Abstract
The mirror-gazing task (MGT) experimentally induces illusions, ranging from simple color changes in the specular image of oneself, to depersonalization-like anomalous self-experiences (ASE) as in experiencing one’s specular image as someone else. The objective was to characterize how connectivity in resting-state networks (RSNs) differed in adolescents reporting such depersonalization-like ASEs during the MGT, in a cross-sectional (Y1) and in a longitudinal manner (a year after). 75 adolescents were recruited; for the cross-sectional analysis, participants were split into 2 groups: those who reported depersonalization-like ASEs on the MGT (ASE), and those who did not (NoASE). For the longitudinal analysis, participants were split into 3 groups whether they experienced MGT depersonalization-like ASEs: only at Y1 (Remitters), both times (Persisters), or never (Controls). Participants also filled out self-reports assessing schizotypal personality (Schizotypal Personality Questionnaire [SPQ]), and underwent resting-state functional MRI procedure (rs-fMRI). A group level Independent Component Analysis (ICA) was conducted and voxel-wise inter-group differences within RSNs were examined. The rs-fMRI analysis revealed lower connectivity of specific visual areas within the primary visual network (PVN), and higher connectivity of regions within the Default Mode Network (DMN) when contrasting the ASE and NoASE groups. The areas that were atypically connected within the PVN further presented differential pattern of connectivity in the longitudinal analysis. Atypical connectivity of visual area within the DMN at Y1 was associated with higher scores on the disorganized dimension of schizotypy at the second evaluation. The present study uncovers a subtle signature in the RSNs of non-clinical adolescents who experienced task-induced ASEs.
Keywords: Schizophrenia, schizotypy, psychosis, mirror task, illusions
Introduction
The growing field of research focusing on early detection of psychotic disorders manifests an increasing interest toward pre-psychotic experiential anomalies that may be observable in the premorbid phases during adolescence and early adulthood. These experiential anomalies of the self have been conceptualized by some authors as anomalous self-experiences (ASE),1 which, when meeting certain frequency and intensity criteria, may also be considered as Basic Symptoms (BS).2 For example, experiencing one’s specular image as that of another person represents a BS measured by the Schizophrenia Proneness interview,3 and is also recognized as an ASE by the Examination of Anomalous Self-Experiences (EASE)4 interview. Although important conceptual and methodological differences exist between the 2 instruments, they both converge in assessing the mirror depersonalization-like phenomena, and conceive of this symptom as representing a potential risk marker for the future onset of psychosis.
Caputo and collaborators introduced an experimental approach to induce such mirror illusions.5 In Caputo’s first study, 66% of 50 healthy young adults participating in the mirror-gazing task (MGT) reported seeing a non-human identity within the 10-minute self-face mirror-gazing task.6 In another study, patients with schizophrenia reported more frequent and intense strange-face apparitions during the same task.7 Subsequently, Fonseca-Pedrero8 and colleagues provided a validation of the MGT in a sample of 110 community adolescents, 34.6% of which presented with clear depersonalization-like phenomena during the task. In particular, the authors found that adolescents experiencing depersonalization-like symptoms during the MGT reported higher schizotypy scores on the positive (cognitive-perceptual) and the disorganization subscales of the Schizotypal Personality Questionnaire (SPQ).9 Schizotypy refers to a set of personality traits that can be measured in the general population.10 Considering that the level of positive schizotypy naturally decreases during adolescence11 but that for youths at increased risk for psychosis, schizotypal expression remains more persistent during this period, conducting research in cohorts of typically developing adolescents may be useful to prevention studies. Indeed, such cohorts provide an opportunity to identify developmental processes implicated in vulnerability to psychosis, without the limitations of medication and other risks factors, such as comorbidities with other psychopathologies and hospitalization. Furthermore, adolescence appears to be a designated period to study the development of ASEs, as it is characterized by a profound change and consolidation of self-identity.12
Importantly, adolescence is the theatre of crucial brain maturation. Today, little is known about the functional brain architecture of adolescents who are vulnerable to depersonalization-like phenomena. Recent neurobiological investigations have focused on examining brain changes associated with the onset of psychosis and along the psychosis spectrum.13 Atypical activations during self-reflective tasks appeared to be involved when participants presented high expression of positive schizotypy,14 as well as in first episode psychosis15 and full blown schizophrenia.16–18 These atypical activations encompass areas such as medial PFC, and other midline cortical structures (anterior cingulate, superior frontal gyrus and posterior cingulate gyri) independently of the sensory modality or stimuli domain.19 Resting state functional MRI (rs-fMRI) studies—which evaluate functional interaction during rest—that investigate typically developing population in relation to schizotypy and distal risk for psychosis are rare. In one study, Lagioia et al,14 found positive correlations between visual network low-frequency fluctuations and adolescents’ schizotypy scores, notably with positive and disorganized dimensions. One way to start broaching the question of neural vulnerability to ASEs is to ask whether those experiencing these illusions differ in cerebral connectivity profiles, when compared to those not experiencing these illusions. To the best of our knowledge, this study is the first to examine resting-state networks (RSNs) connectivity in non-clinical adolescents experiencing task-induced ASEs. Disentangling some of the early neural mechanisms associated to depersonalization-like illusions could help uncovering the neuro-functional patterns sustaining part of the risk for psychosis. In this context, the first aim of the present study is to identify the neural signature in RSNs of adolescents experiencing task-induced ASEs. Secondly, by introducing a longitudinal dimension, we aim to investigate the link between atypical connectivity patterns and schizotypal factors after a 1-year interval, and whether persisting vulnerability to experimentally induced ASEs are linked to consistent atypical connectivity patterns.
Methods
Participants
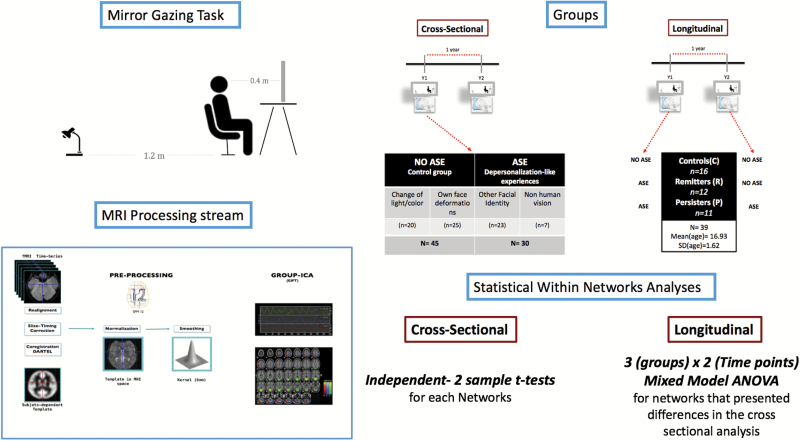
The study included 75 (39 males, 36 female, mean age= 16.85, SD = 2.48) native French-speaking, community adolescents and young adults with normal or corrected to normal vision. Participants were recruited by word of mouth and through advertisement at the University and schools of Geneva. Individuals were included in a longitudinal study, which comprised multiple time points. We were interested in 2 of these time points corresponding to the first time adolescents participated in the task (Y1), and the second time they took part in the same experiment after an interval of 1 year (Y2). The final rs-fMRI analysis at Y1 included the whole sample of 75 adolescents, but the longitudinal rs-fMRI analysis only comprised a subsample of them (N = 39, 22 females and 17 males, mean age = 16.39, SD = 1.5) because 36 participants did not come back for Y2. Participants received a financial compensation, and written consent was obtained from participants or their parents (if they were under 18), under protocols approved by the local ethical commission (Commission Centrale d’éthique de la Recherche des Hôpitaux Universitaires de Genève). The 75 participants included in this study represent a subsample of those comprised in a previously published report8 (N = 110).
Instruments: Self-reported Measures
At both time points, dimensions of schizotypy were measured using the SPQ.9 Adult Self Report (ASR20) and Youth Self Report (YSR21) questionnaires were also assessed to evaluate adaptive behavior in our cohort. Of interest, externalized scores on these scales reflect aggressive and rule breaking behavior, whereas internalizing behaviors include withdrawal, depression, anxiety and somatic complaints. Following Modinos and colleagues’ findings22—exhibiting the impact of depressive and anxiety co-morbidity on the neuroanatomy of individuals at ultra-high risk of psychosis—scores on the 2 dimensions were used as covariates in each of the following statistical analysis. A summary of these measures is presented in table 1 for both the cross-sectional and the longitudinal analyses. Questionnaires are described in the supplementary material.
Table 1.
Descriptive Measures and Statistics Between Groups
| Variables | Whole Sample | No ASE | ASE | Statistics ASE vs NoASE | Effect Size (d) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 75 | 45 | 30 | |||||||||||
| Age | 16.85 ± 2.48 (12–24) | 17.1 ± 2.91 (12.15–24.86) | 16.47 ± 1.61 (12.32–19.47) | t(73) = 1.21, P = .231 | (0.2) | |||||||||
| Gender | 36 Females 39 Males |
22 Females 23 Males |
14 Females 16 Males |
Chi-square(1) = .120, P = .729 | ||||||||||
| Externalizing scores | 54.91 ± 10 (30–76) | 52.67 ± 10.1 (30–73) | 58.46 ± 9.2 (40–76) | t(73) = −2.447, P = .017* | (−0.6) | |||||||||
| Internalizing scores | 52.21 ± 10.6 (32–82) | 51.31 ± 9.98 (32–68) | 53.79 ± 11.54 (32–82) | t(73) = −0.911, P = .365 | (−0.2) | |||||||||
| SPQ-positive | 7.05 ± 6.16 (0–27) | 5.44 ± 4.49 (0–18) | 9.46 ± 7.51 (0–27) | U = 420, Z = −2.766, P = .006** | (−0.6) | |||||||||
| SPQ-disorganized | 5.0 ± 3.70 (0–16) | 4.39 ± 3.45 (0–13) | 6.09 ± 3.92 (0–16) | U = 444, Z = −2.513, P = .012* | (−0.5) | |||||||||
| SPQ-negative | 5.66 ± 4.24 (0–18) | 5.2 ± 4.0 (0–14) | 6.36 ± 4.56 (0–18) | U = 553, Z = −1.324, P = .186 | (−0.3) | |||||||||
| Mean duration | 6.01 ± 15.68 (0–135.98) | 4.63 ± 8.6 (0–40.7) | 8.65 ± 22.84 (0–135) | U = 661, Z = −1.678, P = .038* | (−0.2) | |||||||||
| Cumulative duration | 86.7 ± 126.5 (0–815) | 0.13 ± 0.18 (0–1.04) | 0.18 ± 0.25 (0–1.36) | U = 534, Z = −1.317, P = .188 | (−0.2) | |||||||||
| First onset | 53.7 ± 82.6 (0–480) | 43.05 ± 62.66 (0–276) | 71.82 ± 107.4 (0–480) | U = 527, Z = −1.394, P = .163 | (−0.3) | |||||||||
| Frequency | 16.7 ± 15.6 (0–59) | 0.025 ± 0.028 (0–0.98) | 0.03 ± 0.023 (0–0.08) | U = 515, Z = −1.390, P = .190 | (−0.2) | |||||||||
| Variables | Whole sample(N=39) | Controls (N=32) | Persisters (N=22) | Remitters (N=24) | ||||||||||
| Y1 | Y2 | Stats | d | Y1 | Y2 | Stats | d | Y1 | Y2 | Stats | d | Chi-square Test | ||
| N | 78 | 16 | 16 | 11 | 11 | 12 | 12 | |||||||
| Age | 16.92 ± 1.65 (12.33–20.31) | 16.25 ± 1.4 (12.7–18.8) | 17.78 ± 1.57 (14.2–20.3) | t(15) = −12.76, P = .00** | (−1) | 16.3 ± 1.28 (13.5–17.9) | 17.65 ± 1.36 (14.36–19.12) | t(10) = −12.32, P = .00** | (−1) | 16.35 ± 1.8 (12.33–19.04) | 17.41 ± 1.82 (13.48–20.07) | t(11) = −27.53, P = .00** | (0.6) | |
| Gender | 9 Females 7 Males |
7 Females 4 Males |
6 Females 6 Males | Chi-square = 0.434, P = .805 | ||||||||||
| Externalizing scores | 57.58 ± 9.1 (34–77) | 56.25 ± 8.3 (42–73) | 52.9 ± 8 (34–63) | t(15) = 1.07, P = .30 | (0.4) | 56.45 ± 8.74 (40–70) | 58.09 ± 8.64 (45–71) | t(10) = −0.87, P = .4 | (−0.1) | 61.92 ± 10.6 (38–76) | 60.25 ± 9.3 (48–77) | t(11) = 0.717, P = .48 | (0.2) | |
| Internalizing scores | 53.78 ± 9.42 (32–82) | 54.19 ± 8.49 (40–68) | 50.42 ± 10 (36–69) | t(15) = 0.43, P = .67 | (0.4) | 53.91 ± 14.2 (35–82) | 57.55 ± 6.2 (45–65) | t(10) = −1.15, P = .27 | (−0.3) | 54.58 ± 9.34 (32–66) | 52.25 ± 5.9 (44–67) | t(11) = 0.706, P = .49 | (0.3) | |
| SPQ-positive | 7.65 ± 5.6 (1–27) | 5.75 ± 3.1 (2–12) | 4 ± 2.9 (1–10) | t(11) = 1.57, P = .14 | (0.6) | 12.64 ± 9.3 (1–27) | 7.55 ± 5.13 (2–19) | t(10) = 2.72, P = .02* | (0.7) | 9.5 ± 5.4 (2–21) | 7.5 ± 2.9 (3–12) | t(11) = 1.2, P = .24 | (0.5) | |
| SPQ- disorganized | 5.41 ± 3.46 (0–16) | 4.5 ± 2.7 (0–9) | 2.08 ± 2.15 (0–6) | t(11) = 3.74, P = .003** | (1) | 7.82 ± 4 (2–16) | 6.09 ± 3.3 (2–12) | t(10) = 1.8, P = .1 | (0.5) | 5.67 ± 3.34 (0–13) | 6.83 ± 2.55 (2–11) | t(11) = −1.48, P = .17 | (−0.4) | |
| SPQ-negative | 6.22 ± 4.2 (0–18) | 5.31 ± 3 (0–10) | 5.08 ± 4 (0–15) | t(11) = 0.0, P = 1 | (0.06) | 8.09 ± 5.8 (0–18) | 7 ± 5.4 (0–14) | t(10) = 1.3, P = .22 | (0.2) | 6.08 ± 3.42 (2–11) | 6.25 ± 3.46 (1–13) | t(11) = −0.29, P = .77 | (−0.05) | |
| Mean duration | 4.67 ± 6.44 (0–40.7) | 5.28 ± 9.8 (0–40.7) | 3.54 ± 4.4 (0–12.6) | Z(15) = −0.72, P = .47 | (0.2) | 6.16 ± 8.2 (0–2821) | 3.72 ± 5.6 (0–20.13) | Z(10) = −1.96, P = .05 | (0.3) | 5.06 ± 3.46 (0.48–11.61) | 4.11 ± 3.9 (0–15.05) | Z(11) = −1.02, P = .31 | (0.2) | |
| Cumulative duration | 0.15 ± 0.16 (0–0.7) | 0.15 ± 0.17 (0–0.5) | 0.13 ± 0.15 (0–0.5) | Z(15) = −0.28, P = .78 | (0.12) | 0.19 ± 0.19 (0–0.7) | 0.12 ± 0.16 (0–0.57) | Z(10) = −1.87, P = .06 | (0.4) | 0.17 ± 0.11 (0–0.3) | 0.15 ± 0.18 (0–0.65) | Z(11) = −0.86, P = .38 | (0.1) | |
| First onset | 53 ± 98 (0–480.9) | 39.9 ± 56 (0–229) | 91.8 ± 167 (0–473) | t(15) = −0.16, P = .87 | (−0.4) | 40.74 ± 51.97 (0–180) | 18.13 ± 13.32 (0–38.71) | Z(10) = −0.98, P = .33 | (0.6) | 70.17 ± 131 (6.86–480.9) | 58.07 ± 87.9 (0–309.9) | Z(11) = −0.31, 0, P = .75 | (0.1) | |
| Frequency | 0.03 ± 0.02 (0–0.11) | 0.03 ± 0.03 (0–0.9) | 0.03 ± 0.03 (0–0.11) | Z(15) = −1.0, P = .31 | (0) | 0.03 ± 0.02 (0–0.06) | 0.03 ± 0.02 (0–0.06) | Z(10) = −0.25, P = .8 | (0) | 0.03 ± 0.02 (0–0.08) | 0.03 ± 0.02 (0–0.06) | Z(11) = −0.16, P = .87 | (0) | |
Note: Top: Cross-sectional analysis. Bottom: Longitudinal analysis.
*Correlation is significant at the .05 level (2-tailed); ** Correlation is significant at the .01 level (2-tailed).
Mirror-Gazing Task
Setup of the MGT.
Participants faced a large mirror mounted on a tripod in a parcel of a room where the light was dimed (figure 1). Before the beginning of the task, the experimenter gave the following instructions: “Your task is to look at yourself in the mirror. You should keep staring into your eyes. The task will last 10 minutes”. See supplementary material for more details.
Fig. 1.
Methods.
Qualitative Measures.
In order to characterize the nature of participants’ perceptions changes, they were administered a standardized questionnaire after the mirror-gazing session ended, in which they described what they perceived in the mirror. We used these qualitative measures to identify which adolescents experienced depersonalization-like phenomenon, such as perceiving another facial identity, either human or non-human, in the mirror. The ASE and noASE groups were formed on this basis. See supplementary material for detailed description of the methodology.
Quantitative Measures.
Participants were also informed to press a button every time they experienced a variation in perception and hold it until the change disappeared, their responses were digitally recorded through COGENT software (http://cogent.psyc.bbk.ac.uk). The event-related responses to perceptions of modifications in the specular image were recorded in terms of number and duration of abnormal perceptions (please consult the supplementary material for descriptions of measures).
Partition of Participants in Groups
Groups for the Cross-sectional Analysis.
All 75 participants were distributed into 1 of 2 groups, on the basis of depersonalization-like phenomena they experienced, which were assessed through the questionnaire. The first group included adolescents who experienced only slight changes of color/light and/or deformation of their own faces (participants experiencing no aberrant self-experiences during the MGT = NoASE). The second group reunited participants who perceived another facial identity and/or had non-human visions (participants experiencing aberrant self-experiences during the MGT = ASE). Individuals were included into one of these groups on the basis of the most significant illusion they reported.
Groups for the Longitudinal Analysis.
Only 39 out of the 75 participants initially included in the study came back at Y2. To explore the longitudinal trajectories of our adolescents between Y1 and Y2, we constituted 3 groups: The Control group included participants who did not report any ASE after the MGT in Y1, nor in Y2; the Remitters consisted in adolescents who reported ASE at Y1 MGT, but not during the follow-up visit; the Persisters comprised individuals reporting ASE during the MGT task at both time points. Therefore, participants who experienced ASE at Y1 in the longitudinal analysis (Persisters + Remitters at Y1) constituted a subsample of the 30 participants reporting ASE included in the cross-sectional study. Those who were classified as Controls at Y1 in the longitudinal analysis were a subsample of the 45 who did not experience ASE in the cross-sectional analysis. A schema of the partition of participant into groups is presented in figure 1. Only 2 participants did not experience ASE at Y1 but experienced ASE at Y2: they were excluded from the analyses. There was no group difference in actual time interval between Y1 and Y2 (F(2,36) = 3.41, P = .08). Analysis of participants who did not come back at Y2 compared to those who came back displayed no significant differences (supplementary material).
MRI Acquisition and Pre-processing
Acquisition and pre-processing methods were common to both cross-sectional and longitudinal analyses.
Acquisition.
Anatomical, and functional resting-state imaging data were acquired on a 3T Siemens Trio scanner. For the detailed acquisition parameters, see supplementary material.
fMRI Pre-processing.
Functional MRI data was pre-processed using SPM12 analysis software (http://www.fil.ion.ucl.ac.uk/spm). A standard pre-processing pipeline was used, including slice timing correction, realignment, co-registration, normalization, and smoothing. None of the participants had a range of movement greater than 3 mm translation or 3 degrees of rotation, and movement parameters were regressed out at the individual level, in order to minimize biases from motion artifacts. These criteria have been widely employed in non-clinical population.23–25 Furthermore, Power’s Framewise Displacement (FD) was computed and mean comparison between groups (NoASE: FD = 0.17, ASE: FD = 0.19) did not reveal significant differences (t(73) = 0.877, P = .383). Linear detrending and bandpass filtering (0.001–0.1 Hz) were conducted using DPARSF (http://ffmri.org/DPARSF). Connectivity values were z-transformed (supplementary material). For cross-sectional and longitudinal analysis, and in the correlation analysis, we co-varied for gender, demeaned age (mean centering), and standardized externalized and internalized scores.
Cross-sectional Statistical Analysis of rs-fMRI Data
rs-fMRI Analysis.
Group-level spatial Independent Component Analysis (ICA) was conducted on the entire sample of participant (N = 75) using GIFT toolbox implemented in Matlab (http://mialab.mrn.org/software/gift). ICA technique allows the separation of spatio-temporal BOLD signal into spatially statistically independent components (ICs).26 The Infomax ICA algorithm27 was run 50 times in ICASSO and resulting components were clustered to estimate the reliability of the decomposition—the index Iq, ranging from 0 to 1,28 was greater than 0.9 for each component. Ten components were visually identified as RSNs (supplementary figure 1) and confirmed using correlations computed between the components and RSN templates (http://findlab.stanford.edu/functional_ROIs.htlm, supplementary table 3).
Group Differences Within Networks.
To test for differences among groups within each network, we fed the spatial maps of the ICs from participants into 2 independent samples t test. A full factorial analysis was completed in SPM12 for each component. We generated contrasts between ASE and noASE, from which we extracted significant clusters exhibiting peak activity (t-values) passing FWE-correction29 (family wise error) P < .05.
Group Differences Between Networks.
To investigate variations of inter-network connectivity, we constructed a connectivity matrix per group (Nparticipants × NICA × NICA). Functional connectivity between pairs of ICs was assessed using partial correlations, resulting in a 10 × 10 matrix in which each element represented the connectivity strength between 2 ICs. Statistical analysis was conducted using 2-sample t-test for each connection. Acceptance criteria of the results included a threshold of P < .05, FWE-corrected for multiple comparisons.
Longitudinal Statistical Analysis of rs-fMRI Data
rs-fMRI Analysis.
The rs-fMRI longitudinal analysis was conducted using the same method and parameters we employed for cross-sectional analysis. Group ICA was conducted on 78 sessions because each participant (N = 39) had 2 time points and each time point was considered as a single session. This method—reducing the risk of missing components that are only present at Y2 and decreasing ICA algorithmic variability—was employed in previously published longitudinal study.30
Analysis of the Interaction Groups × Time Points Within Networks.
The longitudinal analysis focused on networks that presented differences in the cross-sectional analysis. We tested for within-network connectivity differences in regions within the primary visual network (PVN) and default mode network (DMN) among groups, time points as well as the effect of their interaction. To do so we fed, on one hand, the spatial maps of the dDMN and on the other hand of the PVN from all participants into 2 separate mixed model ANOVAS.31 The statistical analysis was implemented in SPM12 for each component and consisted in a 3 (groups) × 2 (time points) design. We generated F-contrasts to assess main effects and interactions, and post hoc t-contrasts to identify the direction of those effects. We retained significant clusters exhibiting peak activity (t-values) passing FWE-correction for multiple comparisons at the voxel level, P < .05. Moreover, a Bonferroni32 correction for multiple analyses was computed with a criterion of P < .025.
Correlation With Clinical Data
In order to investigate the relationship between within network connectivity differences at Y1 and the evolution of SPQ dimensions on the 1-year interval, mean voxel values were extracted using Marsbar toolbox from each participant. We extracted these values within ROIs at MNI coordinates corresponding to peak t values from clusters that had passed the FWE correction of P < .05 in the cross-sectional analysis. The mean voxel values thus represented the strength of the cluster expressed at a spatial location within the network. We then computed the difference of scores between Y1 and Y2 for each of the dimensions of the SPQ, which we correlated to the strength values. Spearman partial correlation for non-parametric analysis was used. Results were retained at a threshold of P < .05 and Bonferroni32 correction for multiple comparisons was applied.
Results
Descriptive Measures
Descriptive measures are presented in table 1 for participants of both the cross-sectional and longitudinal analysis (see supplementary material for more details).
Identification of Functionally Connected Neural Networks
After running ICA, we retained 10 networks; their spatial maps are shown in supplementary figure 1 and coordinates of their peak activations are presented in supplementary table 1.
Cross-sectional Results: Group Differences Within and Between Networks
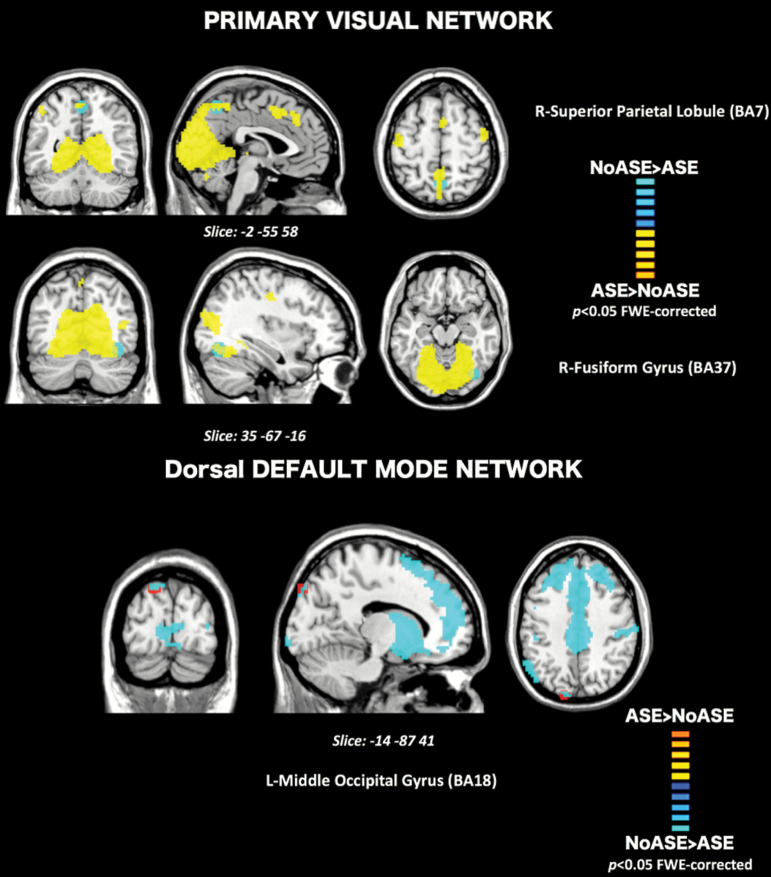
Group contrasts revealed significant differences in functional connectivity between noASE and ASE groups (figure 2). Lower within-network connectivity in the ASE group relative to NoASE (NoASE > ASE) was observed in sub clusters of the PVN, more precisely in the right fusiform gyrus (BA 37, t(73) = 5.33, P = .038) and the right superior parietal lobule (BA7, t(73) = 5.51, P = .016). In contrast, individuals who experienced depersonalization exhibited greater within network connectivity (ASE > noASE) in sub clusters of the ICA component associated with the dorsal default mode network (dDMN), in non-typical regions of the network, the left middle occipital gyrus (BA 18, t(73) = 5.90, P = .003). Between networks analysis did not yield any statistically significant results.
Fig. 2.
Cross-sectional rs-fMRI: Top: Connectivity differences within the Primary Visual Network (PVN) for the contrast NoASE>ASE (Blue) showed connectivity differences in BA7 and BA37. Bottom: Within the Default Mode Network, the contrast ASE>NoASE (Red) showed connectivity differences in BA18. Results are clustered for family wise errors (P < .05) and overlaid on their respective components’ maps (Yellow = PVN, Blue = dDMN).
Longitudinal Results: Group × Time Point Differences Within Networks
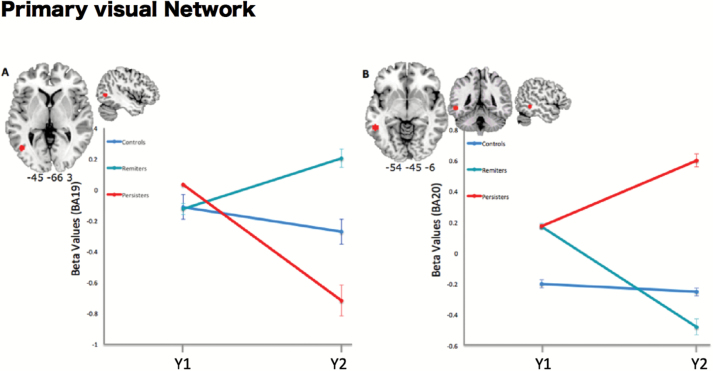
Group contrasts from the mixed model ANOVA revealed a significant effect of group × time points interaction on the connectivity of regions corresponding to 2 Brodmann areas within the PVN. From Y1 to Y2, connectivity of the right lateral occipital gyrus (BA19) was decreased in the Persisters’s group, whereas it was increased in the Remitters’ group (F(2,36) = 5.67, P = .015 FWE-corrected). Furthermore, connectivity of the left posterior inferior temporal gyrus (BA20) within the PVN was increased from Y1 to Y2 in the Persisters and decreased in the Remitters (F(2,36) = 5.34, P = .015 FWE-corrected, figure 3). These results remained significant when applying Bonferroni correction. No significant differences were found when investigating the dDMN.
Fig. 3.
Longitudinal rs-fMRI: Results of contrast time point × groups within the PVN. (A) L-lateral occipital gyrus (BA 19). (B) L-Inferior posterior temporal gyrus (BA 20). Results are displayed for a threshold of P < .05, FWE-corrected.
Correlation With Clinical Data
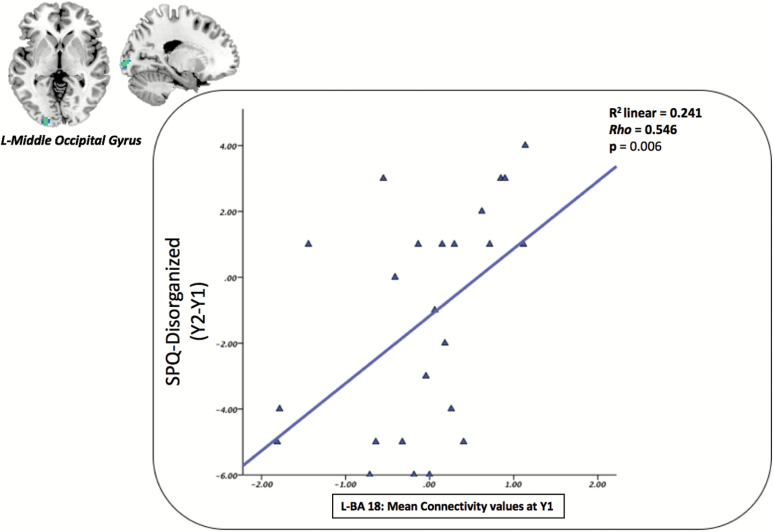
Correlations between mean voxel values extracted from ROIs at Y1 (BA 18, BA 7 and BA 37), and, the evolution of SPQ dimensions during the 1-year interval (Y2-Y1) did not reveal significant association when analyzing the whole sample, or when analyzing only the control group. However, in the ASE group, mean connectivity of area 18 was positively correlated with the SPQ disorganized dimension (ρ = 0.546, P = .006). This result survived the Bonferroni correction for multiple comparisons. Therefore, the atypical connectivity of the middle occipital gyrus related to the ICA component associated with the dorsal DMN of the ASE group observed at Y1 appears to be associated with increasing scores on the disorganized dimension of schizotypy when considering an interval of 1 year (figure 4). No association was found between ROIs resulting from the interaction groups × time points and SPQ dimensions.
Fig. 4.
Correlation with clinical data: In the ASE group; mean connectivity values of the left middle occipital gyrus were positively correlated with scores on SPQ disorganized dimension after 1 year.
Discussion
We employed the MGT to characterize proneness to depersonalization-like ASE, in a cohort of typically developing adolescents. Based on their reports of ASEs during the MGT, adolescents were split into 2 distinct groups for the cross-sectional analyses, and into 3 for the longitudinal analyses. Each participant also underwent a rs-fMRI scan. Using ICA, we identified 3 areas presenting differences between the 2 groups (ASE and noASE): the right fusiform gyrus (FG, BA37) and superior parietal lobule (SPL, BA7) related to the PVN ICA component, and the left middle occipital gyrus (m-OG, BA18) related to the dDMN ICA component. The longitudinal analysis yielded differences between ASEs Persisters and Remitters in the left inferior posterior temporal gyrus (ip-TG, BA20) and the left lateral OG (l-OG, BA19) as related to the PVN component. The following discussion will firstly address the results of the cross-sectional, and secondly those of the longitudinal rs-fMRI analyses, in line with existing literature on rs-networks and self-referential processing in psychosis-prone and patients with psychosis.
Concerning the cross-sectional results, at the network level, we observed a lower connectivity within the PVN-related ICA component involving the right FG and the right SPL. Furthermore, we found a greater connectivity of the m-OG with the DMN-related ICA component of adolescents experiencing ASEs. Empirical evidence suggests that the initial encoding of facial features and subsequent perceptual organization primarily engage the occipital face area (BA18)33 and fusiform face area (BA37).34 Therefore, areas presenting subtle connectivity alterations appear to be implicated in core steps of the face-recognition processing stream.35 This could partly explain why adolescents expressing these subtle alterations would be more prone to experience depersonalization phenomena induced by the MGT. Early perceptual organization36 impairments may disorganize the articulation of sensory information into coherent representations, and constitute an initial step to self-experienced perceptual deficits described by patients with schizophrenia.37
These findings may also be discussed at a conceptual level. From the standpoint of Northoff’s model of self, 3 different layers of processing may be distinguished on the basis of results from meta-analyses19: sensory processing related to the “bodily self” sustained by the sensory cortex; pre-reflective self-referential processing is referred to as the “minimal self” and is associated with medial cortical connectivity; and higher order processing linked to the cognitive aspects of self-processing, such as autobiographical and emotional aspects of self which are related to the lateral cortex. Our results seem to corroborate a connection between self-referential processing and sensory (visual) processing in the experienced modification of one’s own face. We speculate that the MGT could induce a conflict between sensory and self-referential processing, underpinned by the subtle alteration of functional connectivity involving components associated with the PVN and DMN. Alternatively, the atypical connectivity patterns might lead to a disconnection between the 2 aspects of the self in the model, ie, bodily and pre-reflective selves. A recent study38 showed that gazing at one’s self in the mirror increases the consciousness about bodily self. However, in a context of sensory deprivation, in participants experiencing ASE, bottom-up regulation (eg, from bodily to reflective self) could be disconnected, inducing interruption of self-face recognition, while overweighting top-down modulation (reflective to bodily self), in a manner that could generate the illusion.
The longitudinal analysis did not yield a strict anatomical correspondence with respect to the cross-sectional analysis, as differences between groups were found in the left ip-TG (BA20) and the left l-OG (BA19) in relation to the PVN component. However, the corresponding Brodmann areas are functionally consistent with those highlighted in the cross-sectional analysis. Both sets of areas may have complementary though slightly different functions: for instance, the left BA20 has been implicated in visual fixation,39 whereas the right FG determines whether recognized “face-like” features belong to an actual face.40 Area 18 is implicated in the detection of light41 and pattern, while area 19 play a role in human object recognition.42 Importantly, the longitudinal results showed differential patterns of connectivity between the Persisters and the Remitters, meaning that atypical connectivity of these areas implicated in visual processing streams, either persist or remits at the 1-year follow-up.
The link between schizotypy and RSNs was supported by a positive correlation between the connectivity of the R-mOG in relation to the DMN at Y1 and the evolution of scores on the disorganized dimension of schizotypy. These results are consistent with those reported by Lagioia and colleagues on an independent sample, finding positive correlations between VN low-frequency resting-state fluctuations and adolescents’ schizotypy scores, notably pertaining to positive and disorganized dimensions.14
From a theoretical standpoint, it is relevant to invoke and build upon recent Bayesian models of schizophrenia’s positive symptoms in order to interpret our results. In this model, positive symptoms are conceived as reflecting poor precision in prior beliefs about the causes of sensory inputs.43 Trait abnormalities would result from an inadequate relative precision of prior beliefs and sensory evidence.44 We could hypothesize in our context, that cognitive priors about our own face direct attention towards sensory features based on top-down modulation of sensory precision. Considering the MGT as inducing a situation of under-stimulation analogous to sensory deprivation, sensory evidence cannot contribute to posterior information, which implies that perceptual inference is mostly based on priors. The precision of cognitive priors is updated to compensate for the decrease of sensory precision and could be leading to perceptual illusions. Assuming adolescents experiencing ASEs present atypical priors, differences in the degree of failure to attenuate the weight of their own faces’ priors—meaning to attenuate the corollary discharge of self-made face-priors—might explain the varying degrees of depersonalization.
We may notice some of the rs-fMRI results discussed above appear outside of the anatomical regions typically associated with such rs-network ICA components. Inter-group differences were revealed in the m-OG and the SPL, which are not originally included in the traditional definition of respectively, the DMN and the PVN. However, the results appear robust: group-ICA performed on the whole sample of our adolescents ascertained the presence of differences in the signals from the components associated with these network with peak values surviving a stringent statistical threshold (P < .0001 FWE-corrected, supplementary table 1). Analyses were performed per ICA component, and the strength of the resulting contrasts supports the hypothesis that they represented an actual effect rather than noise. The results thus highlight regions that may have a significant functional role in connection to components and networks featuring a core anatomy that does not typically engage them.45,46
These results must be interpreted in light of the following limitations. Firstly, as the task was performed outside the scanner, rs-fMRI represents an indirect measure. Future studies should provide a direct measure of the emergence of ASEs, at the exact moment at which they appear, using an fMRI task. Secondly, our cohort is constituted of typical adolescents, thus, further inquiry is necessary on other risk cohorts, such as individuals experiencing BSs and/or at ultra-high risk states.
Findings of the present study suggest that subtle atypical within-network connectivity, involving sensory and self-referential networks, is linked to susceptibility to experience induced ASEs. Further research concerning the mechanisms at stake in the emergence of ASEs could potentially reveal phenomenological and biological markers for vulnerability to psychosis.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Funding
This work was supported by research grants to M.D. from the Swiss National Science Foundation (100019_159440) and to M.D. and S.E. from the Gertrude Von Meissner Foundation (ME 7871). R.C. was supported by the Beijing Municipal Science & Technology Commission Grant (Z161100000216138), and the Beijing Training Project for Leading Talents in S&T (Z151100000315020).
Acknowledgments
We wish to thank all the participants who kindly volunteered for this study as well as Elodie Toffel, Mathieu Ischer and Lia Antico for their help in experimental setup and data collection. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr Bull. 2003;29:427–444. [DOI] [PubMed] [Google Scholar]
- 2. Schultze-Lutter F, Ruhrmann S, Fusar-Poli P, Bechdolf A, Schimmelmann BG, Klosterkötter J. Basic symptoms and the prediction of first-episode psychosis. Curr Pharm Des. 2012;18:351–357. [DOI] [PubMed] [Google Scholar]
- 3. Fux L, Walger P, Schimmelmann BG, Schultze-Lutter F. The Schizophrenia Proneness Instrument, Child and Youth version (SPI-CY): practicability and discriminative validity. Schizophr Res. 2013;146:69–78. [DOI] [PubMed] [Google Scholar]
- 4. Parnas J, Møller P, Kircher T et al. EASE: examination of anomalous self-experience. Psychopathology. 2005;38:236–258. [DOI] [PubMed] [Google Scholar]
- 5. Caputo GB. Strange-face-in-the-mirror illusion. Perception. 2010;39:1007–1008. [DOI] [PubMed] [Google Scholar]
- 6. Caputo GB. Apparitional experiences of new faces and dissociation of self-identity during mirror gazing. Percept Mot Skills. 2010;110:1125–1138. [DOI] [PubMed] [Google Scholar]
- 7. Caputo GB, Ferrucci R, Bortolomasi M, Giacopuzzi M, Priori A, Zago S. Visual perception during mirror gazing at one’s own face in schizophrenia. Schizophr Res. 2012;140:46–50. [DOI] [PubMed] [Google Scholar]
- 8. Fonseca-Pedrero E, Badoud D, Antico L et al. Strange-Face-in-the-Mirror Illusion and Schizotypy During Adolescence. Schizophr Bull. 2015;41(suppl 2):S475–S482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. [DOI] [PubMed] [Google Scholar]
- 10. Ettinger U, Meyhöfer I, Steffens M, Wagner M, Koutsouleris N. Genetics, cognition, and neurobiology of schizotypal personality: a review of the overlap with schizophrenia. Front Psychiatry. 2014;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Debbané M, Badoud D, Balanzin D, Eliez S. Broadly defined risk mental states during adolescence: disorganization mediates positive schizotypal expression. Schizophr Res. 2013;147:153–156. [DOI] [PubMed] [Google Scholar]
- 12. Goth K, Foelsch P, Schlüter-Müller S et al. Assessment of identity development and identity diffusion in adolescence - theoretical basis and psychometric properties of the self-report questionnaire AIDA. Child Adolesc Psychiatry Ment Health. 2012;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pantelis C, Velakoulis D, Wood SJ et al. Neuroimaging and emerging psychotic disorders: the melbourne ultra-high risk studies. Int Rev Psychiatry. 2007;19:371–381. [DOI] [PubMed] [Google Scholar]
- 14. Lagioia A, Van De Ville D, Debbané M, Lazeyras F, Eliez S. Adolescent resting state networks and their associations with schizotypal trait expression. Front Syst Neurosci. 2010;4:35. doi:10.3389/fnsys.2010.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alonso-Solís A, Corripio I, de Castro-Manglano P et al. Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophr Res. 2012;139:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nelson B, Thompson A, Yung AR. Not all first-episode psychosis is the same: preliminary evidence of greater basic self-disturbance in schizophrenia spectrum cases. Early Interv Psychiatry. 2013;7:200–204. [DOI] [PubMed] [Google Scholar]
- 17. Brunelin J, d’Amato T, Brun P et al. Impaired verbal source monitoring in schizophrenia: an intermediate trait vulnerability marker?Schizophr Res. 2007;89:287–292. [DOI] [PubMed] [Google Scholar]
- 18. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 19. Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. [DOI] [PubMed] [Google Scholar]
- 20. Achenbach TM, Rescorla LA.. Manual for the ASEBA Adult Forms & Profiles. Research Center for Children,Youth, & Families. Burlington, VT: University of Vermont; 2003. [Google Scholar]
- 21. Achenbach TM. Manual for the Youth Self-Report and 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 22. Modinos G, Allen P, Frascarelli M et al. Are we really mapping psychosis risk? Neuroanatomical signature of affective disorders in subjects at ultra high risk. Psychol Med. 2014;44:3491–3501. [DOI] [PubMed] [Google Scholar]
- 23. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi:10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. [DOI] [PubMed] [Google Scholar]
- 27. Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. [DOI] [PubMed] [Google Scholar]
- 28. Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22: 1214–1222. [DOI] [PubMed] [Google Scholar]
- 29. Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003;12:419–446. [DOI] [PubMed] [Google Scholar]
- 30. Damaraju E, Caprihan A, Lowe JR, Allen EA, Calhoun VD, Phillips JP. Functional connectivity in the developing brain: a longitudinal study from 4 to 9months of age. Neuroimage. 2014;84:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beckman RJ, Nachtsheim CJ, Cook RD. Diagnostics for Mixed-Model Analysis of Variance. Technometrics. 1987;29:413. doi:10.2307/1269452. [Google Scholar]
- 32. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 33. Pitcher D, Walsh V, Duchaine B. The role of the occipital face area in the cortical face perception network. Exp Brain Res. 2011;209:481–493. [DOI] [PubMed] [Google Scholar]
- 34. Dien J. A tale of two recognition systems: implications of the fusiform face area and the visual word form area for lateralized object recognition models. Neuropsychologia. 2009;47:1–16. [DOI] [PubMed] [Google Scholar]
- 35. Atkinson AP, Adolphs R. The neuropsychology of face perception: beyond simple dissociations and functional selectivity. Philos Trans R Soc Lond B Biol Sci. 2011;366: 1726–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uhlhaas PJ, Silverstein SM. Perceptual organization in schizophrenia spectrum disorders: empirical research and theoretical implications. Psychol Bull. 2005;131: 618–632. [DOI] [PubMed] [Google Scholar]
- 37. Albers M, Schultze-Lutter F, Steinmeyer EM, Klosterkötter J. Can self-experienced neuropsychological deficits indicate propensity to schizophrenic psychosis? Results of an 8-year prospective follow-up study. International Clinical Psychopharmacology. 1998;13:S75. [Google Scholar]
- 38. Ainley V, Tajadura-Jiménez A, Fotopoulou A, Tsakiris M. Looking into myself: changes in interoceptive sensitivity during mirror self-observation. Psychophysiology. 2012;49:1504–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Richter HO, Costello P, Sponheim SR, Lee JT, Pardo JV. Functional neuroanatomy of the human near/far response to blur cues: eye-lens accommodation/vergence to point targets varying in depth. Eur J Neurosci. 2004;20:2722–2732. [DOI] [PubMed] [Google Scholar]
- 40.Trafton A. How does our brain know what is a face and what’s not?MIT News. MIT News Office, January 9 2012. http://news.mit.edu/2011/face-perception-0109.
- 41. Mentis MJ, Alexander GE, Grady CL et al. Frequency variation of a pattern-flash visual stimulus during PET differentially activates brain from striate through frontal cortex. Neuroimage. 1997;5:116–128. [DOI] [PubMed] [Google Scholar]
- 42. Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41:1409–1422. [DOI] [PubMed] [Google Scholar]
- 43. Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. The computational anatomy of psychosis. Front Psychiatry. 2013;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Corlett PR, Honey GD, Krystal JH, Fletcher PC. Glutamatergic model psychoses: prediction error, learning, and inference. Neuropsychopharmacology. 2011;36:294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soto D, Theodoraki M, Paz-Alonso PM. How the human brain introspects about one’s own episodes of cognitive control. Cortex. 2017; ISSN 0010-9452. doi:https://doi.org/10.1016/j.cortex.2017.10.016. http://www.sciencedirect.com/science/article/pii/S0010945217303623. [DOI] [PubMed] [Google Scholar]
- 46. Philippi CL, Tranel D, Duff M, Rudrauf D. Damage to the default mode network disrupts autobiographical memory retrieval. Soc Cogn Affect Neurosci. 2015;10:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.