Abstract
Background:
No large population-based studies have been done on systemic lupus erythematosus (SLE) mortality trends in the United States.
Objective:
To identify secular trends and population characteristics associated with SLE mortality.
Design:
Population-based study using a national mortality database and census data.
Setting:
United States.
Participants:
All U.S. residents, 1968 through 2013.
Measurements:
Joinpoint trend analysis of annual age-standardized mortality rates (ASMRs) for SLE and non-SLE causes by sex, race/ethnicity, and geographic region; multiple logistic regression analysis to determine independent associations of demographic variables and period with SLE mortality.
Results:
There were 50 249 SLE deaths and 100 851 288 non-SLE deaths from 1968 through 2013. Over this period, the SLE ASMR decreased less than the non-SLE ASMR, with a 34.6% cumulative increase in the ratio of the former to the latter. The non-SLE ASMR decreased every year starting in 1968, whereas the SLE ASMR decreased between 1968 and 1975, increased between 1975 and 1999, and decreased thereafter. Similar patterns were seen in both sexes, among black persons, and in the South. However, statistically significant increases in the SLE ASMR did not occur among white persons over the 46-year period. Females, black persons, and residents of the South had higher SLE ASMRs and larger cumulative increases in the ratio of the SLE to the non-SLE ASMR (31.4%, 62.5%, and 58.6%, respectively) than males, other racial/ethnic groups, and residents of other regions, respectively. Multiple logistic regression showed independent associations of sex, race, and region with SLE mortality risk and revealed significant racial/ethnic differences in associations of SLE mortality with sex and region.
Limitations:
Underreporting of SLE on death certificates may have resulted in underestimates of SLE ASMRs. Accuracy of coding on death certificates is difficult to ascertain.
Conclusion:
Rates of SLE mortality have decreased since 1968 but remain high relative to non-SLE mortality, and significant sex, racial, and regional disparities persist.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with limited treatment options (1). Five- and 10-year survival rates for patients with SLE improved from less than 50% in the 1950s to more than 90% in the 1980s (2). However, the influence of more recent diagnostic and therapeutic developments on SLE mortality in the general population of the United States is unknown.
Previous studies of SLE mortality were based primarily on deaths in patient cohorts (3–6), which do not capture changes in SLE incidence overtime and do not reflect the true burden and trends of SLE mortality in the general population. Several studies have used population-based designs but were limited to specific regions and small samples (7–10). Some studies pooled deaths from multiple years (for example, 9 to 30 years) (3, 7, 10), which obscures changes in SLE mortality trends during the collection periods. These limitations may have contributed to the inconsistency of findings from previous studies (5, 6). We therefore undertook a population-based study of temporal trends and demographic and regional differences in SLE mortality in the United States from 1968 through 2013. We examined SLE mortality trends by sex, race/ethnicity, and geographic region and conducted multiple logistic regression analysis to assess the independent association of these variables with SLE mortality.
Methods
Data Sources
The Centers for Disease Control and Prevention (CDC)’s National Vital Statistics System maintains a mortality database, with data provided by various jurisdictions that are legally responsible for the registration of vital events and information extracted from death certificates. This database encompasses more than 99% of deaths of U.S. residents in all 50 states and the District of Columbia. Using the CDC’s WONDER (Wide-ranging Online Data for Epidemiologic Research) database, we gathered data on SLE deaths from 1968 (the earliest year for which the CDC published county-level mortality data) through 2013(11).
Death certificates in the United States provide the International Classification of Diseases (ICD) code for the underlying cause of death, which is defined as “the disease or injury that initiated the events resulting in death” (12). Deaths were attributed to SLE if an ICD code for SLE was listed as the underlying cause on the death certificate (Appendix Table 1, available at Annals.org). Age, race/ethnicity, and geographic region were ascertained using standard methods (13). Since 1999, race has been classified as white, black or African American, Asian or Pacific Islander (PI), or American Indian (AI) or Alaska Native (AN). Before 1999, AI, AN, Asian, and PI were grouped into an “other” race category. Ethnicity is classified as Hispanic or non-Hispanic, with this information available only after 1999. Geographic region is classified according to U.S. census regional divisions (Appendix Table 2, available at Annals.org).
We used the CDC WONDER database to obtain annual death counts in the entire U.S. population and separately by sex, race/ethnicity, and U.S. geographic region (Northeast, Midwest, South, or West). Counts of SLE deaths for smaller racial groups (Asian, PI, AI, and AN) were collapsed due to aggregated reporting by the CDC to avoid potential threats to confidentiality associated with counts at higher resolutions. Because information on ethnicity was not collected before 1999, we used 2 alternate racial/ethnic classifications. For analyses that included deaths before 1999, we used 3 race categories (white, black, and Asian/PI/AI/AN), and for analyses limited to 1999 through 2013, we used 4 racial/ethnic categories (non-Hispanic white, non-Hispanic black, Hispanic, and Asian/PI/AI/AN). For data from 1999 through 2013, we also obtained death counts for 192 combinations of sex, race/ethnicity, region, and age (≤64 or ≥65 years), pooled across multiple years (into 3 periods) for confidentiality. For calculation of mortality rates, the size of the population (total and each group) was obtained from the U.S. Census Bureau files for each year.
Annual Mortality Rates
We quantified age-specific crude mortality rates for SLE and non-SLE causes for each year from 1968 through 2013 as the number of deaths divided by the number of persons in the U.S. general population. This was done within age strata (10-year ranges, except at the extremes of the age distribution, as shown in Appendix Table 3 [available at Annals.org]) for the total U.S. population and separately for each sex, race, and geographic region.
To calculate the overall age-standardized mortality rate (ASMR) for the population for each year from 1968 through 2013, we combined the yearly age-specific crude mortality rates with the age distribution of the U.S. population in 2000, as described in Appendix Table 3. This was done separately for the total U.S. population and for each sex, race, and geographic region and for both SLE deaths and non-SLE deaths. We then computed the ratio of the SLE ASMR to the non-SLE ASMR for each year.
Statistical Analysis
We used joinpoint regression to fit piecewise-linear (or broken-line) trends to the yearly SLE ASMR, the yearly non-SLE ASMR, and the ratio of the former to the latter over the 46-year period. Joinpoint regression identifies a set of joinpoints (or knots)–the time points (calendar years) at which the change in the slope of the ASMR is statistically significant–and computes the slope (year-to-year percentage change in annual ASMR) and the 95% CI over each linear trend segment between adjacent joinpoints (14). This approach identifies the year when the trend (slope of the increase or decrease) in mortality rate changes significantly and determines the magnitude of the change. Joinpoint regression analyses were conducted using the National Cancer Institute Joinpoint Regression Program, which uses a grid search method (15) to find the best locations for the joinpoints based on a least-squares fit of the data and uses a permutation test to determine the optimal number of joinpoints (16) (Appendix, available at Annals.org).
A second set of analyses was designed to determine the independent relationship of age (≤64 vs. ≥65 years), sex, race/ethnicity, geographic region, and calendar year with SLE mortality. Data were pooled across calendar years into three 5-year periods for this analysis (to allow downloading of death counts without a threat to confidentiality): 1999 through 2003, 2004 through 2008, and 2009 through 2013. We performed multiple logistic regression on the aggregated group-level data (SLE death count and population size for 192 groups defined by 2 age categories, 2 sexes, 4 race/ethnicity categories, and 4 geographic regions, summed over all calendar years in each of the 3 periods) using maximum likelihood estimation with frequency weights (logistic command using the fweight option in Stata, version 13.1 [StataCorp]). Frequency weights represented total SLE deaths or the total population without SLE deaths for each group in each period. In addition to the main effects for each of the demographic, geographic, and time variables, we tested for effect modification by including the pairwise interaction terms of race/ethnicity, sex, geographic location, and calendar year in the model. For interactions that were statistically significant (2-sided P < 0.05), we assessed SLE mortality associations stratified by race/ethnicity. We estimated the odds ratio and 95% CI. We then used the Stata margins command to calculate model-predicted annual mortality for the individual demographic, region, or time characteristics integrated across all other characteristics and the pwcompare option to compute the differences in predicted annual mortality between 2 strata (for example, old vs. young or male vs. female), integrated across all other demographic, region, and time variables.
Sensitivity Analysis
Since 1999, information on the contributing cause of death, which is defined as “other significant conditions contributing to death but not resulting in the underlying cause,” has been available in the CDC WONDER database. To address the possibility that SLE may not be listed as the underlying cause on death certificates of some patients who died of SLE complications and that this coding error may differ across different subpopulations, we performed the multiple logistic regression analyses for cases where SLE was listed as a contributing cause of death.
Role of the Funding Source
The funding agencies had no role in this study.
Results
We identified 50 249 deaths with SLE listed as the underlying cause in the United States from 1968 through 2013. The proportions of SLE deaths among females, nonwhite persons, and residents of the South and West were higher than the proportions of non-SLE deaths (Table 1).
Table 1.
Demographic Characteristics of SLE and Non-SLE Deaths, 1968-2013*
| Characteristic | Deaths† |
Average Population (n = 255 515 709)‡ | |
|---|---|---|---|
| SLE (n = 50 249) | Non-SLE (n = 100 851 288) | ||
| Sex | |||
| Male | 8020 (16.0) | 52 296 499 (51.9) | 124 911 842 (48.9) |
| Female | 42 229 (84.0) | 48 554 789 (48.1) | 130 603 867 (51.1) |
| Race§ | |||
| White | 32 860 (65.4) | 87 289 828 (86.6) | 212 744 356 (83.3) |
| Black | 15 530 (30.9) | 11 949 610 (11.8) | 31 993 449 (12.5) |
| Asian/PI/AI/AN | 1859 (3.7) | 1 611 850 (1.6) | 10 777 904 (4.2) |
| Geographic region | |||
| Northeast | 9069 (18.0) | 22 038 635 (21.9) | 51 697 432 (20.2) |
| Midwest | 10 489 (20.9) | 25 326 347 (25.1) | 61 445 627 (24.0) |
| South | 20 547 (40.9) | 35 635 519 (35.3) | 88 650 732 (34.7) |
| West | 10 144 (20.2) | 17 850 787 (17.3) | 53 758 117 (21.1) |
AI = American Indian; AN = Alaska Native; PI = Pacific Islander; SLE = systemic lupus erythematosus.
Values are numbers (percentages). Percentages may not sum to 100 due to rounding.
Absolute number of deaths from all 50 states and the District of Columbia.
Mean annual population derived from U.S. Census Bureau files.
Information on Hispanic ethnicity was not collected before 1999 and is therefore not shown.
Mortality Trends Overall and by Sex, Race, and Geographic Region
The ASMR for SLE decreased from 0.45 (95% CI, 0.42 to 0.48) per 100 000 persons in 1968 to 0.34 (CI, 0.32 to 0.36) per 100 000 persons in 2013. However, the relative decrease in the SLE ASMR (24.4%) was lower than that for non-SLE causes (43.9%) during the same period (Table 2).
Table 2.
Cumulative Percentage Change in SLE ASMR, Non-SLE ASMR, and Ratio of SLE to Non-SLE ASMR, 1968-2013
| Variable | SLE |
Non-SLE* |
Ratio of SLE to Non-SLE ASMR |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1968 |
2013 |
Change, % | ASMR per 100 000 Persons |
Change, % | Ratio × 10−5 |
Change, % | |||||
| ASMR per 100 000 Persons | Deaths, n | ASMR per 100 000 Persons | Deaths, n | 1968 | 2013 | 1968 | 2013 | ||||
| All | 0.45 | 807 | 0.34 | 1144 | −24.4 | 1303.1 | 731.6 | −43.9 | 34.5 | 46.5 | 34.6 |
| Sex | |||||||||||
| Male | 0.19 | 157 | 0.10 | 160 | −47.4 | 1633.5 | 863.5 | −47.1 | 11.6 | 11.6 | −0.4 |
| Female | 0.70 | 650 | 0.55 | 984 | −21.4 | 1041.9 | 623.0 | −40.2 | 67.2 | 88.3 | 31.4 |
| Race† | |||||||||||
| White | 0.39 | 619 | 0.26 | 732 | −33.3 | 1270.5 | 730.8 | −42.5 | 30.7 | 35.6 | 15.9 |
| Black | 0.98 | 178 | 0.85 | 356 | −13.3 | 1611.3 | 860.0 | −46.6 | 60.8 | 98.8 | 62.5 |
| Asian/PI/AI/AN | NA‡ | 10 | 0.24 | 56 | NA‡ | 929.6 | 435.8 | −53.1 | NA‡ | 59 | NA‡ |
| Geographic region | |||||||||||
| Northeast | 0.47 | 211 | 0.28 | 175 | −40.4 | 1348.2 | 686.9 | −49.1 | 34.9 | 40.8 | 16.9 |
| Midwest | 0.40 | 203 | 0.27 | 201 | −32.5 | 1292.6 | 759.8 | −41.2 | 30.9 | 35.5 | 14.8 |
| South | 0.43 | 233 | 0.40 | 505 | −7.0 | 1328.1 | 778.9 | −41.4 | 32.4 | 51.4 | 58.6 |
| West | 0.53 | 160 | 0.34 | 263 | −35.8 | 1195.4 | 660.5 | −44.7 | 44.3 | 51.5 | 16.1 |
AI = American Indian; AN = Alaska Native; ASMR = age-standardized mortality rate; NA = not available; PI = Pacific Islander; SLE = systemic lupus erythematosus.
Total non-SLE deaths in 1968 and 2013 were 1 929 275 and 2 595 849, respectively.
Information on Hispanic ethnicity was not collected before 1999 and is therefore not shown.
Data not shown because <20 persons died of SLE causes in 1968 in this subpopulation.
The SLE ASMR was lower in 2013 than in 1968 among males and females, among white persons and black persons, and in all geographic regions (Table 2), but the relative cumulative decrease in the SLE ASMR was smaller in females than in males, in black persons than in white persons, and in the South than in other geographic regions. The decrease in the SLE ASMR was smaller than the decrease in the non-SLE ASMR in all subpopulations except in males, where the cumulative change between 1968 and 2013 was similar for SLE and non-SLE causes.
Joinpoint trend analysis showed that although the ASMR for non-SLE causes decreased continuously between 1968 and 2013, the SLE ASMR increased between 1975 and 1999 (Figure). After an initial decrease between 1968 and 1975, the SLE ASMR increased steeply between 1975 and 1981 and continued to increase at a lower rate between 1981 and 1999 before decreasing again starting in 1999. Similar trends in the SLE ASMR were seen in both sexes, except that the reversal of the uptrend in SLE mortality began more than a decade earlier in males than in females (1989 vs. 2000) (Appendix Figure 1, available at Annals.org). Although the SLE ASMR did not statistically significantly increase among white persons and those in the Asian/PI/AI/AN group at any time over the study period, the SLE ASMR among black persons increased between 1975 and 1983, did not change between 1983 and 2002, and decreased between 2002 and 2013. Persons in all 4 geographic regions also experienced a period of increase in the SLE ASMR before a decrease during the most recent period. In contrast to this changing trend for the SLE ASMR, the non-SLE ASMR decreased or stayed stable throughout the study period in all subpopulations (Appendix Figure 1).
Figure.
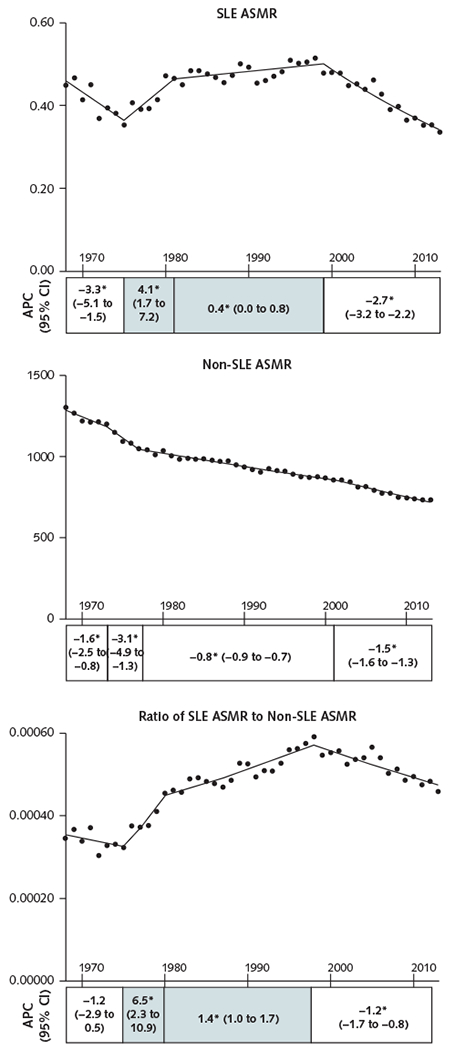
ASMRs for SLE and non-SLE causes and ratio of SLE to non-SLE mortality rates, 1968-2013.
ASMRs per 100 000 persons for SLE and non-SLE causes are shown in the top and middle panels, respectively. Data are displayed per calendar year of death, with lines fitted on the basis of joinpoint analysis. The bottom panel shows the ratio of SLE to non-SLE ASMRs. A positive slope of the ratio indicates increased risk for death from SLE vs. non-SLE causes, and a negative slope indicates decreased risk. The APC for each trend in SLE ASMR, non-SLE ASMR, and the ratio of SLE to non-SLE ASMR is presented as stack bars under each panel. Each stack is segmented at the year in which the change in slope is statistically significant and is aligned with the trend line. Numbers in each stack denote the APC (95% CI). The shaded stacks indicate an increasing trend, and the unshaded stacks represent a decreasing or nonsignificant trend. APC = annual percentage change; ASMR= age-standardized mortality rate; SLE = systemic lupus erythematosus.
* P < 0.05 for slope change.
To highlight the changes in SLE mortality relative to non-SLE mortality over time, we also examined secular trends in the ratio of the SLE ASMR to the non-SLE ASMR (Figure). An increase in the ratio between 1975 and 1998 indicates an increase in the proportion of U.S. deaths from SLE during this period. The ratio showed a sustained decrease after 1998 but was still 34.6% higher in 2013 than in 1968 (Table 2). A similar pattern of increase followed by decrease in the ratio was seen in all subpopulations (Appendix Figure 1), except that the rising trend ended earlier in males than in females (1983 vs. 1998) and in white persons than in black persons (1998 vs. 2004). The relative cumulative change between 1968 and 2013 also differed between males and females, between white persons and black persons, and between the South and other regions (Table 2).
Results of Multiple Logistic Regression
The secular decreases over time since 1999 that were seen in the unadjusted joinpoint analyses (Figure) were supported by the results of the multiple logistic regression (Table 3). After adjustment for age, sex, race/ethnicity, and geographic region, the risk for SLE death was significantly lower in 2004 through 2008 than in 1999 through 2003 and decreased even further in 2009 through 2013. Females had significantly higher risk than males, as did members of racial/ethnic minorities (black, Asian/PI/AI/AN, and Hispanic) relative to white persons, residents of the South or West relative to the Northeast, and persons aged 65 years or older relative to those aged 0 to 64 years.
Table 3.
SLE Mortality Associations With Demographic Characteristics, 1999-2013: Multivariable Logistic Regression Analysis*
| Characteristic | Deaths, n | Predicted Annual Mortality per 100 000 Persons (95% CI)† | Predicted Annual Mortality Difference per 100 000 Persons (95% CI)‡ | P Value | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 2732 | 0.129 (0.124 to 0.134) | Reference | Reference | ||
| Female | 16 134 | 0.686 (0.676 to 0.697) | 0.558 (0.546 to 0.569) | <0.001 | 5.33 (5.12 to 5.55) | <0.001 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 9328 | 0.300 (0.294 to 0.306) | Reference | Reference | ||
| Non-Hispanic black | 6292 | 1.170 (1.140 to 1.200) | 0.873 (0.843 to 0.904) | <0.001 | 3.91 (3.79 to 4.05) | <0.001 |
| Asian/PI/AI/AN | 860 | 0.358 (0.333 to 0.382) | 0.058 (0.033 to 0.083) | <0.001 | 1.19 (1.11 to 1.28) | <0.001 |
| Hispanic | 2386 | 0.409 (0.392 to 0.426) | 0.109 (0.091 to 0.127) | <0.001 | 1.36 (1.30 to 1.43) | <0.001 |
| Geographic region | ||||||
| Northeast | 2866 | 0.349 (0.336 to 0.362) | Reference | Reference | ||
| Midwest | 3474 | 0.372 (0.359 to 0.384) | 0.023 (0.005 to 0.041) | 0.012 | 1.07 (1.01 to 1.12) | 0.012 |
| South | 8346 | 0.454 (0.445 to 0.464) | 0.106 (0.090 to 0.122) | <0.001 | 1.30 (1.25 to 1.36) | <0.001 |
| West | 4180 | 0.475 (0.460 to 0.490) | 0.127 (0.107 to 0.146) | <0.001 | 1.36 (1.30 to 1.43) | <0.001 |
| Calendar period | ||||||
| 1999–2003 | 6621 | 0.473 (0.461 to 0.484) | Reference | Reference | ||
| 2004–2008 | 6439 | 0.434 (0.423 to 0.444) | −0.039 (−0.055 to −0.023) | <0.001 | 0.92 (0.89 to 0.95) | <0.001 |
| 2009–2013 | 5806 | 0.366 (0.357 to 0.375) | −0.107 (−0.121 to −0.092) | <0.001 | 0.77 (0.75 to 0.80) | <0.001 |
| Age | ||||||
| 0–64 y | 13 010 | 0.332 (0.326 to 0.337) | Reference | Reference | ||
| ≥65 y | 5856 | 1.060 (1.040 to 1.090) | 0.731 (0.703 to 0.760) | <0.001 | 3.20 (3.10 to 3.31) | <0.001 |
AI = American Indian; AN = Alaska Native; PI = Pacific Islander; SLE = systemic lupus erythematosus.
Main effects only. The period from 1999 through 2013 was chosen for this analysis because information on Hispanic ethnicity was not available before 1999. The total number of deaths in the sample was 18 866.
Annual mortality for each characteristic is the marginal probability predicted by the model, integrated across all other characteristics. For example, mortality in males and in females is integrated over both age groups, all races/ethnicities, all calendar periods, and all geographic regions.
Mortality differences for the individual-variable comparisons are marginal probability differences among strata, integrated across all other characteristics. For example, age difference is the difference in SLE mortality between the 2 age groups, integrated over all males and females, all races/ethnicities, all calendar periods, and all geographic regions.
Interaction testing for effect modification revealed significant interactions between race/ethnicity and both sex and geographic region, indicating that race/ethnicity modified the relationship among sex, geographic region, and SLE mortality. No significant interactions were found between calendar periods and all other covariates (data not shown), suggesting that the decrease in SLE mortality with time occurred similarly in all demographic groups. The final model included only the interactions between sex and race and between region and race.
Analysis of SLE mortality risk stratified by race/ethnicity revealed that it was higher in females than in males in all racial/ethnic groups, but the adjusted odds ratio and mortality differences differed between various racial/ethnic groups and were largest in non-Hispanic black persons and smallest in non-Hispanic white persons (Table 4; Appendix Table 4, available at Annals.org). For each racial/ethnic group, SLE mortality risk was greater in all other regions relative to the Northeast, except for non-Hispanic black persons in the Midwest and Hispanic persons in the South and Midwest. Hispanic persons in the Midwest had significantly lower SLE mortality than those in the Northeast. In non-Hispanic white persons, residence in the South conferred the highest SLE mortality risk, but in the other 3 racial/ethnic groups, residents of the West had the highest risk. The largest regional disparity in SLE mortality risk was in the Asian/PI/AI/AN group in the West versus the Northeast; the next largest regional disparity was in Hispanic persons in the West versus the Midwest.
Table 4.
SLE Mortality Associations With Sex and Geographic Region, Stratified by Race/Ethnicity, 1999-2013*
| Characteristic | Predicted Annual Mortality per 100 000 Persons (95% CI)† |
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-Hispanic White | P Value | Non-Hispanic Black | P Value | Asian/PI/AI/AN | P Value | Hispanic | P Value | |
| Sex | ||||||||
| Male | 0.107 (0.102 to 0.112) | - | 0.286 (0.265 to 0.306) | - | 0.098 (0.805 to 0.116) | - | 0.105 (0.094 to 0.116) | - |
| Female | 0.494 (0.483 to 0.504) | - | 1.850 (1.800 to 1.900) | - | 0.562 (0.522 to 0.603) | - | 0.611 (0.584 to 0.637) | - |
| Geographic region | ||||||||
| Northeast | 0.247 (0.235 to 0.260) | - | 1.000 (0.940 to 1.070) | - | 0.178 (0.139 to 0.217) | - | 0.336 (0.300 to 0.372) | - |
| Midwest | 0.277 (0.266 to 0.289) | - | 1.030 (0.972 to 1.100) | - | 0.310 (0.247 to 0.373) | - | 0.229 (0.190 to 0.268) | - |
| South | 0.367 (0.355 to 0.378) | - | 1.140 (1.100 to 1.180) | - | 0.280 (0.236 to 0.325) | - | 0.341 (0.318 to 0.365) | - |
| West | 0.331 (0.316 to 0.346) | 1.310 (1.210 to 1.410) | - | 0.435 (0.398 to 0.472) | - | 0.406 (0.382 to 0.429) | - | |
|
Predicted Annual Mortality Difference per 100 000 Persons (95% CI)‡ |
||||||||
| Sex | ||||||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 0.387 (0.374 to 0.399) | <0.001 | 1.570 (1.520 to 1.620) | <0.001 | 0.464 (0.420 to 0.508) | <0.001 | 0.506 (0.477 to 0.534) | <0.001 |
| Geographic region | ||||||||
| Northeast | Reference | Reference | Reference | Reference | ||||
| Midwest | 0.030 (0.013 to 0.047) | <0.001 | 0.030 (−0.058 to 0.118) | 0.50 | 0.132 (0.058 to 0.206) | <0.001 | −0.107 (−0.160 to −0.054) | <0.001 |
| South | 0.120 (0.102 to 0.137) | <0.001 | 0.135 (0.062 to 0.209) | <0.001 | 0.102 (0.043 to 0.161) | 0.001 | 0.005 (−0.038 to 0.486) | 0.81 |
| West | 0.084 (0.064 to 0.103) | <0.001 | 0.303 (0.184 to 0.421) | <0.001 | 0.256 (0.203 to 0.310) | <0.001 | 0.070 (0.026 to 0.113) | 0.002 |
AI = American Indian; AN = Alaska Native; PI = Pacific Islander; SLE = systemic lupus erythematosus.
The period from 1999 through 2013 was chosen for this analysis because information on Hispanic ethnicity was not available before 1999. The total number of deaths in the sample was 18 866. Multivariable logistic regression models included interaction terms. Of the pairwise interactions tested, only race/ethnicity by sex and race/ethnicity by geographic region were statistically significant.
Annual mortality for each characteristic is the marginal probability predicted by the model, integrated across all other characteristics within each racial/ethnic group. For example, mortality in males and in females is integrated over both age groups, all calendar periods, and all geographic regions.
Mortality differences for the individual-variable comparisons are marginal probability differences among strata, integrated across all other characteristics within each racial/ethnic group. For example, age difference is the difference in SLE mortality between the 2 age groups, integrated over all males and females, all calendar periods, and all geographic regions.
We repeated the multiple logistic regression analyses for deaths where SLE was listed as a contributing cause (Appendix Tables 5 and 6, available at Annals.org). Findings were similar to trends observed in cases where SLE was recorded as the underlying cause of death.
Discussion
Analyses of all deaths recorded across the United States over a 46-year period revealed that the reduction in mortality attributed to SLE was less than the reduction in non-SLE mortality, and the ratio of SLE to non-SLE mortality was 34.6% higher in 2013 than in 1968. Although all-cause mortality has decreased continuously over time, SLE mortality decreased initially but then increased before decreasing in the most recent study period. We found differences in SLE mortality trends by sex, race, and region, which were supported by multiple regression analyses that also revealed significant racial/ethnic differences in associations of SLE mortality with sex and region.
After an initial decrease between 1968 and 1975, SLE mortality increased annually for 24 years, followed by a sustained decrease for 14 years starting in 1999. Changes in SLE incidence over time could partially explain the observed changing trends in SLE mortality. In Minnesota, incidence of SLE tripled between 1950 and 1992 (7), and in Spain, incidence increased from 1.9 cases per 100 000 persons in 1987 to 1991 to 4.5 cases per 100 000 persons in 1992 to 1996 before decreasing to 1.6 cases per 100 000 persons in 2002 to 2006 (17). Use of new diagnostic tests, classification criteria, and therapies could also have influenced SLE mortality trends (Appendix Figure 2, available at Annals.org). For example, oral immunosuppressive therapies, including cyclophosphamide and azathioprine, were introduced in the 1970s (18) and were associated with increased drug toxicities that were reduced with the introduction of intravenous pulse cyclophosphamide in the 1980s (19, 20). Subsequent use of mycophenolate (21) and combination therapy with induction and maintenance phases in the 1990s (22) led to further reductions in drug-associated complications and greater efficacy. Increasing use of antimalarial drugs (7) and awareness of cardiovascular complications of SLE since the late 1990s (23) might also have contributed to recent improvements in SLE outcomes. The pattern of changes in mortality that we observed may reflect these therapeutic advances and potential benefits or complications of treatment.
We found significant disparities in SLE mortality among subpopulations based on sex, age, race/ethnicity, and geographic region. Increased SLE mortality in older persons may be related to complications associated with increasing cumulative doses of immunosuppressive medications as well as SLE complications, such as atherosclerosis. Higher mortality in females might reflect the higher prevalence of SLE among them, although sex-associated genetic, hormonal, social, and environmental factors may also influence SLE mortality (24, 25). Increased SLE mortality in black persons might be attributable to more high-risk disease features, such as extensive cellular crescents in the kidneys (26). Similar findings of increased mortality in black persons were reported in previous studies (3, 27). The higher SLE mortality in Hispanic persons than in white persons observed in this study contradicts a previous study that reported lower mortality risk among Hispanic versus white Medicaid patients; however, that study had relatively few SLE deaths among Hispanic persons (27). Furthermore, the significant sex and regional differences that we found in racial/ethnic disparities may have confounded previous studies that did not explicitly look for such interactions.
We found significant regional differences in SLE mortality in each racial/ethnic group. Residence in the West conferred the highest SLE mortality risk in all racial/ethnic groups except white persons, who had the highest risk in the South. A previous study identified clusters of elevated SLE mortality in Alabama, Arkansas, Louisiana, and New Mexico and clusters of low mortality in Minnesota, Vermont, Virginia, and Washington (28). The areas with elevated mortality had higher rates of poverty and/or greater concentrations of Hispanic persons than the areas with lower mortality (28). Further, a study in nonwhite persons suggested a greater effect of poverty than race/ethnicity on SLE mortality (29). Geographic differences in the quality of care of patients with lupus nephritis have also been reported, with more patients in the Northeast receiving standard-of-care medications (30). Interactions between genetic and nongenetic factors associated with race/ethnicity and geographic differences in environment, such as increased sunlight exposure, socioeconomic factors, and access to medical care, might also influence SLE mortality.
Our approach of using direct age standardization to calculate annual mortality rates has advantages over the more commonly used indirect age standardization methods. Previous studies of SLE mortality, which were summarized in 2 recent meta-analyses (5, 6), have reported standardized mortality ratios, which are based on indirect standardization using the age structure of the study population (that is, the SLE patient cohort). However, standardized mortality ratios from different studies are not comparable when the age structure of the study population varies (31). Other strengths of our study include a systematic statistical approach to identifying the calendar years when mortality trends changed; computation of the ratio of the SLE ASMR to the non-SLE ASMR to assess SLE mortality relative to all-cause mortality; and use of multivariate regression analyses with interaction terms to assess the independent effect of demographic characteristics and region on SLE mortality, with allowance for one risk factor modifying the effect of another.
Several limitations of this study should be considered. First, the validity of our findings depends on the accuracy of the physicians’ coding on death certificates, which is difficult to ascertain. Systemic lupus erythematosus was recorded on death certificates in only 60% of deaths in patients with SLE in well-defined patient cohorts from the South (32). Such underreporting of SLE on death certificates, especially in older patients and those without health insurance and with low education levels (32, 33), may result in underestimates of SLE mortality in certain subpopulations. Nevertheless, the large differences we found in SLE mortality by sex, race/ethnicity, and region are unlikely to have resulted from misclassification of cause of death because greater underreporting of SLE as the cause of death in underprivileged groups (32, 33) would lead to greater underestimation of SLE mortality in the groups for which we found higher risk (such as females, black persons, Hispanic persons, and residents of the South). Second, revisions between ICD-8 and ICD-9 and between ICD-9 and ICD-10 may also have influenced the estimation of mortality trends, although studies that measured the effects of ICD revisions have reported good comparability ratios for disease classification between revisions (34, 35). Third, secular changes over time in physicians’ reporting and attributing of SLE as the underlying cause of death could have influenced SLE mortality trend estimates. However, sensitivity analyses of deaths for which SLE was recorded as a contributing cause also showed the same trends (Appendix Tables 5 and 6), suggesting that errors in coding of cause of death did not substantially bias the findings. Finally, data on disease severity; treatment history; organ involvement; and other clinical, laboratory, and social variables were not available in our study.
In conclusion, our analyses of data from the U.S. National Vital Statistics System revealed that SLE mortality decreased in all subpopulations, including females and black persons, during the past decade after periods of increasing rates from the 1970s through the 1990s. Despite improving trends, SLE mortality remains high relative to non-SLE mortality, and disparities persist between subpopulations and geographic regions. Comprehensive examination of SLE mortality using prospective population-based data collection could be helpful in understanding the mechanisms of the disparities in SLE mortality and identifying potentially modifiable risk factors that might inform targeted research and public health programs to promote health equity across subpopulations and regions of the United States.
Acknowledgment:
The authors thank Dr. Kenrik Duru (UCLA Division of General Internal Medicine and Health Services Research and UCLA Center for Health Policy Research) for critically reading the manuscript and the UCLA Institute for Digital Research and Education Statistical Consulting Group for statistical assistance.
Financial Support: This work was supported in part by the National Institutes of Health (P30-AG028748, R01-AI080778, R01-AR056465, S21-MD-000103, U54-MD-008149, U54-MD-007598, and UL1 TR001881-02), the Lupus Foundation of America, and the Rheumatology Research Foundation. Dr. Yen was supported by the National Institutes of Health (T32-DK-07789 and 5T32-HD-007512), the UCLA Children’s Discovery and Innovation Institute, and a Mallinckrodt Research Fellowship Award.
Primary Funding Source: None.
Appendix: Statistical Methods
Joinpoint Regression Program
The joinpoint regression model identifies changes in trend data by fitting a set of joinpoints (points indicating slope changes) over the entire period. The model assumes that its regression mean function is piecewise linear and that the linear segments are continuously connected at the joinpoints. Joinpoint regression is also referred to as segmented regression, piecewise regression, or broken-line regression. Joinpoints are the years in which the changes in the slope are statistically significant. The Joinpoint Regression Program (version 4.2.0.2; National Cancer Institute) has an easy-to-use graphical user interface. The user inputs a formatted data file and selects the minimum and maximum number of joinpoints based on the observed data. For this study, we selected a maximum of 5 joinpoints. The user then selects 1 of 3 options for handling heteroscedastic errors: constant variance (homoscedasticity), SE, or Poisson variance. In addition, the user can select 1 of 2 methods for model fitting: the grid search method or the Hudson method. The grid search method identifies a discrete number of locations for testing for changes in slope, and the Hudson method allows for continuous model fitting but is more computationally intensive. Each trend can be as short as 1 year or can encompass several years, depending on the grid search method. For this study, we provided the SE and used the grid search method. Finally, there are advanced settings, which are described in the instruction manual (40).
The Joinpoint Regression Program uses a permutation test to find the optimal number of joinpoints. For greater consistency in the permutation test P values, the Joinpoint Regression Program runs at least 4499 permutations to select the optimal number of linear segments to fit the model. Because fitting all 4499! (factorial) possible permutations would be computationally intensive, the program uses a Monte Carlo simulation to conduct significance tests on a sample of the 4499! permutations, which are adjusted using the Bonferroni correction to reduce the likelihood of false-positive results (16).
Multivariable Regression Analyses (Stata Codes)
Model 1: Main Effect
a. logistic y i.sex i.race_ethnicity i.geo_region i.calndr_period i.age [fweight=freq].
This was the initial model constructed before interaction terms were added.
i. margins [marginslist], pwcompare (effects) where marginlist is a list of variables or interactions from the logistic model. Margins command allow us to compute the annual mortality difference for the individual demographic variables averaging across all other demographic strata.
Model 2: With Calendar Period × Covariate Interactions
logistic y i.sex i.race_ethnicity i.geo_region i.calndr_period i.age i.sex#i.calndr_period i.race_ethnicity#i.calndr_period i.geo_region#i.calndr_period i.age#i.calndr_period [fweight=freq]
No significant interactions were found between calendar periods and all other covariates (data not shown), suggesting that the decrease in SLE mortality with time occurred similarly in all demographic groups.
Model 3: Model With Race/Ethnicity-by-Sex and Race/Ethnicity-by-Geographic Region Interactions
a. logistic y i.sex i.race_ethnicity i.geo_region i.calndr_period i.age i.race_ethnicity#i.sex i.race_ethnicity#i.geo_region [fweight=freq]
Significant interactions were found between race/ethnicity and sex and race/ethnicity and geographic region.
Appendix Table 1.
ICD Codes for SLE
| ICD-8 (1968-1978) |
| 734.1 SLE* |
| ICD-9 (1978-1998) |
| 710.0 SLE* |
| ICD-10 (1999-2013) |
| M32 SLE† |
| M32.1 SLE with organ or system involvement |
| M32.8 Other forms of SLE |
| M32.9 SLE, unspecified |
ICD = International Classification of Diseases; SLE = systemic lupus erythematosus.
ICD-8 and ICD-9 have a single category code for SLE.
ICD-10 has a single category code for SLE plus subcategory codes.
Appendix Table 2.
Census Regions
| Northeast |
| Connecticut |
| Maine |
| Massachusetts |
| New Hampshire |
| New Jersey |
| New York |
| Pennsylvania |
| Rhode Island |
| Vermont |
| Midwest |
| Illinois |
| Indiana |
| Iowa |
| Kansas |
| Michigan |
| Minnesota |
| Missouri |
| Nebraska |
| North Dakota |
| Ohio |
| South Dakota |
| Wisconsin |
| South |
| Alabama |
| Arkansas |
| Delaware |
| District of Columbia |
| Florida |
| Georgia |
| Kentucky |
| Louisiana |
| Maryland |
| Mississippi |
| North Carolina |
| Oklahoma |
| South Carolina |
| Tennessee |
| Texas |
| Virginia |
| West Virginia |
| West |
| Alaska |
| Arizona |
| California |
| Colorado |
| Hawaii |
| Idaho |
| Montana |
| Nevada |
| New Mexico |
| Oregon |
| Utah |
| Washington |
| Wyoming |
Appendix Table 3.
Example of Direct Age Standardization Calculation: SLE Mortality in the U.S. Female Population for 2013*
| Age Group | SLE Deaths, n | General Population, n | Age-Specific SLE Crude Mortality Rates per 100 000 Persons† | Standard Population (Year 2000), n | Expected Deaths, n‡ |
|---|---|---|---|---|---|
| <1 y | 0 | 1 925 056 | 0.000 | 3 794 901 | 0.0 |
| 1–4 y | 2 | 7 790 614 | 0.026 | 15 191 619 | 3.9 |
| 5–14 y | 2 | 20 159 538 | 0.010 | 39 976 619 | 4.0 |
| 15–24 y | 50 | 21 429 247 | 0.233 | 38 076 743 | 88.7 |
| 25–34 y | 101 | 21 203 096 | 0.476 | 37 233 437 | 177.2 |
| 35–44 y | 110 | 20 307 429 | 0.542 | 44 659 185 | 242.1 |
| 45–54 y | 157 | 22 198 448 | 0.707 | 37 030 152 | 261.8 |
| 55–64 y | 202 | 20 359 676 | 0.992 | 23 961 506 | 237.7 |
| 65–74 y | 176 | 13 419 124 | 1.312 | 18 135 514 | 237.9 |
| 75–84 y | 113 | 7 686 002 | 1.470 | 12 314 793 | 181.0 |
| ≥85 y | 71 | 3 999 007 | 1.775 | 4 259 173 | 75.6 |
| All§ | 984 | 160 477 237 | 0.613 | 274 633 642 | 1509.9 |
SLE = systemic lupus erythematosus.
Rates are standardized to the population from 2000 to eliminate the effect of age variation from year to year. The same method was used to calculate age-standardized mortality rates for all years from 1968 to 2013 and for all subpopulations based on sex, race, and geographic region.
Calculated as SLE Deaths / General Population × 100 000.
Calculated as age-specific SLE crude mortality rates per 100 000 persons × standard population. Total number of deaths expected in the standard population = 1510.
Age-standardized rate per 100 000 persons is equal to 1510 / 274 633 644 × 100 000 = 0.550.
Appendix Figure 1.
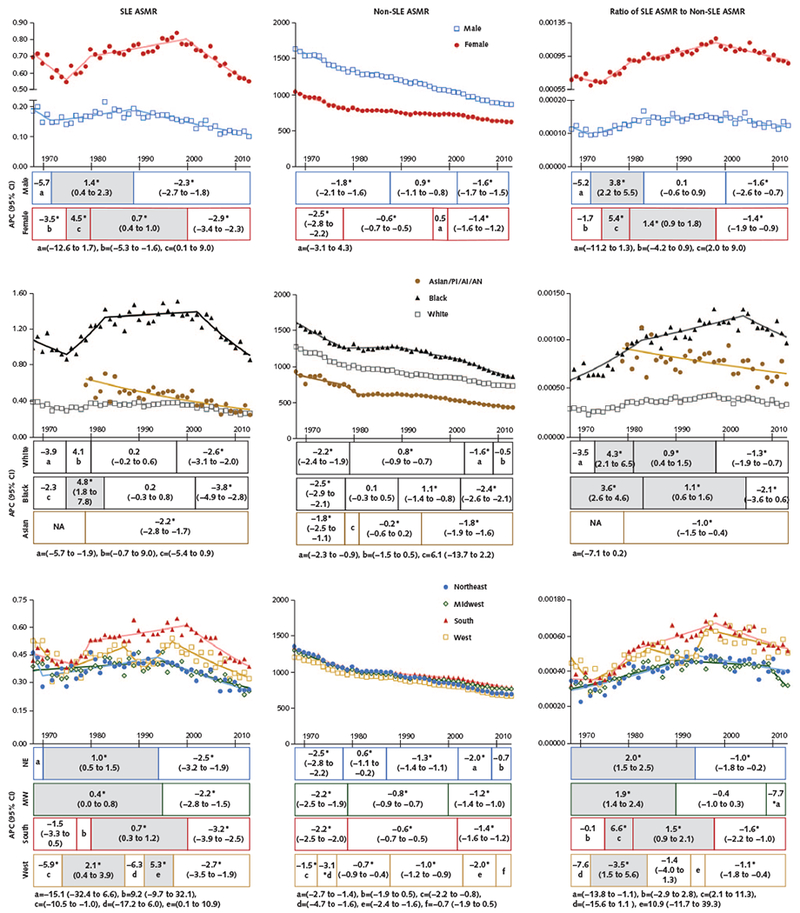
Trends in ASMRs from SLE and non-SLE causes and ratio of SLE to non-SLE mortality rates, by sex, race, and geographic region, 1968-2013.
Results are shown as SLE ASMRs and non-SLE ASMRs per 100 000 persons and the ratio of SLE to non-SLE mortality rates. Data are displayed per calendar year of death, with lines fitted on the basis of joinpoint trend analysis. From 1968 through 2013, the annual number of SLE deaths ranged from 570 to 1210 among females, 126 to 216 among males, 490 to 885 among white persons, 177 to 468 among black persons, 20 to 73 among Asians/PIs/AIs/ANs, 136 to 242 among persons in the Northeast, 180 to 293 among persons in the Midwest, 233 to 630 among persons in the South, and 110 to 317 among persons in the West. Data for Asians/PIs plus AIs/ANs are shown only for 1979 through 2013 because data from before 1979 are unreliable due to a small number of annual SLE deaths (<20) in this subpopulation. Because information on Hispanic ethnicity on death certificates is available only after 1999, ethnicity was not included in the joinpoint analysis. The APC for each trend for each subpopulation is presented as stack bars below each panel. Each stack is segmented at the year in which the change in slope is statistically significant and is aligned with the trend line. The numbers in each stack denote the APC (95% CI). The shaded stacks indicate an increasing trend, and the unshaded stacks represent a decreasing or nonsignificant trend. AI = American Indian; AN = Alaska Native; APC = annual percentage change; ASMR = age-standardized mortality rate; MW = Midwest; NA = not available; NE = Northeast; PI = Pacific Islander; SLE = systemic lupus erythematosus.
* P < 0.05 for slope change.
Appendix Table 4.
SLE Mortality Associations With Sex and Geographic Region, Stratified by Race/Ethnicity, 1999-2013: Adjusted Odds Ratio*
| Characteristic | Adjusted Odds Ratio (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-Hispanic White | P Value | Non-Hispanic Black | P Value | Asian/PI/AI/AN | P Value | Hispanic | P Value | |
| Sex | ||||||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 4.62 (4.37–4.88) | <0.001 | 6.49 (6.02–7.00) | <0.001 | 5.72 (4.71–6.95) | <0.001 | 5.81 (5.19–6.51) | <0.001 |
| Geographic region | ||||||||
| Northeast | Reference | Reference | Reference | Reference | ||||
| Midwest | 1.12 (1.05–1.20) | <0.001 | 1.03 (0.94–1.12) | 0.50 | 1.74 (1.29–2.35) | <0.001 | 0.68 (0.56–0.83) | <0.001 |
| South | 1.48 (1.40–1.58) | <0.001 | 1.14 (1.06–1.22) | <0.001 | 1.57 (1.20–2.06) | 0.001 | 1.02 (0.89–1.15) | 0.81 |
| West | 1.34 (1.25–1.43) | <0.001 | 1.30 (1.18–1.44) | <0.001 | 2.44 (1.93–3.09) | <0.001 | 1.21 (1.07–1.36) | 0.003 |
AI = American Indian; AN = Alaska Native; PI = Pacific Islander; SLE = systemic lupus erythematosus.
The period from 1999 through 2013 was chosen for this analysis because information on Hispanic ethnicity was not available before 1999. Multivariable logistic regression models included interaction terms. Of the pairwise interactions tested, only race/ethnicity by sex and race/ethnicity by geographic region were statistically significant. The total number of deaths in the sample was 18 866.
Appendix Table 5.
Sensitivity Analysis for SLE as a Contributing Cause of Death, 1999-2013: Multiple Logistic Regression Analysis*
| Characteristic | Deaths, n | Predicted Annual Mortality per 100 000 Persons (95% CI)† | Predicted Annual Mortality Difference per 100 000 Persons (95% CI)‡ | P Value | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 2031 | 0.094 (0.090 to 0.098) | Reference | Reference | ||
| Female | 10 563 | 0.436 (0.428 to 0.445) | 0.343 (0.333 to 0.352) | <0.001 | 4.66 (4.43 to 4.89) | <0.001 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 7521 | 0.233 (0.228 to 0.239) | Reference | Reference | ||
| Non-Hispanic black | 3351 | 0.671 (0.647 to 0.694) | 0.438 (0.413 to 0.462) | <0.001 | 2.88 (2.76 to 3.00) | <0.001 |
| Asian/PI/AI/AN | 511 | 0.216 (0.197 to 0.235) | −0.017 (−0.037 to 0.003) | 0.089 | 0.93 (0.85 to 1.01) | 0.100 |
| Hispanic | 1211 | 0.223 (0.210 to 0.236) | −0.010 (−0.024 to 0.004) | 0.168 | 0.96 (0.90 to 1.02) | 0.175 |
| Geographic region | ||||||
| Northeast | 1886 | 0.222 (0.212 to 0.232) | Reference | Reference | ||
| Midwest | 2523 | 0.254 (0.244 to 0.264) | 0.032 (0.018 to 0.047) | <0.001 | 1.15 (1.08 to 1.22) | <0.001 |
| South | 5209 | 0.291 (0.283 to 0.299) | 0.069 (0.056 to 0.082) | <0.001 | 1.31 (1.24 to 1.38) | <0.001 |
| West | 2976 | 0.344 (0.332 to 0.357) | 0.123 (0.106 to 0.139) | <0.001 | 1.55 (1.46 to 1.65) | <0.001 |
| Calendar period | ||||||
| 1999–2003 | 4225 | 0.301 (0.292 to 0.310) | Reference | Reference | ||
| 2004–2008 | 4158 | 0.276 (0.268 to 0.285) | −0.024 (−0.037 to −0.012) | <0.001 | 0.92 (0.88 to 0.96) | <0.001 |
| 2009–2013 | 4211 | 0.266 (0.258 to 0.274) | −0.035 (−0.047 to −0.023) | <0.001 | 0.88 (0.85 to 0.92) | <0.001 |
| Age | ||||||
| 0–64 y | 7023 | 0.181 (0.177 to 0.185) | Reference | Reference | ||
| ≥65 y | 5571 | 0.958 (0.932 to 0.985) | 0.777 (0.751 to 0.804) | <0.001 | 5.29 (5.10 to 5.49) | <0.001 |
AI = American Indian; AN = Alaska Native; CDC = Centers for Disease Control and Prevention; PI = Pacific Islander; SLE = systemic lupus erythematosus; WONDER = Wide-ranging Online Data for Epidemiologic Research.
Main effects only. The period from 1999 through 2013 was chosen for this analysis because information on contributing causes of death was not available in the CDC WONDER database before 1999. The total number of deaths in the sample was 12 463.
Annual mortality for each characteristic is the marginal probability predicted by the model, integrated across all other characteristics. For example, mortality in males and in females is integrated over both age groups, all races/ethnicities, all calendar periods, and all geographic regions.
Mortality differences for the individual-variable comparisons are marginal probability differences between strata, integrated across all other characteristics. For example, age difference is the difference in SLE mortality between the 2 age groups, integrated over all males and females, all races/ethnicities, all calendar periods, and all geographic regions.
Appendix Table 6.
Sensitivity Analysis for SLE as a Contributing Cause of Death, 1999-2013: Mortality Associations With Sex and Geographic Region, Stratified by Race/Ethnicity*
| Characteristic | Predicted Annual Mortality per 100 000 Persons (95% CI)† |
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-Hispanic White | P Value | Non-Hispanic Black | P Value | Asian/PI/AI/AN | P Value | Hispanic | P Value | |
| Sex | ||||||||
| Male | 0.089 (0.084 to 0.094) | - | 0.175 (0.159 to 0.191) | - | 0.054 (0.041 to 0.068) | - | 0.064 (0.055 to 0.073) | - |
| Female | 0.380 (0.370 to 0.389) | - | 0.956 (0.921 to 0.991) | - | 0.333 (0.303 to 0.364) | - | 0.281 (0.264 to 0.299) | - |
| Geographic region | ||||||||
| Northeast | 0.198 (0.186 to 0.209) | - | 0.495 (0.451 to 0.540) | - | 0.083 (0.056 to 0.110) | - | 0.156 (0.131 to 0.180) | - |
| Midwest | 0.226 (0.215 to 0.236) | - | 0.589 (0.543 to 0.636) | - | 0.183 (0.133 to 0.232) | - | 0.113 (0.085 to 0.141) | - |
| South | 0.268 (0.258 to 0.278) | - | 0.595 (0.568 to 0.622) | - | 0.165 (0.131 to 0.200) | - | 0.182 (0.165 to 0.199) | - |
| West | 0.302 (0.288 to 0.317) | - | 0.762 (0.686 to 0.839) | - | 0.264 (0.236 to 0.293) | - | 0.204 (0.187 to 0.221) | - |
|
Predicted Annual Mortality Difference per 100 000 Persons (95% CI)‡ |
||||||||
| Sex | ||||||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 0.291 (0.280 to 0.302) | <0.001 | 0.781 (0.742 to 0.819) | <0.001 | 0.279 (0.246 to 0.313) | <0.001 | 0.217 (0.197 to 0.237) | <0.001 |
| Geographic region | ||||||||
| Northeast | Reference | Reference | Reference | Reference | ||||
| Midwest | 0.028 (0.012 to 0.043) | <0.001 | 0.094 (0.030 to 0.158) | 0.004 | 0.100 (0.044 to 0.156) | <0.001 | −0.042 (−0.080 to −0.005) | 0.026 |
| South | 0.071 (0.056 to 0.086) | <0.001 | 0.100 (0.048 to 0.152) | <0.001 | 0.083 (0.039 to 0.126) | <0.001 | 0.026 (−0.004 to 0.056) | 0.084 |
| West | 0.105 (0.087 to 0.123) | <0.001 | 0.267 (0.179 to 0.356) | <0.001 | 0.181 (0.142 to 0.221) | <0.001 | 0.049 (0.019 to 0.079) | 0.001 |
|
Adjusted Odds Ratio (95% CI) |
||||||||
| Sex | ||||||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 4.28 (4.02 to 4.56) | <0.001 | 5.46 (4.94 to 6.02) | <0.001 | 6.15 (4.73 to 7.99) | <0.001 | 4.38 (3.75 to 5.11) | <0.001 |
| Geographic region | ||||||||
| Northeast | Reference | Reference | Reference | Reference | ||||
| Midwest | 1.14 (1.06 to 1.23) | <0.001 | 1.19 (1.06 to 1.34) | 0.004 | 2.21 (1.44 to 3.37) | <0.001 | 0.73 (0.54 to 0.98) | 0.035 |
| South | 1.36 (1.27 to 1.45) | <0.001 | 1.20 (1.09 to 1.33) | <0.001 | 2.00 (1.36 to 2.94) | <0.001 | 1.17 (0.97 to 1.41) | 0.095 |
| West | 1.53 (1.42 to 1.65) | <0.001 | 1.54 (1.35 to 1.76) | <0.001 | 3.19 (2.26 to 4.50) | <0.001 | 1.31 (1.10 to 1.57) | 0.003 |
AI = American Indian; AN = Alaska Native; CDC = Centers for Disease Control and Prevention; PI = Pacific Islander; SLE = systemic lupus erythematosus; WONDER = Wide-ranging Online Data for Epidemiologic Research.
The period from 1999 through 2013 was chosen for this analysis because information on contributing causes of death was not available in the CDC WONDER database before 1999. The total number of deaths in the sample was 12 463. Multivariable logistic regression models included interaction terms. Of the pairwise interactions tested, only race/ethnicity by sex and race/ethnicity by geographic region were statistically significant.
Annual mortality for each characteristic is the marginal probability predicted by the model, integrated across all other characteristics within each racial/ethnic group. For example, mortality in males and in females is integrated over both age groups, all calendar periods, and all geographic regions.
Mortality differences for the individual-variable comparisons are marginal probability differences between strata, integrated across all other characteristics within each racial/ethnic group. For example, age difference is the difference in SLE mortality between the 2 age groups, integrated over all males and females, all calendar periods, and all geographic regions.
Appendix Figure 2.
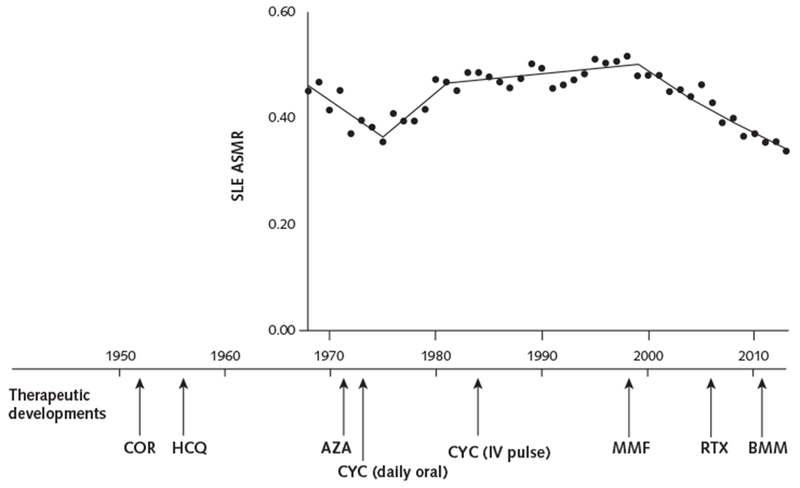
Major SLE treatment milestones in relation to SLE mortality rates.
The ASMR per 100 000 persons for SLE (Figure, top) is shown in relation to major SLE treatment milestones. Corticosteroids and hydroxychloroquine were introduced to treat patients with SLE in the 1950s (36, 37). Immunosuppressive drugs, including azathioprine (38) and daily oral cyclophosphamide (18), were introduced in the 1970s. The superiority of immunosuppressive drugs plus corticosteroids over corticosteroids alone was reported in the 1980s (39). Monthly IV cyclophosphamide was introduced in the 1980s (19, 20). Drugs that have more recently been used to treat SLE include mycophenolate, since the late 1990s (21); rituximab, since the mid-2000s; and belimumab, which was approved by the U.S. Food and Drug Administration to treat SLE in 2011 (22). ASMR = age-standardized mortality rate; AZA = azathioprine; BMM = belimumab; COR = corticosteroids; CYC = cyclophosphamide; HCQ = hydroxychloroquine; IV = intravenous; MMF = mycophenolate; RTX = rituximab; SLE = systemic lupus erythematosus.
Web-Only References
- 36.Mullins JF, Watts FL, Wilson CJ. Plaquenil in the treatment of lupus erythematosus. J Am Med Assoc 1956;161:879–81. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 37.Johnson SA, Meyer OO. The treatment of lupus erythematosus disseminatus with cortisone. Am J Med Sci 1952;223:9–15. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 38.Sztejnbok M, Stewart A, Diamond H, Kaplan D. Azathioprine in the treatment of systemic lupus erythematosus. A controlled study. Arthritis Rheum 1971;14:639–45. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 39.Felson DT, Anderson J Evidence for the superiority of immunosuppressive drugs and prednisone over prednisone alone in lupus nephritis. Results of a pooled analysis. N Engl J Med 1984;311: 1528–33. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 40.National Cancer Institute. Joinpoint Help Manual 4.5.0.1. Bethesda: National Cancer Institute; Accessed at https://surveillance.cancer.gov/joinpoint/Joinpoint_Help_4.5.0.1.pdf on 3 October 2017. [Google Scholar]
Footnotes
Drs. Karlamangla and Singh share senior authorship of this article.
Note: Drs. Yen and Singh had full access to the data and take full responsibility for the integrity of the data and the accuracy of the analysis.
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M17-0102.
Reproducible Research Statement: Study protocol: Not available. Statistical code: Available from Dr. Yen (eyen911@gmail.com). Data set: The data used in this study came from a national mortality database maintained by the CDC.
Current author addresses and author contributions are available at Annals.org.
References
- 1.Singh RR. Systemic lupus erythematosus In: Madhok R, Luthra H, eds. The Year in Rheumatic Disorders. Oxford, United Kingdom: Atlas Medical Publishing; 2007:171–90. [Google Scholar]
- 2.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. ; European Working Party on Systemic Lupus Erythematosus. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore). 2003;82:299–308. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 3.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum 2006;54:2550–7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 4.Urowitz MB, Gladman DD, Tom BD, Ibanez D, Farewell VT. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol 2008;35:2152–8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 5.Yurkovich M, Vostretsova K, Chen W, Aviha-Zubieta JA. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res (Hoboken). 2014;66:608–16. [PMID: ] doi: 10.1002/acr.22173 [DOI] [PubMed] [Google Scholar]
- 6.Lee YH, Choi SJ, Ji JD, Song GG Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727–34. [PMID: ] doi: 10.1177/0961203315627202 [DOI] [PubMed] [Google Scholar]
- 7.Uramoto KM, Michet CJ Jr, Thumboo J, Sunku J, O’Fallon WM, Gabriel SE. Trends in the incidence and mortality of systemic lupus erythematosus, 1950-1992. Arthritis Rheum 1999;42:46–50. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 8.Gudmundsson S, Steinsson K. Systemic lupus erythematosus in Iceland 1975 through 1984. A nationwide epidemiological study in an unselected population. J Rheumatol 1990;17:1162–7. [PMID: ] [PubMed] [Google Scholar]
- 9.Ståhl-Hallengren C, Jönsen A, Nived O, Sturfelt G Incidence studies of systemic lupus erythematosus in Southern Sweden: increasing age, decreasing frequency of renal manifestations and good prognosis. J Rheumatol 2000;27:685–91. [PMID: ] [PubMed] [Google Scholar]
- 10.Björnádal L, Yin L, Granath F, Klareskog L, Ekbom A Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964-95. J Rheumatol 2004;31:713–9. [PMID: ] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. CDC WONDER. Updated 27 June 2017. Accessed at https://wonder.cdc.gov on 1 October 2015.
- 12.Instructions for classifying the underlying cause of death In: Centers for Disease Control and Prevention. ICD-10 Mortality Manual 2a. Hyattsville, MD: Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 13.Centers for Disease Control and Prevention. Technical Appendix from Vital Statistics of United States 1999: Mortality. 2004. Accessed at www.cdc.gov/nchs/data/statab/techap99.pdf on 1 October 2015.
- 14.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med 2009; 28:3670–82. [PMID: ] doi: 10.1002/sim.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerman PM. Fitting segmented regression models by grid search. Appl Stat 1980;29:77–84. [Google Scholar]
- 16.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–51. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 17.Alonso MD, Llorca J, Martinez-Vazquez F, Miranda-Filloy JA, Diaz de Teran T, Dierssen T, et al. Systemic lupus erythematosus in northwestern Spain: a 20-year epidemiologic study. Medicine (Baltimore). 2011;90:350–8. [PMID: ] doi: 10.1097/MD.0b013e31822edf7f [DOI] [PubMed] [Google Scholar]
- 18.Steinberg AD, Kaltreider HB, Staples PJ, Goetzl EJ, Talal N, Decker JL. Cyclophosphamide in lupus nephritis: a controlled trial. Ann Intern Med 1971;75:165–71. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 19.Sessoms SL, Kovarsky J. Monthly intravenous cyclophosphamide in the treatment of severe systemic lupus erythematosus. Clin Exp Rheumatol 1984;2:247–51. [PMID: ] [PubMed] [Google Scholar]
- 20.Malaviya AN, Singh RR, Sindhwani R, Singh YN, Ahuja RK, Bhuyan UN, et al. Intermittent intravenous pulse cyclophosphamide treatment in systemic lupus erythematosus. Indian J Med Res 1992; 96:101–8. [PMID: ] [PubMed] [Google Scholar]
- 21.Glicklich D, Acharya A. Mycophenolate mofetil therapy for lupus nephritis refractory to intravenous cyclophosphamide. Am J Kidney Dis 1998;32:318–22. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 22.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. ; American College of Rheumatology. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken). 2012; 64:797–808. [PMID: ] doi: 10.1002/acr.21664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA Jr, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 1997;145:408–15. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 24.Lu LJ, Wallace DJ, Ishimori ML, Scofield RH, Weisman MH. Review: male systemic lupus erythematosus: a review of sex disparities in this disease. Lupus. 2010;19:119–29. [PMID: ] doi: 10.1177/0961203309350755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reveille JD, Moulds JM, Ahn C, Friedman AW, Baethge B, Rose-man J, et al. Systemic lupus erythematosus in three ethnic groups: I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity at disease onset. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum 1998; 41:1161–72. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 26.Austin HA 3rd, Boumpas DT, Vaughan EM, Balow JE. High-risk features of lupus nephritis: importance of race and clinical and histological factors in 166 patients. Nephrol Dial Transplant 1995;10: 1620–8. [PMID: ] [PubMed] [Google Scholar]
- 27.Gómez-Puerta JA, Barbhaiya M, Guan H, Feldman CH, Alarcón GS, Costenbader KH. Racial/ethnic variation in all-cause mortality among United States Medicaid recipients with systemic lupus erythematosus: a Hispanic and Asian paradox. Arthritis Rheumatol 2015;67:752–60. [PMID: ] doi: 10.1002/art.38981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh SJ, DeChello LM. Geographical variation in mortality from systemic lupus erythematosus in the United States. Lupus. 2001;10: 637–46. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 29.Durán S, Apte M, Alarcón GS; LUMINA Study Group. Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups [Editorial]. J Natl Med Assoc 2007; 99:1196–8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- 30.Yazdany J, Feldman CH, Liu J, Ward MM, Fischer MA, Costenbader KH. Quality of care for incident lupus nephritis among Medicaid beneficiaries in the United States. Arthritis Care Res (Hoboken). 2014;66:617–24. [PMID: ] doi: 10.1002/acr.22182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoenbach VJ, Rosamond WD. Standardization of rates and ratios In: Understanding the Fundamentals of Epidemiology: An Evolving Text. Chapel Hill, NC: Univ North Carolina at Chapel Hill; 2000:129–51. [Google Scholar]
- 32.Calvo-Alén J, Alarcón GS, Campbell R Jr, Fernández M, Reveille JD, Cooper GS. Lack of recording of systemic lupus erythematosus in the death certificates of lupus patients. Rheumatology (Oxford). 2005;44:1186–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 33.Ward MM Education level and mortality in systemic lupus erythematosus (SLE): evidence of underascertainment of deaths due to SLE in ethnic minorities with low education levels. Arthritis Rheum 2004;51:616–24. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 34.Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep 2001;49:1–32. [PMID: ] [PubMed] [Google Scholar]
- 35.Klebba A, Scott J. Estimates of Selected Comparability Ratios Based on Dual Coding of 1976 Death Certificates by the Eighth and Ninth Revisions of the International Classification of Diseases. Hyattsville, MD: National Center for Health Statistics; 1980:1–19. [Google Scholar]


