Abstract
Airborne methicillin-resistant Staphylococcus aureus (MRSA) have previously been found on pig farms, which may lead to nasal deposition of MRSA in humans via inhalation. The anterior nares are the main niche for S. aureus, and S. aureus can cause, e.g. wound infection and pneumonia. The aim of this study was to acquire knowledge about the potential deposition of airborne MRSA, specifically, and of total S. aureus (including both methicillin-sensitive S. aureus and MRSA, in the following called S. aureus) in the different parts of the airways during occupancy on pig farms. Measurements of airborne MRSA and S. aureus were performed on four pig farms using a six and a three-stage sampler during different work tasks, such as high-pressure cleaning and everyday inspection. MRSA were quantified using MRSA-selective agar, and S. aureus were quantified using Staphylococcus selective agar. The identity of the bacteria were confirmed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. The geometric mean (GM) concentrations of MRSA and S. aureus were 447 cfu/m3 air and 1.8 × 103 cfu/m3 air, respectively. The highest concentrations of MRSA and S. aureus were found among pigs in a weaner stable and during high-pressure cleaning of an empty stable, respectively. The lowest concentrations of MRSA and S. aureus were found in a stable with sick pigs and in feed-storages, respectively. Most MRSA and S. aureus were associated with particles between 7 and 12 µm. On average, the particle size fractions potentially depositing in the upper airways constituted 70%, in the primary and secondary bronchi 22%, and in the terminal bronchi and alveoli 8% of the inhalable MRSA and S. aureus concentration. Across the sampled areas, the geometric mean diameter (Dg) of particles with MRSA and S. aureus were 7.2 and 6.4 µm, respectively, and no significant difference was found between these Dgs. The Dg of the airborne particles with the studied bacterium was significantly associated with the different locations on the farms. The largest Dgs were found in the air samples from the aisles and on the fence to the pens, while the smallest Dgs were found in samples from the pens among the pigs and in samples taken at greater distances from the pigs: in the hallway, feed-storage, and entry room. In conclusion, airborne MRSA and S. aureus were found in sample fractions potentially depositing in all six parts of the airways. However, the majority was found to potentially deposit in the upper airways. The concentration of airborne MRSA and S. aureus and MRSA, as well as the fraction potentially depositing in the different parts of the airways, depended on the specific work task being performed and the location on the farm.
Keywords: airway deposition, bioaerosol, dust, MRSA, occupational exposure, particle size, Staphylococcus aureus
Introduction
Airborne methicillin-resistant Staphylococcus aureus (MRSA) have previously been found in pig barns constituting 802 colony forming units (cfu)/m3 air [geometric mean value (GM)] (Friese et al., 2012), and recently, it has been shown that nasal deposition of MRSA on pig farms can occur via inhalation (Angen et al., 2017). The anterior nares are described as the main niche for S. aureus (both resistant and non-resistant to methicillin), and nasal carriage is one of the main risk factors for S. aureus infection (van Belkum et al., 2009). S. aureus is an opportunistic human pathogen (Aires de Sousa and Lencastre, 2004), which can cause, e.g. wound infections (Ruhlmann et al., 2008), and, mainly for weakened individuals, also pneumonia (Witte et al., 2007), thoracic empyema (Lozano et al., 2011), and bacteremia (Berning et al., 2015). The MRSA are of special concern because of their resistance to β-lactam antibiotics, which makes infections difficult to treat.
Exposure to MRSA on farms is an occupational health problem affecting people working on the farms (van Cleef et al., 2010; Geenen et al., 2013). Except for nasal deposition (Létourneau et al., 2010; Angen et al., 2017; Goerge et al., 2017), the knowledge of deposition or potential deposition of MRSA in the different parts of the airways of exposed workers on pig farms is very limited. Yet, this knowledge is important since the place of deposition affects the potential health effects caused by the bacteria. Laboratory generated aerosols of S. aureus have particle sizes between 0.542 and 1.197 µm and aerodynamic particle sizes between 0.723 and 0.777 µm (Lutz, 2010). However, airborne bacteria are often present in clusters and/or associated with other particles. Thus, the deposition of bacteria in the airways depends on the aerodynamic diameter of the particle with which they are associated, and consequently determines the potential site the bacterium may initially infect (Thomas, 2013). In pig houses in the USA, mesophilic bacteria and Staphylococcus spp. have mainly been found on particles larger than 4 µm; in contrast, a slightly larger fraction of the total amount of Lactobacillus spp. was found on particles smaller than 4 µm (Predicala et al., 2002). In the farm environment, we do not expect MRSA to be present only as individual bacterial cells, but also as clusters of bacteria or associated with other particles such as fragments of skin cells from the pigs. MRSA may be aerosolized into the air in the stable directly from the pigs, either from mucus or exfoliated epithelial skin particles (Zhao et al., 2014), but they may also be re-aerosolized from all surfaces in the stable, resulting in a broader range of MRSA particle sizes. Furthermore, differences regarding the size distributions of particles carrying microorganisms within a specific type of environment and even within the same facility may also exist (Clauss, 2015). Therefore, it is important to acquire knowledge of the size distribution of MRSA aerosolized during different work tasks in pig farms.
The aerodynamic diameter of a particle also influences how long time it stays airborne and thus also for how long the potential human exposure of the airways to the airborne bacterium occurs. Furthermore, the particle size influences the ability of the bacterium to penetrate filters of dust masks and to disperse between rooms or to the outside, e.g. through cracks. The Andersen sampler has been used for decades and in several studies to measure airborne bacteria to evaluate how they potentially may deposit in the airways (Beaumont et al., 1985; Buttner and Stetzenbach, 1991; Chang et al., 2001; Uhrbrand et al., 2017). The six-stage Andersen sampler (ASCI) samples airborne particles within six health-relevant size ranges.
In this study, we investigate the size distribution of airborne MRSA, quantified on MRSA-selective agar, and total S. aureus [methicillin-sensitive S. aureus (MSSA) and MRSA, in the following called S. aureus], quantified on S. aureus selective agar, on four pig farms in relation to deposition in the airways. The measurements were performed during miscellaneous work tasks, such as high-pressure cleaning of an empty stable, tail docking, and everyday inspection, and in different locations on the farms, including stables with pigs of different ages.
Materials and Methods
Farms and design
Airborne bacteria were sampled on four pig farms in autumn and winter in 2015–2017 in Jutland and Zealand in Denmark. The farms were specialized in breeding pigs of different stages, and due to concern about disease transmission between sections of the farms we were not allowed to visit all areas of the farms. Also, at least 21 days occurred between visits on the different farms. Farm A, was specialized in fattening pigs and measurements were done in the autumn, 2015, in this section during an everyday inspection, and in a feed-storage room connected by a closed door to a stable with fattening pigs. Measurements on Farm B were done in the winter, 2016, during two consecutive days in three locations: An entry room, a feed-storage room from where the feed was automatically transported into the stables but otherwise connected by a closed door, and a farrowing section where a farmer docked the tails of the piglets. On Farm C, measurements were done in the winter, 2016, during three days in three locations: A weaner and a farrowing section during an everyday inspection, and a hallway outside the weaner section. On Farm D, measurements were done in the winter, 2017, in two locations: A stable with sick pigs of different ages, which had been transferred from other sections, and an empty stable during high-pressure cleaning. In general, the most active pigs were the pigs in the weaner section, followed by pigs in the farrowing section, and then the fattening pigs while the most passive were the sick pigs. All measurements were done between 7 a.m. and 7 p.m. on days with outdoor temperatures between −2 and 11°C.
Sampling of airborne bacteria using the ASCI
In total, 77 air samples were taken using the ASCI (Thermo Fisher Scientific Inc., Waltham, MA, USA). The ASCI is an active size-selective sampler, which samples directly onto six agar plates with a flow rate of 28.3 lpm. Particles of the following sizes were sampled: Stage 1: 7.0–12 µm, Stage 2: 4.7–7.0 µm, Stage 3: 3.3–4.7 µm; Stage 4: 2.1–3.3 µm; Stage 5: 1.1–2.2 µm, and Stage 6: 0.65–1.1 µm. The sum of all stages represents the inhalable bacteria, and the sum of stages 3, 4, 5, and 6 represents the respirable bacteria, while the sum of stages 1 and 2 represents the fraction potentially depositing in the upper airways. The ASCI was mounted with Brilliance MRSA 2 agar plates (in the following called MRSA-agar; Oxoid) for sampling and quantification of MRSA or with SaSelect agar plate (in the following called SA-agar; Bio-RAD, Marnes-la-Coquette, France) for sampling and quantification of airborne S. aureus. The two agar media were chosen as we wanted to obtain knowledge about the concentrations of S. aureus present both as MRSA as well as total culturable S. aureus (which includes MRSA) within the different size fractions. For clinical isolates, MRSA-selective agar has shown a specificity of 94% (Verkade et al., 2011). In clinical samples, MRSA-selective agar media can underestimate the number of MRSA-positive samples (Veenemans et al., 2013). This may also be the case for the farm environment, and, thus, another reason to also include the SA-agar.
Sampling on MRSA-agar was done for 4–20 min and on SA-agar for 2–20 min. The detection limits depend on the duration of each sampling and were between 1.8 and 18 cfu/m3. Other bacteria also grew on both agar types, and all colonies of MRSA and S. aureus were identified by color and subsequently by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). On Farm B, measurements were done using only SA-agar as we knew from a previous study that MRSA only constituted a minor fraction of the S. aureus (Feld et al., 2018).
On farm A and D, samples were taken repeatedly, but with durations of 4, 10, and 20 min to study the effect of sampling time on the measured Dg of MRSA and S. aureus.
Farmers worked either in an upright position, slightly stooping, or stooping all the way down. Consequently, we measured in three heights: (i) Sampling at chest level (1.50 m) was done in the aisles, the feed-storage rooms, a windowsill, and a hallway, (ii) at approximately 1.0 m during high-pressure cleaning, and on the fence between the pen and the aisle, and (iii) 20 cm above floor level among the piglets (in the following called floor level).
Sampling using the DGI and the Respicon
A DGI-1570 Gravimetric Impactor (Dekati, Tampere, Finland) was used to sample particles according to size. Sampling was performed in the aisle once at Farms A, C, and D for 15 min at a height of 1.5 m. The sampler separates particles into four size fractions based on aerodynamic diameters with lower cut points of 0.264, 0.608, 1.200, and 2.968 μm when used at a flow rate of 50 lpm. In addition, particles < 0.264 μm is collected to the backup filter; that filter was not analyzed in this study. Airborne particles were sampled on polycarbonate filters (47 mm diameter, pore size 1 µm, Nuclepore, Whatman). The particles in the two smallest size fractions were pooled for analysis. The filters were extracted as described earlier (Madsen et al., 2014), and aliquots of 500 µl and 100 µl were plated on both SA-agar and MRSA-agar. The detection limit was 27 cfu/m3.
The Respicon (TSI Incorporated, MN, USA) sampled in the aisles for 3 h, at a height of 1.5 m, once at each farm; after 1 h of sampling, a sample was also collected using the ASCI for 10 min. The Respicon has been shown to meet the ACGIH/ISO/CEN particle size-selective sampling criteria (Koch et al., 2002; Tatum et al., 2002). Stage 1 of the device collects respirable particles and stage 2 collects tracheobronchial particles. Extrathoracic particles are collected on stage 3 of the Respicon. Therefore, particles collected with the Respicon sampler are categorized by the appropriate combination of the results from the three stages; thus, thoracic = respirable (stage 1) + tracheobronchial (stage 2), and inhalable = thoracic + extrathoracic (stage 3). The Respicon was fitted with Teflon filters and used at a flow rate of 3.1 lpm. The dust on the filters was extracted in 5.0 ml solution (0.85% NaCl and 0.05% Tween 80) by orbital shaking (500 rpm) for 15 min at room temperature, and aliquots of 500 µl and 100 µl were plated on four SA-agar and four MRSA-agar plates. The detection limit for each agar type was 3.6 cfu/m3.
Species identification by MALDI-TOF MS
To verify the species identity of bacterial colonies with specific morphology on the selective agar plates, we used MALDI-TOF MS. Isolates resembling MRSA and S. aureus were identified from MRSA-agar and SA-agar, respectively (Madsen et al., 2016). On SA plates with samples from Dekati Gravimetric Impactor (DGI) filters, all colonies were identified. A toothpick was used to transfer a small amount of the bacterial colony onto the target plate (MSP 96 target polished steel BC, Bruker Daltonics, Bremen, Germany). The sample was then overlaid with 70% formic acid and allowed to dry before addition of an HCCA matrix solution (α-cyano-4-hydroxycinnamic acid, Bruker Daltonics). The MALDI-TOF MS analysis was performed on a Microflex LT mass spectrometer (Bruker Daltonics) using the Bruker Biotyper 3.1 software with the BDAL standard library. A bacterial test standard (Bruker Daltonics) was used to calibrate the instrument.
In the following, bacteria identified as S. aureus on MRSA-agar are presented as MRSA, and bacteria identified as S. aureus on SA-agar are presented as S. aureus or total S. aureus, even though unknown quantities of the latter are MRSA. The MRSA on all farms were previously determined to be CC398 (Feld et al., 2018).
Treatment of data
SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for statistical analysis. The bacteria measured by the ASCI are presented in several different ways: As the Dg calculated from sizes and quantities; as concentrations (cfu/m3) of the six size fractions; as the percentage each fraction constitutes out of the total concentration; as the fraction depositing in the upper airways (Stages 1 and 2), in the primary and secondary bronchi (Stages 3 and 4), in the terminal bronchi and alveoli (Stages 5 and 6); and as respirable bacteria (Stages 3, 4, 5, and 6). Three samples on SA-agar from the empty stable were overgrown, and data from these samples are presented as minimum values in Fig. 1, but are excluded from the calculations of Dg and percentage each fraction constitute. The data on the percentages, each fraction constituted, were normally distributed. The average, as well as the standard deviation (SD), was calculated from the fraction percentages.
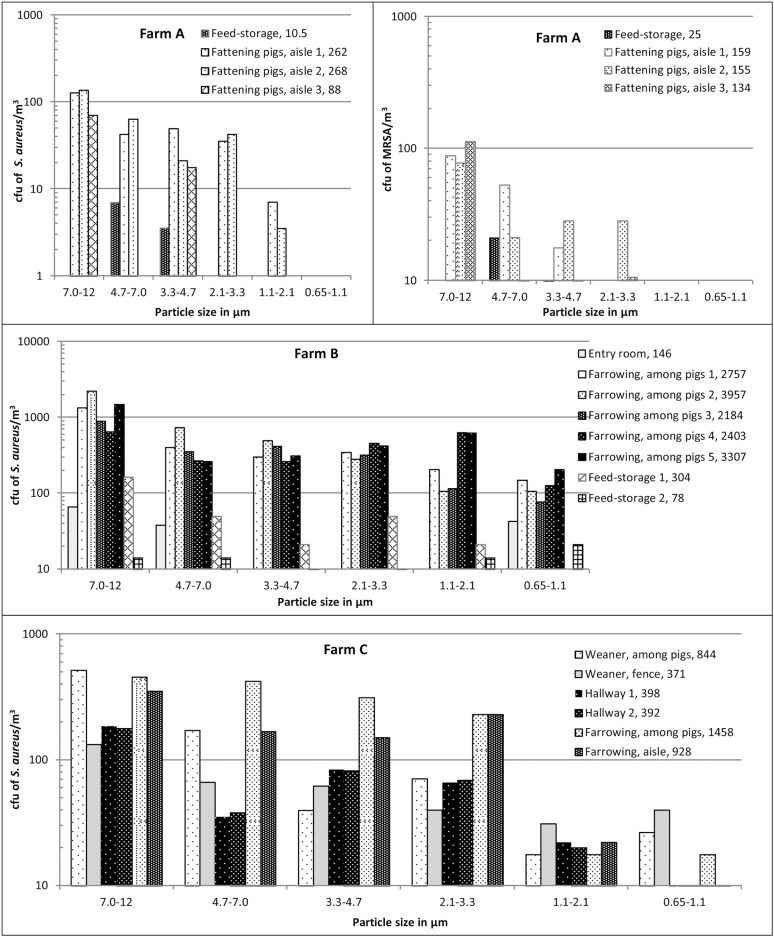
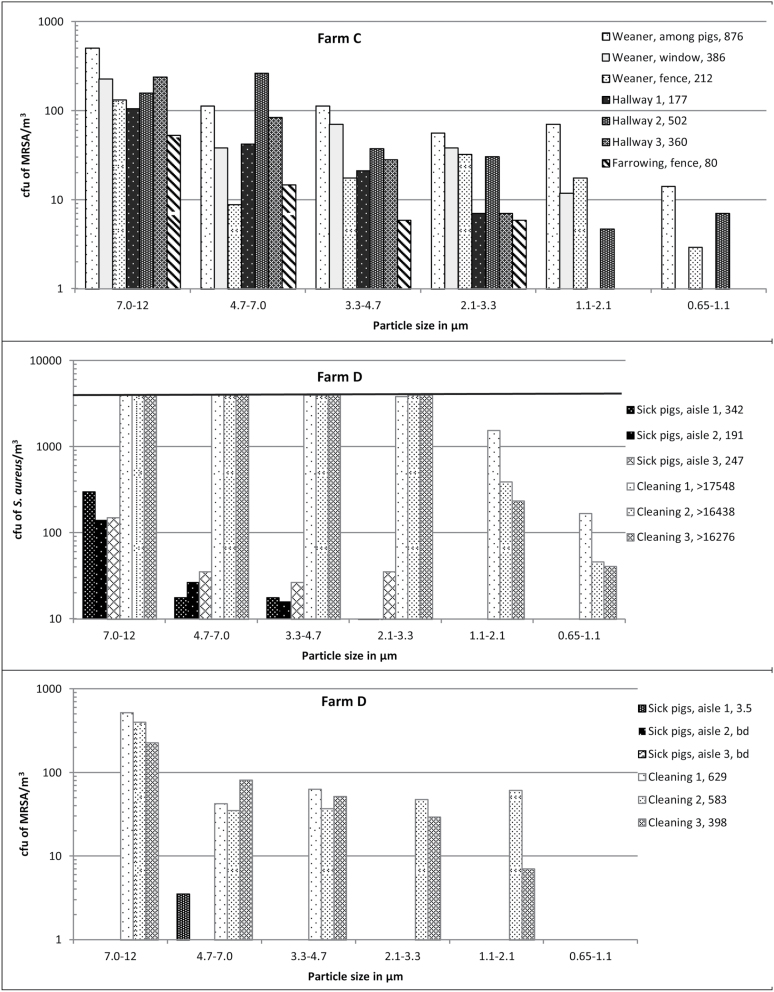
Figure 1.
The concentration of S. aureus (both methicillin-sensitive S. aureus and MRSA) and MRSA as a function of particle size in different areas and during different work tasks on pig farms A to D. On Farm A, measurements were done in a stable with fattening pigs and in a feed-storage next to that stable. On Farm B, measurements were done in an entry room next to the door to the outside, in the farrowing section among the piglets, and in a feed-storage next to the stable. On Farm C, in the windowsill in a weaner section, on the fence between the pig-pen and the aisle in the weaner and farrowing section, in the hallway outside the weaner section, in the farrowing section among the pigs, and in the aisle. On Farm D, in an empty stable during high-pressure cleaning and in a stable with sick pigs of different ages. The last numbers in the legends are the sums (cfu/m3) of the cfu of S. aureus and MRSA in the six size fractions. Where no bars are present, measurements were below the detection level. The solid black line in the S. aureus figure for Farm D illustrates the highest measurable level, and thus 12 measurements were above this level.
The effect of sampling duration (4, 10, and 20 min) on the measured Dg of particles with S. aureus and MRSA was compared for 3 × 4 samples using General Linear Models (GLM). The effect of farm and sampling day on Dg of particles with S. aureus and MRSA as measured among pigs was studied using GLM.
The Dgs of airborne S. aureus and MRSA in different locations were compared across farms. To do this, the sampled locations were divided into the following categories (where n = number of Dgs of total S. aureus + number of Dgs of MRSA): (i) the pen, among piglets, floor level, n = 8 + 4; (ii) the pen fence, n = 8 + 9; (iii), in the aisle between the pens, n = 16 + 14; (iv) in an empty stable during high-pressure cleaning, n = 3 (MRSA only); (v) hallway, between weaner sections, n = 3 + 2; (vi) in the feed-storages and entry room, n = 1 + 4. In total, 72 Dgs were used for the comparison. The Dgs were compared using GLM. Subsequently, some locations were pooled further into three groups based on the Dgs, and using GLM, each size fraction of S. aureus and MRSA as sampled by the ASCI was compared across the groups.
The bacteria measured by the Respicon are presented as the concentrations (cfu/m3) of the three size fractions and as the percentage, each fraction constitutes out of the total concentration. The concentrations and the size fractions (%) of respirable MRSA and S. aureus as measured using the Respicon and the ASCI were compared using a paired t-test. The Pearson’s correlations between the concentrations and the size fractions as measured using the Respicon and the ASCI were calculated. Only values above the detection limits are included in the calculations.
Results
Initial sampling using three samplers
The concentrations as measured using the DGI on farms A, C, and D showed no presence of S. aureus and MRSA, but other Staphylococcus species, S. epidermidis, S. haemolyticus, S. chromogenes, S. simulans, and S. warneri, were present. Most bacteria (80–86%) were found in the fraction with the largest particles (cut point 2.968 μm).
The concentrations as measured using the Respicon and ASCI showed presence of S. aureus on all farms (Table 1). The ASCI and the Respicon sampled in parallel in the same area and at the same time; the Respicon, however, sampled for 3 h, while the ASCI sampled for 10 min. The respirable (P = 0.22, n = 6) and inhalable (P = 0.62, n = 6) concentrations and the respirable fractions (P = 0.82, n = 6) of S. aureus and MRSA were not significantly affected by sampling method. The concentrations (r = 0.99, P < 0.0001, n = 6) and fractions (r = 0.87, P = 0.024, n = 6) of respirable S. aureus and MRSA as sampled by the Respicon correlated significantly with concentrations and fractions obtained by the ASCI. The same was found for the inhalable concentrations (P < 0.0001).
Table 1.
Concentrations (CFU/m3 and percentagea) of inhalable, thoracic, and respirable S. aureus and MRSA as measured using the Respicon, and inhalable and respirable S. aureus and MRSA as measured using the ASCI in the same area in the aisles.
| S. aureusb | MRSA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Respicon | ASCI | Respicon | ASCI | ||||||||
| Farm | Place | Inhalable | Thoracic | Respirable | Inhalable | Respirable | Inhalable | Thoracic | Respirable | Inhalable | Respirable |
| A | Fattening pigs | 227 | 113 46% |
91 37% |
246 | 80 30% |
219 | 95 43% |
61 28% |
266 | 88 33% |
| B | Farrowing | 2.6 × 103 | 850 33% |
732 29% |
2.9 × 103 | 990 34% |
100 | 34 31% |
29 27% |
Nm | Nm |
| C | Weaner | 327 | 194 59% |
138 42% |
441 | 172 45% |
202 | 113 56% |
93 46% |
151 | 85 41% |
| D | Sick pigs | 211 | 62 32% |
32 17% |
188 | 43 19% |
16 | 5.6 36% |
3.6 23% |
Bd | Bd |
aPercentage out of total inhalable S. aureus or MRSA.
bBoth methicillin-sensitive S. aureus and MRSA.
nm = not measured, ASCI = Andersen six-stage sampler, bd = below the detection limit, nm = not measured.
Sampling time did not affect the measured Dg of particles with S. aureus and MRSA as sampled using the ASCI (P = 0.42). Thus, the average Dg for a 4-min sampling was 7.9 µm (SD = 0.50 µm, n = 4), for 10 min 7.3 µm (SD = 0.88 µm, n = 4) and for 20 min 7.7 µm (SD = 0.63 µm, n = 4). Consequently, data obtained using the ASCI but with different sampling durations are treated together.
Concentrations of airborne S. aureus and MRSA as measured using the ASCI
Airborne S. aureus were found in all 44 measurements with the ASCI on the four farms while MRSA were detected in 31 of 33 measurements on three farms. On Farm B, we did not measure MRSA with the ASCI as previous measurements showed a very low concentration of MRSA.
In Fig. 1, concentrations of S. aureus and MRSA are presented for one sampling day at each farm. The GM concentration of inhalable S. aureus and MRSA were 1.8 × 103 cfu/m3 air (average = 5.1 × 103 cfu/m3; range: 77 to > 1.7 × 104 cfu/m3; n = 44) and 447 cfu/m3 air (average = 1.1 × 103 cfu/m3; range: below detection level (bd) to 6.6 × 103 cfu/m3; n = 33), respectively. The highest concentrations of S. aureus and MRSA were found during high-pressure cleaning of an empty stable and among pigs in a weaner stable, respectively. The lowest concentrations were found for S. aureus in feed-storages (average = 131 cfu/m3; SD = 154 cfu/m3; n = 3). For MRSA, the lowest concentration was found in a stable with sick pigs (average = 1.2 cfu/m3; SD = 1.7 cfu/m3; n = 3). In a farrowing section where pigs were being tail-docked, an average concentration of 3.0 × 103 cfu S. aureus/m3 (SD = 642 cfu/m3; n = 5) was found. The concentration of respirable S. aureus and MRSA were between 17.7 and > 9.5 × 103 cfu/m3 and bd and 975 cfu/m3, respectively. As an example, the average concentration of respirable S. aureus in the feed-storages was 48 cfu/m3 (SD = 44 cfu/m3; n = 3).
Size distribution of airborne MRSA and S. aureus as measured using the ASCI
Most airborne S. aureus and MRSA were associated with particles between 7 and 12 µm (Fig. 1). Across the six areas, the GM of the Dgs for S. aureus and MRSA were 6.4 and 7.2 µm, respectively, and no significant difference was found between Dgs for S. aureus and MRSA (P = 0.78). The Dg was measured among pigs in the farrowing section five times at farm B and two times at farm C, and no effect of farm was found (P = 0.30). The Dg was measured on farm C during 2 days in the weaner section among the pigs (P = 0.67; 7 samples), during 3 days in the aisle (P = 0.83; 18 samples), and with the ASCI placed on the pen fence (P = 0.99; 14 samples) and no significant effect of sampling day was found.
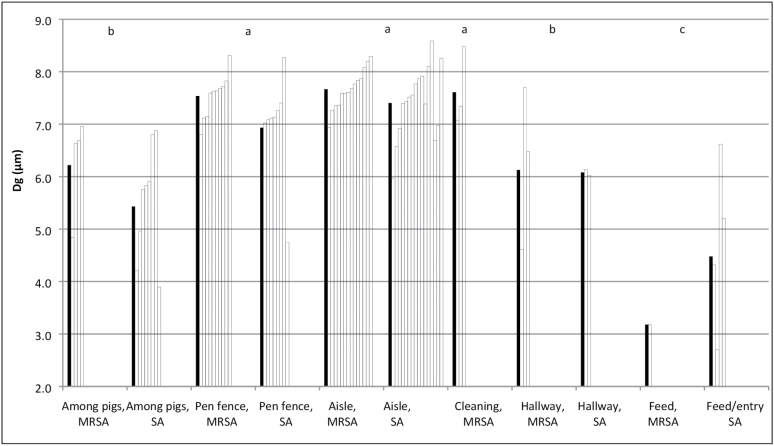
When all data for Dgs of MRSA and S. aureus were studied together in one model, the Dg was not affected by date of sampling (P = 0.92), but by location on the farms (P = 0.0018). The largest Dgs were found in the samples from the aisle, the fence to the pens, and during high pressure cleaning, while the smallest Dgs were found in the samples from the pens among the pigs and samples taken at greater distances from the pigs, such as in the hallway, feed-storage, and entry room (Fig. 2).
Figure 2.
Geometric mean diameter (Dg) of airborne MRSA and S. aureus (SA) in six areas (among pigs, floor level, n = 4 + 8; pen fence, on the fence separating the pens from the aisles, 1.0 m above the floor, n = 9 + 8; Aisle, in the aisle between the pens, 1.5 m above floor, n = 14 + 16; Cleaning, in an empty stable during high-pressure cleaning, MRSA only, n = 3; hallway, between stable sections, 1.5 m above the floor, n = 3 + 2; feed/entry, in the feed-storages and entry room, 1.5 m above floor, n = 1 + 4). The white bars are individual measurements and the black bars are the geometric mean values. Bars with the same letters are not statistically different, analyzed in GLM and the data for Dg of MRSA and SA are considered together.
On average, the fractions of S. aureus and MRSA potentially depositing in the upper airways constituted 70% (SD = 15%; max = 93%), in the primary and secondary bronchi 22% (SD = 12%; max = 41%), and in the terminal bronchi and alveoli 8% (SD = 11%; max = 45%) of the concentration of inhalable S. aureus and MRSA. For the particles depositing in the nose, the fraction of S. aureus and MRSA present in the samples from the aisle and from the fence to the pens was greater than in the samples from inside the pens among the pigs and the samples from the hallway, feed-storage, and entry room considered together. The opposite was seen for the fractions potentially depositing in the pharynx and alveoli (Table 2).
Table 2.
Average fractions (% of total) of MRSA and S. aureus particle size ranges delimited by their potential site of deposition in the six parts of the airways as measured in selected locations on the four farms.
| Aisle and pen fence n = 47 | Among pigs n = 12 | Feed-storage, entry, and hallway n = 10 | P-value** | |
|---|---|---|---|---|
| 7.0–12 µm Nasal cavity |
62.3 a* | 45.4 b | 42.7 b | 0.0047 |
| 4.7–7.0 µm Pharynx |
14.2 b | 19.7 ab | 30.8 a | 0.0097 |
| 3.3–4.7 µm Trachea and primary bronchi |
11.3 a | 13.4 a | 11.8 a | 0.85 |
| 2.1–3.3 µm Secondary bronchi |
8.1 ab | 11.4 a | 6.7 b | 0.16 |
| 1.2–2.1 µm Terminal bronchi |
2.1 b | 7.0 a | 3.4 b | 0.12 |
| 0.65–1.2 µm Alveoli |
1.2 b | 3.2 a | 4.9 a | 0.0067 |
*Numbers in the same row followed by the same letter are not statistically significantly different.
**P-value for the effect of location as measured using GLM.
Discussion
This study shows that airborne MRSA and S. aureus are present on particles with aerodynamic diameters that can be deposited in the human upper airways, the primary, secondary, and terminal bronchi, and the alveoli. S. aureus and MRSA were measured as culturable bacteria, as only viable bacteria are able to colonize and infect the airways. The fact that the largest fractions potentially deposit in the upper airways is interesting since the anterior nares are the main niche for S. aureus, and persistent nasal carriage is a main risk factor for S. aureus infection (Wertheim et al., 2004; van Belkum et al., 2009). It is simple to test for MRSA-carriage in the upper airways, and it has become almost standard to test for presence of MRSA in the upper airways in research studies in occupational settings such as farms (Létourneau et al., 2010; Angen et al., 2017), in population surveys (Abudu et al., 2001), as well as before surgery (Rohr et al., 2004; Matheson et al., 2012). The observation, that the largest fractions of S. aureus and MRSA deposit in the upper airways, is in accordance with what has been found for MRSA in a pig farm (Ferguson et al., 2016), and for culturable, mesophilic bacteria in livestock husbandry (Clauss, 2015). In a Chinese hen house, S. aureus was mainly carried on particles of 2.1–3.2 and 0.6–1.0 µm (reviewed by Clauss, 2015).
On average, 21.5% of the airborne S. aureus and MRSA were of the size potentially depositing in the primary and secondary bronchi. It has been suggested that the bronchi can act as a reservoir of S. aureus which may in time cause pneumonia (Kaye et al., 1990; Bartlett et al., 2000), and it has been shown that nasal and bronchial strains of S. aureus in infected patients usually are identical (Corne et al., 2005). In this study, the concentration of S. aureus potentially depositing in the primary and secondary bronchi exceeded 8 × 103 cfu/m3 during high-pressure cleaning. This means that if a farmer inhales 1.1 m3 air/h during high-pressure cleaning for 4 h without wearing respiratory protection, > 3.6 × 104 cfu S. aureus may deposit in the bronchi. The observation that 22 % of the airborne MRSA and total S. aureus potentially deposit in the bronchi, is also interesting in the relation to how this would affect the microbiota in the bronchi, especially in light of a recent study indicating that pig farming is associated with a special nose microbiota (Kraemer et al., 2018).
We have found no study investigating whether exposure to airborne S. aureus or MRSA of alveolar size can be associated with development of pneumonia. However, it is important to be aware that the bacteria may deposit in other and deeper places in the airways than the nasopharyngeal area. Furthermore, though they may be rare, cases of pneumonia caused by LA-MRSA (Witte et al., 2007; Hartmeyer et al., 2010) and community associated-MRSA (Rubinstein et al., 2008) have been reported. The concentration of airborne S. aureus on particles smaller than 1.1 µm was up to 200 cfu/m3; this level was found in the stable during tail docking. If a farmer with no respiratory protection inhales 1.1 m3 air/h and works for 4 h in the stable under these conditions, 870 cfu S. aureus may potentially deposit in the alveoli. When considering Stages 5 and 6 sampled by the ASCI together, the highest concentration was found during high-pressure cleaning of the empty stable. We found that high-pressure cleaning can cause a concentration of 1.6 × 103 cfu/m3 air, corresponding to a potential deposition of 7.0 × 103 cfu S. aureus in the terminal bronchi and alveoli during 4 h work activity without airway protection.
The highest concentrations of total inhalable MRSA were also found in the empty stable during high-pressure cleaning. A review study concludes that very high exposure to bioaerosols is found during high-pressure cleaning on farms and that the material being cleaned, as well as the degree of dirtiness, highly influences the exposure level (Madsen and Matthiesen, 2013). We have found no other studies measuring concentrations of MRSA during high-pressure cleaning, or any measuring concentrations of MRSA as high as in the present study. The concentration of airborne S. aureus and MRSA was also high in the farrowing section where pigs were having their tails docked. However, this high concentration may partly be caused by a low ventilation rate due to the low outdoor temperature that day (−2°C, data not shown), rather than to the work activity. All the measurements in this study were performed in the winter and autumn on days with outdoor temperatures between −2 and 11°C. If the study had been performed in the summertime, lower concentrations might have been found since seasonal variations in dust concentrations are documented; the highest concentrations are found in the winter, likely due to a lower ventilation rate in the colder weather (Duchaine et al., 2000; O’Shaughnessy et al., 2009; Basinas et al., 2013)— which thus might also have affected the S. aureus and MRSA concentration found in this study. In addition, the activity level as well as the density and sizes of pigs might also have affected the exposure levels to S. aureus and MRSA as these factors seem to affect the dust exposure level in pig farms (Gustafsson, 1999). In this study, the exposure to inhalable S. aureus was higher in the farrowing sections than in the other sections with pigs.
Even in areas where the presence of S. aureus was not expected, such as in the entry rooms and feed-storages, S. aureus was found. Thus, the concentration of airborne, respirable S. aureus in the feed-storage was 48 cfu/m3, which is at the level found in public buildings in China (Li et al., 2015). The presence of S. aureus and MRSA in these, or for MRSA some of these, areas is of importance in relation to further transmission of the bacteria out of the stable—especially in the light of an average 99.9% die-off rate of 66 days for S. aureus and MRSA present in sampled pig farm dust (Feld et al., 2018). In entry rooms and feed-storages, S. aureus and MRSA particles had a smaller Dg than in, e.g. the aisle, which may allow them to stay airborne for a longer time and to pass through small cracks, e.g. between rooms.
Based on our findings in this and previous studies, we find it reasonable to suggest the use of personal dust masks to protect workers in the stable but also to reduce the risk of transmitting the bacterium to other people. This is because nasal MRSA carriage is associated with exposure level to airborne MRSA rather than to direct physical transfer between the hands and the face (Angen et al., 2017), and the concentrations of viable, inhalable, airborne MRSA on pig farms are very high. Coupled with the observation that 94% of 94 volunteers with no respiratory protection were MRSA-positive after 1 h of occupancy in an MRSA-positive stable and 53% still carried MRSA 1 h after leaving the stable (Angen et al., 2017), dust masks provide easily accessible and low-cost protection of workers. Personal protection by dust masks is described as an option to minimize farmers’ exposure to airborne dust (Lee et al., 2005). In Europe, filter dust masks are typically categorized into P1, P2, and P3, where P2 retains about 94% of all airborne particles while P3 retains about 99.95% of airborne particles. The most penetrating particle size is around 0.3 µm, i.e. smaller than S. aureus cells (Lee and Liu, 1980). Thus, only few MRSA-particles may penetrate the filter of a correctly used mask. However, in general, there are problems with the fit of filter masks (Lee et al., 2005; Winter et al., 2010), and this, together with the presence of airborne MRSA as particles smaller than 1.1 µm, may contribute to the explanation of why dust masks sometimes fail to protect farm visitors against nasal deposition of MRSA (Wulf et al., 2008).
The reasons why most of the bacteria were present in the fractions with the largest particles might be because S. aureus and MRSA are mainly associated with larger particles and simply that there is room for more bacteria on larger particles. However, it might also be due to a higher survival rate of S. aureus and MRSA on larger particles, as they may create a more protective environment for the survival of the bacteria. Scanning electron microscopy of dust from pig farms showed that dust particles larger than 5.4 µm were mainly grain meal and only a small fraction was skin (Stroik, 1987; Heber et al., 1988).
S. aureus as measured on SA-agar was in this study present in the same particle size fractions as MRSA. Consequently, the data for S. aureus seem as relevant in relation to the ability to enter apertures, to stay airborne, as well as where in the airways deposition occurs, as the data on MRSA. The ability to stay airborne will affect the concentration of airborne S. aureus and MRSA. In pig farms, an important mechanism to remove dust particles from the air is to allow airborne dust to settle on surfaces (Gustafsson, 1999). The large fraction of S. aureus and MRSA on the 7.0–12 µm particles indicates that settling is also an important mechanism to remove them from the air. Furthermore, the presence of S. aureus and MRSA as particles with a smaller Dg in large distances from the pigs indicates that mainly the smaller particles are transported to these areas.
In this study, all farms were MRSA-positive, but samples from Farms B and D showed considerably lower concentrations on MRSA-agar than on SA-agar. A large fraction of the pig farms, for example in Denmark (Ministry of Environment and Food of Denmark, 2017), Germany (Dahms et al., 2014), Spain (Reynaga et al., 2016), and Poland (Mroczkowska et al., 2017) are MRSA-positive, and the fraction has increased over time. The results of this study are therefore also relevant for other countries.
The ASCI sampler is well described and has been used for decades, and compared to the Respicon and the DGI it has the advantage that the dust extraction step is avoided. On the other hand, the Respicon and DGI samples have the advantage that they can be plated on different agar media and in different dilutions. Overall, the results obtained by the Respicon resembled the results obtained in the same areas using the ASCI. With the ASCI, it is important not to sample for > 40 min, as the agar may dry out and cause particle bouncing (Li, 1999), which causes an overestimation of the smallest particles (Park et al., 2009). In this study, we sampled with the ASCI for up to 20 min, and we saw no effect of sampling duration on the measured Dg. Staphyloccus aureus and MRSA were not found on the filters from the DGI after sampling, which was probably due to the high air flow the bacteria were exposed to during sampling. The sampler has previously been used successfully to sample the spore-forming, gram-positive, airborne bacterium, Bacillus thuringiensis (Madsen et al., 2014). In the present study, we found other Staphylococcus species on the filters, which may indicate that these species are more robust than S. aureus. The observed species have previously been found in farm dust (Feld et al., 2018).
This study was performed on four different farms, in stables with pigs of different ages and during either a variety of work activities or no human activity. Across farms, some general trends were found, such as the Dg of airborne S. aureus and MRSA being larger in the aisles than both inside the pens among the pigs as well as at larger distances from the pigs, such as in the feed-storage and in the entry room. Also, 70% (average) of the airborne S. aureus and MRSA were found to be present in the fraction potentially depositing in the upper airways, 22% (average) in the primary and secondary bronchi, and 8% (average) in the terminal bronchi and alveoli. The results obtained by the ASCI resembled the results obtained in the same areas using the Respicon. In conclusion, airborne S. aureus and MRSA were found in fractions able to deposit in the six studied parts of the airways. Thus, they might colonize or infect susceptible tissue in all parts of the airways. However, most S. aureus and MRSA were found to deposit in the upper airways. The concentration of airborne S. aureus and MRSA, as well as the fraction depositing in the different parts of the airways, depended on the specific location on the farm or the work tasks performed at the farms. Airborne MRSA were not confined to the stable facilities.
Acknowledgements
We wish to thank Margit W. Frederiksen and Mehtap Akyol Polat for valuable laboratory work. We also want to thank the Danish Working Environment Authority for their involvement in the project. Finally, we want to thank the four farms for their participation in the project and Thomas Madsen for facilitating the contact to the farmers.
Funding
Funding for this project was provided by the Danish Working Environment Authority.
Conflict of Interest
The authors declare no conflict of interest relating to the material presented in this Article. Its contents, including any opinions and/or conclusions expressed, are solely those of the authors.
References
- Abudu L, Blair I, Fraise A et al. (2001)Methicillin-resistant Staphylococcus aureus (MRSA): a community-based prevalence survey. Epidemiol Infect; 126: 351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aires de Sousa M, Lencastre H (2004)Bridges from hospitals to the laboratory: genetic portraits of methicillin-resistant Staphylococcus aureus clones. Pathog Dis; 40: 101–11. [DOI] [PubMed] [Google Scholar]
- Angen Ø, Feld L, Larsen J et al. (2017)Transmission of MRSA to human volunteers visiting a swine farm. Appl Environ Microbiol; AEM-01489. doi:10.1128/AEM.01489-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JG, Dowell SF, Mandell LA et al. (2000)Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis; 31: 347–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basinas I, Schlünssen V, Takai H et al. (2013)Exposure to inhalable dust and endotoxin among Danish pig farmers affected by work tasks and stable characteristics. Ann Occup Hyg; 57: 1005–19. [DOI] [PubMed] [Google Scholar]
- Beaumont F, Kauffman HF, van der Mark TH et al. (1985)Volumetric aerobiological survey of conidial fungi in the North-East Netherlands. I. Seasonal patterns and the influence of metereological variables. Allergy; 40: 173–80. [DOI] [PubMed] [Google Scholar]
- Berning C, Lanckohr C, Baumgartner H et al. (2015) Fatal infections caused by methicillin‐resistant Staphylococcus aureus of clonal complex 398: case presentations and molecular epidemiology. JMM Case Reports; 2(2). [Google Scholar]
- van Belkum A, Verkaik NJ, de Vogel CP et al. (2009a) Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis; 199: 1820–6. [DOI] [PubMed] [Google Scholar]
- Buttner MP, Stetzenbach LD (1991)Evaluation of four aerobiological sampling methods for the retrieval of aerosolized pseudomonas syringae. Appl Environ Microbiol; 57: 1268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CW, Chung H, Huang CF et al. (2001)Exposure assessment to airborne endotoxin, dust, ammonia, hydrogen sulfide and carbon dioxide in open style swine houses. Ann Occup Hyg; 45: 457–65. [PubMed] [Google Scholar]
- Clauss M. (2015)Particle size distribution of airborne micro-organisms in the environment - A review. Landbauforsch Appl Agric Forestry Res; 65: 77–100. [Google Scholar]
- van Cleef BA, Verkade EJ, Wulf MW et al. (2010)Prevalence of livestock-associated MRSA in communities with high pig-densities in The Netherlands. PLoS One; 5: e9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corne P, Marchandin H, Jonquet O et al. (2005)Molecular evidence that nasal carriage of Staphylococcus aureus plays a role in respiratory tract infections of critically ill patients. J Clin Microbiol; 43: 3491–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms C, Hübner NO, Cuny C et al. (2014)Occurrence of methicillin-resistant Staphylococcus aureus in farm workers and the livestock environment in Mecklenburg-Western Pomerania, Germany. Acta Vet Scand; 56: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaine C, Grimard Y, Cormier Y (2000)Influence of building maintenance, environmental factors, and seasons on airborne contaminants of swine confinement buildings. AIHAJ; 61: 56–63. [PubMed] [Google Scholar]
- Feld L, Bay H, Angen Ø et al. (2018)Survival of LA-MRSA in dust from swine farms. Ann Work Expo Health; 62: 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DD, Smith TC, Hanson BM et al. (2016)Detection of airborne methicillin-resistant Staphylococcus aureus inside and downwind of a swine building, and in animal feed: potential occupational, animal health, and environmental implications. J Agromedicine; 21: 149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese A, Schulz J, Hoehle L et al. (2012)Occurrence of MRSA in air and housing environment of pig barns. Vet Microbiol; 158: 129–35. [DOI] [PubMed] [Google Scholar]
- Geenen PL, Graat EA, Haenen A et al. (2013)Prevalence of livestock-associated MRSA on Dutch broiler farms and in people living and/or working on these farms. Epidemiol Infect; 141: 1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerge T, Lorenz MB, van Alen S et al. (2017)MRSA colonization and infection among persons with occupational livestock exposure in Europe: prevalence, preventive options and evidence. Vet Microbiol; 200: 6–12. [DOI] [PubMed] [Google Scholar]
- Gustafsson G. 1999. Factors affecting the release and concentration of dust in pig houses. JAER; 74: 379–90. [Google Scholar]
- Hartmeyer GN, Gahrn-Hansen B, Skov RL et al. (2010)Pig-associated methicillin-resistant Staphylococcus aureus: family transmission and severe pneumonia in a newborn. Scand J Infect Dis; 42: 318–20. [DOI] [PubMed] [Google Scholar]
- Heber AJ, Stroik M, Faubion JM et al. (1988)Size distribution and identification of aerial dust particles in swine finishing buildings. Trans ASAE; 31: 882–87. [Google Scholar]
- Kaye MG, Fox MJ, Bartlett JG et al. (1990)The clinical spectrum of Staphylococcus aureus pulmonary infection. Chest; 97: 788–92. [DOI] [PubMed] [Google Scholar]
- Koch W, Dunkhorst W, Lödding H et al. (2002)Evaluation of the respicon as a personal inhalable sampler in industrial environments. J Environ Monit; 4: 657–62. [DOI] [PubMed] [Google Scholar]
- Kraemer JG, Ramette A, Aebi S et al. (2018)Influence of pig farming on the human’s nasal microbiota: The key role of the airborne microbial communities. Appl Environ Microbiol: AEM-02470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Adhikari A, Grinshpun SA et al. (2005)Respiratory protection provided by N95 filtering facepiece respirators against airborne dust and microorganisms in agricultural farms. J Occup Environ Hyg; 2: 577–85. [DOI] [PubMed] [Google Scholar]
- Lee KW, Liu BYH (1980)On the minimum efficiency and the most penetrating particle size for fibrous filters. J Air Pollut Control Assoc; 30: 377–81. [Google Scholar]
- Létourneau V, Nehmé B, Mériaux A et al. (2010)Human pathogens and tetracycline-resistant bacteria in bioaerosols of swine confinement buildings and in nasal flora of hog producers. Int J Hyg Environ Health; 213: 444–9. [DOI] [PubMed] [Google Scholar]
- Li CS. (1999)Sampling performance of impactors for bacterial bioaerosols. Aerosol Sci Technol; 30: 280–87. [Google Scholar]
- Li X, Qiu Y, Yu A et al. (2015)Characteristics of airborne Staphylococcus aureus (including MRSA) in Chinese public buildings. Aerobiologia; 31: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano C, Aspiroz C, Ezpeleta AI et al. (2011)Empyema caused by MRSA ST398 with atypical resistance profile, Spain. Emerg Infect Dis; 17: 138–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz EA. (2010)Human and animal exposure to airborne methicillin-resistant Staphylococcus aureus (MRSA): laboratory evaluations and veterinary hospital pilot study (Doctoral dissertation, The Ohio State University). The Ohio State University; pp. 1–186. [Google Scholar]
- Madsen AM, Alwan T, Ørberg A et al. (2016)Waste workers’ exposure to airborne fungal and bacterial species in the truck cab and during waste collection. Ann Occup Hyg; 60: 651–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen AM, Matthiesen CB (2013)Exposure to aerosols during high-pressure cleaning and relationship with health effects. Ann Agric Environ Med; 20: 420–5. [PubMed] [Google Scholar]
- Madsen AM, Zervas A, Tendal K et al. (2014)Exposure and preventive measure to reduce high and daily exposure to Bacillus thuringiensis in potted plant production. Ann Occup Hyg; 58: 664–76. [DOI] [PubMed] [Google Scholar]
- Matheson A, Christie P, Stari T et al. (2012)Nasal swab screening for methicillin-resistant Staphylococcus aureus–how well does it perform? A cross-sectional study. Infect Control Hosp Epidemiol; 33: 803–8. [DOI] [PubMed] [Google Scholar]
- Ministry of Environment and Food of Denmark (2017)Resultaterne af screening for husdyr-MRSA i svin i 2016. https://www.foedevarestyrelsen.dk/Nyheder/Aktuelt/Documents/MRSA%20ekspertgruppe%20-%20resultatene%20forekomst%20af%20husdyr-MRSA%20i%20svin%202016.pdf [Google Scholar]
- Mroczkowska A, Żmudzki J, Marszałek N et al. (2017)Livestock-associated Staphylococcus aureus on Polish pig farms. PLoS One; 12: e0170745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy PT, Donham KJ, Peters TM et al. (2009)A task-specific assessment of Swine worker exposure to airborne dust. J Occup Environ Hyg; 7: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Rock JC, Wang L et al. (2009)Performance evaluation of six different aerosol samplers in a particulate matter generation chamber. Atm Environ; 43: 280–89. [Google Scholar]
- Predicala BZ, Urban JE, Maghirang RG et al. (2002)Assessment of bioaerosols in swine barns by filtration and impaction. Curr Microbiol; 44: 136–40. [DOI] [PubMed] [Google Scholar]
- Reynaga E, Navarro M, Vilamala A et al. (2016)Prevalence of colonization by methicillin-resistant Staphylococcus aureus ST398 in pigs and pig farm workers in an area of Catalonia, Spain. BMC Infect Dis; 16: 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr U, Wilhelm M, Muhr G et al. (2004)Qualitative and (semi)quantitative characterization of nasal and skin methicillin-resistant Staphylococcus aureus carriage of hospitalized patients. Int J Hyg Environ Health; 207: 51–5. [DOI] [PubMed] [Google Scholar]
- Rubinstein E, Kollef MH, Nathwani D (2008)Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis; 46 (Suppl. 5): S378–85. [DOI] [PubMed] [Google Scholar]
- Ruhlmann CH, Kolmos HJ, Kristiansen JE et al. (2008)Pigs as an infection source for methicillin resistant Staphylococcus aureus infections in humans. Ugeskr Laeger; 170: 3436. [PubMed] [Google Scholar]
- Stroik M. (1987)A quantitative and qualitative analysis of swine finishing house dust with scanning electron and light microscopy. Department of Agricultural Engineering, Kansas State University Manhattan, Kansas, 65 pp. http://krex.k-state.edu/dspace/bitstream/handle/2097/23644/LD2668R4AGE1987S77.pdf?sequence=1 [Google Scholar]
- Tatum V, Ray AE, Rovell-Rixx D (2002)Performance of the RespiCon personal aerosol sampler in forest products industry workplaces. AIHA J (Fairfax, Va); 63: 311–6. [DOI] [PubMed] [Google Scholar]
- Thomas RJ. (2013)Particle size and pathogenicity in the respiratory tract. Virulence; 4: 847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrbrand K, Schultz AC, Koivisto AJ et al. (2017)Assessment of airborne bacteria and noroviruses in air emission from a new highly-advanced hospital wastewater treatment plant. Water Res; 112: 110–9. [DOI] [PubMed] [Google Scholar]
- Veenemans J, Verhulst C, Punselie R et al. (2013)Evaluation of brilliance MRSA 2 agar for detection of methicillin-resistant Staphylococcus aureus in clinical samples. J Clin Microbiol; 51: 1026–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkade E, Ferket M, Kluytmans J (2011)Clinical evaluation of Oxoid Brilliance MRSA Agar in comparison with bioMerieux MRSA ID medium for detection of livestock-associated meticillin-resistant Staphylococcus aureus. J Med Microbiol; 60(Pt 7): 905–8. [DOI] [PubMed] [Google Scholar]
- Wertheim HF, Vos MC, Ott A et al. (2004)Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet; 364: 703–5. [DOI] [PubMed] [Google Scholar]
- Winter S, Thomas JH, Stephens DP et al. (2010)Particulate face masks for protection against airborne pathogens - one size does not fit all: an observational study. Crit Care Resusc; 12: 24–7. [PubMed] [Google Scholar]
- Witte W, Strommenger B, Stanek C et al. (2007)Methicillin-resistant Staphylococcus aureus ST398 in humans and animals, Central Europe. Emerg Infect Dis; 13: 255–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf MW, Sørum M, van Nes A et al. (2008)Prevalence of methicillin-resistant Staphylococcus aureus among veterinarians: an international study. Clin Microbiol Infect; 14: 29–34. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Aarnink AJ, De Jong MC et al. (2014)Airborne microorganisms from livestock production systems and their relation to dust. Crit Rev Environ Sci Technol; 44: 1071–128. [DOI] [PMC free article] [PubMed] [Google Scholar]