Abstract
Intracranial electroencephalography (iEEG) can be perform ed using m inim ally invasive stereo-electroencephalography (SEEG) or by im planting subdural electrodes via a craniotomy or multiple burr holes. There is anecdotal evidence that SEEG is becoming more com m on in the United States, though this has yet to be quantified. To address this question, all SEEG and burr hole/craniotomy subdural iEEG procedures were extracted from the Centers for Medicare and Medicaid Services Part B data files for the years 2000–2016. National trends were compared over time. In 2016, SEEG became the most frequently performed intracranial monitoring procedure in the Medicare population, increasing from 28.8% of total cases in 2000 to 43.1% in 2016 (p = 0.02). The proportion of strip electrode cases (through burr holes) significantly declined, while the frequency of craniotomies for subdural grid placement did not significantly change. These data are consistent with a nationwide increase in the utilization of SEEG with a concomitant decline in burr hole placement of subdural strip electrodes in the United States. The factors driving these changes are unknown, but are likely due in part to the desire for m inim ally invasive surgical options.
Keywords: Epilepsy, Intracranial electroencephalography, Stereoencephalography, Strip electrodes, Electrode array, Depth electrodes
1. Introduction
Epilepsy is one of the most serious and prevalent neurological disorders, affecting approximately 39 million people worldwide [1]. It is estimated that epilepsy affects 3–10 people per 1000 [2,3]. Although the majority of these individuals can be managed medically, some patients fail to respond to such therapy and may be considered candidates for surgical treatment. The goal of such surgery is to achieve seizure freedom with minimal surgical morbidity by resecting or modulating the seizure network. According to a Cochrane review reporting on surgical outcome of more than 16,000 patients [4], 65% of patients demonstrated a good outcome from surgery (i.e., seizure freedom). On the basis of this evidence, which includes multiple randomized controlled trials, international guidelines have been established to refer patients with medically refractory epilepsy early for evaluation at a comprehensive epilepsy center [5,6].
Various noninvasive and invasive diagnostic techniques are utilized in the evaluation of the surgical candidacy of epilepsy patients. For many patients, noninvasive modalities can localize the seizure onset zone; however, it is estimated that 30–40% of patients considered for epilepsy surgery would likely benefit from intracranial electroencephalography (iEEG) evaluation [7]. Different iEEG recording modalities have been employed, including subdural implantation of strip electrodes through burr holes, craniotomy with subdural implantation of grid arrays, and stereoencephalography (SEEG), each with its distinct advantages and limitations.
Despite the importance of these iEEG recordings techniques, the frequency with which each is used remains unknown. Historically, there has been a tendency to prefer subdural grid recordings over SEEG in the United States [8]. There is anecdotal evidence that this trend is changing, but the data have not yet been quantitatively evaluated. To address this question, we undertook an analysis of data from the Centers for Medicare and Medicaid Services.
2. Methods
The Centers for Medicare and Medicaid Services (CMS) Part B National Summary Data File lists the number of procedures billed to CMS, classified by Current Procedural Terminology (CPT) code, which specifically differentiates subdural grids, strips, depth electrodes, and SEEG. International Classification of Disease (1CD-9) codes, like those used in the Nationwide Inpatient Sample (NIS), do not specify whether a iEEG recordings were with subdural grids, strips, depth electrodes, or SEEG, so such databases cannot determine the relative proportion of these techniques.
Using the CMS National Summary Data File, we extracted procedures with CPT codes 61531 (subdural implantation of only strip electrodes through one or more burr or trephine hole(s) for long-term seizure monitoring), 61533 (craniotomy with elevation of bone flap; for subdural implantation of an electrode array, grids, strips, and/or depth electrodes for long-term seizure monitoring), and 61760 (stereotactic implantation of only depth electrodes into the cerebrum for long-term seizure monitoring) that were billed between the years 2000–2016. CMS data also include state-level data, although such values are censored if the number of procedures was low in order to maintain patient protection and anonymity. Because epilepsy surgery is relatively underutilized, we were unable to include state-level data in our analysis. Matlab R2017b (MathWorks; Natick, MA, USA) and SPSS v.25 (IBM Corp.; Armonk, NJ, USA) w ere used for analyses and to generate figures. Trends were modeled with linear regression and significance was determined using ANOVA.
3. Results
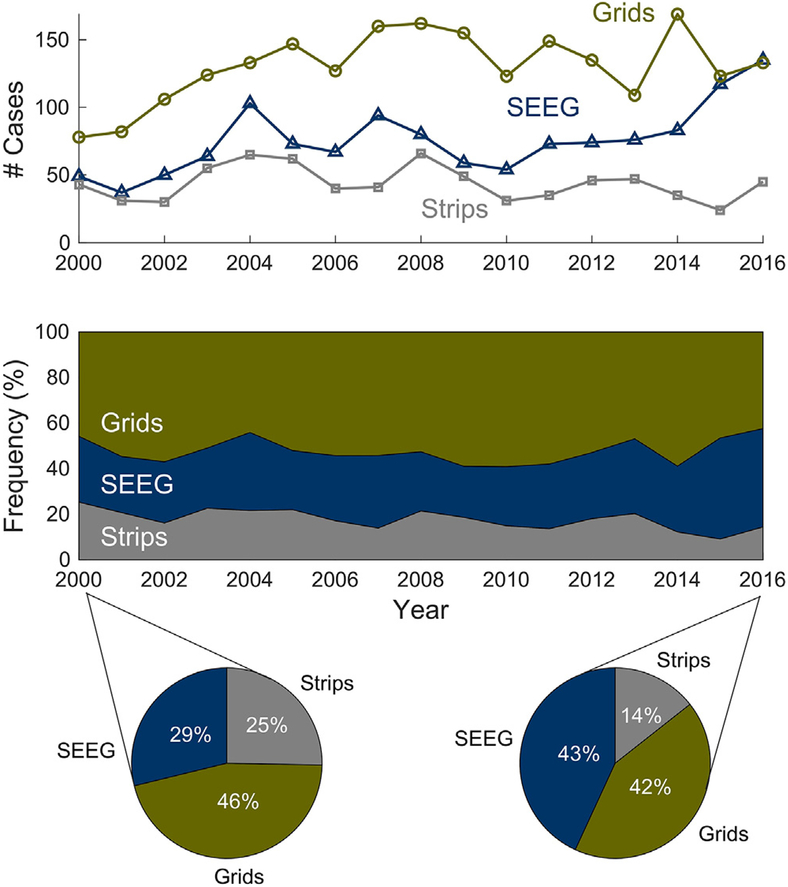
From 2000 to 2016, there was an overall increase in iEEG recordings when techniques w ere pooled (P = 0.024; F = 2.26). SEEG cases alone increased from 28.8% of total cases in 2000 to 43.1% in 2016, corresponding to a 0.6% increase per year (95% confidence interval [CI] 0.12% to 1.2%; F = 6.7; P = 0.02), with the sharpest increase in the last two years (Fig. 1). In absolute numbers, this corresponds to 49 SEEG cases in 2000 with an increase to 135 cases in 2016 (an increase of 3.3 cases/year; 95% CI 1.2 to 5.3 cases; F = 11.0; P = 0.005). The proportion of craniotomy cases for subdural electrode grid placement remained stable (95% CI −0.7% to 0.5%; F =0.51; P = 0.82) while burr hole cases for strip electrode placement gradually declined (−0.6% change per year, 95% CI −0.9% to −0.2%; F =13.7; P = 0.002) (Fig. 1).
Fig. 1.
Trends in iEEG recordings techniques in the United States using national data from CMS. The top panel shows the number of cases per year and the middle panel shows the frequency of cases per year, both demonstrating that SEEG cases significantly increased from 2000 to 2016, while subdural implantation of only strip electrodes through burr holes declined and craniotomy with subdural implantation of electrode arrays, grids, strips, and/or depth electrodes remained stable. The pie charts depict the proportions of iEEG recordings techniques for the first and last year of the study (2000 and 2016).
The average number of cases for subdural implantation of strip electrodes through burr holes from 2000 to 2016 was 43.8 cases, for craniotomy with subdural implantation of electrode array was 130.3 cases, and for SEEG was 75.8 cases. The peak for SEEG case volume was in 2016 (135 cases), while the peak for burr hole placement of subdural strip electrodes was in 2008 (66 cases). The peak for craniotomies with subdural implantation of electrode grid arrays was in 2014 (169 cases).
4. Discussion
Each iEEG technique—SEEG, craniotomy for subdural grids and strips, and burr holes for strip electrode placement—has been reported to have its own inherent advantages and limitations. Subdural electrodes cover a greater density of cortical surface compared with SEEG, thus covering large continuous cortical areas. They can also be utilized for delineation of eloquent cortex via direct cortical stimulation. However, subdural grids are not generally implanted bilaterally. Moreover, intracranial hemorrhage, infections, and elevated intracranial pressure have been reported as potential complications of subdural electrode implantation [9].
SEEG has the advantage of avoiding a craniotomy and is view ed as a minimally invasive option. Other advantages of SEEG are relatively quick removal of electrodes (as compared with a second craniotomy for the grid patients) and easier simultaneous evaluation of bilateral cortical structures. SEEG electrodes readily record from deep structures, but can also record from superficial structures if the contacts span a sufficiently long extent of the electrode. Grids, on the other hand, cannot evaluate deep structures, though they may be paired with depth electrodes as a supplement. The overall complication rate of SEEG appears lower than that of subdural electrodes/grids, with hemorrhage and infection being the most commonly encountered complications [10]. Meta-analyses have provided hemorrhage and neurologic infection rates of 4% and 2.3% with subdural electrodes, compared with 1% and 0.8% with SEEG, respectively [9,10].
It has been noted that SEEG recordings w ere traditionally performed in Europe and Canada, whereas subdural grids and strips were mainly used in the United States [8]; however, a recent anecdotal trend of increased use of SEEG across the U.S. as well has been discussed [8]. The above analysis bears this out: federal nationwide data showed a clear increase in the use of SEEG over the past few years, coupled to a decline in the use of burr holes for subdural strip placement (Fig. 1). Regardless of these changing practice patterns, the surgeon and center should be trained and be comfortable performing and interpreting all of these techniques, as some centers might not have the infrastructure needed for SEEG and/or some cases might be better addressed with a different recording modality. Future studies are therefore needed to shed the light on the differences in outcomes, costs, safety, and utilization-driving factors among these recording strategies.
One limitation to this study is the provenance of the data set. Although the CMS data set captures the Medicare Part B population, it excludes other insurers. Insurance carrier by itself ought not to be a clinically significant determinant of treatment modality. Moreover, if there w ere systematic differences in the Medicare population that made them more appropriate for one modality vs. another (e.g., their age), this would not be expected to change so rapidly over time. Examining these trends using epilepsy center data, regardless of insurance coverage, would allow a more robust estimate of these trends.
5. Conclusion
Nationwide data in the U.S. have demonstrated a recent increase in the utilization of the SEEG technique with a concomitant gradual decline in burr hole placement of subdural iEEG strip electrodes. The use of craniotomy with subdural implantation of electrode arrays has remained stable. Although the driving factors of these changes are largely unknown, they are likely related to the desire for minimally invasive surgical options. Further investigation into the driving factors of increased SEEG utilization is therefore warranted.
Acknowledgment
We thank Kristin Kraus, MSc., for her editorial assistance.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Competing interest statement
None of the authors has any conflict of interest to disclose.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/joocn.2018.04.064.
References
- [1].GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602. 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sander JW. The epidem iology of epilepsy revisited. Curr Opin Neurol 2003;16:165–70. 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- [3].Neligan A, Hauser WA, Sander JW. The epidemiology of the epilepsies. Handb Clin Neurol 2012;107:113–33. 10.1016/B978-0-444-52898-8.00006-9. [DOI] [PubMed] [Google Scholar]
- [4].West S, Nolan SJ, Cotton J, et al. Surgery for epilepsy. Cochrane Database Syst Rev 2015:CD010541 10.1002/14651858.CD010541.pub2. [DOI] [PubMed] [Google Scholar]
- [5].Engel J Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology 2003;60:538–47. [DOI] [PubMed] [Google Scholar]
- [6].Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–77. 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- [7].Kovac S, Vakharia VN, Scott C, Diehl B. Invasive epilepsy surgery evaluation. Seizure 2017;44:125–36. https://doi.org/10.1016lj.seizure.2016.10.016. [DOI] [PubMed] [Google Scholar]
- [8].Engel J Jr The current place of epilepsy surgery. Curr Opin Neurol 2017. 10.1097/WC0.0000000000000528. [DOI] [PMC free article] [PubMed]
- [9].Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA. Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: a systematic review and meta-analysis. Epilepsia 2013;54:828–39. 10.1111/epi.12073. [DOI] [PubMed] [Google Scholar]
- [10].Mullin JP, Shriver M, Alomar S, et al. Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. Epilepsia 2016;57:386–401. 10.1111/epi.13298. [DOI] [PubMed] [Google Scholar]