Abstract
Background
Geometric methods provide an analysis of autonomic modulation using the geometric properties of the resulting pattern, and represent an interesting tool in the analysis of heart rate variability (HRV). The aim of this study was to evaluate the impact of functional training on cardiac autonomic modulation in healthy young women using the geometric indices of HRV.
Methods
Data were analyzed from 29 women, and were stratified into a functional training group (FTG, n = 13; 23.00 ± 2.51 years; 21.90 ± 2.82 kg/m2) and a control group (CG, n = 16; 20.56 ± 1.03 years; 22.12 ± 3.86 kg/m2). The FTG received periodized functional training for 12 weeks. The cardiac autonomic modulation of both groups was evaluated before and after this training, and a qualitative analysis was performed using the Poincaré plot.
Results
There was a significant increase in the difference of the triangular index (RRTri), SD1, SD2, and RR intervals in the FTG as compared to the CG, and the qualitative analysis from the Poincaré plot showed an increase in the dispersion of beat-to-beat and long-term RR intervals in the functional group after training. No changes were observed in the triangular interpolation of RR interval histogram (TINN) or SD1/SD2.
Conclusion
Functional training had a beneficial impact on autonomic modulation, as characterized by increased parasympathetic activity and overall variability, thus highlighting the clinical usefulness of this type of training.
Keywords: Autonomic nervous system, Exercise, Heart rate, Resistance training, Young adult
1. Introduction
Functional training,1, 2, 3, 4, 5 a type of resistance training which has been heavily implemented in recent years,2 provides several benefits with the purpose of adapting training to be more specific for certain movement patterns or actions in the daily activities of athletes and non-athletes.1, 6 Although widely used in clinical practice,1, 3, 4 there are few studies in the literature investigating the cardiovascular parameters in a young population for this specific type of resistance training.7 Furthermore, its effect on autonomic modulation is still unknown, and this represents a gap in the literature.
One method of assessing autonomic modulation is through heart rate variability (HRV), a noninvasive measure with good reproducibility when performed in a standardized manner.8 HRV describes the oscillations in the intervals between consecutive heart beats (RR intervals), which are related to the effects of the autonomic nervous system (ANS) on the sinus node, and can evaluate both cardiac health and the state of the ANS responsible for cardiac regulation.7, 8, 9, 10
The methods used for analyzing HRV include geometric methods the triangular index (RRTri), the triangularinterpolation of RR interval histogram (TINN), and the Poincaré plot which converts the RR intervals into geometric patterns and allows for the analysis of HRV through their geometric properties or graphs of the resulting pattern.9, 11, 12, 13, 14
The RRTri and TINN indices are calculated based on the construction of a density histogram of normal RR intervals containing the length of the RR intervals on the x axis, and the frequency with which they occur on the y axis.9, 11, 12, 13, 14 The Poincaré plot is a 2-dimensional graphical representation of the correlation between consecutive RR intervals, where each interval is plotted against the next interval. The analysis can then be performed qualitatively by assessing the shape formed by the attractor, which shows the degree of complexity of the RR intervals, or quantitatively by fitting an ellipse to the shape formed by the plot, from which the following indices are obtained: SD1, SD2, and the ratio SD1/SD2.10, 15, 16, 17 Furthermore, the analysis of the Poincaré plot is considered by some authors to be based on nonlinear dynamics.9, 10, 18
Nonlinear methods of HRV analysis have been gaining interest, since there is evidence that the mechanisms involved in cardiovascular regulation probably interact in a nonlinear manner.10, 19 Furthermore, nonlinear analysis has given new insights into the abnormalities of heart rate (HR) behavior in various conditions, thus providing additional information for physiological interpretation and prognostics, as compared with traditional methods.20
Considering that HRV analysis using geometric methods can provide clinically relevant information, to better understand the influence of functional training in a healthy population, the aim of this study was to evaluate the effects of functional training on cardiac autonomic modulation in healthy young women.
2. Methods
2.1. Experimental approach
This was a randomized clinical trial, and the training and data collection were performed in a private clinic at the Salus Studio Physical Rehabilitation and Longevity locating in Presidente Prudente, Brazil. Prior to this clinical trial, a pilot study was performed with the same descriptions as noted below. All volunteers underwent an initial assessment which included: anthropometric measurements (height and weight), an assessment of autonomic modulation, and a muscle strength evaluation by means of a 1RM test to determine the load which would be used during training for each of the exercises. The autonomic modulation assessments for both groups were repeated 24 h after the completion of the training period in the functional training group (FTG).
The FTG underwent a periodized functional training program for 12 weeks with a frequency of 3 sessions per week, totaling 30 sessions of training. The loads during the exercises began at 30% of the 1RM load achieved on the assessment test, and were gradually increased until they reached 100% in the final week of training.
2.2. Subjects
The initial sample consisted of 32 healthy volunteers aged 18–26 years who had not performed any regular physical activity or strength training in the previous 6 months.
Volunteers who presented any of the following characteristics were excluded: smokers, alcoholics, users of drugs or medications that could influence ANS activity, subjects with known cardiovascular, respiratory or metabolic diseases, subjects who presented with any inflammatory and/or infectious process or episodes of the musculotendinous junction, and subjects who presented with musculoskeletal injuries of the upper or lower limbs, and/or the spine.
The volunteers were informed about the procedures and aims of this study, and after agreeing to participate, signed a written informed consent form. In addition, each volunteer attached a copy of a medical certificate confirming them to be in sufficient physical condition to perform the exercises. All procedures utilized in this study were approved by the Committee for Ethics and Research of the Institution (CAAE Process. No. 01310212.4.0000.5402).
2.3. Experimental groups
The volunteers were randomly assigned to the following 2 groups consisting of 16 subjects each:
a) Control group (CG): the participants received all evaluations, but were not enrolled in the functional training program;
b) Functional training group (FTG): the participants received all evaluations, and were enrolled in the functional training program.
At the end of the experimental protocol, the CG still contained 16 volunteers. However, the FTG had been reduced by 3 subjects: 1 voluntarily dropped out, and the other 2 presented with a series of RR intervals with errors of more than 5%, and therefore the final FTG was composed of 13 subjects.
The anthropometric values of each group and their p values to verify the homogeneity between control and experimental groups were: age (20.56 ± 1.03 vs. 23.00 ± 2.51 years; p = 0.01), weight (58.42 ± 10.43 vs. 58.32 ± 8.72 kg; p = 0.977), height (1.62 ± 0.04 vs. 1.63 ± 0.05 m; p = 0.0788), and body mass index (BMI) (22.12 ± 3.86 vs. 21.90 ± 2.82 kg/m2; p = 0.862).
2.4. Procedures
2.4.1. Training program
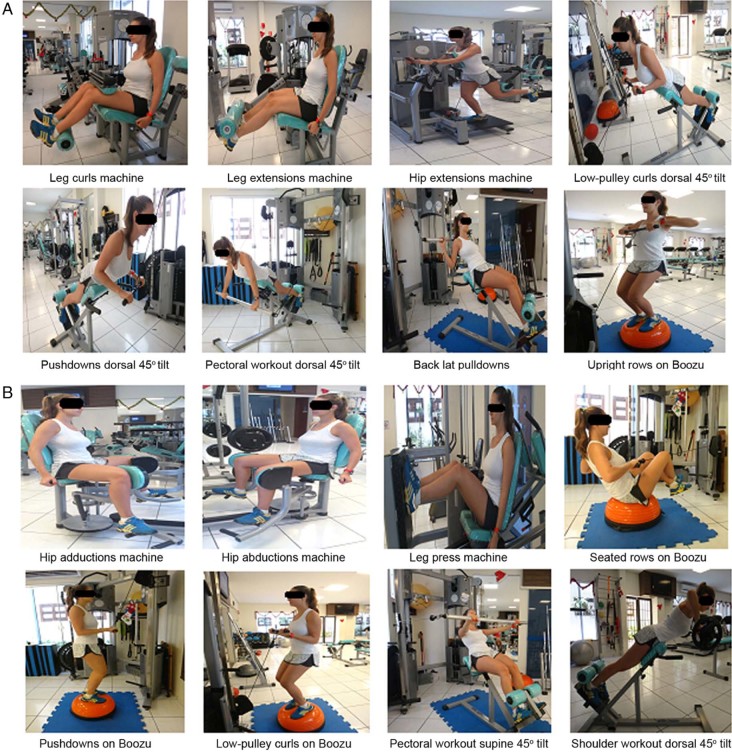
The FTG was enrolled in the periodized training program with a frequency of 3 sessions per week, with 60 min each one, totaling 30 sessions of training with recuperative intervals of 24–72 h between sessions. The program included 2 sets of exercises (A and B), which encompassed the majority of the muscle groups such as: flexors/extensors/adductors/abductors of the leg, hip, arm, and shoulder, scapular stabilizers, gluteals, abdominals, lumbar spine, chest and back muscles. The exercise sets were alternatedevery session. Fig. 1A and B presents exercise sets A and B, respectively.
Fig. 1.
Functional resistance training exercises: (A) set A, (B) set B.
Prior to the start of each exercise program, a 1RM test and familiarization with both the equipment and the exercises were performed. The loads for each exercise were calculated according to a load percentage of the 1RM stipulated for each week of training.
The training was performed in a periodized and progressive manner, starting with loads of between 30% and 40% of the 1RM during the period of force resistance (3 weeks), in which the number of sets and repetitions were increased each week as follows: 2 × 12, 2 × 16, and 2 × 20. The 4th week was allocated for recuperation (untrained). Strength training was performed from the 5th week of training. The load increases from the 5th to the 8th week ranged from 40% to 90% of the 1RM, using increments termed “pyramids”, where for each 10% increase in 1RM workload, there was a decrease of 2 repetitions of the exercise. Three series were performed for each exercise, starting with 16 repetitions in Week 5, 12 in Week 6, 10 in Week 7, and 8 in Week 8, respectively.
The 9th week was also allocated for recuperation, and then the strength training continued with load increments (“pyramids”) as follows: the number of series and repetitions were: 1 × 6, 1 × 4, 1 × 2, 1 × 4, and 1 × 6, and the loads varied according to the number of repetitions, respectively: 80%, 90%, 100%, 90%, and 80% of the 1RM. On the 10th week, a series of 1 × 2 was added with a load of 100% of the 1RM, and on the 12th week, a further series of 1 × 2 with a load of 100% of the 1RM was added. The interval between each set of exercises varied from 40 s to 90 s.
2.4.2. Anthropometric assessment
The body weight was measured digitally (Welmy R/I 200; Welmy Balanças, Santa Bárbara d'Oeste, Brazil) with the volunteers standing barefoot, arms extended along the body. For height measurements (Standard Sanny stadiometer; Sanny, São Bernardo do Campo, Brazil), the volunteers were also barefoot and the measurement was taken during exhalation. The BMI was calculated from the measurements of height and weight (kg/m2).21
2.4.3. Assessment of autonomic modulation
The data collection was carried out in a room with the temperature between 21°C and 23°C and relative humidity between 40% and 60%. The data were collected between 7:00 a.m. and 12:00 a.m. to minimize interference with circadian rhythms.
The volunteers were instructed to abstain from ingesting alcoholic beverages and/or stimulants such as coffee, tea, soft drinks, and chocolate for 24 h prior to performing the assessment. The data were collected individually, and the subjects were instructed to remain at rest and avoid conversation during this procedure. The circulation of people around the room was not permitted during the data collection in order to reduce the anxiety of the volunteers.
The HRV and HR indices were recorded beat-to-beat using a Polar® S810i HR monitor (Polar Electro OY, Kempele, Finland), which had been previously validated for capturing beat-to-beat HR and for the analysis of HRV.22 To capture the HR, a belt containing an HR monitor transmitter was placed on the chest of each volunteer at the level of the distal third of the sternum, just below the breasts, and the capture instrument was placed on the right wrist of the volunteer. After the placement of the HR monitor, the volunteers remained lying supine on a stretcher at rest, breathing spontaneously for 30 min. All collections were carried out in the morning to avoid circadian variations.
The data obtained from the HR monitor were transferred to a computer through Polar Precision Performance SW software, Version 3.0 (Polar Electro OY). To calculate the HRV indices, Kubios HRV software, Version 2.0 (Kubios; Biosignal Analysis and Medical Image Group, Department of Physics, University of Kuopio, Kuopio, Finland) was used.
To analyze the data, excerpts were selected from a series of RR intervals (1000 consecutive RR intervals between the 5th up to the 25th min),23 which contained more than 95% sinus beats before the filtering procedures. After this selection, the excerpt was subjected to a filtering process. Initially, the series were digitally filtered by Polar Precision Performance SW software, and then supplemented by manual inspection using Microsoft Excel software (Microsoft Office, Redmond, WA, USA). After these procedures, the visual inspection of the series did not reveal any interference that could influence the outcome.
The RRtri and TINN indices, together with the quantitative and qualitative analyses from the Poincaré plot and the RR intervals, were used to analyze HRV.
The triangular index was calculated from the construction of a density histogram of the normal RR intervals, and consisted of the integral of the histogram (i.e., the total number of RR intervals) divided by the maximum density distribution (modal frequency of RR intervals), measured on a discrete scale with boxes of 7.8125 ms (1/128 s).9 The TINN consisted of the width of the line at the base of the measured distribution as the base of a triangle, approximating the distribution of all RR intervals, and using the difference of the least squares to determine the triangle.9
For the quantitative assessment of the Poincaré plot, by adjusting the ellipse of the shape formed by the attractor, 3 indices were obtained: the SD1, SD2, and the ratio SD1/SD2. The SD1 index represents the standard deviation (SD) of the instantaneous variability of the continuous RR intervals, determined by the width of the ellipse formed by the Poincaré plot, and can be considered a marker of parasympathetic modulation. The SD2 index corresponds to the SD of the long-term continuous RR intervals, determined by the length of the plot, and is a marker of parasympathetic and sympathetic modulation. The SD1/SD2 index shows the relationship between the short and long variations of the intervals.8, 9, 13
The qualitative analysis of the plot was performed by analyzing the figures formed by the attractor, and the following patterns were considered for this analysis: 1) a pattern where an increase in the dispersion of beat-to-beat RR intervals was observed; 2) a pattern with a small beat-to-beat global dispersion (SD1) and without any increase in the dispersion of the long-term RR intervals; 3) a parabolic or complex pattern in which 2 or more distinct ends were separated from the main body of the plot, with at least 3 points included at each end.9, 13
2.4.4. Statistical analysis
The data analysis of the population profile was checked for normality by means of the Shapiro–Wilk test, and since all of the data presented in a normal distribution, we applied the Student's t test to check the homogeneity between the groups. To evaluate the effects of the functional training, the differences between groups (final values – baseline) were compared, and for this the normality of the data was tested using the Shapiro–Wilk test. After this, the Student's t test for paired data was used when the distribution was normal, and the Mann–Whitney test for data whose distribution was not normal. The level of significance was set at p < 0.05 for all tests. The calculation for the power of this study, with the number of subjects analyzed and a significance level of 5% (2-tailed test), ensured a power greater than 80% for detecting differences between the variables.
3. Results
Table 1 presents the geometric HRV indices and the RR intervals analyzed pre- and post-training. A comparison between the differences obtained from the final and initial values from each group indicated that the FTG showed a significant (p < 0.05) increase in values of these indices:RR intervals, RRtri, SD1, and SD2, as compared to the CG. No statistically significant differences were observed between the TINN indices and the SD1/SD2 ratio.
Table 1.
Means ± SD, means (95%CI), and p values for RR intervals and analysis of geometric heart rate variability indices in the control and functional training groups pre- and post-training.
| Control | FTG | p value | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Difference | Pre | Post | Difference | ||
| RR intervals | 856.98 ± 92.79 | 851.53 ± 71.87 | −5.45 ± 104.25 | 815.78 ± 143.13 | 869.58 ± 112.27 | 53.80 ± 72.96 | 0.048 |
| 839.75(807.55–906.42) | 835.90(813.24–889.82) | −1.10(−302.20–156.90) | 768.30(729.28–902.29) | 886.50(801.73–937.43) | 79.90(−122.19–134.10) | ||
| RRtri | 12.68 ± 2.67 | 14.04 ± 4.15 | 1.72 ± 2.94 | 12.06 ± 4.06 | 17.29 ± 4.84 | 5.23 ± 3.74 | 0.009 |
| 12.95(11.25–14.10) | 13.40(12.19–16.61) | 0.71(−1.94–7.87) | 11.91(9.61–14.51) | 16.71(14.36–20.22) | −0.48(−1.47– 12.24) | ||
| TINN | 221.56 ± 74.89 | 247.19 ± 53.48 | 25.62 ± 104.40 | 184.62 ± 61.12 | 251.15 ± 58.88 | 66.53 ± 76.74 | 0.050 |
| 215.00(181.66–261.46) | 237.50(218.70–275.68) | 15.00(−105.00–365.00) | 180.00(141.05–225.18) | 260.00(215.57–286.74) | 65.00(−80.00–195.00) | ||
| SD1 | 30.19 ± 8.77 | 31.13 ± 10.86 | 0.91 ± 5.94 | 26.00 ± 17.85 | 38.06 ± 16.92 | 12.06 ± 8.22 | 0.001 |
| 29.55(25.51–34.86) | 30.40(25.32–36.90) | −0.45(−7.2–10.80) | 21.90(15.21–36.79) | 33.80(27.84–48.29) | 10.50(0.90–26.30) | ||
| SD2 | 68.06 ± 18.59 | 74.74 ± 23.99 | 6.67 ± 22.61 | 60.85 ± 15.68 | 94.90 ± 21.75 | 34.05 ± 21.38 | 0.003 |
| 61.65(58.16–99.97) | 70.80(61.96–87.52) | 7.05(−35.10–62.10) | 63.10(51.37–70.33) | 89.30(81.76–108.50) | 28.60(5.70–79.00) | ||
| SD1/SD2 | 0.44 ± 0.08 | 0.42 ± 0.13 | −0.01 ± 0.15 | 0.40 ± 0.21 | 0.38 ± 0.11 | −0.01 ± 0.16 | 0.287 |
| 0.46(0.31–0.59) | 0.38(0.26–0.73) | −0.06(−0.24–0.33) | 0.34(0.17–0.94) | 0.36(0.22–0.67) | 0.00(−0.48–0.16) | ||
Abbreviations: RR intervals = intervals between consecutive heart beats; RRtri = triangular index; TINN = triangular interpolation of RR intervals; SD1 = standard deviation of instantaneous beat-to-beat variability; SD2 = standard deviation of the long-term variability; SD1/SD2 = ratio between SD1 and SD2; 95%CI = 95% confidence intervals.
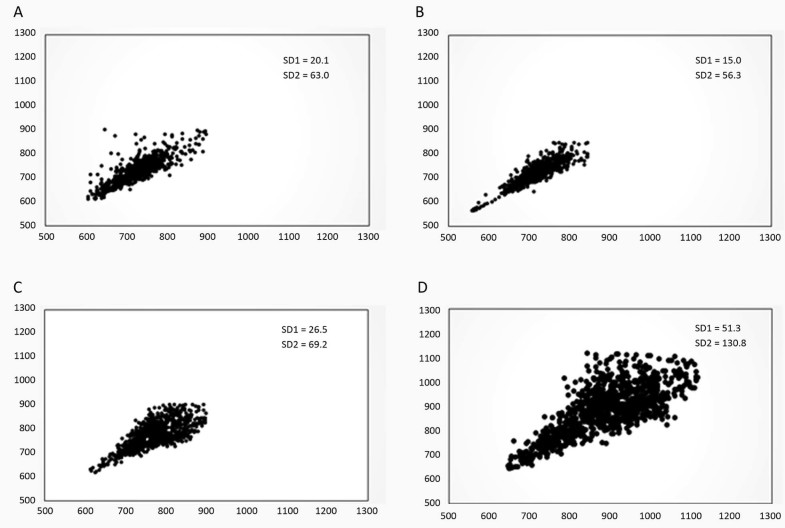
Fig. 2 provides a visual representation of the pattern of the Poincaré plot pre and post the functional training protocol for both experimental and control groups. The plot of 1 subject from each group (CG vs. FTG) pre and post is shown in Fig. 2. This subject was chosen according to the mean values of the SD1 and SD2 indices of the groups.
Fig. 2.
Example of a Poincaré plot observed in the control group pre (A) and post (B), and in the experimental group pre (C) and post (D) the functional training program.
4. Discussion
The present study investigated the impact of functional training on cardiac autonomic modulation in healthy young women using geometric indices of HRV. These results showed a statistically significant increase (p < 0.05) in the value of the indices RRTri, SD1, SD2, and RR intervals in the FTG as compared to the CG. In addition, the qualitative analysis of the Poincaré plot showed an increase in the dispersion of the beat-to-beat and long-term RR intervals in the FTG after training. These findings suggest that functional training over 12 weeks was able to promote improvements in autonomic modulation in healthy young women.
This is the first study which evaluated the effects of a period of functional training on autonomic modulation using geometric indices of HRV, with an emphasis on the analysis of the Poincaré plot, considered by some authors as a nonlinear index.9, 10, 18
HRV analysis using nonlinear methods enables a broader understanding of the physiological impact of functional training on autonomic modulation, since there is evidence that the mechanisms involved in cardiovascular regulation probably interact in a nonlinear manner.10, 19
There was an increase in the SD1 index in the trained volunteers as compared to the controls between the pre- and post-training values, suggesting that functional training promoted an increase in the parasympathetic cardiac component branch of the ANS.
It is known that decreased vagal activity is associated with an increased risk of morbidity and mortality from various causes, as well as the development of risk factors for cardiovascular disease.24 Therefore, it can be suggested that functional training is a good strategy for preventing such occurrences, because it promotes an increased parasympathetic cardiac component.
Performing functional training also promoted an increase in the overall variability, as supported by the increases in the SD2 and RRTri indices. The TINN index is also an indicator of overall ANS activity. Although it was higher in the training group with values close to being statistically significant, the TINN index showed no statistically significant differences between the groups.
The magnitude of HRV provides important information about the functioning of autonomic nervous control and the ability of the heart to respond to different stimuli.11 Considered as a whole, these results suggest that functional training increases the responsiveness of the heart to external stimuli.
Regarding the SD1/SD2 ratio, no statistically significant differences were observed when comparing the functional group with the controls, which could be explained by the increases in both SD1 and SD2 indices in the group which underwent training.
In the qualitative analysis of the Poincaré plot, both greater global beat-to-beat dispersion and an increase in the dispersion of long-term RR intervals were observed after training, suggesting that functional training increased HRV.
The present results demonstrated that the geometric indices were able to identify an improvement in autonomic modulation induced by functional training. This is an important result, because the main advantage of geometric methods lies in their sensitivity to the analytical quality of a series of intervals. Furthermore, both RRTri and TINN indices are highly insensitive to artefacts and ectopic beats, because they are left out of the triangle, thus reducing the need for pre-processing the recorded data.7
5. Limitation and conclusion
One limitation of this study is the fact that the groups being compared were not homogeneous in age. However, despite being statistically significant, the age difference between the groups was small. We do not believe that this influenced the results. Other studies have investigated the effects of functional training in different age groups and populations, and may further contribute to its use in a clinical setting.
The results of this study show that functional training performed for 12 weeks in healthy young women promoted a beneficial impact on cardiac autonomic modulation, as characterized by an increase in the parasympathetic cardiac component and overall variability, and that these changes could be identified by examining the geometric indices of HRV. The results obtained in this study indicate that functional training could be an interesting strategy for promoting the benefits of cardiac autonomic modulation in healthy young adult women.
Authors' contributions
MPCRB and BMC were responsible for all procedures involving the clinical trial, as sessions and data collection. JNJ and CMP developed and coordinated the training protocol. LCMV oversaw the clinical trial with MPCRB, and was responsible for the complete preparation of the manuscript. AKFS, AFBB, FMV, JNJ, and CMP were responsible for reviewing the article. All authors have read and approved the final version of the manuscript and agree with the order of presentation of the authors.
Competing interests
None of the authors declare competing financial interests.
Acknowledgment
We would like to thank the Coordination for Improvement of Higher Education Personnel – CAPES for their financial support, and all volunteers who participated in this clinical trial for their dedication.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Tomljanović M., Spasić M., Gabrilo G., Uljević O., Foretić N. Effects of five weeks of funcional vs. tradicional resistance training on anthropometric and motor performance variables. Kinesiology. 2011;43:145–154. [Google Scholar]
- 2.Weiss T., Kreitinger J., Wilde H., Wiora C., Steege M., Dalleck L. Effect of functional resistance training on muscular training on muscular fitnees outcomes in young adults. J Exerc Sci Fit. 2010;8:113–122. [Google Scholar]
- 3.Kibele A., Behm D.G. Seven weeks of instability and traditional resistance training effects on strength, balance and functional performance. J Strength Cond Res. 2009;23:2443–2450. doi: 10.1519/JSC.0b013e3181bf0489. [DOI] [PubMed] [Google Scholar]
- 4.Legally K.M., Cordero J., Good J., Brown D.D., Mccaw S.T. Physiologic and metabolic responses to a continuous functional resistance exercise workout. J Strength Cond Res. 2009;23:373–379. doi: 10.1519/JSC.0b013e31818eb1c9. [DOI] [PubMed] [Google Scholar]
- 5.Milton D., Porcari J.P.P., Foster C., Gibson M., Udermann B. The effect of functional exercise training on functional fitness levels of older adults. Gundersen Med J. 2008;5:4–8. [Google Scholar]
- 6.Pacheco M.M., Teixeira L.A.C., Franchini E., Takito M.Y. Functional vs. strength training in adults: specific needs define the best intervention. Int J Sports Phys Ther. 2013;8:34–43. [PMC free article] [PubMed] [Google Scholar]
- 7.Rezende Barbosa M.P.C., Silva A.K.F., Bernardo A.F.B., Souza N.M., Junior J.N., Pastre C.M. Influence of resistance training on cardiac autonomic modulation: literature review. Med Express. 2014;1:284–288. [Google Scholar]
- 8.Acharya U.R., Joseph K.P., Kannathal N., Lim C.M., Suri J.S. Heart rate variability: a review. Med Biol Eng Comput. 2006;44:1031–1051. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 9.Da Silva S.P.F., Guida H.L., Antônio A.M.S., Vanderlei L.C., Ferreira L.L., Abreu L.C. Auditory stimulation with music influences the geometric indices of heart rate variability in men. Int Arch Med. 2014;7:27. doi: 10.1186/1755-7682-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshi R.A., Pastre C.M., Vanderlei L.C., Godoy M.F. Poincaré plot indexes of heart rate variability: relationships with other nonlinear variables. Auton Neurosci. 2013;177:271–274. doi: 10.1016/j.autneu.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 12.Vanderlei L.C., Pastre C.M., Freitas I.F., Godoy M.F. Geometric indexes of heart rate variability in obese and eutrophic children. Arq Bras Cardiol. 2010;95:35–40. doi: 10.1590/s0066-782x2010005000082. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho T.D., Pastre C.M., Rossi R.C., Abreu L.C., Valenti V.E., Vanderlei L.C. Geometric index of heart rate variability in chronic obstructive pulmonary disease (Índices geométricos de variabilidade da frequência cardíaca na doença pulmonar obstrutiva crônica) Rev Port Pneumol. 2011;17:260–265. doi: 10.1016/j.rppneu.2011.06.007. [in Portuguese] [DOI] [PubMed] [Google Scholar]
- 14.Tulppo M.P., Makikallio T.H., Takala T.E.S., Seppanen T., Huikuri H.V. Quantitative beat-to-beat analysis of heart rate dynamics during exercise. Am J Physiol Circ Physiol. 1996;271:H244–52. doi: 10.1152/ajpheart.1996.271.1.H244. [DOI] [PubMed] [Google Scholar]
- 15.De Carvalho T.D., Wajnsztejn R., de Abreu L.C., Marques Vanderlei L.C., Godoy M.F., Adami F. Analysis of cardiac autonomic modulation of children with attention deficit hyperactivity disorder. Neuropsychiatr Dis Treat. 2014;10:613–618. doi: 10.2147/NDT.S49071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi R.C., Vanderlei F.M., Bernardo A.F., Souza N.M., Goncalves A.C., Ramos E.M. Effect of pursed-lip breathing in patients with COPD: linear and nonlinear analysis of cardiac autonomic modulation. COPD. 2014;11:39–45. doi: 10.3109/15412555.2013.825593. [DOI] [PubMed] [Google Scholar]
- 17.Vanderlei L.C.M., Pastre C.M., Hoshi R.A., Carvalho T.D., Godoy M.F. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc. 2009;24:205–217. doi: 10.1590/s0102-76382009000200018. [DOI] [PubMed] [Google Scholar]
- 18.Karmakar C.K., Gubbi J., Khandoker A.H., Palaniswami M. Analyzind temporal variabililty of standard descriptors of Poincaré plots. J Electrocardiol. 2010;43:719–724. doi: 10.1016/j.jelectrocard.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Godoy M.F., Takakura I.T., Correa P.R. Relevance of the analysis of nonlinear dynamic behavior (chaos theory) as a prognostic factor of morbidity and mortality in patients undergoing coronary artery bypass grafting. Arq Cienc Saúde. 2005;12:167–171. [Google Scholar]
- 20.Khaled A.S., Owis M.I., Mohamed A.S.A. Employing time-domain methods and Poincaré plot of heart rate variability signals to detect congestive heart failure. BIME J. 2006;6:35–41. [Google Scholar]
- 21.World Health Organization . World Health Organization; Geneva: 2000. Obesity: preventing and managing the global epidemic. Report of a World Health Organization consultation. WHO obesity technical report series. p.284. [PubMed] [Google Scholar]
- 22.Vanderlei L.C.M., Silva R.A., Pastre C.M., Azevedo F.M., Godoy M.F. Comparison of the polar S810i monitor and the ECG for the analysis of heart rate variability in the time and frequency domains. Braz J Med Biol Res. 2008;41:854–859. doi: 10.1590/s0100-879x2008005000039. [DOI] [PubMed] [Google Scholar]
- 23.de Rezende Barbosa M.P.C., Silva N.T., de Azevedo F.M., Pastre C.M., Vanderlei L.C. Comparison of Polar® RS800G3TM heart rate monitor with Polar® S810iTM and electrocardiogram to obtain the series of RR intervals and analysis of heart rate variability at rest. Clin Physiol Funct Imaging. 2016;36:112–117. doi: 10.1111/cpf.12203. [DOI] [PubMed] [Google Scholar]
- 24.Thayer J.F., Lane R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]