatment. Breast Cancer Res. , 395–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nabilsi N. H., Broaddus R. R., Loose D. S. (2009) DNA methylation inhibits p53-mediated survivin repression. Oncogene , 2046–2050 [DOI] [PubMed] [Google Scholar]
- 57.Hattori M., Sakamoto H., Satoh K., Yamamoto T. (2001) DNA demethylase is expressed in ovarian cancers and the expression correlates with demethylation of CpG sites in the promoter region of c-erbB-2 and survivin genes. Cancer Lett. , 155–164 [DOI] [PubMed] [Google Scholar]
- 58.Esteve P. O., Chin H. G., Pradhan S. (2005) Human maintenance DNA (cytosine-5)- methyltransferase and p53 modulate expression of p53-repressed promoters. Proc. Natl. Acad. Sci. U. S. A. , 1000–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hopfer O., Komor M., Koehler I. S., Schulze M., Hoelzer D., Thiel E., Hofmann W. K. (2007) DNA methylation profiling of myelodysplastic syndrome hematopoietic progenitor cells during in vitro lineage-specific differentiation. Exper. Hematol. , 712–723 [DOI] [PubMed] [Google Scholar]