Abstract
Background
Cholera has caused 7 global pandemics, including the current one which has been ongoing since 1961. A systematic review of risk factors for symptomatic cholera infection has not been previously published.
Methods
In accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we performed a systematic review and meta-analysis of individual and household risk factors for symptomatic cholera infection.
Results
We identified 110 studies eligible for inclusion in qualitative synthesis. Factors associated with symptomatic cholera that were eligible for meta-analysis included education less than secondary level (summary odds ratio [SOR], 2.64; 95% confidence interval [CI], 1.41–4.92; I2 = 8%), unimproved water source (SOR, 3.48; 95% CI, 2.18–5.54; I2 = 77%), open container water storage (SOR, 2.03; 95% CI, 1.09–3.76; I2 = 62%), consumption of food outside the home (SOR, 2.76; 95% CI, 1.62–4.69; I2 = 64%), household contact with cholera (SOR, 2.91; 95% CI, 1.62–5.25; I2 = 89%), water treatment (SOR, 0.37; 95% CI, .21–.63; I2 = 74%), and handwashing (SOR, 0.29; 95% CI, .20–.43; I2 = 37%). Other notable associations with symptomatic infection included income/wealth, blood group, gastric acidity, infant breastfeeding status, and human immunodeficiency virus infection.
Conclusions
We identified potential risk factors for symptomatic cholera infection including environmental characteristics, socioeconomic factors, and intrinsic patient factors. Ultimately, a combination of interventional approaches targeting various groups with risk-adapted intensities may prove to be the optimal strategy for cholera control.
Keywords: cholera, V. cholerae, risk factors, predictor, neglected tropical diseases
Cholera, the acute watery diarrheal illness caused by toxigenic Vibrio cholerae, is endemic to the Indian subcontinent and has caused 7 recorded global pandemics [1]. The seventh pandemic has been ongoing since 1961 and has extended throughout Asia into Africa, Europe, and the Americas. In 1855, John Snow first identified contaminated water as an individual risk factor for symptomatic cholera [2, 3]. Since then, many studies have explored other potential risk factors in a variety of settings, and yet overall rates of cholera have not measurably decreased, with the 172454 cholera cases reported in 2015 representing a fraction of the estimated 1.3–4.0 million annual cases worldwide [4, 5]. As the oral cholera vaccine continues to be more widely used as a targeted tool for cholera control [6], it will be important to consider if the evidence that exists for groups and individuals at high risk for cholera is being used to full capacity to inform implementation of this (and other) interventions. In this systematic review and meta-analysis, we thus sought to identify and summarize all known individual and household risk factors for symptomatic cholera.
METHODS
We conducted this study in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, which provide evidence-based recommendations for conducting and reporting systematic reviews and meta-analyses [7].
Eligibility Criteria
We searched for peer-reviewed articles assessing individual or household risk factors for symptomatic cholera infection, either with microbiologic confirmation or as defined by the World Health Organization (WHO) case definition of cholera: a patient ≥5 years of age with acute watery diarrhea, with or without vomiting, in an area with a known cholera epidemic [8]. We considered studies focused on the following to be beyond the scope of this review: subclinical cholera infection; neighborhood-, district-, or national-level risk factors for cholera; risk factors for cholera severity or mortality; and studies assessing protection from cholera provided by oral cholera vaccine, the subject of a recent meta-analysis [6].
Search Strategy
We searched PubMed, Embase, and the Cochrane Library using the following terms: (“cholera” OR “Vibrio cholerae”) AND (“risk” or “predict” or “risk factor”). We manually reviewed reference lists of related reviews and all included articles.
Study Selection and Data Collection
After elimination of duplicate records, 2 reviewers independently screened abstracts of all records for full-text review. After screening, 2 reviewers independently applied eligibility criteria to each full-text article and proceeded to data extraction for eligible studies using a standardized form created for the study (Supplementary Table 1). Disagreements were settled by discussion among all authors.
Assessment of Bias
We assessed risk of bias within nonrandomized studies using the Newcastle–Ottawa Scale [9]. There were no randomized studies included in this review. We generated funnel plots and visually inspected for publication bias.
Data Analysis
All included studies were summarized in a qualitative synthesis. We considered a risk factor for meta-analysis if it was assessed in an analogous way by >1 study, and included studies that reported an effect measure, implemented a multivariable model, and used community or household controls. We included effect measures generated in multivariable models when available. We generated summary odds ratios (SORs) using random effects models and a generic inverse variance approach to allow for inclusion of odds ratios controlled for measured confounders. We assessed heterogeneity with the Cochran Q test and the I2 statistic.
We performed a prespecified sensitivity analysis for risk factors included in meta-analysis by assessing for a subgroup difference between studies with and without microbiologic confirmation of cholera infection. We also evaluated for a subgroup difference between studies that took place in endemic settings compared to epidemic settings.
Data were analyzed using Review Manager (RevMan) version 5.3 (The Cochrane Collaboration, Copenhagen: Nordic Cochrane Center).
RESULTS
Study Selection
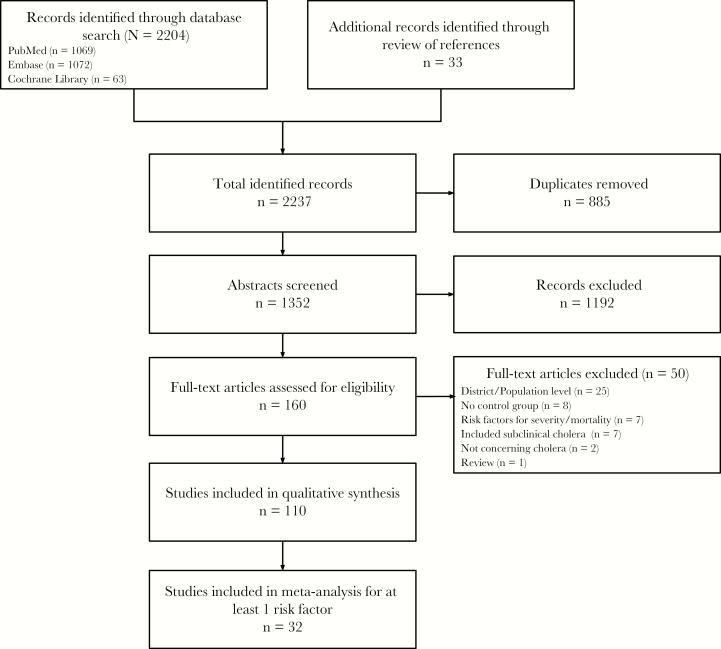
The database search was performed on 18 January 2018 and is summarized in Figure 1. After removal of 885 duplicates, 1352 abstracts were screened, yielding 160 full-text articles for review. After applying inclusion and exclusion criteria, there were 110 remaining articles for inclusion in the qualitative synthesis (Supplementary Table 2) [10–119]. Of these, 32 studies ultimately qualified to be included in meta-analysis for at least 1 potential risk factor for cholera.
Figure 1.
Flow diagram summarizing literature search.
Demographics
The majority of studies were both age- and sex-matched analyses, but a subset assessed risk for cholera based on these characteristics. Fifteen studies assessed differential risk by age [13, 26, 32, 33, 35, 47, 53, 70, 82, 85, 97, 101, 104, 110, 118], 10 of which implemented a multivariable model [26, 32, 33, 35, 82, 97, 101, 104, 110, 118]. Age was measured heterogeneously and findings were mixed. Four studies found that younger patients were at higher risk for cholera [26, 35, 101, 110], and 1 study found that older children had higher risk than children <1 year old [32]. Other studies, however, found older people to be at higher risk [33, 97], or no difference in risk based on age [82, 104, 118].
The role of sex was evaluated in 15 studies [13, 26, 32, 33, 35, 37, 43, 70, 72, 73, 90, 101, 104, 116, 118], of which 11 implemented a multivariable model [32, 33, 35, 37, 72, 73, 90, 101, 104, 116, 118]. Seven of these studies met criteria for meta-analysis (Supplementary Figure 1) [35, 72, 73, 90, 101, 116, 118]. The SOR for risk of cholera for females was 1.22 (95% confidence interval [CI], .71–2.12), with I2 = 80% (Q test P < .0001).
Socioeconomic Factors
Seven studies directly assessed the relationship between income and risk of cholera [13, 18, 32, 33, 39, 43, 116]. Three of these implemented multivariable models: 2 found that people with cholera were more likely to come from lower-income households compared to those with noncholera diarrhea [32, 33], and 1 found no difference in cholera risk by income [116].
Seven studies considered socioeconomic status as measured by asset ownership or composite wealth index [17, 41, 49, 54, 70, 85, 90]. Four implemented a multivariable analysis [41, 49, 54, 90], of which 3 found lower socioeconomic status to be independently associated with risk of cholera [49, 54, 90].
Eight studies evaluated household building materials [10, 32, 33, 41, 43, 73, 101, 110]. Seven of these implemented multivariable models [10, 32, 33, 41, 73, 101, 110], 5 of which found that higher-quality housing was associated with lower risk of cholera [10, 32, 33, 101, 110]. One study found that risk of cholera independently increased with population density surrounding a household [101].
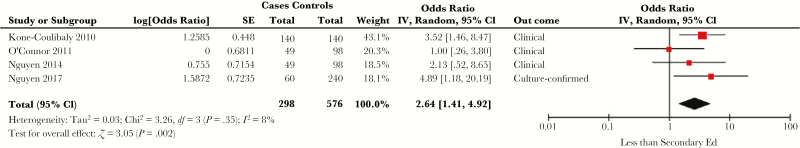
Fourteen studies explored the role of education [18, 26, 32, 33, 41, 43, 56, 65, 73, 82, 83, 85, 86, 101], of which 11 implemented a multivariable model [26, 32, 33, 41, 56, 65, 73, 82, 83, 85, 86]. Four studies looked specifically at whether an individual had some secondary education and met criteria for meta-analysis (Figure 2) [65, 82, 83, 86]. The SOR for cholera with less than some secondary education was 2.64 (95% CI, 1.41–4.92), with I2 = 8% (Q test P = .35).
Figure 2.
Forest plot of studies included in meta-analysis assessing whether an individual having less than secondary education was associated with symptomatic cholera. The summary odds ratio was calculated using random effects models. Heterogeneity is described using the Cochran Q test and the I2 statistic. Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
There were mixed findings by 8 studies assessing the relationship between number of household members and risk of cholera [18, 33, 49, 54, 73, 101, 116, 118]. Of the studies that implemented multivariable models, 3 found higher risk with more members [33, 54, 118], 1 found lower risk with more members [49], and 3 found no association [73, 101, 116].
Four studies found no relationship between cholera and electricity in the household [70, 73, 86, 118], 3 found no relationship with literacy [26, 41, 116], and 2 found no relationship with household size [17, 43].
Water
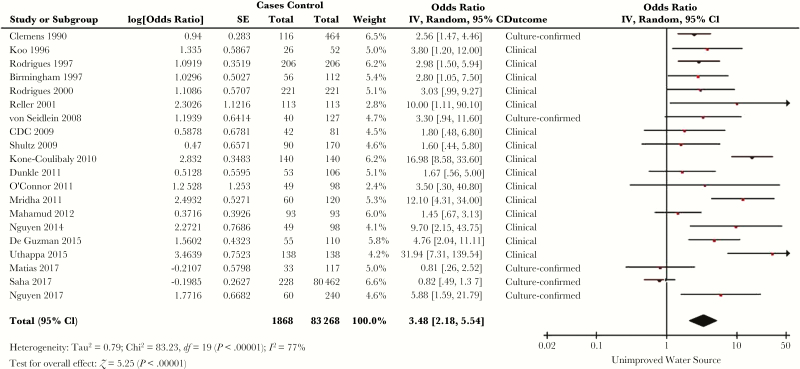
Fifty-six studies evaluated risk of cholera based on source of water, with many identifying a specific culprit [17–19, 21, 22, 25–27, 30, 32, 33, 36, 37, 41–43, 47, 50, 59–61, 63, 65, 66, 68, 70, 72–74, 76, 78, 79, 81–83, 85–88, 91, 92, 94, 95, 97, 101–108, 110, 113, 116, 118]. Thirty-one studies implemented a multivariable model [19, 26, 27, 30, 32, 33, 36, 37, 41, 42, 60, 65, 66, 68, 72, 73, 78, 79, 82, 83, 86, 91, 92, 94, 95, 101, 104–106, 116, 118]. Of these, 20 measured the water source in a way that could be classified as improved (piped household, protected well or spring, or collected rainwater) or unimproved, and met criteria for meta-analysis (Figure 3) [19, 27, 30, 37, 41, 65, 66, 72, 73, 78, 82, 83, 86, 91, 94, 95, 101, 106, 116, 118]. The SOR for risk of cholera with an unimproved water source was 3.48 (95% CI, 2.18–5.54), with I2 = 77% (Q test P < .00001). Of the 11 studies excluded from meta-analysis, 1 found a significant association but did not report an effect measure [92], 6 used hospital controls [32, 33, 60, 68, 104, 105], and 4 did not define water source in way comparable to the others [26, 42, 79, 110].
Figure 3.
Forest plot of studies included in meta-analysis assessing whether exposure to an unimproved water source was associated with symptomatic cholera. The summary odds ratio was calculated using random effects models. Heterogeneity is described using the Cochran Q test and the I2 statistic. Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
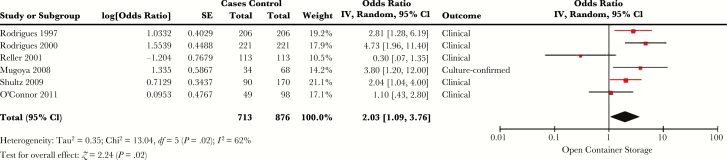
Methods of water storage were assessed by 17 studies [17, 19, 21, 25, 35, 50, 54, 64, 79, 86, 88, 91, 92, 94, 95, 106, 112]. Of the 12 that included multivariable analyses [19, 35, 54, 64, 79, 86, 91, 92, 94, 95, 106, 112], 6 looked at storing water in a bucket or open container and were eligible for meta-analysis (Figure 4) [79, 86, 91, 94, 95, 106]. The SOR for risk of cholera with storing water in a bucket or open container was 2.03 (95% CI, 1.09–3.76), with I2 = 62% (Q test P = .02).
Figure 4.
Forest plot of studies included in meta-analysis assessing whether water storage with an open container or bucket was associated with symptomatic cholera. The summary odds ratio was calculated using random effects models. Heterogeneity is described using the Cochran Q test and the I2 statistic. Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
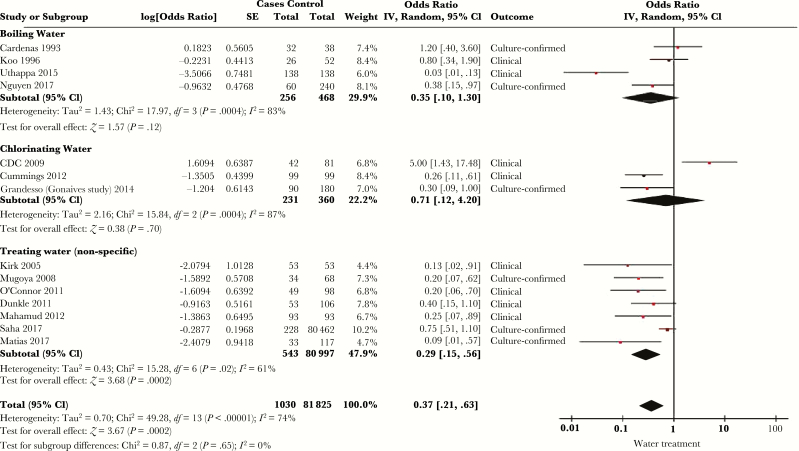
Thirty-nine studies considered the relationship between risk of cholera and household water treatment, typically in the form of either chlorination or boiling [10, 13, 17, 20, 26, 27, 32–35, 37, 39–41, 47, 49, 50, 56, 64, 66, 72, 73, 79, 80, 82, 86, 88, 91, 92, 96, 101–103, 108, 112, 114–116, 119]. Twenty-seven of these studies used a multivariable model [10, 26, 27, 32, 33, 35, 37, 40, 41, 49, 56, 64, 66, 72, 73, 79, 80, 82, 86, 91, 92, 96, 101, 112, 115, 116, 119], 15 of which met criteria for meta-analysis after stratification into type of water treatment (chlorination, boiling water, or nonspecific) (Figure 5) [26, 27, 35, 41, 49, 64, 66, 72, 73, 79, 82, 86, 101, 115, 116]. The overall SOR for cholera with water treatment was 0.37 (95% CI, .21–.63), with I2 = 74% (Q test P < .00001). There was no significant difference between the subgroups (P = .65). Of the 12 multivariable analyses excluded from meta-analysis, 8 did not report an effect measure (3 found a significant relationship between water treatment and cholera, and 5 did not) [37, 40, 80, 91, 92, 96, 112, 119]. Three studies directly assessed chlorine concentration in household water; none found an association with cholera risk [25, 49, 86].
Figure 5.
Forest plot of studies included in meta-analysis assessing whether water treatment was associated with symptomatic cholera. Studies were stratified by whether they assessed chlorination, boiling, or nonspecific report of water treatment. The summary odds ratio was calculated using random effects models. Heterogeneity is described using the Cochran Q test and the I2 statistic. Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
Distance to a water source was assessed in 5 studies which implemented multivariable models [10, 27, 30, 32, 33]. Three studies found that increased distance from a water source was associated with cholera risk [10, 32, 33], 1 found the opposite [27], and 1 study found that proximity to a contaminated river was associated with increased cholera risk [30].
Two studies found no relationship between availability of water and risk of cholera [26, 85].
Food
Reported exposure to a specific food was commonly assessed. In particular, the role of seafood was evaluated in 23 studies [10, 15, 17, 19, 21, 23, 42, 43, 46, 47, 56, 60, 63, 69, 70, 74, 83, 95, 109, 112, 113, 118, 119]. Implicated seafood items in multivariable analyses included raw or partially cooked seafood [56, 119], crabs or shellfish [83, 95], dried fish [10], and seafood salad [42]. Thirty studies looked at other types of food exposures [13, 17, 19, 21, 40, 42, 47, 54, 55, 60, 61, 66, 69, 70, 72, 75, 80, 82, 83, 86, 87, 94, 95, 97, 105, 109, 111, 112, 114, 118]. Notable among these were 6 multivariable analyses with mixed results on vegetable exposure; some found a significant relationship between cholera and raw vegetable exposure [40, 75, 80], others did not [83, 105], and 1 study found steamed vegetables to be associated with reduced risk of cholera [82].
Twenty-four studies assessed risk of cholera with exposure to food from street vendors or outside the home [26, 35, 41, 49, 56, 57, 66, 68, 70, 72, 73, 78, 79, 83–85, 88, 91, 92, 103, 109, 115, 118, 119]. Seventeen studies implemented multivariable models [35, 41, 49, 56, 66, 68, 72, 73, 78, 79, 83, 91, 92, 109, 115, 118, 119], and 12 could be summarized using meta-analysis (Supplementary Figure 2) [35, 41, 49, 66, 72, 73, 78, 79, 83, 109, 115, 118]. The SOR of cholera risk with exposure to street vendor food or food from outside the home was 2.76 (95% CI, 1.62–4.69), with I2 = 64% (Q test P = .0008). Of the 5 controlled studies not eligible for meta-analysis, 3 did not report an effect measure [91, 92, 119].
There were mixed results when looking at cholera risk based on hot meal preparation or exposure to leftover food [27, 35, 41, 60, 65, 66, 75, 76, 80, 83, 88, 91, 92, 106, 109, 113, 118]. Of the 14 studies that used multivariable models, 4 found increased risk of cholera with a cold meal whereas 2 found no difference in risk [27, 35, 65, 83, 91, 118], and 3 found increased risk of cholera with leftover food whereas 2 found no difference in risk [41, 60, 66, 75, 80, 92, 106, 109].
The possible protective role of breastfeeding in the setting of cholera was first noted in 1979, when bottle-fed children in a matched case-control study had significantly higher risk of cholera [50]. This relationship has been further explored [30, 32, 43, 89], including by 2 multivariable analyses that confirm the association between breastfeeding status and reduced risk of cholera [30, 32].
One study found that retinol deficiency was associated with a higher likelihood of developing symptomatic disease among people growing V. cholerae in their stool [53], and another found that prior retinol supplementation was associated with risk of cholera in children, hypothesized by the authors to reflect an underlying deficiency [32].
No studies evaluated risk of cholera based on access to food or food security, although one multivariable analysis found dietary diversity to be associated with a reduced risk of cholera [41]. No studies assessed nutritional status and risk of cholera.
Latrines
Access to a flush toilet, latrine, or open defecation was included as a potential risk factor in 27 studies [10, 18, 20, 27, 32, 33, 35, 37, 39, 41, 43, 49, 54, 64, 73, 79, 82, 85–87, 90, 94, 97, 101–103, 107]. Of these, 18 implemented a multivariable model, with mixed results [10, 27, 32, 33, 35, 37, 41, 49, 54, 64, 73, 79, 82, 86, 90, 94, 97, 101]. Seven studies found no significant difference in cholera risk with access to a latrine [35, 37, 41, 49, 79, 86, 90], 2 found increased risk with latrines [54, 73], 2 found increased risk with open defecation whereas 1 found no significant difference [10, 27, 97], and 4 found decreased risk with a flush toilet while 2 found no difference [32, 33, 64, 82, 94, 101].
Six studies assessed whether sharing a latrine was a risk factor for cholera [40, 43, 70, 72, 106, 118]. Four of these were multivariable analyses [40, 72, 106, 118], of which 2 found that a communal latrine was associated with risk of cholera [72, 106].
Cholera Contacts and Proximity to Other Cases
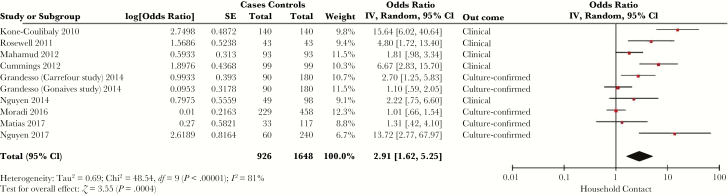
The risk of cholera with a household contact with cholera was evaluated in 25 studies [20, 26, 32, 33, 35, 45, 47, 49, 51, 56, 57, 61, 63, 65, 72, 73, 75, 82, 83, 87, 96, 97, 105, 107, 110]. Fifteen studies of household contact included a multivariable model [26, 32, 33, 35, 49, 56, 65, 72, 73, 75, 82, 83, 96, 97, 105], of which 9 were eligible for meta-analysis (Figure 6) [35, 49, 65, 72, 73, 75, 82, 83, 96]. The SOR of cholera with a household contact with cholera was 2.91 (95% CI, 1.62–5.25), with I2 = 81% (Q test P < .00001). The 6 studies excluded from meta-analysis used hospital or clinic controls with either noncholera diarrhea or without diarrhea [26, 32, 33, 56, 97, 105], and all reported an association between cholera and a household contact with cholera.
Figure 6.
Forest plot of studies included in meta-analysis assessing whether presence of a household contact with cholera was associated with symptomatic cholera. The summary odds ratio was calculated using random effects models. Heterogeneity is described using the Cochran Q test and the I2 statistic. Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
Two studies looked specifically at sharing a latrine with a person with cholera; 1 found a significantly increased risk and 1 found no significant association [49, 105].
Two studies found that individuals living in close proximity to other cases had the greatest risk of cholera [38, 71].
Hygiene
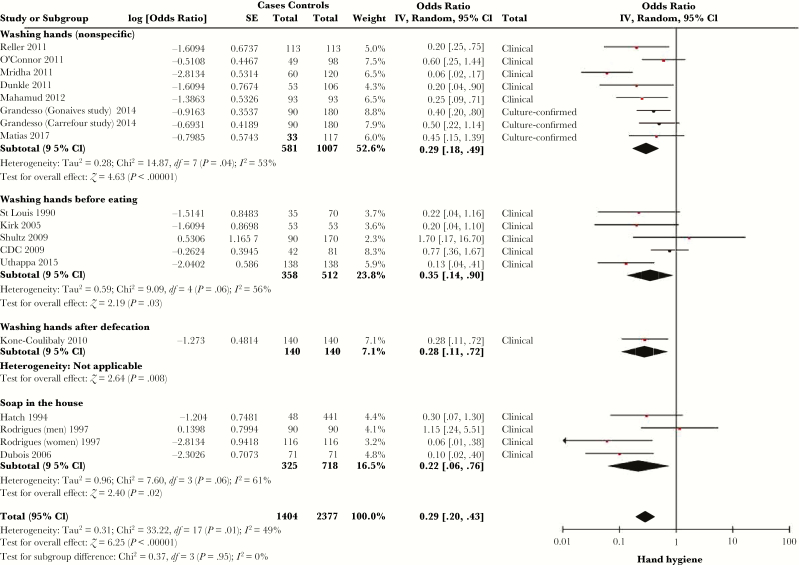
Hand hygiene was assessed in a number of ways, including handwashing before eating [20, 27, 54, 56, 60, 64, 79, 87, 94, 97, 102, 106, 109, 116], handwashing after defecation [20, 27, 35, 37, 40, 65, 79, 102, 106, 110], nonspecific handwashing [25, 39–41, 49, 61, 72, 73, 78, 86, 88, 91], and presence of soap in the home [54, 64, 72, 94, 97, 113, 119]. The vast majority of studies measuring handwashing relied on self-report. Sixteen studies, stratified by type of hand hygiene measured, met criteria for meta-analysis (Figure 7) [27, 40, 41, 49, 54, 64, 65, 72, 73, 78, 86, 91, 94, 106, 109, 116]. The SOR of cholera with hand hygiene was 0.29 (95% CI, .20–.43), with I2 = 49% (Q test P = .01), with no significant difference between the subgroups (P = .95). Three other studies that included handwashing after defecation in multivariable analyses but did not report effect measures found no significant association with cholera [35, 37, 79].
Figure 7.
Forest plot of studies included in meta-analysis assessing whether handwashing was associated with symptomatic cholera. Studies were stratified by whether they assessed handwashing before eating, nonspecific handwashing, or presence of soap in the household. The summary odds ratio was calculated using random effects models. Heterogeneity is described using the Cochran Q test and the I2 statistic. Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
Of 4 studies evaluating the role of bathing in unsafe water, 2 found increased risk of cholera and 2 found no significant association [10, 19, 82, 109]. One multivariable analysis found no significant association between washing utensils with unsafe water and cholera risk [96].
Attending a Gathering or Funeral
Fourteen included studies evaluated risk of cholera after attending a large gathering or funeral [10, 27, 51, 56, 63, 65, 79, 83, 96, 97, 105, 107, 112, 116]. Seven of these met criteria for meta-analysis (Supplementary Figure 3) [27, 65, 79, 83, 96, 105, 116]. The SOR of cholera after attending a large gathering or funeral was 2.42 (95% CI, 1.43–4.09), with I2 = 38% (Q test P = .14).
Blood Type and Genetic Risk
Multiple studies found higher risk of symptomatic cholera among patients with blood group O [16, 28, 29, 48, 53], although those with blood group O appear to have lower risk of initial colonization by V. cholerae [52]. Among people with blood group A or B, the Lewis blood group Le(a+b–) was associated with symptomatic cholera in 1 study [14].
One study further explored the role of genetics in the risk of symptomatic cholera [62]. In a genome-wide association study, the authors noted evidence of natural selection in a Bengali population on genes in the NF-κB signaling pathway, which is implicated in proinflammatory response to V. cholera lipopolysaccharide. They went on to conduct a case-control study, finding that these genes were strongly associated with susceptibility to symptomatic cholera.
Gastric Acidity
Decreased gastric acid levels have been implicated as a risk factor for cholera infection in several ways. In an experimental trial of cholera inoculation, buffering gastric acid led to a lower required infectious dose of V. cholerae, from 108 organisms to 104 [58]. Observational studies have found lower gastric acid levels in symptomatic cholera compared to noncholera diarrhea both during and after infection [44, 99, 117]. In one multivariable model, a positive Helicobacter pylori immunoglobulin G was associated with risk of cholera [31]. One study found higher risk of cholera among those who have had gastric surgery, although another did not [15, 69]. Antacid use was associated with cholera risk in 1 study, but not in 2 others where antacid use was low [69, 73, 111].
Four multivariable analyses assessed use of acidic additives to food. Three of these reported an effect measure and were candidates for meta-analysis (Supplementary Figure 4) [94, 95, 109]. The summary odds ratio of cholera with not using the acidic additive was 7.49 (95% CI, 2.10–26.68), with I2 = 72% (Q test P = .03).
Human Immunodeficiency Virus
Two case-control studies, 1 using hospital controls and 1 with community controls, found an increased risk of cholera among people with human immunodeficiency virus (HIV) infection [104, 118].
Prior Infection and Natural Immunity
A trial of experimental inoculation of volunteers with V. cholerae O1 demonstrated natural immunity to clinical infection when rechallenged 3 years later [67]. A case-control study in Bangladesh found a reduced risk of subsequent V. cholerae O1 infection with prior V. cholerae O1 infection, but no significant protection for V. cholerae O139 or cross-protection between serogroups [12]. In 2 studies, higher baseline vibriocidal titers were associated with protection from clinical infection of V. cholerae O1 in household contacts of cholera cases, but not for V. cholerae O139 [77, 100]. Another study found that vibriocidal titers did not predict risk of asymptomatic vs symptomatic illness among cholera household contacts with a positive stool culture for cholera, although lipopolysaccharide-specific antibodies were higher in symptomatic patients [53].
Sensitivity Analysis
There were no significant subgroup differences for any of the variables included in meta-analysis when stratifying by whether studies used microbiologic confirmation of cholera cases or a clinical case definition, or whether the studies took place in an endemic setting or an epidemic setting.
Bias
A summary of bias within studies can be found in Supplementary Table 3. Funnel plots were generated for all variables included in meta-analysis (Supplementary Figures 5–14). Visual inspection of the funnel plots suggested the presence of publication bias for the following variables: sex, improved water source, water storage, water treatment, street vendors, attendance of a gathering/funeral, and household contact with cholera.
DISCUSSION
This systematic review of 110 studies and >22000 people with cholera identified factors associated with symptomatic infection related to water, sanitation, and hygiene (collectively referred to as WASH), other sources of exposure, socioeconomic status, and intrinsic patient characteristics.
Use of an unimproved water source conferred >3-fold increase in the odds of cholera in meta-analysis, highlighting the importance of a safe water source in cholera control. There was significant heterogeneity between the effects of water source on cholera risk among studies included in meta-analysis, probably reflecting differing degrees of contamination or exposure in different water sources. Use of an open container or bucket for water storage was also significantly associated with cholera, potentially through higher risk of water contamination. Water treatment, either by chlorination or boiling, was associated with lower risk of cholera. There was substantial heterogeneity among studies assessing water treatment, possibly reflecting differences in practice or reporting.
While access to adequate sanitation is likely to minimize risk of water supply contamination, use of latrines did not convincingly impact an individual’s risk of cholera, with the majority of well-controlled studies assessing individual latrine use finding no association with risk of cholera. Access to and use of hygiene measures, sanitation infrastructure, and sharing may impact cholera risk with latrine use, although none of this was definitively evident from the available information.
Handwashing, either before meals or generally, had a similar effect measure for decreased cholera risk as water treatment. This finding is consistent with a recent systematic review of the use of handwashing for prevention of diarrhea more generally [120]. Handwashing after defecation did not convincingly reduce cholera risk for the handwashing person, with 3 of 4 multivariable analyses assessing this variable finding no relationship but not reporting an effect measure. The majority of studies assessing handwashing relied on self-report rather than direct observation, introducing reporting and social desirability bias, such that the findings, while fitting with public health principles, should be interpreted with caution in terms of their specific usefulness to interrupt cholera epidemics. More specific microbiological studies may be needed to better understand the impact of handwashing on the interruption of cholera epidemics. Notably, despite the range of WASH factors that are associated with symptomatic cholera infection by such self-reported studies, a recent systematic review of WASH interventions found that it is not clear which interventions have impact in any given context [121].
Other non-water-related sources of exposure were also implicated in the risk of cholera. Food items, and seafood in particular, were associated with cholera in a number of studies. More generally, eating street food was associated with a 5-fold increase in the odds of cholera in meta-analysis, indicating that interventions with street vendors may be an important pathway to interrupt transmission during an outbreak, although current programmatic guidelines on how to address this are nonspecific [122]. Similarly, attending a large gathering or funeral during an outbreak was associated with cholera risk, and these gatherings may serve as a focus for ongoing cholera transmission. Additionally, having a household contact with cholera was associated with significantly increased risk of cholera in well-controlled studies. There was considerable heterogeneity between studies assessing both street vendor food and a household contact with cholera, indicating that these factors likely vary by setting. For example, local practices surrounding caring for an ill household contact, type of street food, and vendor practices may alter the magnitude of association between these factors and cholera risk.
Several socioeconomic factors were independently associated with risk of cholera. Both direct income and composite wealth were closely linked to cholera risk in all multivariable analyses assessing these variables. Education (in particular, less than secondary education) was associated with cholera risk. Taken together, these findings strongly point to the need to explore how poverty reduction, social support, and educational infrastructure could be used as interventions with a goal of cholera control.
Some intrinsic patient factors were also associated with cholera risk. Several genetic features, related to blood group O and genes in the NF-κB pathway, are associated with increased risk of symptomatic cholera, and appear to have contributed to natural selection in parts of Asia. Risk of cholera by age varied substantially between studies and may reflect differences in populations living in epidemic vs endemic areas, where natural immunity may play a role. Breastfeeding infants showed evidence of protection from cholera, probably as a result of passive immunity from breast milk and reduced likelihood of direct exposure. Reduced gastric acidity, from H. pylori infection or otherwise, decreased the required infectious dose of cholera, thus increasing the risk of symptomatic infection. Finally, HIV infection may increase risk of cholera, although there are only 2 studies assessing this association and both were at high risk for selection bias.
There was evidence of publication bias for some variables included in meta-analysis, including sex, water source, water treatment, water storage, street vendor food exposure, attending a large gathering or funeral, and household cholera contacts. The summary effect measures for these risk factors may thus be biased away from the null because negative associations are less likely to be reported or published. In some cases, studies that would have otherwise been eligible for meta-analysis did not report an effect measure. While generally there were relatively equal numbers of these studies reporting a null or significant association in one direction or the other, this may have introduced some bias into the resulting summary effect measures. We hypothesized that there might be differential risk associated with some variables when stratifying by whether or not cases were culture-confirmed, but this was not the case for any assessed risk factor included in meta-analysis. Regional differences may also contribute to heterogeneity in risk factors, although we did not find any differences based on epidemic or endemic setting in particular.
In sum, we identified potential risk factors for symptomatic cholera infection ranging from environmental characteristics directly impacting exposure risk, to socioeconomic factors such as wealth or education, to intrinsic patient factors such as specific genetic features or gastric acidity. Many of these potential risk factors can and have been intervened upon in cholera control efforts [122], although the relative efficacy and effectiveness of interventions targeting these factors as public health interventions remains a key gap in the literature [121]. Future studies should deepen our understanding of specific risk factors in water, sanitation, personal hygiene, and food hygiene, so that public health campaigns can go beyond generic advice often known as “key messages” and get deeper into specific recommendations that are known to reduce the risk of cholera. Additionally, our findings suggest that some high-risk groups (eg, people living with HIV) may warrant special attention during the cholera response. Ultimately, a combination of interventional approaches that target various groups with risk-adapted intensities may prove to be the most effective strategy.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by grants from the Bill & Melinda Gates Foundation (grant number OPP1148213 to L. C. I.) and the National Institute of Allergy and Infectious Diseases (grant number R01AI099243 to L. C. I.).
Supplement sponsorship. This work is part of a supplement sponsored by the Bill and Melinda Gates Foundation.
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Clemens JD, Nair GB, Ahmed T, Qadri F, Holmgren J. Cholera. Lancet 2017; 390:1539–49. [DOI] [PubMed] [Google Scholar]
- 2. Snow J. On the mode of communication of cholera. 2nd ed. London: John Churchill, 1855. [Google Scholar]
- 3. Brody H, Rip MR, Vinten-Johansen P, Paneth N, Rachman S. Map-making and myth-making in Broad Street: the London cholera epidemic, 1854. Lancet 2000; 356:64–8. [DOI] [PubMed] [Google Scholar]
- 4. Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis 2015; 9:e0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Cholera, 2015. Wkly Epidemiol Rec 2016; 38:433–40. [PubMed] [Google Scholar]
- 6. Bi Q, Ferreras E, Pezzoli L, et al. Oral Cholera Vaccine Working Group of the Global Task Force on Cholera Control Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17:1080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162:777–84. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization Global Task Force on Cholera Control. Cholera outbreak: assessing the outbreak response and improving preparedness. Geneva, Switzerland: WHO, 2004. [Google Scholar]
- 9. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 5 January 2018.
- 10. Acosta CJ, Galindo CM, Kimario J, et al. Cholera outbreak in southern Tanzania: risk factors and patterns of transmission. Emerg Infect Dis 2001; 7:583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alam AN, Goff PA, Abdal NM, Rashid MA, Rahaman MM. Serum ferritin and cholera. A prospective study. Trop Geogr Med 1991; 43:12–6. [PubMed] [Google Scholar]
- 12. Ali M, Emch M, Park JK, Yunus M, Clemens J. Natural cholera infection-derived immunity in an endemic setting. J Infect Dis 2011; 204:912–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anh DD, Lopez AL, Thiem VD, et al. Use of oral cholera vaccines in an outbreak in Vietnam: a case control study. PLoS Negl Trop Dis 2011; 5:e1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arifuzzaman M, Ahmed T, Rahman MA, et al. Individuals with Le(a+b-) blood group have increased susceptibility to symptomatic Vibrio cholerae O1 infection. PLoS Neglected Trop Dis 2011; 5:e1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baine WB, Mazzotti M, Greco D, et al. Epidemiology of cholera in Italy in 1973. Lancet 1974; 2:1370–4. [DOI] [PubMed] [Google Scholar]
- 16. Barua D, Paguio AS. ABO blood groups and cholera. Ann Hum Biol 1977; 4:489–92. [DOI] [PubMed] [Google Scholar]
- 17. Beatty ME, Jack T, Sivapalasingam S, et al. An outbreak of vibrio cholerae O1 infections on Ebeye Island, Republic of the Marshall Islands, associated with use of an adequately chlorinated water source. Clin Infect Dis 2004; 38:1–9. [DOI] [PubMed] [Google Scholar]
- 18. Bhunia R, Ramakrishnan R, Hutin Y, Gupte MD. Cholera outbreak secondary to contaminated pipe water in an urban area, West Bengal, India, 2006. Indian J Gastroenterol 2009; 28:62–4. [DOI] [PubMed] [Google Scholar]
- 19. Birmingham ME, Lee LA, Ndayimirije N, et al. Epidemic cholera in Burundi: patterns of transmission in the Great Rift Valley Lake region. Lancet 1997; 349:981–5. [DOI] [PubMed] [Google Scholar]
- 20. Biswas DK, Bhunia R, Maji D, Das P. Contaminated pond water favors cholera outbreak at Haibatpur village, Purba Medinipur district, West Bengal, India. J Trop Med 2014; 2014:764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blake PA, Rosenberg ML, Costa JB, Ferreira PS, Guimaraes CL, Gangarosa EJ. Cholera in Portugal, 1974.I. modes of transmission. Am J Epidemiol 1977; 105:337–43. [DOI] [PubMed] [Google Scholar]
- 22. Blake PA, Rosenberg ML, Florencia J, Costa JB, do Prado Quintino L, Gangarosa EJ. Cholera in Portugal, 1974. II. Transmission by bottled mineral water. Am J Epidemiol 1977; 105:344–8. [DOI] [PubMed] [Google Scholar]
- 23. Blake PA, Allegra DT, Snyder JD, et al. Cholera—a possible endemic focus in the United States. N Engl J Med 1980; 302:305–9. [DOI] [PubMed] [Google Scholar]
- 24. Boyce TG, Mintz ED, Greene KD, et al. Vibrio cholerae O139 Bengal infections among tourists to Southeast Asia: an intercontinental foodborne outbreak. J Infect Dis 1995; 172:1401–4. [DOI] [PubMed] [Google Scholar]
- 25. Burrowes V, Perin J, Monira S, et al. Risk factors for household transmission of vibrio cholerae in Dhaka, Bangladesh (CHoBI7 Trial). Am J Trop Med Hyg 2017; 96:1382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cardenas V, Saad C, Varona M, Linero M. Waterborne cholera in Riohacha, Colombia, 1992. Bull Pan Am Health Organ 1993; 27:313–30. [PubMed] [Google Scholar]
- 27. Centers for Disease Control and Prevention. Cholera outbreak—southern Sudan, 2007. MMWR Morb Mortal Wkly Rep 2009; 58:337–41. [PubMed] [Google Scholar]
- 28. Chaudhuri A, DasAdhikary CR. Possible role of blood-group secretory substances in the aetiology of cholera. Trans R Soc Trop Med Hyg 1978; 72:664–5. [DOI] [PubMed] [Google Scholar]
- 29. Clemens JD, Sack DA, Harris JR, et al. ABO blood groups and cholera: new observations on specificity of risk and modification of vaccine efficacy. J Infect Dis 1989; 159:770–3. [DOI] [PubMed] [Google Scholar]
- 30. Clemens JD, Sack DA, Harris JR, et al. Breast feeding and the risk of severe cholera in rural Bangladeshi children. Am J Epidemiol 1990; 131:400–11. [DOI] [PubMed] [Google Scholar]
- 31. Clemens J, Rao M, Sack D, et al. Impaired immune response to natural infection as a correlate of vaccine failure in a field trial of killed oral cholera vaccines. Am J Epidemiol 1995; 142:759–64. [DOI] [PubMed] [Google Scholar]
- 32. Colombara DV, Cowgill KD, Faruque AS. Risk factors for severe cholera among children under five in rural and urban Bangladesh, 2000–2008: a hospital-based surveillance study. PLoS One 2013; 8:e54395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colombara DV, Faruque AS, Cowgill KD, Mayer JD. Risk factors for diarrhea hospitalization in Bangladesh, 2000–2008: a case-case study of cholera and shigellosis. BMC Infect Dis 2014; 14:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Conroy RM, Meegan ME, Joyce T, McGuigan K, Barnes J. Solar disinfection of drinking water protects against cholera in children under 6 years of age. Arch Dis Child 2001; 85:293–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cummings MJ, Wamala JF, Eyura M, et al. A cholera outbreak among semi-nomadic pastoralists in northeastern Uganda: epidemiology and interventions. Epidemiol Infect 2012; 140:1376–85. [DOI] [PubMed] [Google Scholar]
- 36. Datta SS, Ramakrishnan R, Murhekar MV. A rapidly-progressing outbreak of cholera in a shelter-home for mentally-retarded females, amta-II block, Howrah, West Bengal, India. J Health Popul Nutr 2012; 30:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Guzman A, de los Reyes VC, Sucaldito MN, Tayag E. Availability of safe drinking-water: the answer to cholera outbreak? Nabua, Camarines Sur, Philippines, 2012. Western Pac Surveill Response J 2015; 6:12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Debes AK, Ali M, Azman AS, Yunus M, Sack DA. Cholera cases cluster in time and space in Matlab, Bangladesh: implications for targeted preventive interventions. Int J Epidemiol 2016; 45:2134–9. [DOI] [PubMed] [Google Scholar]
- 39. Deepthi R, Sandeep SR, Rajini M, Rajeshwari H, Shetty A. Cholera outbreak in a village in south India—timely action saved lives. J Infect Public Health 2013; 6:35–40. [DOI] [PubMed] [Google Scholar]
- 40. DuBois AE, Sinkala M, Kalluri P, Makasa-Chikoya M, Quick RE. Epidemic cholera in urban Zambia: hand soap and dried fish as protective factors. Epidemiol Infect 2006; 134:1226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dunkle SE, Mba-Jonas A, Loharikar A, et al. Epidemic cholera in a crowded urban environment, Port-au-Prince, Haiti. Emerg Infect Dis 2011; 17:2143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eberhart-Phillips J, Besser RE, Tormey MP, et al. An outbreak of cholera from food served on an international aircraft. Epidemiol Infect 1996; 116:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Emch M. Diarrheal disease risk in Matlab, Bangladesh. Soc Sci Med 1999; 49:519–30. [DOI] [PubMed] [Google Scholar]
- 44. Evans CA, Gilman RH, Rabbani GH, Salazar G, Ali A. Gastric acid secretion and enteric infection in Bangladesh. Trans R Soc Trop Med Hyg 1997; 91:681–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fatiregun AA, Ajayi IO, Isere EE. Cholera outbreak in a southwest community of Nigeria: investigation of risk factors and evaluation of a district surveillance system. West Afr J Med 2013; 32:173–9. [PubMed] [Google Scholar]
- 46. Finelli L, Swerdlow D, Mertz K, Ragazzoni H, Spitalny K. Outbreak of cholera associated with crab brought from an area with epidemic disease. J Infect Dis 1992; 166:1433–5. [DOI] [PubMed] [Google Scholar]
- 47. Fukuda JM, Yi A, Chaparro L, Campos M, Chea E. Clinical characteristics and risk factors for Vibrio cholerae infection in children. J Pediatr 1995; 126:882–6. [DOI] [PubMed] [Google Scholar]
- 48. Glass RI, Holmgren J, Haley CE, et al. Predisposition for cholera of individuals with O blood group. Possible evolutionary significance. Am J Epidemiol 1985; 121:791–6. [DOI] [PubMed] [Google Scholar]
- 49. Grandesso F, Allan M, Jean-Simon PS, et al. Risk factors for cholera transmission in Haiti during inter-peak periods: insights to improve current control strategies from two case-control studies. Epidemiol Infect 2014; 142:1625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gunn RA, Kimball AM, Pollard RA, et al. Bottle feeding as a risk factor for cholera in infants. Lancet 1979; 2:730–2. [DOI] [PubMed] [Google Scholar]
- 51. Gunnlaugsson G, Einarsdottir J, Angulo FJ, Mentambanar SA, Passa A, Tauxe RV. Funerals during the 1994 cholera epidemic in Guinea-Bissau, West Africa: the need for disinfection of bodies of persons dying of cholera. Epidemiol Infect 1998; 120:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Harris JB, Khan AI, LaRocque RC, et al. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infect Immun 2005; 73:7422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Harris JB, LaRocque RC, Chowdhury F, et al. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis 2008; 2:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hatch DL, Waldman RJ, Lungu GW, Piri C. Epidemic cholera during refugee resettlement in Malawi. Int J Epidemiol 1994; 23:1292–9. [DOI] [PubMed] [Google Scholar]
- 55. Haus-Cheymol R, Theodose R, Quilici ML, et al. A cluster of acute diarrhea suspected to be cholera in French travelers in Haiti, December 2010. J Travel Med 2012; 19:189–91. [DOI] [PubMed] [Google Scholar]
- 56. Hoge CW, Bodhidatta L, Echeverria P, Deesuwan M, Kitporka P. Epidemiologic study of Vibrio cholerae O1 and O139 in Thailand: at the advancing edge of the eighth pandemic. Am J Epidemiol 1996; 143:263–8. [DOI] [PubMed] [Google Scholar]
- 57. Holmberg SD, Harris JR, Kay DE, et al. Foodborne transmission of cholera in Micronesian households. Lancet 1984; 1:325–8. [DOI] [PubMed] [Google Scholar]
- 58. Hornick RB, Music SI, Wenzel R, et al. The broad street pump revisited: response of volunteers to ingested cholera vibrios. Bull N Y Acad Med 1971; 47:1181–91. [PMC free article] [PubMed] [Google Scholar]
- 59. Hughes JM, Boyce JM, Levine RJ, et al. Epidemiology of eltor cholera in rural Bangladesh: importance of surface water in transmission. Bull World Health Organ 1982; 60:395–04. [PMC free article] [PubMed] [Google Scholar]
- 60. Hutin Y, Luby S, Paquet C. A large cholera outbreak in Kano City, Nigeria: the importance of hand washing with soap and the danger of street-vended water. J Water Health 2003; 1:45–52. [PubMed] [Google Scholar]
- 61. Ishaku A, Shadrack BE, Ajumobi O, Olayinka A, Nguku P. Investigation of cholera outbreak in an urban north central Nigerian community—the Akwanga experience. Public Health Research 2014; 4:7–12. [Google Scholar]
- 62. Karlsson EK, Harris JB, Tabrizi S, et al. Natural selection in a Bangladeshi population from the cholera-endemic Ganges river delta. Sci Transl Med 2013; 5:192ra86 -ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Killewo JZ, Amsi DM, Mhalu FS. An investigation of a cholera epidemic in Butiama village of the Mara region, Tanzania. J Diarrhoeal Dis Res 1989; 7:13–7. [PubMed] [Google Scholar]
- 64. Kirk MD, Kiedrzynski T, Johnson E, Elymore A, Wainiqolo I. Risk factors for cholera in Pohnpei during an outbreak in 2000: lessons for Pacific countries and territories. Pacific Health Dialog 2005; 12:17–22. [PubMed] [Google Scholar]
- 65. Kone-Coulibaly A, Tshimanga M, Shambira G, et al. Risk factors associated with cholera in Harare City, Zimbabwe, 2008. East Afr J Public Health 2010; 7:311–7. [DOI] [PubMed] [Google Scholar]
- 66. Koo D, Aragon A, Moscoso V, et al. Epidemic cholera in Guatemala, 1993: transmission of a newly introduced epidemic strain by street vendors. Epidemiol Infect 1996; 116:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Levine MM, Black RE, Clements ML, Cisneros L, Nalin DR, Young CR. Duration of infection-derived immunity to cholera. J Infect Dis 1981; 143:818–20. [DOI] [PubMed] [Google Scholar]
- 68. Lim-Quizon MC, Benabaye RM, White FM, Dayrit MM, White ME. Cholera in metropolitan Manila: foodborne transmission via street vendors. Bull World Health Organ 1994; 72:745–9. [PMC free article] [PubMed] [Google Scholar]
- 69. Lowry PW, McFarland LM, Peltier BH, et al. Vibrio gastroenteritis in Louisiana: a prospective study among attendees of a scientific congress in New Orleans. J Infect Dis 1989; 160:978–84. [DOI] [PubMed] [Google Scholar]
- 70. Lucas ME, Deen JL, von Seidlein L, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med 2005; 352:757–67. [DOI] [PubMed] [Google Scholar]
- 71. Luquero FJ, Banga CN, Remartinez D, Palma PP, Baron E, Grais RF. Cholera epidemic in Guinea-Bissau (2008): the importance of “place.” PLoS One 2011; 6:e19005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mahamud AS, Ahmed JA, Nyoka R, et al. Epidemic cholera in Kakuma refugee camp, Kenya, 2009: the importance of sanitation and soap. J Infect Dev Ctries 2012; 6:234–41. [DOI] [PubMed] [Google Scholar]
- 73. Matias WR, Teng JE, Hilaire IJ, Harris JB, Franke MF, Ivers LC. Household and individual risk factors for cholera among cholera vaccine recipients in rural Haiti. Am J Trop Med Hyg 2017; 97:436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McIntyre RC, Tira T, Flood T, Blake PA. Modes of transmission of cholera in a newly infected population on an atoll: implications for control measures. Lancet 1979; 1:311–4. [DOI] [PubMed] [Google Scholar]
- 75. Moradi G, Rasouli MA, Mohammadi P, Elahi E, Barati H. A cholera outbreak in Alborz Province, Iran: a matched case-control study. Epidemiol Health 2016; 38:e2016018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Moren A, Stefanaggi S, Antona D, et al. Practical field epidemiology to investigate a cholera outbreak in a Mozambican refugee camp in Malawi, 1988. Am J Trop Med Hyg 1991; 94:1–7. [PubMed] [Google Scholar]
- 77. Mosley WH, Ahmad S, Benenson AS, Ahmed A. The relationship of vibriocidal antibody titre to susceptibility to cholera in family contacts of cholera patients. Bull World Health Organ 1968; 38:777–85. [PMC free article] [PubMed] [Google Scholar]
- 78. Mridha P, Biswas AK, Ramakrishnan R, Murhekar MV. The 2010 outbreak of cholera among workers of a jute mill in Kolkata, West Bengal, India. J Health Popul Nutr 2011; 29:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mugoya I, Kariuki S, Galgalo T, et al. Rapid spread of Vibrio cholerae O1 throughout Kenya, 2005. Am J Trop Med Hyg 2008; 78:527–33. [PubMed] [Google Scholar]
- 80. Mujica OJ, Quick RE, Palacios AM, et al. Epidemic cholera in the Amazon: the role of produce in disease risk and prevention. J Infect Dis 1994; 169:1381–4. [DOI] [PubMed] [Google Scholar]
- 81. Mukherjee R, Halder D, Saha S, et al. Five pond-centred outbreaks of cholera in villages of West Bengal, India: evidence for focused interventions. J Health Popul Nutr 2011; 29:421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nguyen TV, Pham QD, Do QK, et al. Cholera returns to southern Vietnam in an outbreak associated with consuming unsafe water through iced tea: a matched case-control study. PLoS Negl Trop Dis 2017; 11:e0005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nguyen VD, Sreenivasan N, Lam E, et al. Cholera epidemic associated with consumption of unsafe drinking water and street-vended water—Eastern Freetown, Sierra Leone, 2012. Am J Trop Med Hyg 2014; 90:518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Noorhaida U, Kamaluddin A, Fadzilah K, et al. Cholera outbreak in Pantai Rombang village, Tanjung Kling, Melaka, Malaysia, 2007. Medical Journal of Malaysia 2010; 65:78. [Google Scholar]
- 85. Nsagha DS, Atashili J, Fon PN, Tanue EA, Ayima CW, Kibu OD. Assessing the risk factors of cholera epidemic in the Buea Health District of Cameroon. BMC Public Health 2015; 15:1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. O’Connor KA, Cartwright E, Loharikar A, et al. Risk factors for disease early in the 2010 Haiti cholera epidemic. Emerg Infect Dis 2011; 17:2136–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Opare J, Ohuabunwo C, Afari E, et al. Outbreak of cholera in the East Akim municipality of Ghana following unhygienic practices by small-scale gold miners, November 2010. Ghana Med J 2012; 46:116–23. [PMC free article] [PubMed] [Google Scholar]
- 88. Quick RE, Thompson BL, Zuniga A, et al. Epidemic cholera in rural El Salvador: risk factors in a region covered by a cholera prevention campaign. Epidemiol Infect 1995; 114:249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Qureshi K, Molbak K, Sandstrom A, et al. Breast milk reduces the risk of illness in children of mothers with cholera: observations from an epidemic of cholera in Guinea-Bissau. Pediatr Infect Dis J 2006; 25:1163–6. [DOI] [PubMed] [Google Scholar]
- 90. Rahman KM, Duggal P, Harris JB, et al. Familial aggregation of Vibrio cholerae–associated infection in Matlab, Bangladesh. J Health Popul Nutr 2009; 27:733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Reller ME, Mong YJ, Hoekstra RM, Quick RE. Cholera prevention with traditional and novel water treatment methods: an outbreak investigation in Fort-Dauphin, Madagascar. Am J Public Health 2001; 91:1608–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ries AA, Vugia DJ, Beingolea L, et al. Cholera in Piura, Peru: a modern urban epidemic. J Infect Dis 1992; 166:1429–33. [DOI] [PubMed] [Google Scholar]
- 93. Riley LW, Waterman SH, Faruque AS, Huq MI. Breast-feeding children in the household as a risk factor for cholera in rural Bangladesh: an hypothesis. Trop Geogr Med 1987; 39:9–14. [PubMed] [Google Scholar]
- 94. Rodrigues A, Brun H, Sandstrom A. Risk factors for cholera infection in the initial phase of an epidemic in Guinea-Bissau: protection by lime juice. Am J Trop Med Hyg 1997; 57:601–4. [DOI] [PubMed] [Google Scholar]
- 95. Rodrigues A, Sandström A, Cá T, Steinsland H, Jensen H, Aaby P. Protection from cholera by adding lime juice to food—results from community and laboratory studies in Guinea-Bissau, West Africa. Trop Med Int Health 2000; 5:418–22. [DOI] [PubMed] [Google Scholar]
- 96. Rosewell A, Dagina R, Murhekar M, et al. Vibrio cholerae O1 in 2 coastal villages, Papua New Guinea. Emerg Infect Dis 2011; 17:154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rosewell A, Addy B, Komnapi L, et al. Cholera risk factors, Papua New Guinea, 2010. BMC Infect Dis 2012; 12:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ryder RW, Rahman AS, Alim AR, Yunis MD, Houda BS. An outbreak of nosocomial cholera in a rural Bangladesh hospital. J Hosp Infect 1986; 8:275–82. [DOI] [PubMed] [Google Scholar]
- 99. Sack GH Jr, Pierce NF, Hennessey KN, Mitra RC, Sack RB, Mazumder DN. Gastric acidity in cholera and noncholera diarrhoea. Bull World Health Organ 1972; 47:31–6. [PMC free article] [PubMed] [Google Scholar]
- 100. Saha D, LaRocque RC, Khan AI, et al. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J Infect Dis 2004; 189:2318–22. [DOI] [PubMed] [Google Scholar]
- 101. Saha A, Hayen A, Ali M, et al. Socioeconomic risk factors for cholera in different transmission settings: an analysis of the data of a cluster randomized trial in Bangladesh. Vaccine 2017; 35:5043–9. [DOI] [PubMed] [Google Scholar]
- 102. Sasaki S, Suzuki H, Igarashi K, Tambatamba B, Mulenga P. Spatial analysis of risk factor of cholera outbreak for 2003–2004 in a peri-urban area of Lusaka, Zambia. Am J Trop Med Hyg 2008; 79:414–21. [PubMed] [Google Scholar]
- 103. Seas C, Alarcon M, Aragon JC, et al. Surveillance of bacterial pathogens associated with acute diarrhea in Lima, Peru. Int J Infect Dis 2000; 4:96–9. [DOI] [PubMed] [Google Scholar]
- 104. Sema Baltazar C, Langa JP, Dengo Baloi L, et al. Multi-site cholera surveillance within the African cholera surveillance network shows endemicity in Mozambique, 2011–2015. PLoS Negl Trop Dis 2017; 11:e0005941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shapiro RL, Otieno MR, Adcock PM, et al. Transmission of epidemic Vibrio cholerae O1 in rural western Kenya associated with drinking water from Lake Victoria: an environmental reservoir for cholera?Am J Trop Med Hyg 1999; 60:271–6. [DOI] [PubMed] [Google Scholar]
- 106. Shultz A, Omollo JO, Burke H, et al. Cholera outbreak in Kenyan refugee camp: risk factors for illness and importance of sanitation. Am J Trop Med Hyg 2009; 80:640–5. [PubMed] [Google Scholar]
- 107. Siddiqui FJ, Bhutto NS, von Seidlein L, et al. Consecutive outbreaks of Vibrio cholerae O139 and V. cholerae O1 cholera in a fishing village near Karachi, Pakistan. Trans R Soc Trop Med Hyg 2006; 100:476–82. [DOI] [PubMed] [Google Scholar]
- 108. Sinclair GS, Mphahlele M, Duvenhage H, Nichol R, Whitehorn A, Küstner HG. Determination of the mode of transmission of cholera in Lebowa. An epidemiological investigation. S Afr Med J 1982; 62:753–5. [PubMed] [Google Scholar]
- 109. St Louis ME, Porter JD, Helal A, et al. Epidemic cholera in West Africa: the role of food handling and high-risk foods. Am J Epidemiol 1990; 131:719–28. [DOI] [PubMed] [Google Scholar]
- 110. Sur D, Deen JL, Manna B, et al. The burden of cholera in the slums of Kolkata, India: data from a prospective, community based study. Arch Dis Child 2005; 90:1175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Swaddiwudhipong W, Kunasol P. An outbreak of nosocomial cholera in a 755-bed hospital. Trans R Soc Trop Med Hyg 1989; 83:279–81. [DOI] [PubMed] [Google Scholar]
- 112. Swerdlow DL, Mintz ED, Rodriguez M, et al. Waterborne transmission of epidemic cholera in Trujillo, Peru: lessons for a continent at risk. Lancet 1992; 340:28–33. [DOI] [PubMed] [Google Scholar]
- 113. Swerdlow DL, Malenga G, Begkoyian G, et al. Epidemic cholera among refugees in Malawi, Africa: treatment and transmission. Epidemiol Infect 1997; 118:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tauxe RV, Holmberg SD, Dodin A, Wells JV, Blake PA. Epidemic cholera in Mali: high mortality and multiple routes of transmission in a famine area. Epidemiol Infect 1988; 100:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ujjiga TT, Wamala JF, Mogga JJ, et al. Risk factors for sustained cholera transmission, Juba County, South Sudan, 2014. Emerg Infect Dis 2015; 21:1849–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Uthappa CK, Allam RR Nalini C, et al. An outbreak of cholera in Medipally village, Andhra Pradesh, India, 2013. J Health Popul Nutr 2015; 33:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Van Loon FP, Clemens JD, Shahrier M, et al. Low gastric acid as a risk factor for cholera transmission: application of a new non-invasive gastric acid field test. J Clin Epidemiol 1990; 43:1361–7. [DOI] [PubMed] [Google Scholar]
- 118. von Seidlein L, Wang XY, Macuamule A, et al. Is HIV infection associated with an increased risk for cholera? Findings from a case-control study in Mozambique. Trop Med Int Health 2008; 13:683–8. [DOI] [PubMed] [Google Scholar]
- 119. Weber JT, Mintz ED, Canizares R, et al. Epidemic cholera in Ecuador: multidrug-resistance and transmission by water and seafood. Epidemiol Infect 1994; 112:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ejemot-Nwadiaro RI, Ehiri JE, Arikpo D, Meremikwu MM, Critchley JA. Hand washing promotion for preventing diarrhoea. Cochrane Database Syst Rev 2015:1–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Taylor DL, Kahawita TM, Cairncross S, Ensink JH. The impact of water, sanitation and hygiene interventions to control cholera: a systematic review. PLoS One 2015; 10:e0135676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. United Nations Children’s Fund. Cholera toolkit https://www.unicef.org/cholera_toolkit/Cholera-Toolkit-2017.pdf. Accessed 26 March 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.