Abstract
The Clinical Assessment Interview for Negative Symptoms (CAINS) was designed in accordance with the recent theory and research in social affective neuroscience and to address the psychometric and conceptual limitations of other instruments assessing negative symptoms. The present study aimed to provide a large-scale validation of the CAINS in China and examine its applicability and validity evidence across the schizophrenia spectrum. Using confirmatory factor analysis, our results replicated the original findings in the US development samples that the CAINS possesses a stable 2-factor structure, namely “motivation/pleasure” and “expression”. We also found significant correlations between the CAINS and other negative symptom measures. The CAINS demonstrated good discriminant validity in differentiating negative symptoms in people with schizophrenia, nonpsychotic first-degree relatives and people with social anhedonia. People with schizophrenia exhibited significantly higher CAINS subscale scores than first-degree relatives and healthy controls. In addition, first-degree relatives had higher “motivation/pleasure” scores than healthy controls. The “motivation/pleasure” subscale scores of individuals with social anhedonia were also significantly higher than healthy controls.
Keywords: negative symptoms, schizotypy, schizophrenia spectrum disorders
Introduction
Historically, positive symptoms such as delusions and hallucinations have received much attention in the research and treatment of schizophrenia.1–6 However, negative symptoms play an important role in prognosis and predicting functional and occupational outcome in people with schizophrenia.7,8 More importantly, negative symptoms are often resistant to both conventional and second generation antipsychotic medications.9 The development of a sensitive, psychometrically sound, and specific clinical assessment of negative symptoms has been one of the most important areas in schizophrenia research in the last decade.10–13
Recent reformulation of an anticipatory-consummatory construct of anhedonia from affective neuroscience has also led to a corresponding change in the clinical evaluation of negative symptoms.14–16 The Clinical Assessment Interview for Negative Symptoms (CAINS) was designed in accordance with this theoretical framework to address the limitations of other instruments measuring negative symptoms in this clinical group.17–19
Kring et al19 demonstrated that the CAINS comprises 2 factors, namely “expression” and “motivation/pleasure” in people with schizophrenia. Good psychometric properties in terms of reliability, convergent, and discriminant validity as well as relevance to real-world social and vocational functioning have been demonstrated. This 2-factor structure of the CAINS has also been validated in German,20 Spanish,21 and Korean22 samples. Preliminary findings from a Chinese sample also suggested a similar 2-factor structure.23 However, the factor structure in the Chinese sample was slightly different, in that 2 items evaluating motivation and pleasure loaded more highly onto the “expression” factor. This difference may reflect a cultural difference or it may reflect something specific to that preliminary and small sample. Thus, we sought to further examine the factor structure, reliability, and validity of the CAINS in a large Chinese sample.
Insel24 argued for the importance of broadening the neurodevelopmental model of schizophrenia and emphasized the unique and common features of people in different “stages” or with different manifestations of the disorder. Negative symptoms have been observed in people with first-episode schizophrenia,25 in the prodrome before the onset of illness,26,27 and in people with social anhedonia.28 First-degree relatives with genetic risk of schizophrenia often show similar but far less severe psychotic symptoms.29,30 Negative symptoms may be one of the important precursors that predict the emergence of at-risk mental states. Only a few recent studies have focused on negative symptoms in people with clinical prodrome or at-risk mental states.31–33 These findings highlighted the possible predictive role of negative symptoms in people before the onset of schizophrenia. To date, no study has specifically examined negative symptoms in high-risk groups using the 2-factor structure of “expression” and “motivation/pleasure” inherent in the CAINS.
In the present study, we sought to conduct a large scale validation study of the CAINS in the Chinese setting. Based on the preliminary findings of the Chinese validation of the CAINS,23 we hypothesized that a stable 2-factor structure of “expression” and “motivation/pleasure” would be found in people with schizophrenia as indicated by confirmatory factor analysis. We also examined negative symptoms across the schizophrenia spectrum to further evaluate the discriminant validity of the CAINS, including people with schizophrenia, nonpsychotic first-degree relatives and people with social anhedonia. We hypothesized that negative symptoms captured by the CAINS would also be found in individuals with social anhedonia and nonpsychotic first-degree relatives of people with schizophrenia, with the schizophrenia group exhibiting the highest level of negative symptoms.
Methods
Participants
We recruited 185 (94 men) people with schizophrenia of Han ethnicity from the Community Health Service Centre of the Institute of Mental Health (the 6th Affiliated Hospital of Peking University) in Haidian District, Beijing, and Castle Peak Hospital of Hong Kong. All participants were diagnosed with schizophrenia according to the DSM-IV based on structured clinical interviews.34 All participants were receiving antipsychotic medications during the assessment, and the mean dosage was 290.96 mg/day (chlorpromazine equivalence, SD = 199.92). The mean age of the sample was 41.16 years (SD = 13.23), the mean duration of illness was 14.58 years (SD = 11.52), and the mean number of years of education was 11.83 years (SD = 2.74).
We also recruited 43 nonpsychotic first-degree relatives of the schizophrenia participants (mean age = 56.33 years, SD = 9.73; 18 men; birth place: 32 from the city, 11 from the countryside) and 44 healthy controls without any family history of psychosis (mean age = 54.54 years, SD = 13.22; 14 men; birth place: 28 from the city, 16 from the countryside) from the Haidian District of Beijing. Finally, 37 people with social anhedonia (mean age = 19.00 years, SD = 3.46; 27 men; family income: mean = 5275.86 ¥/month, SD = 3865.11) and 36 healthy volunteers without any family history of psychosis (mean age = 18.83 years, SD = 3.32; 22 men; family income: mean = 5519.23 ¥/month, SD = 3448.13) (screened by the Chapman Social Anhedonia Scale,35 M. L. Eckblad, L. J. Chapman, J. P. Chapman, M. Mishlove, unpublished data; Chinese version36) were also recruited from the central sample pool of a local college. Consistent with prior studies, we adopted a cut-off of higher than 1.96 SDs above the mean on the Chapman Social Anhedonia Scale to classify individuals as having social anhedonia. Healthy volunteers who scored lower than the mean were considered to be individuals without social anhedonia. None of the above participants had any mental illness according to the assessment by experienced psychiatrists.
Participants with neurological disorder, head injury, substance abuse, substance dependence or IQ less than 80 were not invited to participate. The CAINS and self-report measures were administered on the same day, while other clinical measures were assessed by experienced psychiatrists within 1 week. The CAINS was rated by 2 raters who had attended training and rated 6 people with schizophrenia together. Twenty-three people with schizophrenia from the 185 person sample participated in a CAINS assessment again 2 weeks later to assess test–retest reliability. This study was approved by the Ethics Committees of the Institute of Psychology, the Institute of Mental Health (the 6th Affiliated Hospital of Peking University), and the New Territories West Cluster of the Hong Kong Hospital Authority. All participants provided informed consent before taking part in the study.
Measures
The CAINS
The CAINS is a semi-structured interview assessing negative symptoms and contains 13 items. Four items comprise the “expression” factor (eg, facial expression, vocal expression, and gestures) and 9 items make up the “motivation/pleasure” factor (eg, expected pleasure and motivation in social and vocational domains). Each CAINS item is rated on a 5-point scale (0–4) with high numbers indicating greater impairment. The Chinese version of the CAINS23 was developed according to standard guidelines for translation and adaption.37,38
Other Clinical Measures
Chinese versions of the Scale for Assessment of Negative Symptoms (SANS39) and the Positive and Negative Syndrome Scale (PANSS40) were also administered by trained psychiatrists to compare the CAINS with these other negative symptoms measures in the schizophrenia sample.
The Temporal Experience of Pleasure Scale (TEPS)
The TEPS is a well-validated self-report scale capturing consummatory and anticipatory pleasure.41,42 The present study used the Chinese version of the TEPS that has 4 subscales with 20 items, namely the “abstract consummatory pleasure”, “concrete consummatory pleasure”, “abstract anticipatory pleasure”, and “concrete anticipatory pleasure” factors.41,43 Alpha reliability coefficients ranged from 0.60 to 0.72.
The Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS)
The ACIPS is a measure designed to assess social and interpersonal aspects of pleasure experience.44 The Chinese version45 of this instrument was adopted. Its ordinal α coefficient was 0.95.
The Emotional Expressivity Scale (EES)
The EES assesses emotional expressivity.46 The Chinese version consists of 2 factors, namely “emotional suppression” and “emotional expression.”47 Alpha coefficients were 0.84 and 0.79.
The Chapman Social Anhedonia Scale (CSAS)
The CSAS35,36 was adopted to assess the social aspects of pleasure experience and its alpha coefficient was 0.95.
Data Analysis
Data was analyzed with LISREL 8.72,48R,49 and SPSS 17.0.50 We tested 3 models using confirmatory factor analysis (CFA) in the sample of 185 people with schizophrenia to examine the latent factor structure of the CAINS. Specifically, we tested a unitary factor model; the 2-factor model from the US developmental sample of the CAINS19 and the slightly different 2-factor model from the pilot study of the Chinese version of the CAINS.23 Models were rerun following modification indices that indicated they would improve model fit. We computed ordinal α coefficients using the polychoric correlation matrix. We computed test–retest reliability coefficients for the subsample (n = 23) of people with schizophrenia who were administered the CAINS twice. Correlations were computed between the derived factor scores of the CAINS and the other measures.
To examine negative symptoms across the schizophrenia spectrum, we conducted multivariate analysis of variance (MANOVA). A univariate analysis of variance was also performed to compare people with schizophrenia (44 people from the original sample of 185 who were matched demographically to the other groups), nonpsychotic first-degree relatives of people with schizophrenia and healthy controls. We then conducted an independent sample t-test to compare people with social anhedonia and matched controls.
Results
The CAINS Structure
Table 1 shows all the models tested. The CFA results show that a 2-factor structure fits well with the CAINS data set. According to the modification indices, items assessing the same domains (items 5 and 6: vocational; items 3 and 4: social; items 8 and 9: recreation) or symptom (items 1 and 5, items 1 and 7: motivation; items 4 and 6, items 4 and 9: expected pleasure; items 11 and 12: expression) shared variance. This is not particularly surprising as these shared domains or symptoms may share variance outside of that explained by the “motivation/pleasure” latent factor.
Table 1.
Fit Indices for Confirmatory Factor Analysis of the CAINS in 185 People with Schizophrenia
| χ2 | df | RMSEA | NNFI | CFI | Model AIC | |
|---|---|---|---|---|---|---|
| Unitary model | 937.56 | 65 | 0.270 | 0.78 | 0.81 | 989.56 |
| Two-factor model 1a (Chan et al23) | 280.55 | 64 | 0.136 | 0.91 | 0.92 | 334.55 |
| Two-factor model 1b (item 3 with item 4) | 230.99 | 63 | 0.120 | 0.92 | 0.94 | 286.99 |
| Two-factor model 2a (Kring et al19) | 241.29 | 64 | 0.123 | 0.93 | 0.94 | 295.29 |
| Two-factor model 2b (item 5 with item 6) | 197.10 | 63 | 0.108 | 0.95 | 0.96 | 253.10 |
| Two-factor model 2c (item 3 with item 4) | 153.18 | 62 | 0.089 | 0.96 | 0.97 | 211.18 |
| Two-factor model 2d (item 1 with item 5) | 134.39 | 61 | 0.081 | 0.97 | 0.97 | 194.39 |
| Two-factor model 2e (item 4 with item 6) | 125.41 | 60 | 0.077 | 0.97 | 0.98 | 187.41 |
| Two-factor model 2f (item 8 with item 9) | 110.93 | 59 | 0.069 | 0.98 | 0.98 | 174.93 |
| Two-factor model 2g (item 1 with item 7) | 100.66 | 58 | 0.063 | 0.98 | 0.98 | 166.66 |
| Two-factor model 2h (item 4 with item 9) | 91.06 | 57 | 0.057 | 0.98 | 0.99 | 159.06 |
| Two-factor model 2i (item 11 with item 12) | 85.02 | 56 | 0.053 | 0.99 | 0.99 | 155.02 |
Note: RMSEA, root mean squared error of approximation; NNFI, nonnormed fit index; CFI, comparative fix index; AIC, Akaike’s information criterion.
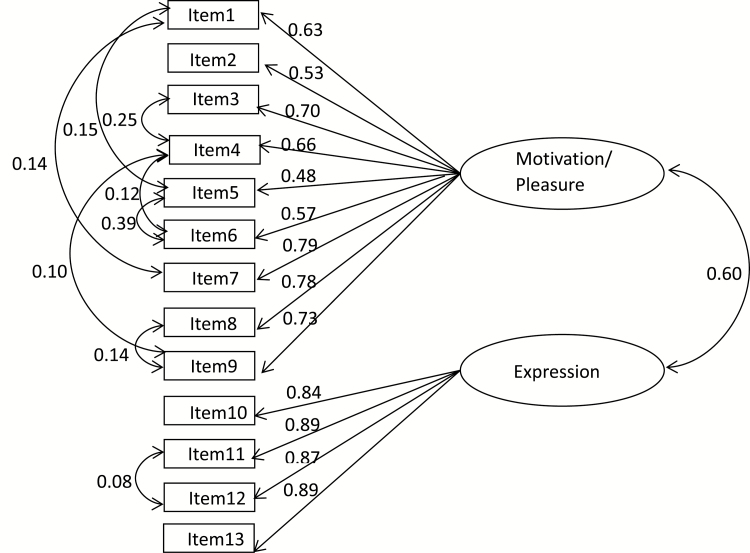
In particular, the 2-factor model from Kring et al19 fit the data better than the other models including the Chan et al23 model. The final best-fitting model (2-factor model 2i: χ2 = 85.02, df = 56, RMSEA = 0.053, NNFI = 0.99, CFI = 0.99, Model AIC = 155.02) consists of the same 2 factors reported in the 2013 development sample, namely “motivation/pleasure” and “expression” (shown in figure 1). The 2 CAINS factors correlated with each other significantly [r (183) = 0.54, P < .01).
Fig. 1.
Schematic diagram of 2-factor Model 2i.
Convergent and Discriminant Validity
Table 2 summarizes the correlations between the CAINS and other clinical ratings. Both CAINS subscales correlated significantly with the negative symptoms scores from the PANSS and the subscales of the SANS, suggesting good convergent validity evidence. The correlations between the CAINS and the SANS were slightly higher than those reported by Kring et al.19Table 3 indicates that the CAINS demonstrates discriminant validity evidence with self-report measures. Specifically, the “motivation/pleasure” subscale rather than the “expression” subscale was inversely correlated with the ACIPS and the TEPS subscales, especially the consummatory pleasure subscales. However, there was no correlation between the CAINS and the EES subscales.
Table 2.
Correlations between the CAINS and Other Clinical Ratings
| M | SD | Motivation/ Pleasure | Expression | |
| PANSS_negative symptoms | 14.00 | 6.53 | 0.39* | 0.61* |
| PANSS_positive symptoms | 10.14 | 3.89 | 0.23 | 0.11 |
| PANSS_general psychopathology | 24.25 | 6.91 | 0.35* | 0.37* |
| SANS_affective blunting | 6.07 | 6.61 | 0.39* | 0.65* |
| SANS_alogia | 3.79 | 4.54 | 0.42* | 0.65* |
| SANS_avolition | 5.37 | 5.06 | 0.43* | 0.59* |
| SANS_anhedonia | 5.43 | 5.85 | 0.43* | 0.54* |
| SANS_attention | 1.71 | 2.03 | 0.25 | 0.37* |
| SANS_total | 22.37 | 21.89 | 0.44* | 0.64* |
Note: PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms.
*Adjusted Bonferroni P < .05.
Table 3.
Correlations between the CAINS and Self-Report Measures
| M | SD | Motivation/ Pleasure | Expression | |
| TEPS_abstract anticipatory | 17.48 | 3.84 | −0.29* | −0.09 |
| TEPS_concrete anticipatory | 16.59 | 4.65 | 0.04 | 0.10 |
| TEPS_abstract consummatory | 26.05 | 6.01 | −0.26* | −0.13 |
| TEPS_concrete consummatory | 15.75 | 4.03 | −0.21 | −0.18 |
| TEPS_anticipatory | 34.07 | 6.95 | −0.14 | 0.02 |
| TEPS_consummatory | 41.80 | 9.06 | −0.27* | −0.17 |
| ACIPS_total | 71.51 | 15.27 | −0.35* | −0.15 |
| EES_expression | 15.43 | 4.44 | −0.08 | −0.03 |
| EES_suppression | 32.30 | 8.10 | 0.15 | 0.15 |
Note: TEPS, Temporal Experience of Pleasure Scale; EES, Emotional Expressivity Scale; ACIPS, Anticipatory and Consummatory Interpersonal Pleasure Scale.
*Adjusted Bonferroni P < .05.
Reliability of the Scores and Rater Agreement
The ordinal α coefficients of the “motivation/pleasure” subscale, the “expression” subscale, and the total CAINS scale were 0.89, 0.92, and 0.91, respectively. The intraclass correlations between the 2 raters for the “motivation/pleasure” subscale, the “expression” subscale and the total scale were 0.79, 0.91, and 0.93, respectively. Test–retest reliability after a 2-week interval for the above subscales and the total CAINS scale were 0.68, 0.63, and 0.68, respectively, similar to those reported by Kring et al.19
CAINS across the Schizophrenia Spectrum
MANOVA revealed significant group differences in CAINS scores between people with schizophrenia, nonpsychotic first-degree relatives, and healthy controls [Wilks Lambda = 0.33, F(4, 254) = 47.14, P < .001, ƞ2 = 0.43]. The main effect of group was significant for both the “motivation/pleasure” [F(2, 128) = 100.16, P < .001, ƞ2 = 0.61] and the “expression” subscales [F(2, 128) = 47.18, P < .001, ƞ2 = 0.42]. Bonferroni post hoc testing revealed that people with schizophrenia received significantly higher CAINS subscale scores than first-degree relatives and healthy controls. In addition, first-degree relatives had higher “motivation/pleasure” subscale scores than controls. The one-way ANOVA results are shown in table 4.
Table 4.
Comparison of CAINS Scores of the Schizophrenia Group, First-Degree Relative Group and Control Group
| SZ (n = 44) | REL (n = 43) | HC (n = 44) | F/χ2 | Bonferroni | ||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |||
| Age | 53.75 | 8.23 | 56.33 | 9.73 | 54.54 | 13.22 | 0.67 | |
| Gender (male/female) | 24/20 | 18/25 | 14/30 | 4.66 | ||||
| Education | 11.51 | 2.47 | 11.24 | 3.83 | 11.55 | 3.04 | 0.12 | |
| Medicationa | 290.96 | 199.92 | ||||||
| Duration of illness | 20.08 | 9.85 | ||||||
| PANSS | ||||||||
| Negative symptoms | 14.24 | 6.44 | ||||||
| Positive symptoms | 10.24 | 4.03 | ||||||
| General psychopathology | 24.35 | 6.81 | ||||||
| SANS_total | 23.28 | 21.85 | ||||||
| CAINS_motivation/pleasure | 18.77 | 6.73 | 9.93 | 5.57 | 2.93 | 2.59 | 100.16*** | SZ > REL > HC |
| CAINS_expression | 5.09 | 4.40 | 0.53 | 0.83 | 0.20 | 0.80 | 47.18*** | SZ > REL, HC |
Note: SZ, schizophrenia; REL, first-degree relatives of people with schizophrenia; HC, healthy controls.
aChlorpromazine equivalence, mg/day.
***P < .001.
Group difference in the CAINS subscales were also found between people with social anhedonia and healthy controls [Wilks Lambda = 0.60, F(2, 70) = 22.92, P < .001, ƞ2 = 0.40]. Individuals with social anhedonia received higher CAINS scores on the “motivation/pleasure” subscale [F(1, 71) = 46.13, P < .001, ƞ2 = 0.39] and the “expression” subscale [F(1, 71) = 10.34, P = .002, ƞ2 = 0.13). Table 5 shows the independent sample t-tests results.
Table 5.
Comparison of CAINS Scores between Individuals with Social Anhedonia and Controls
| SA (n = 37) | HC (n = 36) | t/χ2 | |||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Age | 19.00 | 3.46 | 18.83 | 3.32 | 0.21 |
| Gender (male/female) | 27/10 | 22/14 | 1.16 | ||
| Education | 12.61 | 2.50 | 12.76 | 0.92 | −0.35 |
| CAINS_motivation/pleasure | 9.03 | 5.68 | 2.25 | 1.90 | 6.87*** |
| CAINS_expression | 1.65 | 2.29 | 0.36 | 0.72 | 3.26** |
Note: SA, individuals with social anhedonia; HC, healthy controls; CSAS, the Chapman Social Anhedonia Scale.
**P < .01; ***P < .001.
Discussion
The present study provides additional validity evidence for the Chinese version of the CAINS using a large sample of people with schizophrenia in the Chinese setting. Our findings replicated the 2-factor model of the CAINS reported in the original US development samples and in cross-cultural validation studies of the scale in other western countries. To the best of our knowledge, it is the first study adopting CFA to examine the latent factor structure of the CAINS in a large sample of people with schizophrenia. Moreover, we also found that the CAINS subscales were able to discriminate between groups across the schizophrenia spectrum, showing that nonpsychotic first-degree relatives and individuals with social anhedonia had attenuated negative symptoms.
The 2-factor structure, namely “motivation/pleasure” and “expression”, was confirmed in the present Chinese sample. These findings are consistent with the 2-factor model of the CAINS generated from the western-based samples.19–21 Blanchard et al.51 had also demonstrated that the CAINS could be valid and reliable across different settings. We also found additional convergent validity evidence in that the CAINS subscales were significantly correlated with other negative symptom measures (SANS, PANSS) as well as self-report pleasure experience measures (TEPS and the ACIPS).
Concerning the correlation of the CAINS subscale scores with the self-report measures of pleasure experience and emotion expression, the CAINS, especially the “motivation/pleasure” subscale, was significantly correlated with the consummatory pleasure rather than anticipatory pleasure on the TEPS. These results are generally similar to previous results showing a modest correlation between self-report scales and observer-rated measures or performance-based measures in people with schizophrenia.52,53 However, the “motivation/pleasure” subscale was correlated significantly with the ACIPS, an instrument designed to capture socially oriented pleasure experience rather than physical aspect of anhedonia, the latter of which is assessed by the TEPS. The nonsignificant correlation between the “expression” subscale of the EES with the CAINS subscales, especially the “expression” subscale, may be related to the fact that the EES items are mainly concerned with more general emotional expression, whereas the CAINS specifically assesses facial expressions, prosody of speech, and body gestures. In the CAINS development sample, the “expression” ratings were significantly correlated with observer ratings of facial expressions exhibited during the interview.19 Future studies could examine these relationships in different cultural contexts.
Another main finding of the present study is that the CAINS subscales were able to distinguish groups across the schizophrenia spectrum. Of particular relevance to this special issue are the findings regarding social anhedonia. Many prior studies have found that people with schizotypy share other similarities with people with schizophrenia,54 including negative symptoms.55 Other studies have shown that people with social anhedonia exhibit deficits in motivation and pleasure.56 Consistent with these findings, we found that people with social anhedonia were rated more highly on both CAINS subscales than people without social anhedonia, thus indicating that negative symptoms are present across the spectrum. That people with social anhedonia have impairment in motivation/pleasure domain is consistent with recent theory and data in schizotypy. For example, others have argued that negative schizotypy, which includes anhedonia, may not reflect an inability to experience pleasure per se but instead a problem with perceiving or recognizing that possibly pleasurable events may in fact be pleasurable.54,57,58 This notion is similar to the concept of anticipatory pleasure whereby people with schizophrenia or schizotypy experience pleasure in the presence of stimuli, but not when predicting whether something in the future might be pleasurable.59 However, Grant et al deepen this conceptualization by suggesting that dealing with pleasurable events may just be too overwhelming. This opens up the intriguing hypothesis that the “overwhelmingness” may translate into a type of “shutting down” that would be manifest by diminished expression that is so often central to the diagnosis of schizophrenia and is also observed in schizotypy. Together, these findings seem to support and strengthen the final common model hypothesis proposed by Howes and Kapur.60
Our findings also indicate that negative symptoms, albeit mild ones, are observed in first-degree relatives of people with schizophrenia. Nonpsychotic first-degree relatives, at-risk biologically,61,62 may have difficulties in transforming emotional experience into motivated behaviors.63 As there were no significant difference in the “expression” subscale scores between first-degree relatives and controls, the results suggest that negative symptoms captured by the CAINS, especially the “motivation/pleasure” subscale, may be an important common feature across the schizophrenia spectrum. This finding is consistent with the extant literature suggesting that avolition and anhedonia are already present in the early phases of the schizophrenia spectrum including people with prodrome and first-episode schizophrenia.25,26,28 It is also consistent with data suggesting that anticipatory pleasure is a stable trait across the different stages of schizophrenia, while consummatory pleasure may fluctuate with the severity of negative symptoms.64
There are several limitations for the present study that warrant further consideration in future studies. Given that the psychosis spectrum consists of other disorders such as bipolar disorder, further study should examine the clinical manifestations of negative symptoms using the CAINS in these psychiatric populations. In addition, the present study used self-report measures of anhedonia to examine convergent and divergent validity of the CAINS; future studies might use behavioral or other measure to further validate the CAINS.
In conclusion, the present study provides additional evidence for the 2-factor structure of the CAINS in China. Moreover, the study demonstrates that although people with schizophrenia have more negative symptoms than first-degree relatives or people with social anhedonia, negative symptoms are nevertheless present across the schizophrenia spectrum. It has been found that different subdomains of negative symptoms correspond with different interventions65,66 and distinct pathophysiological mechanism.67–70 Thus, distinguishing the 2 core domains of negative symptoms by the CAINS makes it possible to explore the underlying pathology of each dimension and search for neurobiological markers of these distinct dimensions.
Funding
This study was supported by the National Science Fund China (81571317), National Key Research and Development Programme (2016YFC0906402), the Beijing Training Project for the Leading Talents in Science and Technology (Z151100000315020), the Beijing Municipal Science & Technology Commission grant (Z161100000216138), and the CAS/SAFEA International Partnership Programme for Creative Research Teams (Y2CX131003), and a grant from the Key Laboratory of Mental Health, Institute of Psychology. The funding agents had no further role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the manuscript; and in the decision to submit the article for publication.
Supplementary Material
References
- 1. Bell V, Halligan PW, Ellis HD. Explaining delusions: a cognitive perspective. Trends Cogn Sci. 2006;10:219–226. [DOI] [PubMed] [Google Scholar]
- 2. Censits DM, Ragland JD, Gur RC, Gur RE. Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: a longitudinal study. Schizophr Res. 1997;24:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10:48–58. [DOI] [PubMed] [Google Scholar]
- 4. Freeman D, Garety PA. Connecting neurosis and psychosis: the direct influence of emotion on delusions and hallucinations. Behav Res Ther. 2003;41:923–947. [DOI] [PubMed] [Google Scholar]
- 5. Rotarska-Jagiela A, van de Ven V, Oertel-Knöchel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117:21–30. [DOI] [PubMed] [Google Scholar]
- 6. Zimmermann G, Favrod J, Trieu VH, Pomini V. The effect of cognitive behavioral treatment on the positive symptoms of schizophrenia spectrum disorders: a meta-analysis. Schizophr Res. 2005;77:1–9. [DOI] [PubMed] [Google Scholar]
- 7. Brüne M, Schaub D, Juckel G, Langdon R. Social skills and behavioral problems in schizophrenia: the role of mental state attribution, neurocognition and clinical symptomatology. Psychiatry Res. 2011;190:9–17. [DOI] [PubMed] [Google Scholar]
- 8. Rabinowitz J, Levine SZ, Garibaldi G, Bugarski-Kirola D, Berardo CG, Kapur S. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr Res. 2012;137:147–150. [DOI] [PubMed] [Google Scholar]
- 9. Marder SR, Rabinowitz J, Kapur S. Clinical trials for negative symptoms—emerging directions and unresolved issues. Schizophr Res. 2013;150:327. [DOI] [PubMed] [Google Scholar]
- 10. Arango C, Garibaldi G, Marder SR. Pharmacological approaches to treating negative symptoms: a review of clinical trials. Schizophr Res. 2013;150:346–352. [DOI] [PubMed] [Google Scholar]
- 11. Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daniel DG. Issues in selection of instruments to measure negative symptoms. Schizophr Res. 2013;150:343–345. [DOI] [PubMed] [Google Scholar]
- 13. Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative symptoms and cognitive deficits: what is the nature of their relationship?Schizophr Bull. 2006;32:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience?Brain Res Brain Res Rev. 1998;28:309–369. [DOI] [PubMed] [Google Scholar]
- 15. Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. [DOI] [PubMed] [Google Scholar]
- 16. Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blanchard JJ, Kring AM, Horan WP, Gur R. Toward the next generation of negative symptom assessments: the collaboration to advance negative symptom assessment in schizophrenia. Schizophr Bull. 2011;37:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res. 2011;132:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Engel M, Fritzsche A, Lincoln TM. Validation of the German version of the Clinical Assessment Interview for Negative Symptoms (CAINS). Psychiatry Res. 2014;220:659–663. [DOI] [PubMed] [Google Scholar]
- 21. Valiente-Gómez A, Mezquida G, Romaguera A et al. Validation of the Spanish version of the Clinical Assessment for Negative Symptoms (CAINS). Schizophr Res. 2015;166:104–109. [DOI] [PubMed] [Google Scholar]
- 22. Jung SI, Woo J, Kim YT, Kwak SG. Validation of the Korean-version of the Clinical Assessment Interview for Negative Symptoms of Schizophrenia (CAINS). J Korean Med Sci. 2016;31:1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan RC, Shi C, Lui SS et al. Validation of the Chinese version of the Clinical Assessment Interview for Negative Symptoms (CAINS): a preliminary report. Front Psychol. 2015;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. [DOI] [PubMed] [Google Scholar]
- 25. Hovington CL, Bodnar M, Joober R, Malla AK, Lepage M. Identifying persistent negative symptoms in first episode psychosis. BMC Psychiatry. 2012;12:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dworkin RH, Lewis JA, Cornblatt BA, Erlenmeyer-Kimling L. Social competence deficits in adolescents at risk for schizophrenia. J Nerv Ment Dis. 1994;182:103–108. [DOI] [PubMed] [Google Scholar]
- 27. Tsuang MT, Stone WS, Faraone SV. Towards the prevention of schizophrenia. Biol Psychiatry. 2000;48:349–356. [DOI] [PubMed] [Google Scholar]
- 28. Wang Y, Lui SS, Zou LQ et al. Individuals with psychometric schizotypy show similar social but not physical anhedonia to patients with schizophrenia. Psychiatry Res. 2014;216:161–167. [DOI] [PubMed] [Google Scholar]
- 29. Calkins ME, Iacono WG, Curtis CE. Smooth pursuit and antisaccade performance evidence trait stability in schizophrenia patients and their relatives. Int J Psychophysiol. 2003;49:139–146. [DOI] [PubMed] [Google Scholar]
- 30. Lawrie SM, Whalley HC, Abukmeil SS et al. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol Psychiatry. 2001;49:811–823. [DOI] [PubMed] [Google Scholar]
- 31. Corcoran CM, Keilp JG, Kayser J et al. Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: a neurodevelopmental perspective. Psychol Med. 2015;45:2959–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kantrowitz JT, Woods SW, Petkova E et al. D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: a pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. Lancet Psychiatry. 2015;2:403–412. [DOI] [PubMed] [Google Scholar]
- 33. Nelson B, Yuen HP, Wood SJ et al. Long-term follow-up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry. 2013;70:793–802. [DOI] [PubMed] [Google Scholar]
- 34. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition (DSM-IV). Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 35. Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. [DOI] [PubMed] [Google Scholar]
- 36. Chan RC, Wang Y, Yan C et al. A study of trait anhedonia in non-clinical Chinese samples: evidence from the Chapman Scales for Physical and Social Anhedonia. PLoS One. 2012;7:e34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25:3186–3191. [DOI] [PubMed] [Google Scholar]
- 38. Muniz J, Elosua P, Hambleton RK. International Test Commission Guidelines for test translation and adaptation: second edition. Psicothema. 2013;25:151–157. [DOI] [PubMed] [Google Scholar]
- 39. Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. [DOI] [PubMed] [Google Scholar]
- 40. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 41. Chan RC, Shi YF, Lai MK, Wang YN, Wang Y, Kring AM. The Temporal Experience of Pleasure Scale (TEPS): exploration and confirmation of factor structure in a healthy Chinese sample. PLoS One. 2012;7:e35352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Pers. 2006; 40: 1086–1102. [Google Scholar]
- 43. Chan RC, Wang Y, Huang J et al. Anticipatory and consummatory components of the experience of pleasure in schizophrenia: cross-cultural validation and extension. Psychiatry Res. 2010;175:181–183. [DOI] [PubMed] [Google Scholar]
- 44. Gooding DC, Pflum MJ. The assessment of interpersonal pleasure: Introduction of the Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS) and preliminary findings. Psychiatry Res. 2014;215:237–243. [DOI] [PubMed] [Google Scholar]
- 45. Chan RC, Yang ZY, Li Z, Xie DJ, Gooding DC. Validation of the Chinese version of the Anticipatory and Consummatory Interpersonal Pleasure Scale. Psych J. 2016;5:238–244. [DOI] [PubMed] [Google Scholar]
- 46. Kring AM, Smith DA, Neale JM. Individual differences in dispositional expressiveness: development and validation of the Emotional Expressivity Scale. J Pers Soc Psychol. 1994;66:934–949. [DOI] [PubMed] [Google Scholar]
- 47. Chan RCK, Wang Y, Li H et al. A 2-stage factor analysis of the Emotional Expressivity Scale in the Chinese context. Psychologia. 2010; 53: 44–50. [Google Scholar]
- 48. Jöreskog KG, Sörbom D.. LISREL 8.72: Interactive LISREL for MS Windows. Lincolnwood, IL: Scientific Software International; 2005. [Google Scholar]
- 49. Bivand RS, Pebesma EJ, Gomez-Rubio V, Pebesma EJ.. Applied Spatial Data Analysis with R (Vol. 747248717). New York: Springer; 2008. [Google Scholar]
- 50. George D. SPSS for Windows Step by Step: A Simple Study Guide and Reference, 17.0 update, 10/e. London: Pearson Education India; 2011. [Google Scholar]
- 51. Blanchard JJ, Bradshaw KR, Garcia CP et al. Examining the reliability and validity of the Clinical Assessment Interview for Negative Symptoms within the Management of Schizophrenia in Clinical Practice (MOSAIC) multisite national study. Schizophr Res. 2017;185:137–143. [DOI] [PubMed] [Google Scholar]
- 52. Chan RC, Wang Y, Ma Z et al. Objective measures of prospective memory do not correlate with subjective complaints in schizophrenia. Schizophr Res. 2008;103:229–239. [DOI] [PubMed] [Google Scholar]
- 53. Stip E, Caron J, Renaud S, Pampoulova T, Lecomte Y. Exploring cognitive complaints in schizophrenia: the subjective scale to investigate cognition in schizophrenia. Compr Psychiatry. 2003;44:331–340. [DOI] [PubMed] [Google Scholar]
- 54. Barrantes-Vidal N, Grant P, Kwapil TR. The role of schizotypy in the study of the etiology of schizophrenia spectrum disorders. Schizophr Bull. 2015;41(suppl 2):S408–S416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sarin F, Wallin L. Cognitive model and cognitive behavior therapy for schizophrenia: an overview. Nord J Psychiatry. 2014;68:145–153. [DOI] [PubMed] [Google Scholar]
- 56. Campellone TR, Elis O, Mote J, Sanchez AH, Kring AM. Negative symptoms in psychometrically defined schizotypy: the role of depressive symptoms. Psychiatry Res. 2016;240:181–186. [DOI] [PubMed] [Google Scholar]
- 57. Grant P, Green MJ, Mason OJ. Models of schizotypy: the importance of conceptual clarity. Schizophr Bull. 2018. doi:10.1093/schbul/sby012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grant P. Is schizotypy per se a suitable endophenotype of schizophrenia?—Do not forget to distinguish positive from negative facets. Front Psychiatry. 2015;6:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kring AM, Elis O. Emotion deficits in people with schizophrenia. Annu Rev Clin Psychol. 2013;9:409–433. [DOI] [PubMed] [Google Scholar]
- 60. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Galderisi S, Rossi A, Rocca P et al. ; Italian Network for Research on Psychoses Pathways to functional outcome in subjects with schizophrenia living in the community and their unaffected first-degree relatives. Schizophr Res. 2016;175:154–160. [DOI] [PubMed] [Google Scholar]
- 62. Cavus SY, Darcin AE, Dilbaz N, Kaya H. Comparison of the schizotypal features of first-degree relatives of schizophrenic patients with those of healthy controls. Noropsikiyatri Ars. 2012; 49: 266–271. [Google Scholar]
- 63. Xie DJ, Lui SS, Geng FL et al. Dissociation between affective experience and motivated behaviour in schizophrenia patients and their unaffected first-degree relatives and schizotypal individuals. Psychol Med. 2017; 1–13. [DOI] [PubMed] [Google Scholar]
- 64. Li Z, Lui SS, Geng FL et al. Experiential pleasure deficits in different stages of schizophrenia. Schizophr Res. 2015;166:98–103. [DOI] [PubMed] [Google Scholar]
- 65. Bodkin JA, Siris SG, Bermanzohn PC, Hennen J, Cole JO. Double-blind, placebo-controlled, multicenter trial of selegiline augmentation of antipsychotic medication to treat negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 2005;162:388–390. [DOI] [PubMed] [Google Scholar]
- 66. Conley RR, Boggs DL, Kelly DL et al. The effects of galantamine on psychopathology in chronic stable schizophrenia. Clin Neuropharmacol. 2009;32:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jamadar SD, Pearlson GD, O’Neil KM, Assaf M. Semantic association fMRI impairments represent a potential schizophrenia biomarker. Schizophr Res. 2013;145:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee JS, Park HJ, Chun JW et al. Neuroanatomical correlates of trait anhedonia in patients with schizophrenia: a voxel-based morphometric study. Neurosci Lett. 2011;489:110–114. [DOI] [PubMed] [Google Scholar]
- 69. Stip E, Fahim C, Liddle P et al. Neural correlates of sad feelings in schizophrenia with and without blunted affect. Can J Psychiatry. 2005;50:909–917. [DOI] [PubMed] [Google Scholar]
- 70. Wolf DH, Satterthwaite TD, Kantrowitz JJ et al. Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures. Schizophr Bull. 2014;40:1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.