Abstract
Background
A previous study has reported a 50% reduction in disuse atrophy of the quadriceps during the first 14 days after anterior cruciate ligament (ACL) reconstruction. A follow-up trial is needed to confirm these promising results. The present study aims to investigate the effect of an occlusion stimulus on quadriceps atrophy after ACL reconstruction.
Methods
A total of 24 subjects participated in the study. They were randomized into two groups. Starting the 2nd day after surgery, the occlusion group received an occlusion stimulus for 5 min, followed by removal of the occlusive pressure for 3 min. This was repeated five times in one training session, twice daily. During the period of occlusive stimulus, the subjects performed 20 low load exercises for the quadriceps. The control group followed the same exercise protocol, but without the occlusion stimulus. Changes in quadriceps anatomical cross section area (ACSA) were measured using axial magnetic resonance (MR) images at 40% and 50% of the length of the femur.
Results
Both groups had a significant reduction of quadriceps ACSA from 2 days before surgery to 16 days after surgery. During the intervention period, the occlusion group lost 13.8% ± 1.1% (mean ± SEM) and the control group lost 13.1% ± 1.0% of their quadriceps ACSA, respectively. There was no significant difference between the occlusion and control groups with regards to atrophy of the quadriceps muscles.
Conclusion
In conflict with other studies using a similar protocol, application of blood flow restriction the first 14 days after ACL reconstruction did not reduce quadriceps ACSA muscle atrophy measured by MR in a population of athletes.
Keywords: ACL reconstruction, Ascular occlusion, Hypoxia ischemia, Quadriceps atrophy
1. Introduction
Recovery from lower leg trauma or surgery often requires a period of unloading, resulting in loss of muscle mass. In the thigh, the quadriceps is most affected, while the muscle mass of the hamstrings and adductors are mainly maintained.1 Even short periods of disuse have been shown to cause substantial loss of muscle mass in the anti-gravity muscles.2, 3, 4, 5 For example, in healthy subjects, a reduction in quadriceps muscle volume of 8.4% after 14 days of lower limb unloading has been reported.3 Rate of atrophy is higher after arthroscopic knee surgery. Gerber et al.6 found a reduction in quadriceps muscle volume of 20%–33% from time of injury to 3 weeks after surgery. Muscle mass lost due to short term unloading can be restored relatively quickly with active recovery. The quadriceps muscle mass lost due to 3 weeks of unloading can be restored in the same amount of time in uninjured subjects using traditional resistance training.5 However, the quadriceps atrophy after anterior cruciate ligament (ACL) reconstruction is harder to regain. A difference of 8.6% in quadriceps anatomical cross section area (ACSA) of the injured limb compared to the un-injured limb 49 months after ACL reconstruction has been reported.7
Despite conflicting evidence, a majority of studies suggest that specific atrophy among the individual muscle bellies of the quadriceps does not occur.1, 5 However, disuse atrophy is not uniformly distributed along the length of the muscle. Loss of muscle mass is greater around the region of the peak ACSA, rather than towards the proximal and distal ends of the muscle.8, 9 Persistent atrophy of the quadriceps muscles following ACL reconstruction can be a major challenge. Preventive measures, such as neuromuscular electrical stimulation (NMES), are often used in an attempt to reduce muscle atrophy after ACL reconstruction. A recent study investigating the effect of NMES on prevention of quadriceps atrophy after 5 days of lower limb unloading, found no significant change in quadriceps ACSA in the group using NMES for 40 min twice daily. In the control group, ACSA of the quadriceps was reduced by 3.5% ± 0.5%.2 A number of studies reporting hypertrophic changes in skeletal muscle after low load resistance training with restricted blood flow have been published in the past decade.10 Blood flow restriction has also been used to reduce muscle atrophy and loss of muscle strength after lower limb surgery and disuse. Studies have shown impressive results using a protocol consisting of five repetitions of vascular occlusion for 5 min, followed by release of occlusion for 3 min, twice daily. They reported a reduction in quadriceps atrophy after ACL surgery by 50%,11 and maintenance of thigh circumference and strength after unloading.12
To our knowledge, the effect of this intervention has not been evaluated on a population of athletes before. The aim of this study was to repeat these promising results in a population of athletes during the first 16 days after ACL reconstruction. Our hypothesis was that the occlusion group would have less relative reduction in quadriceps ACSA compared to the control group.
2. Methods
2.1. Subjects
A total of 24 patients planned for ACL reconstruction surgery were included in the study. All subjects were physically active, and had sustained their injury while participating in sports. Subjects between 18 and 40 years of age, with no prior knee injuries, ACL injury not more than 6 months before surgery, and planned for reconstruction using hamstring tendon graft were eligible to participate in the study. Prior to inclusion, all participants were given information about the purpose of the study, and gave their written informed consent. The research project was conducted according to the Declaration of Helsinki and was approved by the Norwegian Ethics Committee.
2.2. Experimental procedure
A total of 24 patients participated in the study. Twelve patients (7 men, 5 women) were randomized to intermittent blood flow restriction and exercises (occlusion group), and 12 patients (7 men, 5 women) were randomized to exercises only (control group). There was no statistical difference between the groups at baseline (Table 1). During the pre-operation visit, all subjects were instructed in how to perform the exercise protocol. In addition, the occlusion group were trained in operating the occlusion cuff. Magnetic resonance (MR) imaging was performed on the injured leg 2 days before, and 16 days after surgery. The intervention started at Day 2 after surgery. In the experimental group, a 14-cm wide contoured pneumatic occlusion cuff (Delphi low pressure cuff 9-7450-003) was applied to the most proximal part of the thigh. Inflation of the cuff was administered with a portable blood pressure hand pump (Trigger Aneroid DS66; Welch Allyn, Skaneateles Falls, NY, USA) fitted to the cuff. The cuff was inflated to give an occlusion stimulus for 5 min, followed by removal of the occlusive pressure for 3 min. This was repeated five times in one training session. Subjects were sitting with their upper body inclined to about 45° during the session. Starting pressure was set at 130 mmHg with an increase in pressure of 10 mmHg every second day, up to a maximum pressure of 180 mmHg. If the occlusive stimulus caused unbearable pain, the subjects were instructed to use the highest tolerable pressure. The experimental group performed the occlusion protocol twice daily. During the occlusion intervals the patients performed quadriceps exercises consisting of isometric quadriceps contractions, progressing to leg extension over a knee-roll, and straight leg-raises. The patient performed 20 repetitions during each 5-min occlusion period. Adding up to 100 repetitions per training session, and 200 repetitions per day. The occlusion stimulus was terminated 14 days after surgery, to avoid muscle swelling to interfere with the MR measurements. The control group followed the same exercise regime, but without the occlusion stimulus. Patients in the occlusion group received training in the use of the occlusion cuff prior to surgery. After surgery they performed the occlusion protocol as a home exercise. All patients had two consultations during the experimental period, 5 and 10 days after surgery. During these consultations degree of swelling and knee mobility was subjectively evaluated. In addition, performance of the quadriceps exercises, and use of the occlusion cuff was controlled. All participants recorded every training session, and the occlusion group also recorded cuff pressure.
Table 1.
Baseline characteristics of subjects (mean ± SD).
| Characteristic | Occlusion group | Control group |
|---|---|---|
| Age (year) | 24.9 ± 7.4 | 29.8 ± 9.3 |
| Height (cm) | 176.9 ± 7.9 | 178.9 ± 7.8 |
| Weight (kg) | 76.9 ± 12.1 | 77.6 ± 9.6 |
| IKDC score | 65.3 ± 14.1 | 67.4 ± 13.5 |
| Time from injury to surgery (month) | 2.5 ± 1.1 | 5.4 ± 3.8 |
Abbreviation: IKDC = International Knee Documentation Committee.
2.3. MR
To obtain anatomical cross sectional images, MR was performed using a Toshiba Excelart Vantage Atlas (1.5 T, Toshiba Medical Systems Corp., Tochigi, Japan). During the first MR, a coronal-plane T1-weighted echo-sequence of the entire femur was performed. When necessary, markers attached to the skin were used as a reference for the measurement of the length of the femur. The MR investigations were performed 2 days before surgery, and 16 days after surgery. During the MR investigation, a sagittal-plane T1-weighted spin echo-sequence was performed. This sequence covered the distal 2/3 of the femur, and proximal 1/3 of the calf of the injured leg. In addition, an axial-plane T1-weighted spin echo-sequence with 10-mm sectional thickness was performed. This sequence was started at the lateral joint line of the knee, and covered 2/3 of the distal thigh. The following variables were used: TR 775 ms, TE 15 ms, FOV 25 × 30. The patient was placed in a supine position. Fixation and cushions were used to avoid movement during the examination. The length of the femur was determined using the lateral joint line as reference point. Axial images at 40% and 50% of the femur length, measured distal to proximal from the lateral joint line of the knee, were obtained. ACSA of the quadriceps were measured using software included in Sectra PACS. An experienced radiologist (A.L.) blinded to group allocation performed the ACSA calculations.
2.4. Statistical analysis
Descriptive data are presented as means ± SD. All analyses were performed using statistical software (Prism 6; GraphPad Software, Inc., La Jolla, CA, USA). Comparison between changes in variables obtained form the occlusion and control groups were made with a parametric unpaired t test. Our null hypothesis was that there would be no difference between the occlusion group and the control group with regards quadriceps ACSA 16 days after surgery.
3. Results
At baseline, 2 days before surgery, there was no statistically significant difference between the groups in quadriceps ACSA. From 2 days before surgery to 16 days after surgery there was a statistically significant reduction in quadriceps ACSA for both the occlusion (p < 0.0001) and control groups (p < 0.0001).
MR taken at 40% the femur length, with reference to the lateral joint line of the knee, showed a reduction in quadriceps ACSA of −9.7 ± 1.0 cm2 (mean ± SEM) and −9.2 ± 0.8 cm2 for the occlusion and control groups, respectively. MR taken at the midpoint of the femur showed a reduction in quadriceps ACSA of −13.7 ± 0.9 cm2 and −11.5 ± 0.7 cm2 for the occlusion and control groups, respectively. Mean change in quadriceps ACSA (40% and 50% of the length of the femur) was −13.8% ± 1.1% and −13.1% ± 1.0% for the occlusion and control groups, respectively. Thus, there was no significant difference in loss of quadriceps ACSA between the groups after the intervention period (p = 0.6265) (Fig. 1).
Fig. 1.
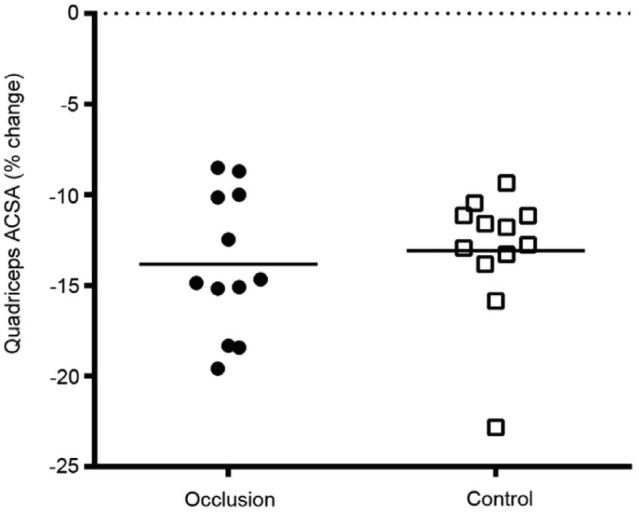
Mean % change in quadriceps anatomical cross sectional area (ACSA).
When analyzing men and women separately, in the occlusion group, men lost 12.8% ± 1.1%, and women lost 15.3% ± 2.1% of their quadriceps ACSA, with no statistical difference (p = 0.28). In the control group, men lost −12.2% ± 0.8%, and women lost −14.3% ± 2.2%, with no statistically significant difference (p = 0.31). Changes in quadriceps ACSA are summarized in Table 2.
Table 2.
Change in anatomical cross sectional area (ACSA) of quadriceps from baseline to 16 days after surgery for the occlusion and control groups (mean ± SD).
| ACSA 40%a (cm2) | ACSA 50%a (cm2) | Mean change in quadriceps ACSA (%) | |
|---|---|---|---|
| Occlusion group | |||
| Baseline | 77.5 ± 2.5 | 87.0 ± 3.6 | |
| Post test | 67.7 ± 2.7 | 73.9 ± 3.5 | |
| Reduction | −9.7 ± 1.0 | −13.7 ± 0.9 | −13.8 ± 1.1 |
| Control group | |||
| Baseline | 75.4 ± 3.2 | 82.8 ± 3.4 | |
| Post test | 66.1 ± 3.3 | 71.3 ± 3.2 | |
| Reduction | −9.2 ± 0.8 | −11.5 ± 0.7 | −13.1 ± 1.0 |
Of the length of the femur, measured distal to proximal with reference to lateral joint line of the knee.
4. Discussion
Our main finding was that intermittent restriction of blood flow does not reduce quadriceps atrophy during the first 16 days after ACL reconstruction in an athletic population. This is in contrast with previous studies using the same inflation/deflation protocol.11, 12 For example, Takarada et al.11 reported a reduction in quadriceps ACSA of 9.4% ± 1.6% and 20.9% ± 2.2% in the occlusion and control groups, respectively. In our study, we did not find a significant difference between groups. From 2 days before surgery to 16 days after surgery, the occlusion group had a reduction of 13.8% ± 1.1%, and the control group a reduction of 13.1% ± 1.0% in quadriceps ACSA. The rate of quadriceps atrophy in our study was slightly more than reported in studies using unloading of the lower limb to study the effects of disuse. This is expected as the surgical procedure traumatizes the knee far more than pure unloading. However, the amount of quadriceps atrophy in our study was markedly less than what was previously reported after ACL surgery.6 Previous studies have shown a slight difference between male and female with regards to quadriceps atrophy after ACL surgery, with female losing more muscle mass.11, 13 There was a tendency towards women losing more muscle mass in our study, however this was not statistically significant.
We used a wider cuff than the previous study investigating the effect of vascular occlusion on muscle atrophy after ACL reconstructive surgery. Takarada et al.11 used a 90-mm wide cuff with a maximal pressure of 238 ± 8 mmHg. They assumed that the occlusive pressure effectively inhibits both the arterial inflow and venous outflow through the occluded area. Wide occlusion cuffs restrict arterial blood flow at a lower pressure than narrow cuffs.14, 15, 16 Laurentino et al.17 using a 140-mm wide cuff reported cessation of the tibial artery pulse at pressures ∼130 mmHg. However, the same cuff may not restrict the same amount of blood flow on all individuals. Thigh circumference has been shown to be the largest determinant of arterial occlusion pressure.16 We did not use individual adjustment of the restrictive cuff pressure in our study, which must be taken in to account when interpreting our data.
A recent study18 reported that the intensity of the exercises during blood flow restricted training is important. They suggest that approximately 10% of maximal strength is the minimum intensity for muscle hypertrophy to occur. This is linked to both muscle activation and muscle cell swelling. Thus, it is possible that the training intensity in our study is below or at the lower border necessary to obtain a reduction in quadriceps atrophy. Based on the number of studies showing effect on muscle hypertrophy, a research model combining NMES with sufficient muscle activation and blood flow restriction may be indicated.
We used two ACSA measurements to quantify change in quadriceps muscle size, at 40% and 50% of the length of the femur with reference to the lateral joint line of the knee. Thus, we should be able to detect area specific atrophy. However, muscle volume has been suggested as a more accurate method to estimate muscle size.1 Future studies should use muscle volume rather than ACSA as outcome measure for quadriceps atrophy. Another limitation is the low number of subjects in the study, particularly as there was a substantial variation in atrophy between subjects (−8.5% to −22.8%). A larger RCT is needed to establish the effect of intermittent blood flow restriction on reduction in quadriceps atrophy the first 2 weeks after ACL reconstruction.
5. Conclusion
In conflict with other studies using a similar protocol, application of blood flow restriction the first 14 days after ACL reconstruction did not reduce quadriceps ACSA muscle atrophy measured by MR in a population of athletes.
Authors' contributions
EI conceived of the study, participated in the design of the study, contributed to the patient intervention, performed the statistical analyses, and drafted the manuscript. VR participated in the design of the study, contributed to the patient intervention, and helped to draft the manuscript. AL designed the MR protocol and performed the ACSA measurements. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
None of the authors declare any competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Akima H., Furukawa T. Atrophy of thigh muscles after meniscal lesions and arthroscopic partial menisectomy. Knee Surg Sports Traumatol Arthrosc. 2005;13:632–637. doi: 10.1007/s00167-004-0602-9. [DOI] [PubMed] [Google Scholar]
- 2.Dirks M.L., Wall B.T., Snijders T., Ottenbros C.L., Verdijk L.B., van Loon L.J. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol. 2014;210:628–641. doi: 10.1111/apha.12200. [DOI] [PubMed] [Google Scholar]
- 3.Wall B.T., Dirks M.L., Snijders T., Senden J.M., Dolmans J., van Loon L.J. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. 2014;210:600–611. doi: 10.1111/apha.12190. [DOI] [PubMed] [Google Scholar]
- 4.Wall B.T., van Loon L.J. Nutritional strategies to attenuate muscle disuse atrophy. Nutr Rev. 2013;71:195–208. doi: 10.1111/nure.12019. [DOI] [PubMed] [Google Scholar]
- 5.Campbell E.L., Seynnes O.R., Bottinelli R., McPhee J.S., Atherton P.J., Jones D.A. Skeletal muscle adaptations to physical inactivity and subsequent retraining in young men. Biogerontology. 2013;14:247–259. doi: 10.1007/s10522-013-9427-6. [DOI] [PubMed] [Google Scholar]
- 6.Gerber J.P., Marcus R.L., Leland E.D., Lastayo P.C. The use of eccentrically biased resistance exercise to mitigate muscle impairments following anterior cruciate ligament reconstruction: a short review. Sports Health. 2009;1:31–38. doi: 10.1177/1941738108327531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arangio G.A., Chen C., Kalady M., Reed J.F., 3rd Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26:238–243. doi: 10.2519/jospt.1997.26.5.238. [DOI] [PubMed] [Google Scholar]
- 8.Akima H., Kubo K., Imai M., Kanehisa H., Suzuki Y., Gunji A. Inactivity and muscle: effect of resistance training during bed rest on muscle size in the lower limb. Acta Physiol Scand. 2001;172:269–278. doi: 10.1046/j.1365-201x.2001.00869.x. [DOI] [PubMed] [Google Scholar]
- 9.Miokovic T., Armbrecht G., Felsenberg D., Belavy D.L. Heterogeneous atrophy occurs within individual lower limb muscles during 60 days of bed rest. J Appl Physiol (1985) 2012;113:1545–1559. doi: 10.1152/japplphysiol.00611.2012. [DOI] [PubMed] [Google Scholar]
- 10.Wernbom M., Augustsson J., Raastad T. Ischemic strength training: a low-load alternative to heavy resistance exercise? Scand J Med Sci Sports. 2008;18:401–416. doi: 10.1111/j.1600-0838.2008.00788.x. [DOI] [PubMed] [Google Scholar]
- 11.Takarada Y., Takazawa H., Ishii N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc. 2000;32:2035–2039. doi: 10.1097/00005768-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Kubota A., Sakuraba K., Sawaki K., Sumide T., Tamura Y. Prevention of disuse muscular weakness by restriction of blood flow. Med Sci Sports Exerc. 2008;40:529–534. doi: 10.1249/MSS.0b013e31815ddac6. [DOI] [PubMed] [Google Scholar]
- 13.Arvidsson I., Arvidsson H., Eriksson E., Jansson E. Prevention of quadriceps wasting after immobilization: an evaluation of the effect of electrical stimulation. Orthopedics. 1986;9:1519–1528. doi: 10.3928/0147-7447-19861101-08. [DOI] [PubMed] [Google Scholar]
- 14.Reilly C.W., McEwen J.A., Leveille L., Perdios A., Mulpuri K. Minimizing tourniquet pressure in pediatric anterior cruciate ligament reconstructive surgery: a blinded, prospective randomized controlled trial. J Pediatr Orthop. 2009;29:275–280. doi: 10.1097/BPO.0b013e31819bcd14. [DOI] [PubMed] [Google Scholar]
- 15.Younger A.S., McEwen J.A., Inkpen K. Wide contoured thigh cuffs and automated limb occlusion measurement allow lower tourniquet pressures. Clin Orthop Relat Res. 2004;428:286–293. doi: 10.1097/01.blo.0000142625.82654.b3. [DOI] [PubMed] [Google Scholar]
- 16.Loenneke J.P., Fahs C.A., Rossow L.M., Sherk V.D., Thiebaud R.S., Abe T. Effects of cuff width on arterial occlusion: implications for blood flow restricted exercise. Eur J Appl Physiol. 2012;112:2903–2912. doi: 10.1007/s00421-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurentino G., Ugrinowitsch C., Aihara A.Y., Fernandes A.R., Parcell A.C., Ricard M. Effects of strength training and vascular occlusion. Int J Sports Med. 2008;29:664–667. doi: 10.1055/s-2007-989405. [DOI] [PubMed] [Google Scholar]
- 18.Abe T., Loenneke J.P., Fahs C.A., Rossow L.M., Thiebaud R.S., Bemben M.G. Exercise intensity and muscle hypertrophy in blood flow-restricted limbs and non-restricted muscles: a brief review. Clin Physiol Funct Imaging. 2012;32:247–252. doi: 10.1111/j.1475-097X.2012.01126.x. [DOI] [PubMed] [Google Scholar]


