Abstract
The concept of subjective value is central to current neurobiological views of economic decision-making. Much of this work has focused on signals in the ventromedial frontal lobe (VMF) that correlate with the subjective value of a variety of stimuli (e.g., food, monetary gambles), and are thought to support decision-making. However, the neural processes involved in assessing and integrating value information from the attributes of such complex options remain to be defined. Here, we tested the necessary role of VMF in weighting attributes of naturalistic stimuli during value judgments. We asked how distinct attributes of visual artworks influenced the subjective value ratings of subjects with VMF damage, compared to healthy participants and a frontal lobe damaged control group. Subjects with VMF damage were less influenced by the energy (emotion, complexity) and color radiance (warmth, saturation) of the artwork, while they were similar to control groups in considering saliency, balance and concreteness. These dissociations argue that VMF is critical for allowing certain affective content to influence subjective value, while sparing the influence of perceptual or representational information. These distinctions are important for better defining the often-underspecified concept of subjective value and developing more detailed models of the brain mechanisms underlying decision behavior.
Keywords: decision-making, neuropsychology, orbitofrontal cortex, subjective value, ventromedial prefrontal cortex
Introduction
Whether a fresh apple or a favorite painting, the things we enjoy in daily life come in the form of complex experiences with multiple, distinct attributes. Even apparently simple decisions, such as between an apple and an orange, require integrating expectations of multiple sensory features (crisp acidity, juicy sweetness) as well as considerations of cost, health, and so on. How this type of information is weighed and integrated shapes individual preferences, in turn affecting behavior. For example, successful dieters give greater emphasis to health attributes of foods (Hare et al. 2009), and attention to the arousing properties of rewards increases impulsive behavior (Mischel et al. 1989).
Economic models propose that decision-making is guided by a common currency representation of subjective value, allowing disparate options to be judged and compared. Neuroscientists have sought evidence for such value representations in the brain (Kable and Glimcher 2009). A number of studies have shown that activity in the ventromedial frontal lobe (VMF), encompassing ventromedial prefrontal (vmPFC) and orbitofrontal cortex (OFC), reflects the subjective value of diverse options ranging from sips of water to attractive faces (Bartra et al. 2013; Clithero and Rangel 2014), and can predict choice behavior (Tusche et al. 2010; Brown et al. 2011; Levy et al. 2011). These findings support the hypothesis that this area represents value in a common currency, enabling choices that are consistent with subjective preferences (Padoa-Schioppa and Cai 2011; Levy and Glimcher 2012).
However, this view of VMF as central to subjective value representation is not fully consistent with evidence from lesion studies. People with VMF damage make value-based choices that are somewhat internally inconsistent, though these effects are relatively subtle, and the underlying mechanism is unclear (Fellows and Farah 2007; Camille et al. 2011; Henri-Bhargava et al. 2012). In a recent study of the effects of frontal lobe damage on value-based judgment and choice, we found that subjects with VMF damage provided consistent value ratings for artwork over the course of the experiment, and made choices between artworks that were consistent with these ratings. However, the correlation between the value ratings of the VMF and the healthy control group was lower than those between controls and other groups with frontal lobe damage (Vaidya and Fellows 2015). These results argue that VMF is not critical for forming a subjective value estimate for these naturalistic stimuli in a general sense, but may affect what stimuli are considered valuable. Taken with other similar findings (Xia et al. 2015), we hypothesized that VMF damage alters the information used to arrive at a value judgment: that is, we propose that there are dissociable components of subjective value, only some of which rely critically on VMF.
Here we tested whether VMF damage alters the attributes that are drawn upon to construct a value judgment for complex stimuli. We first characterized potentially value-predictive attributes of the artwork stimuli from our original experiment. We then examined the extent to which the value ratings of healthy controls, subjects with VMF damage and frontal lobe damaged controls correlated with these underlying attributes, asking if the weights given to these attributes differed systematically between groups.
Materials and Methods
Subjects
Detailed information regarding the frontal lobe damaged subjects and healthy, demographically matched control subjects who participated in this study has been reported previously in Vaidya and Fellows (2015). Briefly, subjects with focal lesions involving the frontal lobes (N = 33) were recruited from the Cognitive Neuroscience Research Registry at McGill University (Fellows et al. 2008). They were eligible if they had a fixed lesion primarily affecting the frontal lobes, and were classified into groups based on the location of damage by a neurologist who was blind to task performance. The groupings followed broad divisions of this region often used in neuropsychological studies of frontal damage (Stuss et al. 2005). The VMF group consisted of subjects with damage near the medial wall of the frontal lobe beneath the genu of the corpus callosum, including medial and central OFC, frontal polar cortex, and rostral cingulate cortex, consistent with past work using this definition (Stuss and Levine 2002; Fellows 2007). The hypothesized region-of-interest here was the VMF. Patients with frontal lobe damage sparing VMF were thus assigned to a single frontal control (FC) group (N = 20). In the VMF group, there were 2 subjects with bilateral lesions, 2 with lesions of the left hemisphere, and 9 with right hemisphere lesions. In the FC group there were 4 patients with bilateral lesions, 9 with left hemisphere lesions, and 6 with right hemisphere lesions. Lesion location and overlap for these groups are shown in Supplementary Figure 1. Lesion subjects were tested a minimum of 5 months after the injury (median, 4.76 years; range: 5 months to 48 years). The VMF and FC group were comparable in lesion volume (Table 1).
Table 1.
Demographic information for healthy matched controls (HC), frontal lobe damaged controls (FC), and ventromedial frontal lobe damaged subjects (VMF)
| Group | Age (years) | Sex (M/F) | Education (years) | BDI-II | AMNART IQ a | Lesion volume (cc) |
|---|---|---|---|---|---|---|
| HC (N = 27) | 58.8 (12.9) | 9/18 | 16.4 (3.1) | 4.2 (4.9) | 121 (5) | — |
| FC (N = 20) | 56.2 (10.2) | 6/14 | 15.0 (3.8) | 8.8 (4.9)* | 118 (6) | 23 (3–96) |
| VMF (N = 13) | 58.8 (12.0) | 5/8 | 15.8 (2.9) | 8.2 (4.9)* | 119 (6) | 16 (7–77) |
Values represent means with standard deviations in parentheses, except for lesion volume where the median and range are provided.
aNot all subjects were able to complete the AMNART. *P < 0.05, two-tailed t-test against healthy control scores, uncorrected.
Age- and education-matched healthy control subjects (N = 27) were recruited through local advertisement in Montreal. They were free of neurological or psychiatric disease and were not taking any psychoactive drugs. Frontal lobe damaged groups and healthy controls were matched for age and education. FC and VMF groups scored higher than healthy controls in the Beck Depression Inventory (BDI-II), but did not differ from each other on this measure (Table 1).
All participants were asked about visual problems, and corrected visual acuity was assessed with a hand-held Snellen chart at the time of testing. Most subjects had 20/20 vision in one or both eyes. A similar proportion of each group had visual acuity worse than 20/20 (ranging from 20/25 to 20/70) (11 healthy control subjects, 6 FC subjects and 5 VMF subjects; χ2 (2) = 0.74, P = 0.7). Three subjects reported red-green color blindness (2 healthy controls, 1 VMF). Potential effects of decreased acuity on the relationship between attributes and value ratings were tested in control analyses.
A separate group of 15 healthy, artistically experienced subjects (10 females) were recruited to provide artwork attribute ratings. These subjects were recruited by advertisement in the local community. All of these artistically experienced subjects had taken at least 2 art classes (studio art, art history, or art theory) at a high school level, or above. These subjects completed a questionnaire created by Chatterjee et al. (2010) to gauge art experience. Demographic information for these subjects and the results of this questionnaire are provided in Supplementary Table 1. Artistically experienced subjects had no history of neurological or psychiatric illness and were not using psychoactive drugs. All artistically experienced subjects had normal or corrected to normal vision.
All subjects provided written, informed consent in accordance with the Declaration of Helsinki and were paid a nominal fee for their time. The study protocol was approved by the McGill University Research Ethics Board.
Lesion Analysis
Individual lesions were traced from the most recent clinical computed tomography or magnetic resonance imaging onto the standard Montreal Neurological Institute (MNI) brain using MRIcro software (Rorden and Brett 2000) (freely available at www.mccauslandcenter.sc.edu/mricro/) by a neurologist experienced in imaging analysis and blind to task performance. A related software tool (MRIcron) was used to generate lesion overlap images and estimate lesion volumes. FC lesions were due to tumor resection in 13 cases, ischemic stroke in 5 cases, aneurysm rupture in 1 case and hemorrhagic stroke in 1 case. Lesions affecting VMF were due to tumor resection in 9 cases, aneurysm rupture in 3 cases, and hemorrhagic stroke in 1 case.
Neuropsychological Screening
All frontal lobe damaged subjects underwent neuropsychological screening to assess cognitive functions more generally. These subjects completed a task that tested visual memory for faces without explicit instructions (incidental memory) (Bower and Karlin 1974), 2 tests of verbal fluency (Fluency-F, Animals) (Benton et al. 1989), a test of working memory (backwards digit span) (Lezak et al. 2012), and a test of the ability to understand and follow one, two, and three-step verbal instructions (sentence comprehension, similar to the Token Test (Derenzi and Vignolo 1962)). Frontal lobe damaged groups were comparable in their performance on neuropsychological screening tests (Table 2).
Table 2.
Performance on neuropsychological screening tests for frontal lobe damaged controls (FC) and ventromedial frontal lobe damaged subjects (VMF)
| Group | Incidental memory P (Correct) | Fluency –animals | Fluency-F | Backwards digit span | Sentence comprehension P (Correct) |
|---|---|---|---|---|---|
| FC (N = 20) | 0.78 (0.14)a | 19.5 (8.0)a | 11.4 (5.1)a | 2.6 (1.2)a | 0.96 (0.07)a |
| VMF (N = 13) | 0.87 (0.09)a | 20.0 (3.8) | 10.4 (3.9) | 3.3 (1.3) | 0.98 (0.06)a |
Values represent means with standard deviations inparentheses.
aData missing from one patient.
Apparatus
All experimental tests were programmed using E-Prime 1.2 (Psychology Software Tools, Inc.). Stimuli were presented on a 19-inch monitor.
Value Rating Task
Frontal lobe damaged subjects and matched healthy controls were asked to judge how much they wanted 175 visual artworks, presented one at a time. The artwork was sampled from a wide range of styles and periods, including work from both famous and lesser-known artists. This wide range was intended to be potentially appealing to very diverse tastes in art. We tested the reliability of subjects’ responses by asking them to re-rate the value of a subset of 50 artworks after a delay with intervening tasks (mean delay = 46 min, SD = 9 min).
Subjects were asked to rate how much they wanted to have each artwork on a seven-point scale from −3 to 3. On each trial, a central fixation cross was presented for 500 ms. Subjects would then see the artwork in the center of the screen, as well as a prompt above the artwork reading “How much do you want this artwork?” The scale was presented below the artwork, labeled −3 (“Not at all”), 0 (“Indifferent”), and 3 (“Very much.”) Subjects verbally reported their rating to the experimenter, who would then click the corresponding number using a computer mouse. Responses were made verbally to allow for a second manual response condition considered during pilot testing. In the end, for simplicity, this additional manual response condition was not included during data collection in the full study. The first 125 artworks presented to subjects in the rating task were used to generate pairs of artwork for the choice task (see below). The remaining 50 artworks were presented to subjects again after the choice task in the retest phase. The order of artwork presentation was randomized for every subject. This task is described in detail in Vaidya and Fellows (2015).
Assessment of Art Attributes
The Assessment of Art Attributes is an instrument designed by Chatterjee et al. (2010) for quantitative measurement of the component attributes of visual artwork. Artistically experienced subjects were asked to judge artworks on 6 perceptual (balance, color saturation, color temperature, depth, complexity, and stroke), and 6 conceptual-representational attributes (abstraction, animacy, emotion, realism, objective accuracy, and symbolism). This instrument was intended primarily to provide a stronger empirical basis for neuropsychological studies of art production, and hence focuses on attributes thought more likely to be affected by brain damage. Thus, this instrument was well suited for the current investigation of the effects of frontal lobe damage on the weighting of these attributes during value judgment. While artistically experienced raters have better insight into artwork variables, these same attributes are detectable to healthy, artistically naïve subjects, albeit with somewhat lower reliability (Chatterjee et al. 2010). There is thus reason to believe that these attributes were perceptible to healthy controls and frontal lobe damaged groups, even though these subjects were not asked to judge artworks for these features.
Artistically experienced subjects judged the attributes of 175 artworks from the value rating task, as well as 24 artworks from Chatterjee et al. (2010) for the purpose of validating attribute ratings. Before beginning the first block, artistically experienced subjects were shown the 24 artworks from Chatterjee et al. (2010), each displayed serially for 2500 ms, to familiarize subjects with the approximate range of artworks in the study. In each block, artistically experienced subjects rated the entire series of 199 artworks on a five-point scale for a single attribute, with labels below the ends of the scale corresponding to the current attribute (e.g., “less animate” and “more animate”). On each trial, the artwork was preceded by a central fixation cross for 500 ms, followed by central presentation of the artwork below a prompt asking the subjects to judge the current attribute (e.g., “How animate is this artwork?”), and the rating scale. Before each block, artistically experienced subjects were given written instructions and 2 extreme examples for the current attribute (not included in the 199 artworks described above), as in Chatterjee et al. (2010). The experimenter verbally confirmed that the artistically experienced subjects understood the meaning of the attribute in question before the block began. A paper copy of the training examples for the current attribute was placed on the desk in front of the artistically experienced subjects to use as a reference for their ratings throughout the block.
The experiment was separated into 2 experimental sessions, where artistically experienced subjects rated 6 attributes in each session (3 conceptual-representational, 3 perceptual). The sequence of these sessions alternated between subjects. Artistically experienced subjects took 1 h and 20 min to complete each session on average (range: 1–2.5 h). The order of blocks within each session, and the order of artwork presentation within each block, were randomized.
Analysis of Artwork Attribute Ratings
Artwork attribute ratings were averaged across subjects to obtain assessments on all 12 attributes for each artwork. Pearson correlations were used to test if the average attribute ratings of artistically experienced subjects in the current study were related to the average ratings of artistically experienced subjects in Chatterjee et al. (2010) for 24 artworks used in both studies. Inter-rater reliability was assessed through pairwise Pearson correlations between individual artistically experienced subjects’ attribute ratings and the average ratings of the rest of the group with that subject removed.
Pearson correlations were used to test for relationships between attributes for the main set of 175 artworks. As there were high correlations between several of these attributes, a principal components analysis (PCA) was used to reduce these attributes to components that captured a large proportion of the variance (www.R-project.org). An initial parallel analysis indicated that the 12 artwork attributes could be reduced to 3 principal components, capturing 70% of the variance. However, the attribute “balance” was not strongly correlated with other attributes and had low communality with these components (0.36). After removing this attribute, 75% of the variance in the remaining attributes was captured by 3 components.
Saliency Analysis
In addition to the Assessment of Art Attributes, we tested if value ratings were related to the visual saliency of artwork perceptual features. Saliency ratings for each artwork were calculated using the SaliencyToolbox, an open-access Matlab (Mathworks) toolbox (Walther and Koch 2006). The sum of these saliency maps was calculated and this value was corrected for the area of the image. A detailed description of this procedure is provided in Vaidya and Fellows (2015). Artwork saliency values were converted to z-scores for the purpose of analysis here.
Characteristics of Value Ratings
We tested for differences in the distribution and intragroup consistency of value ratings for the 175 artworks. The mean and standard deviation of value ratings for each subject were calculated, and groups were compared on these measures with one-way ANOVAs. Intragroup consistency was measured by calculating the Pearson correlation of each subject’s 175 value ratings with the average ratings of the group with that subject removed. These correlation coefficients were also compared between groups using a one-way ANOVA. Follow-up post-hoc tests were carried out with two-way Bonferroni corrected t-tests (α = 0.017 for P = 0.05).
Relationship of Artwork Attributes and Value Ratings
We examined the relationship of artwork components, balance and saliency with the value ratings given by healthy controls and tested if these relationships were different in frontal lobe damaged subjects. Ordinal generalized estimating equations (GEEs), as implemented in SAS (version 9.4, SAS Institute Inc.), were used for these comparisons. GEEs are similar to mixed regression models, but are less sensitive to assumptions about the underlying correlation structure of the data (Hubbard et al. 2010). Value ratings at the extremes of the scale (−3 or 3) were not made by all subjects (N = 7), and were generally less frequent. Thus, to improve the fit of our GEE model at the ends of the rating scale, we collapsed these responses with the nearest response (−2 and 2) for all subjects. A model was also tested using subjects’ original ratings, yielding the same pattern of results as the model using collapsed responses described below.
A simple cumulative logit GEE model with 5 predictor attributes (concreteness, energy, color, balance, and saliency) was first tested in healthy controls alone. Effects of frontal lobe damage were then assessed through interactions between group status and these attributes, referenced to the healthy control group. Significant interactions between attributes and group status were followed up by assessing differences between GEE parameter estimates of patient groups using two-way Bonferroni corrected t-tests (α = 0.017 for P = 0.05).
We also tested the relationship of value ratings with the original 11 artwork attributes in the instrument (excluding “balance”). Attribute ratings were converted to z-scores, and GEEs were used to test these relationships first in the healthy control group, and then to test for interactions with group status to compare these estimates with the FC and VMF groups. These analyses were carried out separately for each attribute, as there were many strong interattribute correlations, and the threshold for significance was corrected for multiple comparisons (Bonferroni correction: α = 0.0045 for P = 0.05).
Individual Coefficients
Coefficients for the relationship between the 5 attributes and value ratings for 175 artworks from the rating task were estimated for individual subjects using ordinal regression analyses. To estimate the intragroup variance in these coefficients, we calculated the root-squared differences of subjects’ coefficients from the group mean coefficient in each of our 5 model attributes. Effects of group status were tested using a non-parametric Kruskal–Wallis test, as these data were not normally distributed. McFadden’s pseudo R2 values for this model were also estimated for each subject to determine the variance in value ratings captured by these attributes. These data were also compared using a non-parametric Kruskal–Wallis test.
Reliability of Attribute Coefficients
To examine the reliability of attribute coefficients, we compared coefficients for all 5 model attributes estimated for the 50 artworks presented in the test and retest period. Ordinal regression analyses were used to separately estimate these coefficients in individual subjects in the test and retest phase. The absolute difference between coefficients for each phase was then calculated for each subject. Group comparisons were carried out using non-parametric Kruskal–Wallis tests.
Voxel-based Lesion Symptom Mapping
The Non-Parametric Mapping (NPM, version 6 June 2013) software (freely available at www.mccauslandcenter.sc.edu/mricro/npm/) was used for voxel-based lesion symptom mapping (VLSM) analysis. This analysis does not hinge on a priori lesion group categorization, providing insights into the specific subregions where damage may be critical for a behavioral effect identified in the region-of-interest analysis described above, as well as the possibility of identifying effects of damage to areas not predicted by our hypothesis, such as to subregions within the FC group (Fellows 2012). Voxel-wise comparisons of the coefficients for energy and color radiance components were carried out using non-parametric Brunner–Munzel (BM) tests (Brunner and Munzel 2000) in all voxels where there were 3 or more patients with lesion damage.
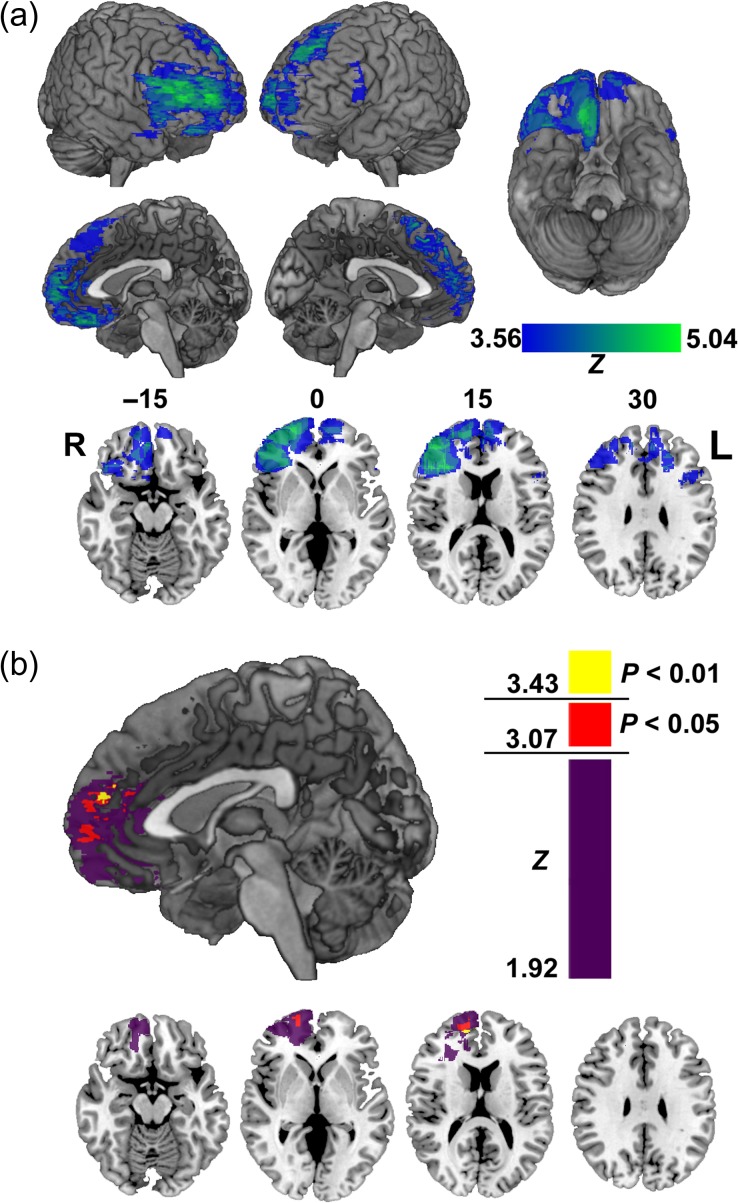
The power map shown in Figure 4a indicates where there was sufficient lesion overlap to test for VLSM effects, and is a guide to the regional power for detecting these effects in the current sample. Notably, power is uneven across the frontal lobes, and asymmetric between hemispheres (there was generally more lesion overlap in the right hemisphere than the left). Any apparent lateralization of the VLSM results in this study may be a consequence of this idiosyncratic distribution of lesion damage.
Figure 4.
Voxel-based lesion symptom mapping (VLSM) analysis. (a) Power map for VLSM analysis shown on 3D views of MNI brain and in representative axial slices. Color bar indicates maximum detectable Wilcoxon rank-sum Z score for each voxel included in this analysis. Numbers above the axial slices correspond to z-coordinates in MNI space. R, Right L, Left. (b) VLSM results showing where damage was associated with a reduced relationship between energy component and value ratings at an uncorrected threshold in three-dimensional sagittal view, and in axial slices. Color scale indicates Brunner–Munzel Z-scores. Voxels in red indicate where this effect was P < 0.05, and in yellow at P < 0.01, corrected with permutation tests.
To control for multiple comparisons, a null distribution of BM Z-scores was calculated from the same dataset using permutation tests (3000 permutations) (Nichols and Holmes 2002). This method provides an assumption-free means of controlling for multiple comparisons that is also more powerful than commonly used corrections like the Bonferroni method (Kimberg et al. 2007). Images of the results of this analysis were created using the software MRICron. A cluster threshold of k = 50 voxels was applied to statistical maps from this analysis for the images produced here.
Results
Artwork Attribute Ratings
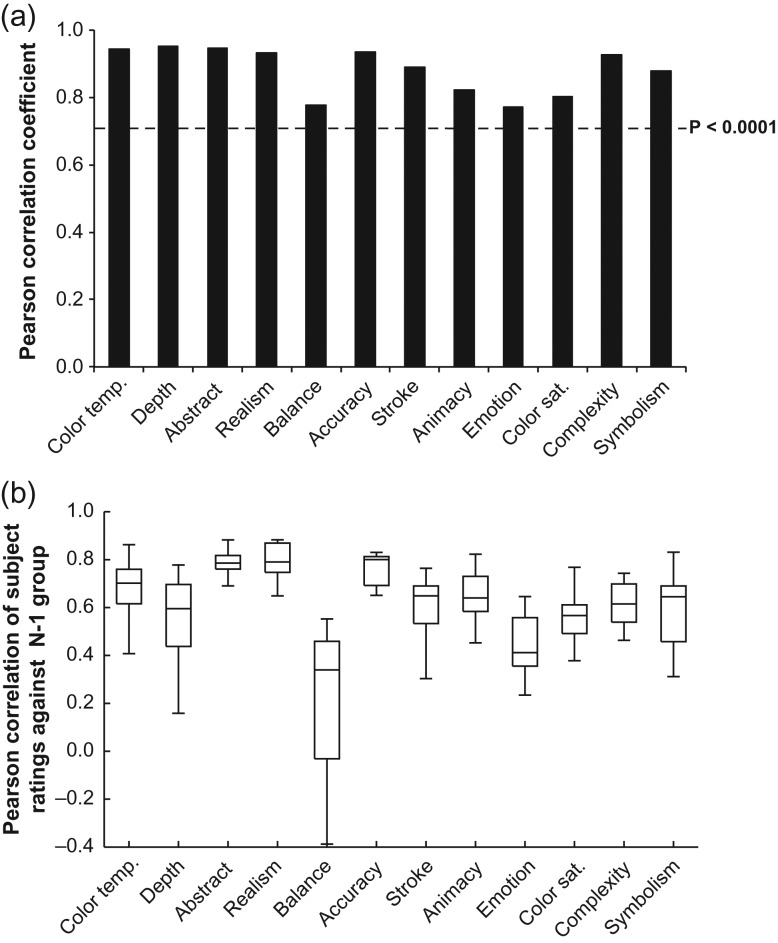
As a validity check for the instrument used here to characterize the attributes of artworks, we compared the correlation between the average ratings of our artistically experienced subjects and a similar group in Chatterjee et al. (2010), for the same 24 artworks. Correlations between ratings of these artworks were high across the 2 studies for all 12 attributes (r’s (23) ≥ 0.77, P’s < 0.0001; Fig. 1a), and inter-rater reliability was also high for most attributes in the current study (Fig. 1b).
Figure 1.
Validation of artistically experienced subjects’ ratings of artwork attributes. (a) Pearson correlation coefficients for relationship between average attributes ratings of artistically experienced subjects in the current study and artistically experienced subjects tested by Chatterjee et al. (2010) for the same set of 24 artworks. (b) Pearson correlations of individual artistically experienced subjects’ attribute ratings for all 199 artworks with the average attribute ratings of the rest of this group. Box plots show the 10th, 25th, 50th, 75th, and 90th percentiles of data.
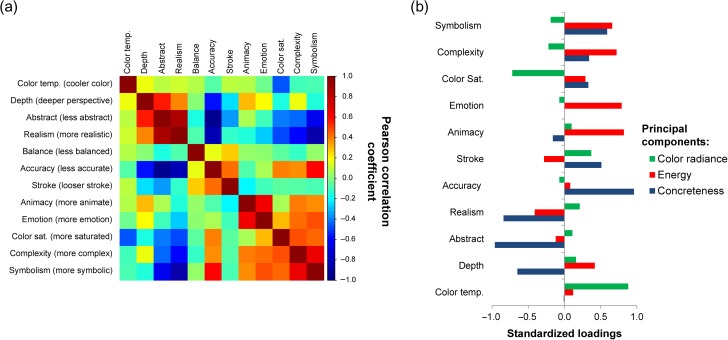
We next examined the correlation between these attributes to assess their independence in the set of 175 artworks that was the focus here. We found that several attributes were strongly correlated with each other (e.g., accuracy and realism; Fig. 2a), so we submitted the attribute ratings to a principal components analysis (PCA) to reduce data redundancy. As the balance attribute was mostly independent, it was removed from this analysis (see Methods). Parallel analysis and comparison of component eigenvalues with simulated data indicated that 3 principal components should be retained (see Supplementary Fig. 2). These 3 components captured a cumulative 75% of the variance of the remaining 11 attributes. The component loadings of these attributes are shown in Figure 2b. The first component loaded on attributes pertaining to the “concreteness” of the artwork (i.e., abstractness, accuracy, and realism). The second loaded on attributes related to the artwork “energy” (i.e., emotion, animacy, and complexity), while the third loaded on information about “color radiance” of the artwork (i.e., color saturation and temperature). In addition to these subject rated attributes, we also included a measure of the objectively defined visual saliency of these stimuli, as this has also been shown to predict preference-based choice (Towal et al. 2013; Vaidya and Fellows 2015).
Figure 2.
Relationship of artwork attributes. (a) Correlation matrix showing the strength of relationships between 12 artwork attributes rated by artistically experienced subjects for 175 artworks. Direction of scale is indicated by parentheticals on the vertical axis. (b) Standardized loadings of artwork attributes for 3 principal components.
Relationship of Artwork Attributes with Value Ratings
To characterize the subjective value ratings of healthy controls and lesion-damaged groups, we compared the mean and standard deviation of value ratings of artworks between groups, as well as the intragroup reliability of value ratings (see Supplementary Table 2). There were no group differences in the mean (F2,57 = 0.76, P = 0.5), or standard deviation (F2,57 = 1.11, P = 0.3), of value ratings between groups. However, there was a significant effect of group status on intragroup rating reliability (F2,57 = 6.67, P = 0.003). Post-hoc tests found that the VMF group had lower intragroup consistency than healthy controls (P < 0.005, Bonferroni corrected t-test), and no other group differences. While groups did not differ in their use of the value rating scale, VMF damaged subjects’ preferences were more variable as a group.
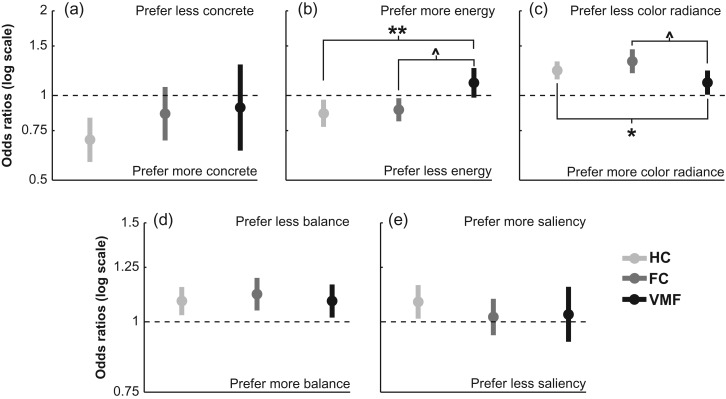
We tested the relationship of 5 model attributes (3 principal components, balance, and visual saliency) with the subjective value ratings of healthy control participants from the previously published study (Vaidya and Fellows 2015). This group gave higher value ratings to artworks that were more concrete (OR = 0.69, CI: 0.58–0.83, P < 0.0001, i.e., lower odds of giving high ratings to less concrete art), lower in energy (OR = 0.86, CI: 0.77–0.96, P = 0.01), lower in color radiance (OR = 1.23, CI: 1.14–1.32, P < 0.0001), less balanced (OR = 1.09, CI: 1.03–1.15, P = 0.004), and more salient (OR = 1.08, CI: 1.01–1.16, P = 0.02). Thus, the subjective value ratings of healthy control subjects were systematically related to distinct attributes of these complex stimuli.
To examine the effects of VMF damage on the consideration of option attributes, we compared frontal lobe damaged groups and healthy controls in their weighting of the concreteness, energy, color radiance, balance, and saliency of these artworks (Fig. 3). Relative to healthy controls, the VMF group gave significantly less weight to the energy (group × energy interaction, VMF: OR = 1.27, CI: 1.08–1.49, P = 0.004), and color radiance components (group × color radiance interaction, VMF: OR = 0.88, CI: 0.78–0.98, P = 0.03) in their subjective value ratings, but did not differ in their weighting of concreteness, balance or salience (P’s > 0.1). There were no significant differences between the healthy control and FC group in the relationships between value ratings and any of these 5 attributes (group × all attribute interactions, FC: P’s ≥ 0.1). Post-hoc t-tests comparing FC and VMF groups also revealed that the VMF group gave less weight to energy (P = 0.02, Bonferroni corrected) and color radiance (P = 0.05, Bonferroni corrected) than this group.
Figure 3.
Odds ratios for relationships between artwork components with value ratings in healthy controls (HC), frontal lobe damaged controls (FC), and the ventromedial frontal lobe damaged group (VMF). (a) Concreteness component. (b) Energy component. (c) Color radiance component. (d) Balance attribute, (e) Visual saliency. Error bars indicate the 95% confidence interval for estimated odds ratios. **P < 0.005, *P < 0.05 against healthy controls. ^P ≤ 0.05, post-hoc t test, Bonferroni corrected.
We also took a more granular, exploratory approach to examine which of the individual attributes rated in the Assessment of Art Attributes instrument were weighted differently by the frontal lobe damaged subjects compared to healthy controls, testing the effects of group status on each attribute separately (see Supplementary Table 3). We found significant relationships between controls’ value judgments and several of these attributes, which aligned with the analysis using principal components. There were no significant interactions of group status for the relationships of value ratings with these original attributes after stringent correction for multiple comparisons. However, there were differences at an uncorrected threshold between the VMF group and healthy controls for the emotion, complexity, symbolism, realism, and color saturation attributes. There were no significant differences between the FC group and healthy controls at either statistical threshold.
Given that the VMF group had less intragroup consistency in their value ratings, we tested if these subjects were less consistent as a group in using any of these artwork attributes during their value judgments. We carried out separate ordinal regression analyses for each subject with all 5 attributes. Odds ratios for individual subjects for each of these attributes are plotted in Supplementary Figure 3a–e, showing the variance within each group for these relationships. Given that VMF damaged subjects had less internally consistent value ratings as a group, we compared the within-group variance of attribute–value coefficients to test if there were group differences in the heterogeneity of these relationships within any particular attribute. This test revealed no significant effects of group status on this measure for any of the 5 attributes tested here (between-subjects Kruskal–Wallis tests, χ2 (2) ≤ 2.93, P ≥ 0.2).
One potential explanation for the reduced weights of artwork attributes in the VMF group is that the value ratings of individual VMF damaged subjects are more random and less coherently linked to the underlying artwork attributes in general. We calculated McFadden’s pseudo R2 values for the full ordinal regression model run on individual subjects to estimate the variance in each subject’s value ratings explained by these artwork attributes (see Supplementary Fig. 3f). There was no significant difference between groups in pseudo R2 values between groups (between-subjects Kruskal–Wallis test, χ2 (2) = 0.48, P = 0.8). These data argue that VMF damage did not cause a more generic disruption in utilizing information from artwork attributes to form value ratings.
Voxel-based Lesion Symptom Mapping
The anatomical specificity of the region-of-interest analysis is limited by the a priori region definition. We therefore also applied VLSM to explore the relationship between location of damage and altered attribute–value relationships at a finer anatomical resolution (Bates et al. 2003). VLSM power depends on the specific patterns of lesion overlap in any given sample. Figure 4a shows where there was statistical power for testing lesion effects here. VLSM revealed that damage near the border of the rostral anterior cingulate cortex and frontal pole in the right hemisphere (BA 32/10; MNI: 15, 54, 19) was significantly associated with decreased coefficients for the energy component (Z = 3.61, P < 0.01, permutation corrected; Fig. 4b). No voxel cluster reached the permutation threshold for the color radiance component coefficient, however the pattern of results above the uncorrected threshold was similar to that of artwork energy (see Supplementary Fig. 4).
VLSM does not account for demographic or clinical variables beyond lesion location. Therefore, we tested whether age, education or BDI-II scores were correlated with coefficients for the energy and color radiance components in healthy controls. None of these factors were significantly related to these coefficients (see Supplementary Table 4), making it unlikely that they contribute to the VLSM findings.
Reliability of Value-attribute Relationships
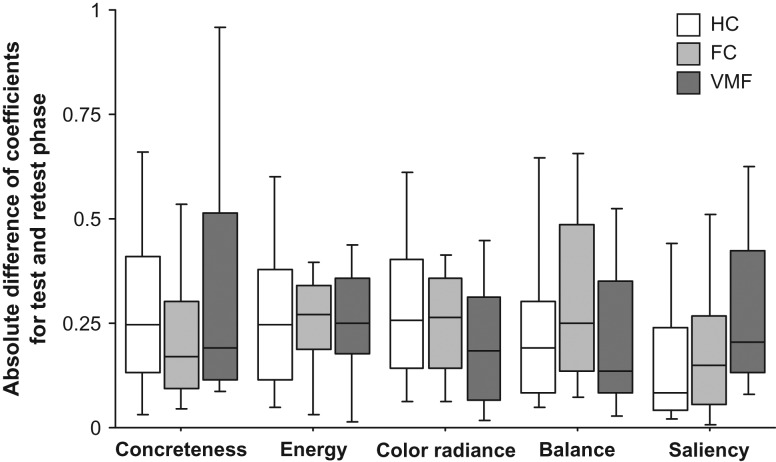
In our previous study, we found that the value ratings of VMF damaged subjects were consistent over the course of an experimental session (Vaidya and Fellows 2015), in contrast to the increased intransitivity of choices over option pairs in preference-based choice observed in prior work (Fellows and Farah 2007; Camille et al. 2011; Henri-Bhargava et al. 2012). One possibility for this difference is that the weights given to certain attributes are noisier for VMF damaged patients, resulting in inconsistent choices across option pairs, but the effects of this noise are not evident when attribute information is pooled together into a single value rating. To test this possibility, we compared the absolute difference of individual-level coefficients for 50 artworks that each participant rated twice over the course of the experiment. There were no effects of group status on this measure for any of these predictor attributes (Kruskal–Wallis tests: χ2’s (2) ≤ 4.96, P’s ≥ 0.08; Fig. 5). Thus, the within-session reliability of attribute weights was similar across groups.
Figure 5.
Consistency of relationship between value ratings and artwork components in healthy controls (HC), frontal lobe damaged controls (FC), and the ventromedial frontal lobe damaged group (VMF). Absolute difference between individual coefficients estimated for relationship between components during test and retest phase of the value rating task. Box plots show the 10th, 25th, 50th, 75th and 90th percentiles of data.
Visual-perceptual Effects
Given that not all subjects had normal or corrected to normal vision, and that 3 subjects reported red-green color blindness, we undertook control analyses to ensure that none of the effects were due to impaired vision. We tested if there was any relationship between low visual acuity and the association of value ratings with each of the 5 model attributes, comparing all participants divided into normal acuity and lower acuity groups. There were no significant interactions between vision group status and any of the 5 predictors (all P’s > 0.7), arguing against any role for visual acuity in explaining our results. The single VMF patient reporting color blindness had a relationship between color radiance and value ratings that was similar to the VMF group mean (subject OR = 1.08, mean VMF OR = 1.15, range: 0.72–1.64), indicating that this patient was not driving the group effect.
Discussion
We found that VMF damage alters how certain option attributes influence value judgments for complex, naturalistic stimuli. Compared to healthy and frontal lobe damaged controls, participants with VMF damage were less influenced by 2 components that we have termed energy and color radiance, but were swayed to the same extent as controls by concreteness, and more basic perceptual attributes like saliency and balance. These findings argue that VMF is critically involved in valuation, but only with respect to a subset of the information that healthy people rely on. That is, some aspects of valuation are affected, but others remain intact after VMF damage. These dissociations suggest a need to refine common currency models of valuation, at least in relation to how these are implemented in the brain, and provide a starting point for specifying the value information that requires VMF.
VMF, as defined here, encompasses a broad region, reflecting the inherent limitations in spatial resolution of human lesion studies. VLSM allows the brain basis of the observed effects to be tentatively mapped at a finer resolution. This analysis revealed that underweighting of the energy component was most closely associated with damage in an area of ventromedial prefrontal cortex that has been a major focus in studies of economic decision-making (Levy and Glimcher 2012), emotional stimulus processing (Roy et al. 2012; Winecoff et al. 2013), and powerful esthetic experiences (Blood and Zatorre 2001; Vessel et al. 2013). The statistical peak observed here was somewhat more dorsal and anterior than commonly found in studies of value-based decision-making (Bartra et al. 2013; Clithero and Rangel 2014), though significant effects were also found ventrally in the frontal pole. Functional imaging studies have suggested ventral-to-dorsal, and posterior-to-anterior gradients for value coding within medial PFC, with more abstract value information (i.e., goals, secondary reinforcers) encoded in more dorsal and anterior sectors (McNamee et al. 2013; Sescousse et al. 2013). Our VLSM findings may reflect this distinction, given the higher-order nature of the artwork energy component. However, the anatomical specificity of this result should be interpreted cautiously, given the limitations of regional power for testing VLSM effects in this sample, and the potential for spurious localization inherent to this analytic approach, due to the non-independence of damage across voxels (Mah et al. 2014).
The underweighting of the energy component by the VMF group may stem from deficits in detecting affective content, or discounting of this information during value judgment, or a combination of the two. We cannot distinguish between these possible explanations in the current experiment, as the artwork attribute ratings were provided by an independent sample of subjects with artistic experience, not the VMF-damaged subjects themselves. The existing literature provides some support for either possibility: In a previous study, we found that VMF damaged subjects differed in how social attributes predicted value-based choices. In that work, we established that VMF damage did not alter the ability to rate the attribute (a higher-order social attribute), but did affect its influence on choice (Xia et al. 2015). This result argues that VMF damaged subjects may perceive artwork energy, but discount this information during value judgments. However, VMF damage can impair the ability to detect subtle emotion from facial expressions (Heberlein et al. 2008; Tsuchida and Fellows 2012; Jenkins et al. 2014), which might relate to a more general difficulty in detecting and interpreting affective information in visual stimuli (Stone et al. 1998; Adolphs 2002).
We also found that VMF damaged subjects differed in weighing a component we termed “color radiance.” While ostensibly perceptual, color preferences strongly relate to the emotional valence of common environmental associations that are likely learned (Palmer and Schloss 2010; Taylor et al. 2013). Thus, the altered weighting of this component by the VMF group may be a consequence of impaired retrieval of these emotional associations, though further work will be needed to test this interpretation.
The preferences of VMF damaged subjects were also more heterogeneous as a group compared to those of the healthy and frontal control groups. The source of this heterogeneity as not clear, however it did not appear to arise from greater variance in the weights given to the option attributes tested here. Moreover, the total variance explained by our full model did not differ between groups, arguing that these predictors captured roughly equivalent variance in the VMF group as in control groups. Our main findings are thus not readily explained by within-group differences in the consistency of relationships between value ratings and these predictors. However, it is possible that the within-group variance of these subjects’ preferences may arise from other aspects of these artworks that were not well captured in the attributes measured here.
Artwork preferences in artistically naïve subjects are shaped by familiarity and normative ideas about esthetics (Palmer et al. 2013), which are reflected in the correlated value judgments of a population (Eysenck 1940). VMF damage affects retrieval of schema knowledge (Moscovitch and Melo 1997; Spalding et al. 2015), which may impair option judgment and generation in realistic decision-making tasks (Peters et al. 2017). Similarly, a deficit in representing normative schema knowledge about artwork could explain the increased heterogeneity of preferences in the VMF group.
These findings indicate that VMF damage affects the underlying information considered during value judgment, but not the ability to form a subjective value judgment per se. We previously showed, in the same sample, that these value judgments guide choice to the same extent, and show the same reliability, in VMF damaged subjects and healthy controls (Vaidya and Fellows 2015). In a separate study of social decision-making (political choice) we observed a similar pattern: people with VMF damage made use of less information to guide value-based choice (Xia et al. 2015), consistent with the claim that these patients draw on an impoverished representation of option attributes during valuation. In the context of political choice, subjects with VMF damage were influenced by attractiveness of the candidates, but not the more complex (and arguably more pertinent) impression of competence. Here, the artwork value judgments of VMF damaged subjects reflected external information like balance, saliency and representational concreteness. Valuation of artwork energy likely depends on higher-order analysis and inference about the latent information in the stimulus (Leder et al. 2004; Chatterjee and Vartanian 2014). The judgments of VMF damaged subjects may have been limited to information that was more easily accessible, or directly observable. This distinction between levels of esthetic analysis echoes suggestions that VMF is involved in inferring latent task variables, or forming conceptual representations for guiding attentional selection (Wilson et al. 2014; Mack et al. 2017). Our findings suggest that VMF may similarly contribute to subjective preferences by providing value information based on complex, higher level attributes.
The dissociable effects of VMF damage on attribute weights suggest that value judgment may be dissected into component processes. Lesion studies have played an important role in demonstrating that concepts or percepts that are experienced as a unified whole in subjects with healthy brains (e.g., vision), may dramatically break down with focal damage. These phenomena are now understood as originating from dissociable processes (e.g., object and spatial vision), reliant on different neural substrates (e.g., ventral and dorsal visual streams) (Mishkin et al. 1983; Goodale and Milner 1992). We argue that subjective value might similarly be decomposed into component valuation processes, with the values of different types of information processed in distinct brain areas. Our current results point to potential divisions between perceptual features and more complex latent affective information, although more work is needed to better specify the relevant categories of value information that might be represented in distinct brain circuits. Imaging work has shown that dissociable value ratings for visual and semantic attributes of a stimulus correlate with areas involved in processing this information (fusiform and posterior superior temporal gyri, respectively) (Lim et al. 2013), arguing that value information for different attributes may be tagged on representations in multiple brain areas. Subjective value judgment may arise from a parallel, competitive process involving multiple brain areas, including VMF, rather than a serial process where information is integrated into a common code for comparison (Cisek 2012; Hunt and Hayden 2017). Further study will be needed to test the extent to which latent and directly experienced attributes are used during value judgment in other domains (e.g., foods, social stimuli), and the extent to which value information for these components is regionally dissociable.
Functional imaging work has suggested that vmPFC integrates the subjective value of options across multiple attributes (Philiastides et al. 2010; Kahnt et al. 2011; Lim et al. 2013). In the current study, the value ratings of VMF damaged subjects, both as individuals and as a group, were stably related to multiple attributes over the course of the testing session, indicating an ability to utilize different information sources during value judgment. Other work from our lab has found that these subjects are affected by the incidental values of irrelevant stimulus dimensions during reinforcement learning to the same extent as healthy controls (Vaidya and Fellows 2016), also arguing that VMF damaged subjects are utilizing value associations in multiple dimensions. However, both these analyses average data over several trials and cannot determine whether these subjects are necessarily integrating information across dimensions in individual value judgments. Notably, we have also found that VMF damage affects how these subjects explore option attributes within a trial, suggesting that the process of integrating value information may be affected by VMF damage in more subtle ways (Fellows 2006).
Previous studies of esthetic preferences have found that judgments of visual artwork are shaped by several attributes. This work has shown that preferences are affected by the visual properties of the artwork, representational depiction (i.e., abstract or concrete), content and semantic meaning (Kettlewell et al. 1990; Vessel and Rubin 2010; Lim et al. 2013; see review by Palmer et al. 2013). The subjective value of an artwork therefore depends on attributes that vary in complexity, from visual features to higher-order information embedded in the content and meaning of the art. Consistent with these studies, we found that the preferences of control subjects were correlated with definable component attributes. Brain lesions and neurodegenerative disease in visual artists can also affect distinct visual and conceptual attributes during artistic expression, indicating links between artwork components and functionally related neural systems (reviewed in Chatterjee 2004; Zaidel 2005). These stimuli are thus a rich source of information for subjective value judgment, with features that may map to separate neural substrates.
It is notable that despite drawing on systematically different information, subjects with VMF damage made value judgments nonetheless, and did not express any difficulty in doing so, pointing to the inherent flexibility of valuation. In addition to underlining the need to better define valuation processes in decision neuroscience, these observations raise questions for normative ethical and legal frameworks for judging decision-making capacity in people with neurological and psychiatric disorders (Grisso and Applebaum 1998). To be legitimate, must a value judgment include considerations of emotional meaning, if people without brain injury typically rely on such information? Fundamental work on the mechanisms of valuation could provide new models and measurement tools to better characterize, and perhaps remediate, decisional disability in people suffering from brain disorders.
The idiosyncrasy of subjective value makes this concept appealing in explaining variability in motivated behavior, but its subjectivity also poses both experimental and practical challenges. Contrary to the old adage, here we show that there is “accounting for taste,” and that defining what elements go into subjective value construction is crucial for understanding the brain mechanisms of decision-making. Our findings provide a window into the mechanisms of value construction, and demonstrate that components of subjective value are dependent on distinct neural substrates. A more complete understanding of how values are formed will require further dissection of the construct of subjective value, with a fuller investigation of the brain bases that underlie valuation of options attributes, and how this information is eventually combined and utilized in a decision. The current study is a step in this direction, pointing to the complex representational machinery under the hood of subjective value.
Supplementary Material
Notes
We would to thank Arlene Berg and Christine Déry for help with subject recruitment and patient screening, and Uku Vainik for help with data analysis. We would also like to express gratitude to the many McGill-affiliated physicians who refer patients to this research program, and the patients and healthy volunteers for their participation. Conflict of Interest: None declared.
Funding
We gratefully acknowledge funding from CIHR (MOP 97821), a Fonds de Recherche en Santé du Québec Chercheur-Boursier award (to L.K.F.), and a Desjardins Outstanding Student Award (to A.R.V.).
References
- Adolphs R. 2002. Neural systems for recognizing emotion. Curr Opin Neurobiol. 12:169–177. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. 2013. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 76:412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. 2003. Voxel-based lesion-symptom mapping. Nat Neurosci. 6:448–450. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Sivan AIC. 1989. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates. [Google Scholar]
- Blood AJ, Zatorre RJ. 2001. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 98:11818–11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower GH, Karlin MB. 1974. Depth of processing pictures of faces and recognition memory. J Exp Psychol. 103:751–757. [Google Scholar]
- Brown S, Gao X, Tisdelle L, Eickhoff SB, Liotti M. 2011. Naturalizing aesthetics: brain areas for aesthetic appraisal across sensory modalities. Neuroimage. 58:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner E, Munzel U. 2000. The nonparametric Behrens-Fisher problem: asymptotic theory and a small-sample approximation. Biometrical J. 42:17–25. [Google Scholar]
- Camille N, Griffiths CA, Vo K, Fellows LK, Kable JW. 2011. Ventromedial frontal lobe damage disrupts value maximization in humans. J Neurosci. 31:7527–7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. 2004. The neuropsychology of visual artistic production. Neuropsychologia. 42:1568–1583. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Vartanian O. 2014. Neuroaesthetics. Trends Cogn Sci. 18:370–375. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Widick P, Sternscein R, Smith WB, Bromberger B. 2010. The assessment of art attributes. Emprical Studies of the Arts. 28:207–222. [Google Scholar]
- Cisek P. 2012. Making decisions through a distributed consensus. Curr Opin Neurobiol. 22:927–936. [DOI] [PubMed] [Google Scholar]
- Clithero JA, Rangel A. 2014. Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci. 9:1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derenzi E, Vignolo LA. 1962. Token test – a sensitive test to detect receptive disturbances in aphasics. Brain. 85:665–678. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. 1940. The general factor in aesthetic judgments. Br J Psychol. 31:94–102. [Google Scholar]
- Fellows LK. 2006. Deciding how to decide: ventromedial frontal lobe damage affects information acquisition in multi-attribute decision making. Brain. 129:944–952. [DOI] [PubMed] [Google Scholar]
- Fellows LK. 2007. The role of orbitofrontal cortex in decision making: a component process account. Ann N Y Acad Sci. 1121:421–430. [DOI] [PubMed] [Google Scholar]
- Fellows LK. 2012. Group studies in experimental neuropsychology In: Cooper H, Camic PM, Long DL, Panter AT, Rindskopf D, Sher KJ, editors. APA handbook of research methods in psychology, Vol 2: Research designs: quantitative, qualitative, neuropsychological, and biological. Washington, DC, USA: American Psychological Association; p. 647–659. [Google Scholar]
- Fellows LK, Farah MJ. 2007. The role of ventromedial prefrontal cortex in decision making: judgment under uncertainty or judgment per se? Cereb Cortex. 17:2669–2674. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Stark M, Berg A, Chatterjee A. 2008. Patient registries in cognitive neuroscience research: advantages, challenges, and practical advice. J Cogn Neurosci. 20:1107–1113. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. 1992. Separate visual pathways for perception and action. Trends Neurosci. 15:20–25. [DOI] [PubMed] [Google Scholar]
- Grisso T, Applebaum PS. 1998. Assessing competence to consent to treatmeant. New York: Oxford University Press. [Google Scholar]
- Hare TA, Camerer CF, Rangel A. 2009. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 324:646–648. [DOI] [PubMed] [Google Scholar]
- Heberlein AS, Padon AA, Gillihan SJ, Farah MJ, Fellows LK. 2008. Ventromedial frontal lobe plays a critical role in facial emotion recognition. J Cogn Neurosci. 20:721–733. [DOI] [PubMed] [Google Scholar]
- Henri-Bhargava A, Simioni A, Fellows LK. 2012. Ventromedial frontal lobe damage disrupts the accuracy, but not the speed, of value-based preference judgments. Neuropsychologia. 50:1536–1542. [DOI] [PubMed] [Google Scholar]
- Hubbard AE, Ahern J, Fleischer NL, Van der Laan M, Lippman SA, Jewell N, Bruckner T, Satariano WA. 2010. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology. 21:467–474. [DOI] [PubMed] [Google Scholar]
- Hunt LT, Hayden BY. 2017. A distributed, hierarchical and recurrent framework for reward-based choice. Nat Rev Neurosci. 18:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LM, Andrewes DG, Nicholas CL, Drummond KJ, Moffat BA, Phal P, Desmond P, Kessels RP. 2014. Social cognition in patients following surgery to the prefrontal cortex. Psychiatry Res. 224:192–203. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. 2009. The neurobiology of decision: consensus and controversy. Neuron. 63:733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Heinzle J, Park SQ, Haynes JD. 2011. Decoding different roles for vmPFC and dlPFC in multi-attribute decision making. Neuroimage. 56:709–715. [DOI] [PubMed] [Google Scholar]
- Kettlewell N, Lipscomb S, Evans L, Rosston K. 1990. The effect of subject matter and degree of realism on aesthetic preferences for paintings. Emprical Studies of the Arts. 8:85–93. [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF. 2007. Power in voxel-based lesion-symptom mapping. J Cogn Neurosci. 19:1067–1080. [DOI] [PubMed] [Google Scholar]
- Leder H, Belke B, Oeberst A, Augustin D. 2004. A model of aesthetic appreciation and aesthetic judgments. Br J Psychol. 95:489–508. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. 2012. The root of all value: a neural common currency for choice. Curr Opin Neurobiol. 22:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Lazzaro SC, Rutledge RB, Glimcher PW. 2011. Choice from non-choice: predicting consumer preferences from blood oxygenation level-dependent signals obtained during passive viewing. J Neurosci. 31:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M, Howieson DB, Bigler ED, Tranel D. 2012. Neuropsychological assessment. New York: Oxford University Press. [Google Scholar]
- Lim SL, O’Doherty JP, Rangel A. 2013. Stimulus value signals in ventromedial PFC reflect the integration of attribute value signals computed in fusiform gyrus and posterior superior temporal gyrus. J Neurosci. 33:8729–8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack ML, Preston AR, Love BC. 2017. Medial prefrontal cortex compresses concept representations through learning. In. 2017 International Workshop on Pattern Recognition in Neuroimaging (PRNI): IEEE.
- Mah YH, Husain M, Rees G, Nachev P. 2014. Human brain lesion-deficit inference remapped. Brain. 137:2522–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee D, Rangel A, O’Doherty JP. 2013. Category-dependent and category-independent goal-value codes in human ventromedial prefrontal cortex. Nat Neurosci. 16:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. 1989. Delay of gratification in children. Science. 244:933–938. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. 1983. Object vision and spatial vision – 2 cortical pathways. Trends Neurosci. 6:414–417. [Google Scholar]
- Moscovitch M, Melo B. 1997. Strategic retrieval and the frontal lobes: evidence from confabulation and amnesia. Neuropsychologia. 35:1017–1034. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. 2002. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Cai X. 2011. The orbitofrontal cortex and the computation of subjective value: consolidated concepts and new perspectives. Ann N Y Acad Sci. 1239:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SE, Schloss KB. 2010. An ecological valence theory of human color preference. Proc Natl Acad Sci USA. 107:8877–8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SE, Schloss KB, Sammartino J. 2013. Visual aesthetics and human preference. Annu Rev Psychol. 64:77–107. [DOI] [PubMed] [Google Scholar]
- Peters SL, Fellows LK, Sheldon S. 2017. The ventromedial frontal lobe contributes to forming effective solutions to real-world problems. J Cogn Neurosci. 29:991–1001. [DOI] [PubMed] [Google Scholar]
- Philiastides MG, Biele G, Heekeren HR. 2010. A mechanistic account of value computation in the human brain. Proc Natl Acad Sci USA. 107:9430–9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M. 2000. Stereotaxic display of brain lesions. Behav Neurol. 12:191–200. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. 2012. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 16:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Caldu X, Segura B, Dreher JC. 2013. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 37:681–696. [DOI] [PubMed] [Google Scholar]
- Spalding KN, Jones SH, Duff MC, Tranel D, Warren DE. 2015. Investigating the neural correlates of schemas: ventromedial prefrontal cortex is necessary for normal schematic influence on memory. J Neurosci. 35:15746–15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. 1998. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 10:640–656. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, Borowiec A, Katz DI. 2005. Multiple frontal systems controlling response speed. Neuropsychologia. 43:396–417. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B. 2002. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol. 53:401–433. [DOI] [PubMed] [Google Scholar]
- Taylor C, Schloss K, Palmer SE, Franklin A. 2013. Color preferences in infants and adults are different. Psychon Bull Rev. 20:916–922. [DOI] [PubMed] [Google Scholar]
- Towal RB, Mormann M, Koch C. 2013. Simultaneous modeling of visual saliency and value computation improves predictions of economic choice. Proc Natl Acad Sci USA. 110:E3858–E3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. 2012. Are you upset? Distinct roles for orbitofrontal and lateral prefrontal cortex in detecting and distinguishing facial expressions of emotion. Cereb Cortex. 22:2904–2912. [DOI] [PubMed] [Google Scholar]
- Tusche A, Bode S, Haynes JD. 2010. Neural responses to unattended products predict later consumer choices. J Neurosci. 30:8024–8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya AR, Fellows LK. 2015. Testing necessary regional frontal contributions to value assessment and fixation-based updating. Nature Commun. 6:10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya AR, Fellows LK. 2016. Necessary contributions of human frontal lobe subregions to reward learning in a dynamic, multidimensional environment. J Neurosci. 36:9843–9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessel EA, Rubin N. 2010. Beauty and the beholder: highly individual taste for abstract, but not real-world images. J Vis. 10:18.1–18.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessel EA, Starr GG, Rubin N. 2013. Art reaches within: aesthetic experience, the self and the default mode network. Front Neurosci. 7:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther D, Koch C. 2006. Modeling attention to salient proto-objects. Neural Netw. 19:1395–1407. [DOI] [PubMed] [Google Scholar]
- Wilson RC, Takahashi YK, Schoenbaum G, Niv Y. 2014. Orbitofrontal cortex as a cognitive map of task space. Neuron. 81:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A, Clithero JA, Carter RM, Bergman SR, Wang L, Huettel SA. 2013. Ventromedial prefrontal cortex encodes emotional value. J Neurosci. 33:11032–11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Stolle D, Gidengil E, Fellows LK. 2015. Lateral orbitofrontal cortex links social impressions to political choices. J Neurosci. 35:8507–8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel D. 2005. Neuropsychology of art: neurological, cognitive and evolutionary perspectives. New York: Psychology Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.