Abstract
Rationale.
Psychological stress-induced cortisol elevations appear to contribute to preterm birth. Yet, some studies suggest that the biological ramifications of racial discrimination-associated stress are unique and may involve development of decreased glucocorticoid sensitivity despite normalized cortisol levels.
Objective.
In this study, we examined the effects of racial discrimination on maternal cortisol output, leukocyte glucocorticoid sensitivity, and the degree of correspondence between cortisol levels and birth timing in an African American cohort.
Method.
A generally healthy prospective cohort was enrolled at 28 to 32 weeks gestation (n=91). The Experiences of Discrimination scale was administered, whole blood collected, and plasma cortisol levels, cytokine levels, and leukocyte counts quantified for examination of patterns of endogenous feedback.
Results.
Racial discrimination in the mid-tertile was associated with greater maternal cortisol levels than the bottom tertile among women reporting internalizing responses (b*=0.68, p=0.001). Decreased leukocyte glucocorticoid sensitivity was witnessed at greater frequencies of experiences of racial discrimination, as evidenced by decreased correspondence between maternal cortisol levels and plasma IL-8 levels, monocyte counts, and lymphocyte counts (ps≤0.043). The association between maternal cortisol levels and birth timing differed by discrimination tertile (p values≤0.005), with greater cortisol levels predictive of earlier birth among women without (b*=−0.59, p<0.001) but not with racial discrimination (ps≥0.497).
Conclusion.
We provide novel evidence of decreased glucocorticoid sensitivity at increasing frequency of exposure to racial discrimination. Our findings suggest that the biology of preterm birth may depend upon racial discriminatory exposures, favoring pathways dependent upon glucocorticoid-induced increases in leukocyte tissue surveillance versus glucocorticoid resistance-associated inflammatory aberrations at increasing levels of exposure. Precision approaches to prenatal care are sorely needed to combat preterm birth, particularly among African American women, with efforts dependent upon further research examining the pathways contributing to the syndrome dependent upon the totality of an individual’s exposures.
Keywords: Cortisol, Cytokines, Disparity, Glucocorticoids, Pregnancy, Discrimination, Stress, Psychological
1. Introduction
African American women exhibit the highest U.S. rates of preterm birth, which contributes to a two-fold increased risk for mortality among African American versus European American infants (Martin, Hamilton, Osterman, Driscoll, & Mathews, 2017; Matthews, MacDorman, & Thoma, 2015). There is strong evidence to suggest that a disproportionate burden of traditional risk factors (e.g., socioeconomic inequities; Colen, Ramey, Cooksey, & Williams, 2018) do not fully account for racial disparities witnessed in health, with discriminatory exposures related to racial minority status implicated as an important contributing factor. In fact, both perceived and objectively quantified exposure to racial discrimination have been linked to increased odds of preterm birth in African American samples (Chae et al., 2018; Rankin, David, & Collins, 2011; Ruiz, Shah, Lewis, & Theall, 2014). Consistent with the conceptualization of racial discrimination as a psychological stressor (Berger & Sarnyai, 2015), a large literature also links alternative forms of psychological stress to preterm birth (Manuck et al., 2015; Staneva, Bogossian, Pritchard, & Wittkowski, 2015).
More recent work has aimed to identify mechanisms by which psychological stress affects birth timing, with several studies now showing that greater maternal output of the glucocorticoid cortisol mediates associations between several forms of stress and earlier birth (Gillespie, Christian, Alston, & Salsberry, 2017; Hoffman, Mazzoni, Wagner, Laudenslager, & Ross, 2016; Mancuso, Schetter, Rini, Roesch, & Hobel, 2004). Yet, emerging evidence also suggests that the biological ramifications of discrimination are unique, with greater perceptions of lifetime racial discrimination associated with perturbations in diurnal patterns of cortisol but not with elevations in average output by adulthood (Adam et al., 2015). Such findings are consistent with studies assessing cortisol levels among pregnant African American versus European American women (Christian, Mitchell, Gillespie, & Palettas, 2016; Simon et al., 2016) and may explain why no study has linked racial discrimination to birth timing via elevations in maternal cortisol output. We are also unaware of any studies publishing null findings.
Of relevance, some forms of psychological stress, particularly those social in nature, have been shown to induce decreased glucocorticoid sensitivity that persists despite normalization of total glucocorticoid output (Jarcho, Slavich, Tylova-Stein, Wolkowitz, & Burke, 2013; G. E. Miller, Cohen, & Ritchey, 2002; G. E. Miller, Gaudin, Zysk, & Chen, 2009; Powell et al., 2009; Walsh et al., 2017). Glucocorticoid sensitivity among stressed groups has been evaluated using several well-developed methods, including by identifying deviations from expected associations among in vivo glucocorticoid levels, cytokine levels, and patterns of leukocyte trafficking (e.g., Cohen et al., 2012; Cole, 2008; Cole, Mendoza, & Capitanio, 2009).
To our knowledge, leukocyte glucocorticoid sensitivity has been the topic of only one study of pregnant women, with results providing support for an association between minority status and decreased sensitivity to cortisol’s anti-inflammatory effects (Corwin et al., 2013). Whether racial discrimination is associated with decreased leukocyte glucocorticoid sensitivity remains to be determined, though the progressive development of this aberration may help to explain the exaggerated, prolonged inflammatory response to social threat witnessed among African Americans reporting more discriminatory exposures (Lucas et al., 2017). This holds important implications for the study of racial disparities in disease processes with inflammatory underpinnings, such as preterm birth (reviewed by Romero, Dey, & Fisher, 2014).
In this study, we examined the effects of racial discrimination on maternal cortisol output and leukocyte glucocorticoid sensitivity among pregnant African American women. We hypothesized that while discrimination would not be associated with cortisol output, women with racial discriminatory exposures would show evidence of decreased leukocyte glucocorticoid sensitivity. We also tested the hypothesis that maternal cortisol levels would show diminished correspondence with birth timing among women with increasing frequencies of experiences of racial discrimination, which may be attributable to the presence of decreased glucocorticoid sensitivity
2. Method
2.1. Study Design and Participants
This prospective cohort study recruited a convenience sample of pregnant women attending two large Midwestern prenatal clinics. Pregnant community members were also recruited by advertisement. Enrollment occurred from September 2013 to June 2015, with participants attending a single study visit at 28 weeks 0 days’ to 32 weeks 6 days’ gestation and followed prospectively to birth to allow for medical record review and clinical data abstraction.
Eligible women were aged 18 to 34 and self-reported African American race, non-Hispanic ethnicity, and birth in the United States. Women reporting tobacco or marijuana use beyond the first trimester or other illicit drug use at any point in pregnancy were not eligible. Women reporting height and pre-pregnancy weight consistent with underweight (body mass index < 18.5) or obese class III (body mass index ≥ 40) classifications were not eligible (World Health Organization, 2000). To enhance accuracy of gestational age estimates, ultrasound dating at < 15 weeks of pregnancy was required for participation (American College of Obstetricians and Gynecologists, 2014). To reduce the potential influence of confounding factors and promote the opportunity to observe women undergoing spontaneously-initiated birth, those with chronic endocrine- or immune-impacting conditions (e.g., sickle cell disease, inflammatory bowel disease), regularly taking endocrine- or immune-impacting medications (e.g., corticosteroids), diagnosed with a major complication of pregnancy (e.g., fetal anomaly, gestational diabetes, gestational hypertensive disorders), or receiving interventions to prolong pregnancy (e.g., cervical cerclage, progesterone) were ineligible. Yet, several women were diagnosed with complications of pregnancy following enrollment.
All participants completed informed consent and received modest payment in the form of a gift card at the time of enrollment. The study was approved following review by The Ohio State University Biomedical Institutional Review Board and the OhioHealth Institutional Review Board.
2.2. Sample Demographic, Health, and Psychosocial Characteristics
The demographics and health behaviors of participants were assessed by self-report. Height measured at the study visit and self-reported pre-pregnancy weight were used to calculate pre-pregnancy body mass index (BMI; kg/m2) according to the definition set forth by the World Health Organization (2000). Non-smoking was defined as no smoking during pregnancy, including during early pregnancy, as all participants were required to report quitting by the second trimester to be eligible for participation. Sleep quality was also assessed using the Pittsburgh Sleep Quality Index (PSQI), which produces a global sleep quality score ranging from 0-21 with higher scores indicative of worse sleep quality. The PSQI has a Cronbach’s α of 0.83 at the individual item level and test-retest reliability of 0.85 for global scores (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). During pregnancy, sleep quality assessed by the PSQI appears to be susceptible to both the physiologic changes associated with the growing fetus (Sedov, Cameron, Madigan, & Tomfohr-Madsen, 2017) and psychological factors (Francis, Klebanoff, & Oza-Frank, 2017). The 14-item Perceived Stress Scale (PSS) was administered to quantify perceptions of stress within the month prior to the study visit (Cohen, Kessler, & Underwood Gordon, 1995). With a range of 0-56, higher scores on the PSS are indicative of greater perceived stress. The psychometrics of the scale have been extensively tested across various populations, including during pregnancy (Bann et al., 2017).
2.3. Racial Discrimination
The Experiences of Discrimination Scale (EOD) was administered to assess lifetime exposure to discrimination on the basis of race, ethnicity, or color. Participants are asked how many times (0, 1, 2, or 3+) they have experienced discrimination in each of nine life situations (e.g., at school, at work, getting medical care), producing a score with a range of zero to 27. Among African American samples, Cronbach’s α reaches 0.86 and test-retest reliability 0.70 (Krieger, Smith, Naishadham, Hartman, & Barbeau, 2005), which is consistent with a Cronbach’s α of 0.82 witnessed for the current study. Due to the extreme positive distribution of EOD frequency scores, a fertile split was applied to produce groups reflective of EOD frequency = 0 (Tertile 1), EOD frequency =1 – 2 (Tertile 2), or EOD frequency ≥ 3 (Tertile 3).
2.4. Biological Parameters
Antecubital or distal venipuncture was performed between 1100 and 1600 hours at a single time point to obtain heparinized and EDTA-preserved whole blood. For participants reporting recent cold- or flu-like illness during visit confirmations, study visits were rescheduled at least one week removed from resolution of symptoms. Participants were asked to refrain from exercise or caffeine use on the day of the visit and to wake at least 2.5 hours prior to the study visit (Bessinger, McMurray, & Hackney, 2002; R. Miller et al., 2016; Tsubouchi et al., 2006).
As reported previously in detail (Gillespie et al., 2017), in batches, total plasma cortisol levels (ng/ml) were assayed in duplicate by solid phase competitive enzyme-linked immunosorbent assay (Calbiotech, Spring Valley, CA) and spectrophotometry (BioTek PowerWave Microplate Spectrophotometer, Winooski, VT), producing intra- and inter-assay coefficients of variation of 3.7% and 9.7%, respectively.
Plasma cytokine levels (pg/ml) were quantified in duplicate using the MULTI-SPOT Pro-Inflammatory II 4-plex Immunoassay and Sector Imager 2400 per manufacturer instructions (Meso Scale Discovery, Gaithersburg, MD). Briefly, plasma of unknown cytokine concentrations was added to capture antibody-coated wells, with spots defined within each well according to the cytokines of interest. Following a two-hour incubation with shaking, the plate was washed and a solution of detection antibodies conjugated with electrochemiluminescent labels was added. Following an additional two-hour incubation with shaking, the plate was washed and read buffer added to support the electrochemiluminescent detection of bound analytes through a sandwich immunoassay format. Intra-assay coefficients of variation were 7.1%, 3.9%, 15.5%, and 4.0% for interleukin (IL)-6, tumor necrosis factor (TNF)-α, IL-1 β, and IL-8, respectively. Inter-assay coefficients of variation were 4.0%, 7.2%, 2.3%, and 3.6% for IL-6, TNF-α, IL-1 β, and IL-8, respectively.
Leukocyte counts (k/μl) were also determined in K2EDTA-preserved whole blood. Samples were transported to the core medical center laboratory immediately after completion of the study visit, with complete blood counts with differential determined using volume, conductivity, and scatter technology within 48 hours of receipt (Beckman Coulter, Fullerton, CA).
The sensitivity of leukocytes to the endogenous effects of cortisol were determined by examining concurrent relationships among total plasma cortisol levels, plasma cytokine levels, and leukocyte counts. Deviations from expected positive (i.e., IL-8, neutrophil) and negative (i.e., IL-6, TNF-α, IL-1β, monocyte, lymphocyte) associations among cortisol and the assessed biological correlates were operationalized as indicative of decreased leukocyte glucocorticoid sensitivity.
2.5. Birth Timing
Post-birth, a comprehensive prenatal, labor and delivery, and newborn medical record review was completed to extract data surrounding the health of the pregnancy and circumstances and timing of birth. Specifically, births were categorized as initiated through spontaneous mechanisms (i.e., spontaneous rupture of membranes, spontaneous labor) or by medical intervention (i.e., induction of labor, Caesarean section) and days gestation at birth was calculated according to the best obstetric estimate of due date and actual date of birth.
2.6. Statistical Analyses
All analyses were performed using STATA 12.0 (College Station, TX). Data distributions and data missingness were examined and Little’s (1988) χ2 tests for missing completely at random (MCAR) and covariate-dependent missingness (CDM) examined. To identify variables with potential for confounding, which were addressed through sensitivity analyses, participant demographic, health, psychosocial, and obstetric characteristics were examined and compared among the racial discrimination tertiles using analysis-of-variance (ANOVA), χ2, or Fisher’s exact tests, as appropriate.
To address the primary objectives of the study, we first built a linear regression model to examine the association between racial discrimination and maternal cortisol output, also considering the potential for interaction by internalizing responses. We then examined the association between racial discrimination and leukocyte glucocorticoid sensitivity, with decreased sensitivity operationalized as deviations from expected associations among circulating maternal cortisol levels, plasma cytokine levels, and leukocyte counts. Specifically, we regressed, in separate models, IL-6, TNF-α, IL-1 β, and IL-8 level, neutrophil, monocyte, and lymphocyte count (Y) onto plasma maternal cortisol levels (X1), racial discrimination tertile (X2), and a plasma cortisol level × racial discrimination tertile interaction term (X1×X2). Finally, to determine whether the association between maternal cortisol level and birth timing differed according to past discriminatory experiences, we regressed days gestation at birth (Y) onto plasma cortisol levels (X1), racial discrimination tertile (X2), and a plasma cortisol level × racial discrimination status interaction term (X1×X2).
Each multiple linear regression model was examined for satisfaction of assumptions. The positively skewed criterion variables of plasma cortisol, IL-6, TNF-α, IL-1 β, IL-8, and neutrophil count and negatively skewed criterion variable of birth timing were noted to result in non-normality of error terms and multiple instances of heteroskedasticity. As such, results produced for each model were confirmed with transformed variables (following reflection in the case of birth timing) serving as criteria, which corrected skewness and heteroskedasticity of error terms for all models. Given the consistency in findings, results are reported for non-transformed variables for ease of interpretation. Residual, leverage, and influence diagnostics for each multiple linear regression model were also carefully examined. Two participants showed outlying plasma cortisol levels (i.e., > M + 3SD), with one data point exerting high leverage across all models (hat value = 0.76) and significant influence over estimated regression coefficients in multiple models according to an a prior definition of Cook’s distance ≥ 1 (Cook & Weisberg, 1982); this participant was excluded from all analyses. Among the five outliers noted across plasma cytokine distributions, only one data point reflective of plasma IL-6 exerted significant influence on regression estimates (Cook’s d = 1.89). Therefore, this data point was excluded from the model examining associations between plasma cortisol and IL-6 levels only. No outlying values were noted for the measures of leukocyte count.
3. Results
3.1. Sample Characteristics
Of 363 potentially eligible women, 222 were screened for eligibility. One-hundred forty-two were confirmed eligible, with 96 enrolling at 28 weeks 0 days’ to 32 weeks 6 days’ gestation (median 30.3 weeks [IQ range 29.3 – 31.9]) and followed prospectively to birth. All data were available for participant demographic, health, and psychosocial characteristics. Of the 96 enrolled participants, there were two instances of data missingness for biological parameters (unsuccessful venipuncture) and one instance of data missingness for birth outcome data (loss to follow-up). We failed to reject the null hypothesis of MCAR (χ2 = 6.15, p = 0.725) and CDM (χ2 = 4.11, p = 0.942), suggesting that no patterns existed in the missing data and variables with missing values were not dependent upon sample demographic, health, or psychosocial characteristics, respectively. Thus, missing data were addressed through list-wise deletion. In addition, one woman was excluded from analyses due to venipuncture occurring outside of the designated study window and one woman was excluded from analyses due to an outlying plasma cortisol level exerting significant influence on regression estimates (as described above). In the end, there was an analytical sample of 91.
Demographic, health, and psychosocial characteristics for the analytic sample are presented in Table 1. Per the study’s inclusion criteria, all women reported African American race and non-Hispanic ethnicity. No participant reported tobacco use beyond the first trimester of pregnancy, though nearly one in four participants reported tobacco use in early pregnancy. Participant obstetric course is summarized in Table 2. The enrollment criteria were largely successful in producing a relatively low-risk sample amenable to the observation of the natural progression of pregnancy, as few women (n = 8; 8.8%) required induction of labor or Caesarean section prior to reaching full term (i.e., 39 weeks gestation). Among these women, indications for induction of labor or Caesarean section included newly diagnosed, uncontrolled chronic hypertension (n = 1), gestational hypertension (n = 3), preeclampsia (n = 1), oligohydramnios (n = 1), and non-reassuring fetal wellbeing (n = 2).
Table 1.
Sample demographic, health, and psychosocial characteristics
| Variable | Analytic sample (n = 91) | Experiences of racial discrimination |
|||
|---|---|---|---|---|---|
| Tertile 1 (n = 46) | Tertile 2 (n = 19) | Tertile 3 (n = 26) | p | ||
| Maternal Age | 26.0 (22.0 – 31.0) | 26.0 (22.0 – 29.0) | 25.0 (23.0 – 32.0) | 26.0 (25.0 – 32.0) | 0.344 |
| Married | 22 (24.2%) | 9 (19.6%) | 6 (31.6%) | 7 (26.9%) | 0.546 |
| ≥ Bachelor’s Degree | 24 (26.4%) | 10 (21.7%) | 5 (26.3%) | 9 (34.6%) | 0.470 |
| Pre-pregnancy BMI | 28.6 (23.0 – 32.1) | 29.1 (24.4 – 32.1) | 30.0 (24.0 – 32.4) | 24.3 (22.1 – 32.0) | 0.566 |
| Non-smoker | 69 (75.8%) | 36 (78.3%) | 16 (84.2%) | 17 (65.4%) | 0.324 |
| Sleep quality (PSQI) | 5.0 (1.0 – 7.0) | 4.0 (2.0 – 6.0) | 5.0 (1.0 – 7.0) | 6.0 (2.0 – 10.0) | 0.056 |
| Perceived stress (PSS-14) | 23.0 (18.0 – 28.0) | 22.0 (17.0 – 27.0) | 24.0 (18.0 – 34.0) | 24.5 (19.0 – 31.0) | 0.032 |
BMI = Body mass index. PSQI = Pittsburgh Sleep Quality Index. PSS = Perceived Stress Scale.
Note: Tertile 1 = 0 experiences, Tertile 2 = 1 – 2 experience, Tertile 3 = ≥ 3 experience of racial discrimination. Continuous variables presented as median (interquartile range). Dichotomized variables presented as n (%). p values for comparisons across tertiles by ANOVA, χ2, or Fisher exact test, as appropriate.
Table 2.
Obstetric course
| Variable | Analytic sample (n = 91) | Experiences of racial discrimination |
|||
|---|---|---|---|---|---|
| Tertile 1 (n = 46) | Tertile 2 (n = 19) | Tertile 3 (n = 26) | p | ||
| Primiparous pregnancy | 29 (31.9%) | 15 (32.6%) | 8 (42.1%) | 6 (23.1%) | 0.396 |
| Gestational diabetes | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| Gestational hypertension | 9 (9.9%) | 4 (8.7%) | 3 (15.8%) | 2 (7.7%) | 0.650 |
| Preeclampsia | 2 (2.2%) | 1 (2.2%) | 0 (0%) | 1 (3.9%) | 1.000 |
| Oligo/Polyhydramnios | 8 (8.8%) | 4 (8.7%) | 2 (10.5%) | 2 (7.7%) | 1.000 |
| Induction or C/S before full term | 8 (8.8%) | 4 (8.7%) | 3 (15.8%) | 1 (3.9%) | 0.386 |
| Gestational age at birth (weeks) | 39.3 (38.4 – 40.0) | 39.2 (38.6 – 40.1) | 38.9 (38.4 – 39.7) | 39.4 (38.7 – 40.0) | 0.451 |
C/S = Caesarean section.
Note: Tertile 1 = 0 experiences, Tertile 2 = 1 – 2 experience, Tertile 3 = ≥ 3 experience of racial discrimination. Continuous variables presented as median (interquartile range). Dichotomized variables presented as n (%). Primiparous pregnancy reflects a status of nullipara converting to primipara for the assessed pregnancy. p values for comparisons across tertiles by ANOVA, χ2, or Fisher’s exact test, as appropriate.
3.2. Racial discrimination
Forty-six (50.6%), 19 (20.1%), and 26 (28.6%) of participants reported experience of racial discrimination frequencies of 0 (Tertile 1), 1 – 2 (Tertile 2), and ≥ 3 (Tertile 3), respectively. Overall, the median frequency of experiences with racial discrimination was 0 (IQ range 0 – 3.5; range 0 – 18). Among those who did report discrimination, the median frequency was 3.5 (IQ range 2.5 – 7.5). Experiences of racial discrimination were reported most frequently in association with getting service in a store or restaurant (28.6%), followed by from police or in the courts (19.8%), at school (14.3%), at work (13.2%), on the street or in a public setting (12.1%), getting hired or getting a job (11.0%), getting credit, bank loans, or a mortgage (4.4%), getting housing (2.2%), and getting medical care (2.2%). The majority of women stated that, if they felt they had been treated unfairly, they would try to do something about it instead of accepting it (69.2%) and talk to other people about it instead of keeping it to themselves (83.5%). Spearman rank-order associations for the primary variables of interest, stratified by racial discrimination tertile, are also presented in Table 3.
Table 3.
Spearman rank-order associations by experiences of racial discrimination tertile
| Variable | Racial discrimination tertile | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 (n = 46) | Tertile 2 (n = 19) | Tertile 3 (n = 26) | |||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1. Cortisol | 1 | -- | -- | -- | -- | -- | -- | -- | -- | 1 | -- | -- | -- | -- | -- | -- | -- | -- | 1 | -- | -- | -- | -- | -- | -- | -- | -- |
| 2. IL-6 | −0.17 | 1 | -- | -- | -- | -- | -- | -- | -- | −0.16 | 1 | -- | -- | -- | -- | -- | -- | -- | 0.25 | 1 | -- | -- | -- | -- | -- | -- | -- |
| 3. TNF-α | −0.16 | 0.25 | 1 | -- | -- | -- | -- | -- | -- | 0.05 | 0.15 | 1 | -- | -- | -- | -- | -- | -- | 0.17 | 0.42 | 1 | -- | -- | -- | -- | -- | -- |
| 4. IL-1β | −0.14 | 0.12 | 0.15 | 1 | -- | -- | -- | -- | -- | 0.08 | 0.16 | 0.15 | 1 | -- | -- | -- | -- | -- | 0.20 | 0.08 | 0.23 | 1 | -- | -- | -- | -- | -- |
| 5. IL-8 | 0.20 | 0.11 | 0.13 | 0.15 | 1 | -- | -- | -- | -- | −0.25 | −0.12 | 0.34 | 0.28 | 1 | -- | -- | -- | -- | −0.06 | 0.09 | 0.22 | 0.10 | 1 | -- | -- | -- | -- |
| 6. Neutrophil | −0.08 | 0.09 | 0.18 | 0.18 | −0.19 | 1 | -- | -- | -- | 0.03 | 0.03 | 0.19 | −0.18 | 0.16 | 1 | -- | -- | -- | 0.32 | −0.05 | −0.04 | 0.11 | −0.15 | 1 | -- | -- | -- |
| 7. Monocyte | −0.18 | 0.03 | 0.09 | 0.16 | −0.12 | 0.48 | 1 | -- | -- | 0.22 | −0.36 | 0.04 | −0.45 | −0.05 | 0.57 | 1 | -- | -- | 0.30 | 0.00 | −0.07 | −0.00 | −0.23 | 0.59 | 1 | -- | -- |
| 8. Lymphocyte | −0.32 | 0.03 | 0.08 | −.07 | −0.07 | 0.42 | 0.33 | 1 | -- | 0.27 | 0.33 | 0.16 | 0.03 | 0.13 | 0.03 | 0.19 | 1 | -- | −0.36 | −0.09 | 0.12 | 0.24 | 0.26 | 0.18 | 0.08 | 1 | -- |
| 9. GA at birth | −0.38 | 0.05 | 0.07 | −0.08 | −0.45 | 0.01 | 0.23 | 0.15 | 1 | −0.13 | −0.27 | 0.17 | 0.07 | 0.37 | −0.33 | −0.27 | −0.35 | 1 | 0.21 | 0.16 | −0.06 | 0.38 | 0.21 | 0.19 | 0.09 | −0.13 | 1 |
Note. Tertile 1 = 0 experiences, Tertile 2 = 1 – 2 experience, Tertile 3 = > 3 experience of racial discrimination. Cortisol and cytokine levels derived from plasma. Neutrophil, monocyte, and lymphocyte values reflective of count. Rho values with p < 0.05 are underlined. IL = interleukin; TNF = tumor necrosis factor; GA = gestational age
As shown in Tables 1 and 2, age, marital status, education, pre-pregnancy BMI, early pregnancy smoking status, and primiparity did not differ by racial discrimination tertile. A trend toward differences in sleep quality (F(2,88) = 2.99, p = 0.056) and significant differences in perceived stress (F(2,88) = 3.59, p = 0.032) were noted in tertile comparisons. Post-hoc comparisons by Sidak’s method revealed at trend toward poorer sleep quality (t(89) = 2.41, p = 0.053) and significantly greater perceived stress (t(89) = 2.58, p = 0.034) among women in the third versus first tertile of experiences of racial discrimination. We also examined whether group differences were present according to time of sampling, noting the potential for differences in sampling time of day (F(2,88) = 2.28, p = 0.108) but not sampling gestational age (F(2,88) = 0.33, p = 0.718). Of note, findings did not suggest an association between racial discrimination tertile and birth timing among the assessed sample of all African American women (F(2,88) = 0.80, p = 0.451).
3.2. Racial Discrimination and Maternal Cortisol Levels
Linear regression demonstrated no main effect of racial discrimination tertile on maternal cortisol levels (p values ≥ 0.238). We considered further the possibility that exposures to discrimination may be more strongly related to cortisol output when responses to discrimination are internalizing, which was supported by a significant interaction in comparisons of Tertile 2 vs. 1 (b* = 0.42, p = 0.002). Among women reporting accepting racial discriminatory exposures as a fact of life and/or keeping these exposures to themselves (n = 34), classification in Tertile 2 was associated with significantly higher cortisol levels than classification in Tertile 1 (b* = 0.68, p = 0.001). This relationship was not present among women that did not report internalizing responses (b* = −0.07, p = 0.594).
3.3. Racial Discrimination and Leukocyte Glucocorticoid Sensitivity
Next, the modifying effect of racial discrimination tertile on associations among maternal plasma cortisol levels and plasma IL-6, TNF-α, IL-1 β, and IL-8 levels was examined in separate multiple linear regression models. Results demonstrated differential associations among plasma cortisol and IL-8 levels among women in the second (b* = −0.83, p = 0.017) and third (b* = −1.04, p = 0.011) tertiles as compared to the first. Shown in Figure 1, consistent with appropriate leukocyte glucocorticoid sensitivity, greater maternal plasma cortisol levels predicted greater plasma IL-8 levels among women reporting no discrimination (b* = 0.36, p = 0.030). The correspondence between cortisol and IL-8 levels progressively deviated from the expected direction among women in the second (b* = −0.21, p = 0.217) and third (b* = −0.36, p = 0.110) tertiles of experiences of racial discrimination, suggesting decreasing levels of glucocorticoid sensitivity. Associations among plasma cortisol and IL-6, TNF-α, and IL-1 β levels did not significantly differ by racial discrimination tertile (p values ≥ 0.266), with maternal plasma cortisol levels failing to significantly predict plasma IL-6 (b* = −0.06, p = 0.570), plasma TNF-α (b* = −0.06, p = 0.592), or plasma IL-1 β (b* = 0.004, p = 0.966) among the full sample.
Fig. 1. Plasma cortisol and interleukin-8 levels by discrimination tertile.
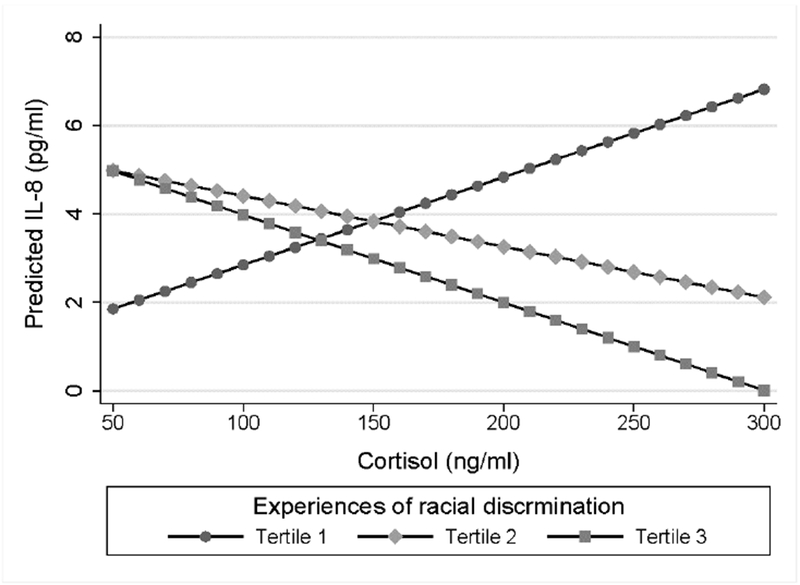
Estimated marginal effects for the prediction of IL-8 levels according to cortisol levels. African American women in the first tertile showed a significant positive association (b* = 0.36, p = 0.030) as compared to non-significant negative associations among women in the second and third tertiles (p values ≥ 0.110).
As shown in Figure 2, multiple linear regression models demonstrated that the association between plasma cortisol level and monocyte count also significantly differed among women in the third tertile of experiences of racial discrimination as compared to the first (b* = 0.83, p = 0.042). Approximating appropriate leukocyte sensitivity, cortisol levels were negatively but non-significantly associated with monocyte count among women in the first tertile (b* = −0.24, p = 0.148). The correspondence between cortisol and monocyte count progressively deviated from the expected direction among women in the second (b* = 0.21, p = 0.208) and third (b* = 0.33, p = 0.141) tertiles of experiences of racial discrimination, suggesting decreasing levels of glucocorticoid sensitivity in a dose-response pattern. Interestingly, cortisol level and lymphocyte count were also dependent upon discrimination tertile but with the second tertile differing significantly from the first tertile (b* = −0.77, p = 0.043; Figure 3) and non-significantly but in the same direction from the third tertile (b* = −0.72, p = 0.078). Consistent with appropriate leukocyte sensitivity, cortisol levels were negatively associated with lymphocyte count among women in the first tertile (b* = −0.35, p = 0.033). Effect estimates for associations among cortisol levels and lymphocyte counts among women in the second and third tertiles were b* = 0.13, p = 0.455 and b* = −0.37, p = 0.099, respectively. With no difference by tertile (p values ≥ 0.123), maternal cortisol levels did not show a significant association with neutrophil count (b* = 0.04, p = 0.705).
Fig. 2. Plasma cortisol levels and monocyte count by discrimination tertile.
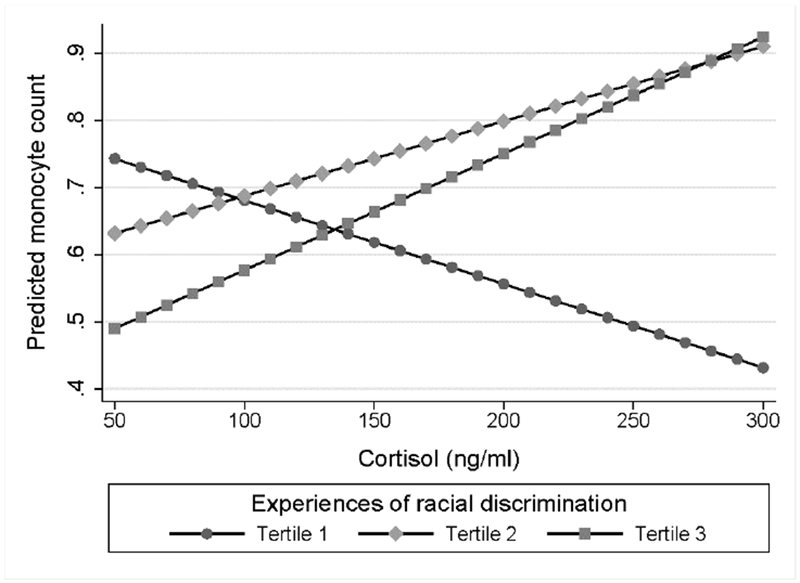
Estimated marginal effects for the prediction of monocyte count according to cortisol levels. African American women in the first tertile showed a non-significant negative association (b* = −0.24, p = 0.149) as compared to non-significant positive associations among women in the second and third tertiles (p values ≥ 0.141).
Fig. 3. Plasma cortisol levels and lymphocyte count by discrimination tertile.
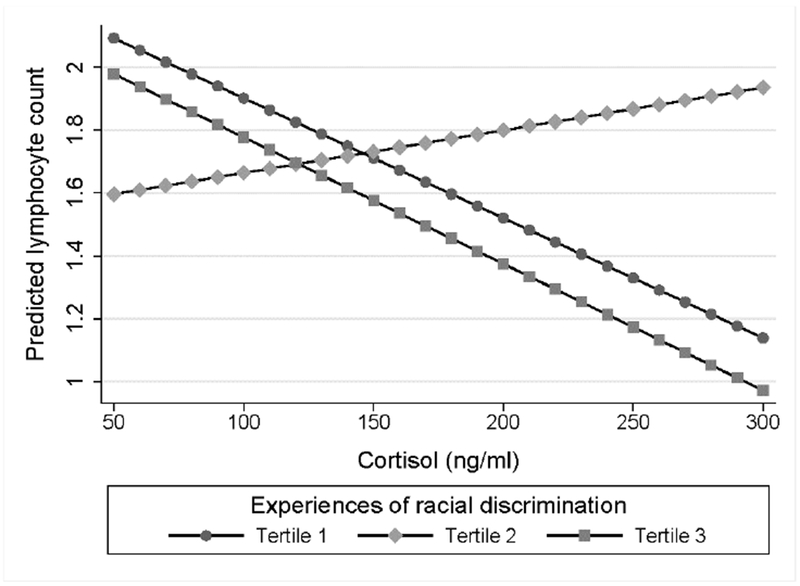
Estimated marginal effects for the prediction of lymphocyte count according to cortisol levels. African American women in the first tertile showed a significant negative association (b* = −0.35, p = 0.033) as compared to a non-significant positive association among women in the second tertiles (b* = 0.13, p = 0.455) and non-significant negative association among women in the third tertile (b* = −0.37, p = 0.099).
3.4. Maternal Cortisol Levels and Birth timing by Racial Discrimination Tertile
Next, we tested the hypothesis of effect modification by racial discrimination tertile in the prediction of birth timing according to maternal cortisol level. Results from the interacting multiple linear regression model indicated that the association between maternal cortisol level and birth timing differed among those in the second (b* = 0.97, p = 0.005) and third (b* = 1.07, p = 0.007) tertile of experiences of racial discrimination as compared to the first. As shown in Figure 4, each ng/ml positive difference in maternal cortisol level predicted birth 0.15 days earlier among women in the first tertile (b* = −0.59, p < 0.001) but did not provide predictive power among women in the second (b* = 0.06, p = 0.690) or third tertile (b* = 0.15, p = 0.497).
Fig 4. Plasma cortisol levels and birth timing by discrimination tertile.
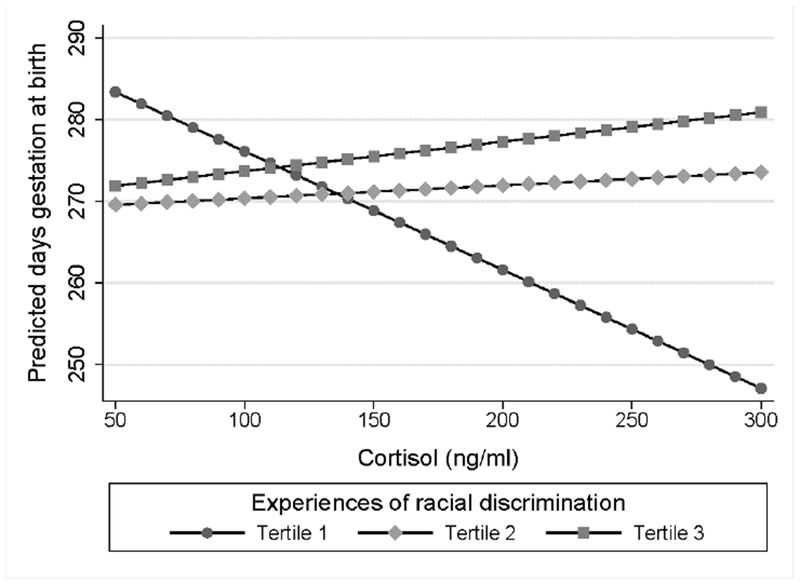
Estimated marginal effects for the prediction of birth timing according to cortisol levels. African American women in the first tertile showed a significant negative association (b* = −0.59, p < 0.001) as compared to non-significant positive associations among women in the second and third tertile (p values ≥ 0.497).
Given our focus on spontaneously-initiated birth timing, we also repeated this analysis excluding cases of induction of labor or Caesarean section prior to 39 weeks gestation (n = 8), again noting maternal cortisol level × racial discrimination interaction effects (Tertile 2 vs. 1: b* = 0.94, p = 0.007; Tertile 3 vs. 1: b* = 1.11, p = 0.007) and the presence (b* = −0.60, p = 0.001) and absence (p values ≥ 0.448) of statistically significant cortisol-birth timing association among women in the first versus second and third tertiles of experiences with racial discrimination, respectively.
3.5. Sensitivity Analyses
To determine whether the above findings were susceptible to confounding, we repeated all analyses controlling for sleep quality, perceived stress, and sampling time of day, each of which were significantly or suggested potential association with racial discrimination tertile in our sample. Results remained consistent, including for the identification of a significant racial discrimination × internalizing response interaction in the prediction of cortisol levels and maternal cortisol level × racial discrimination tertile interactions in the prediction of plasma IL-8 levels, monocyte counts, lymphocyte counts, and birth timing without and with exclusions applied. The presence versus absence of predictive power for birth timing according to cortisol level among women in the first versus second and third tertiles was again noted.
4. Discussion
4.1. Racial Discrimination and Leukocyte Glucocorticoid Sensitivity
To our knowledge, this prospective cohort study is the first to demonstrate an association between experiences of racial discrimination and patterns of endogenous feedback consistent with decreased leukocyte glucocorticoid sensitivity. To our knowledge, few reports have assessed the effects of discrimination on related biological parameters, though our findings are generally consistent with those from Berger et al. (2017) who report blunted cortisol responses to acute psychological stress as a function of self-reported racial discrimination among Indigenous and non-Indigenous Australian young adults. Our findings are also in line with but extend prior work examining the effects of alternative forms of psychological stress on glucocorticoid sensitivity using similar methods to our own (e.g., repeated social defeat in animal models, caregiver stress, long-term threat; Cohen et al., 2012; G. E. Miller et al., 2002; Powell et al., 2009; Walsh et al., 2017).
This report also adds to a small literature examining psychoneuroimmune/endocrine contributions to glucocorticoid signaling during the prenatal period. Specifically, our findings lend support to reports from Corwin et al. (2013) and Katz et al. (2012), which noted the presence of decreased prenatal leukocyte glucocorticoid sensitivity among minority/low income versus nonminority/high income women and as a factor of higher maternal depressive symptoms, respectively. Interestingly, a positive association between maternal distress and placental expression of the glucocorticoid receptor has also been reported, which may contribute to greater sensitivity to the hormone at the level of the placenta (Mina, Raikkonen, Riley, Norman, & Reynolds, 2015; Reynolds et al., 2015). Nonetheless, some evidence does suggest that this link is present among Caucasian but not African American women (Capron, Ramchandani, & Glover, 2018). Additional prospective cohort studies among perinatal samples of appropriate scale are needed to determine whether the biological ramifications of psychological stress are dependent upon the stress construct, the endocrine hormone, the cell, and/or population under study. Considering our findings and given the dearth of evidence, there remains the possibility that psychological stress-induced decreases in glucocorticoid sensitivity can be witnessed in response to various hormones (e.g., corticotropic-releasing hormone) across various cells and tissues (e.g., the placenta), which holds important implications for the study of complications of pregnancy (Smith & Nicholson, 2007).
4.2. Implications for Birth Timing
In addition, we provide novel evidence of effect modification by experiences of racial discrimination tertile on associations among prenatal cortisol levels and future birth timing, with a negative association noted among African American women reporting no experiences but not among those in the mid- and upper tertiles of exposure. A growing body of literature implicates prenatal cortisol elevations, including in response to various forms of psychological stress, in accelerated birth timing (e.g., Gillespie et al., 2017; Giurgescu, 2009; Hoffman et al., 2016), with our findings suggesting that it may be true among some but not all women. This pattern is of critical importance considering that an accurate depiction of the biologic pathways to shortened gestation is a prerequisite to the design of targeted, effective preventive interventions, particularly among African American women bearing a disproportionate burden of preterm birth in the United States. (Martin et al., 2017).
While the spontaneous initiation of labor processes by means of cortisol elevations may be related to aberrations in immune or neuroendocrine feedback circuits, it is notable that this study simultaneously witnessed deviations from expected biologic effects of cortisol on specific aspects of leukocyte feedback as well as birth timing dependent upon experiences of racial discrimination. Specifically, African American women showed progressive deviation from expected associations among maternal cortisol levels and plasma IL-8 levels (expected positive association) and monocyte counts (expected negative association) in progressing from the 1st to 3rd tertile of experiences of racial discrimination. Interestingly, the expected pattern of association among maternal cortisol levels and lymphocytes counts (expected negative association) was maintained among those without experiences of discrimination, with significant differences witnessed specifically among the second tertile (i.e., those reporting 1 – 2 experiences). The meaning behind this finding, as opposed to those above suggesting a dose-response, is not immediately clear but is certainly worth further attention.
IL-8, noted for its role in neutrophil recruitment, is tightly regulated during healthy gestation and is seen in higher concentrations peripherally during preterm versus term labor (e.g., Christian & Porter, 2014; Gillespie, Porter, & Christian, 2016; Herrera-Munoz, Fernandez-Alonso, Fischer-Suarez, Chedraui, & Perez-Lopez, 2017). Moreover, the coinciding diurnal patterns of cortisol levels and monocyte/lymphocyte counts, in which the nadir meets the trough and vice versa, have long been appreciated in studies of animals (e.g., Dhabhar, Miller, Stein, McEwen, & Spencer, 1994) and humans (e.g., Thomson, McMahon, & Nugent, 1980). As described by Dhabhar and colleagues (2012), rising cortisol levels appear to induce monocyte and lymphocyte trafficking to peripheral tissues as opposed to decreasing cell numbers. Given that these immune cells rapidly influx maternal-fetal tissues during labor, it may be that enhanced cortisol-induced tissue surveillance increases likelihood for expedited initiation of the well-described inflammatory cascade of labor (Hamilton et al., 2012). This will be an interesting area of inquiry for future work.
It is also critical to note that, while there is biologic plausibility that racial discrimination-associated differences in sensitivity to the biologic effects of cortisol diminishes associations between the hormone’s levels and birth timing, exposure to salient psychological stressors such as racial discrimination do not protect against but heighten risk for preterm birth (Rankin et al., 2011; Ruiz et al., 2014). One explanation for these seemingly discordant findings is that the development of decreased leukocyte glucocorticoid sensitivity, in and of itself, increases risk for preterm birth – essentially shifting the biologic pathway underlying the syndrome but increasing risk nonetheless.
Decreased glucocorticoid sensitivity may lead to subtle but important changes in the maternal inflammatory milieu, impairing a woman’s ability to maintain pregnancy. Indeed, this study and others have noted that exposure to racial discrimination alone does not consistently predict greater total cortisol output (Busse, Yim, Campos, & Marshburn, 2017; Korous, Causadias, & Casper, 2017), which is likely related to an increasing ability to normalize total cortisol output as time passes following stressor first emergence (G. E. Miller, Chen, & Zhou, 2007). However, our findings certainly suggest that these relationships may be complicated. Specifically, our report suggests that both experiences of and responses to racial discrimination may be important in determining biological effects, as moderate exposures to racial discrimination predicted elevated cortisol levels only among those exhibiting internalizing responses. This is consistent with work showing that resultant distress may be a particularly important mediator linking discriminatory exposures to cortisol levels (Lee et al., 2018). Moreover, glucocorticoid resistance can persist undetected, resulting in failure to dampen inflammation upon environmental challenge through loss of important transactivation and transrepressive effects at the level of the leukocyte (e.g., Jarcho et al., 2013; Walsh et al., 2017). In fact, while research in this area is sparse, Maranville and colleagues (2013) noted in a small pilot that, compared to European Americans, African Americans show decreased glucocorticoid-mediated inhibition of lymphocyte proliferation, which appears to be related to decreased transrepression of NFKB1, which codes a subunit of the positive inflammatory regulator, nuclear factor kappa B.
4.3. Strengths
Major strengths of the current study include its prospective cohort design, which provided the opportunity to assess exposure to racial discrimination and the biologic parameters of interest during asymptomatic pregnancy in advance of birth. Enrollment of a generally healthy sample also reduced the potential for confounding. The EOD has been extensively studied and is a well validated tool for use among African American women, including for the prediction of outcomes of interest during pregnancy (Francis et al., 2017). Also of note, biologic parameters were standardized to gestational age and sampling time of day windows (removed from awakening, exercise, or caffeine intake), with further statistical controls applied as appropriate to account for prenatal and diurnal fluctuations in cortisol output. The examination of patterns of endogenous glucocorticoid feedback, using concurrent sampling of cortisol, inflammatory markers, and leukocyte counts, also helped to remove the potential effects of these fluctuations. Though, of course, given the diurnal nature of cortisol fluctuations, future studies may wish to expand their sampling beyond a single concurrent draw, which may provide more power to note subtle differences such as in cortisol trajectories as a function of experiences of racial discrimination. The accuracy of gestational age estimates were bolstered through the requirement of ultrasound determination or confirmation of estimated due dates, which is accurate within seven days when performed at or before 15 weeks of pregnancy (American College of Obstetricians and Gynecologists, 2014).
4.4. Limitations
This study is limited by lack of ability to examine the potential effects of alternative forms of racism on the outcomes of interest, as it is highly likely that individual encounters with racial discrimination fail to fully account for the social inequalities thought to contribute to racial health disparities (e.g., Geronimus, 1992; Geronimus, 1996). For example, Chae and colleagues (2018) recently reported increasing prevalence of preterm birth as a function of greater area racism, estimated according to Google search patterns. Supported by our findings as well as prior literature (Chae et al., 2018; e.g., Corwin et al., 2013), future perinatal research will benefit from examining the effects of individual-level racial discrimination as well as more subtle but perhaps equally important discriminatory exposures on patterns of glucocorticoid sensitivity and birth outcomes. On a related note, it must be reiterated that, among women without experiences of racial discrimination, expected significant associations among maternal cortisol levels and the assessed biological parameters were only noted for prediction of IL-8 and lymphocytes and approximated for prediction of monocytes. Maternal cortisol levels failed to predict IL-6, TNF-α, or IL-1 β for the full cohort, with no differences by discrimination tertile. As such, though greater frequency of experiences of racial discrimination was generally consistent with progressive deviations from expected patterns of association, it is difficult to draw solid conclusions in this regard. Larger studies powered to examine the multiple exposures with potential importance for glucocorticoid sensitivity, as discussed above, may help to clarify this issue further.
Further, the appropriateness of categorization of continuously-measured predictors, regardless of their distribution, has been long debated in the psychological sciences (e.g., MacCallum, Zhang, Preacher, & Rucker, 2002). In this case, the patterns in the data prompted us to create distinct racial discrimination categories, which decreases the likelihood of assigning spurious prediction at rare levels of a significantly skewed variable. Prior studies applying similar categorization have also reported effects of racial discrimination on biological parameters and birth outcomes (Christian, Iams, Porter, & Glaser, 2012; Collins et al., 2000; Giurgescu et al., 2016); however, the potential for spurious results based on false categorization cannot be ruled out. In addition, the current study lacked a repeated measures design, which prohibited examination of potential gestational age effects on patterns of leukocyte glucocorticoid sensitivity. The absence of alternative measures of glucocorticoid resistance, such as through ex vivo or in vivo dexamethasone suppression testing, was also a limitation. Unmeasured factors, including binding proteins, receptor number, and activity, may also provide further explanations for findings.
5. Conclusion
In conclusion, the current study provides new data showing an association between racial discrimination and cortisol levels during pregnancy among women with internalizing responses to this important and salient exposure. Moreover, we report associations among racial discrimination and biologic profiles consistent with decreased leukocyte glucocorticoid sensitivity among pregnant African American women. Specifically, effects of racial discrimination were seen in examining concurrent associations among maternal cortisol level, plasma IL-8 level, monocyte count, and lymphocyte count. We also provide novel evidence showing that greater maternal cortisol levels predict earlier birth among African American women without but not with a history of exposure to racial discrimination. These findings suggest that the biology of preterm birth may differ dependent upon this important exposure, favoring pathways dependent upon glucocorticoid-induced increases in leukocyte tissue surveillance versus glucocorticoid resistance-associated aberrations in inflammatory regulation, respectively. Precision approaches to the management of prenatal care are sorely needed to combat the high rates of preterm birth witnessed worldwide, particularly among African American women. These efforts are dependent upon further research examining the intricate and potentially diverse biologic pathways that may contribute to the syndrome dependent upon the totality of an individual’s exposures.
Highlights.
Racial discrimination predicts lower maternal leukocyte glucocorticoid sensitivity.
Elevated cortisol levels predict earlier birth in women without past discrimination.
Findings suggest biology of preterm birth may be unique based on prior exposures.
Acknowledgements
We appreciate the contributions of our Undergraduate Research Assistants, Amy Kole and Patricia Do, to data collection and thank the women who participated in this study and our supportive leadership and colleagues.
Role of Funding Sources
This work was supported by the National Institute of Nursing Research of the National Institutes of Health (F31NR01460; SLG); Association of Women’s Health, Obstetric and Neonatal Nurses (SLG); Midwest Nursing Research Society (SLG); Sigma Theta Tau International, Epsilon (SLG); Cola-Cola Critical Difference for Women and Department of Women’s, Gender and Sexuality Studies, Graduate School, and Office of Diversity and Inclusion of The Ohio State University (SLG). Resources supported by the National Center for Advancing Translational Sciences (UL1TR001070) of the National Institutes of Health were utilized. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The funding agencies had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Declaration
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from the publisher.
References
- Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, … Eccles JS (2015). Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: A 20-year prospective study. Psychoneuroendocrinology, 62, 279–291. doi: 10.1016/j.psyneuen.2015.08.018 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. (2014). Committee opinion no 611: Method for estimating due date. Obstetrics and Gynecology, 124(4), 863–866. doi: 10.1097/01.AOG.0000454932.15177.be [doi] [DOI] [PubMed] [Google Scholar]
- Bann CM, Parker CB, Grobman WA, Willinger M, Simhan HN, Wing DA, … NuMoM2b study. (2017). Psychometric properties of stress and anxiety measures among nulliparous women. Journal of Psychosomatic Obstetrics and Gynaecology, 38(1), 53–62. doi: 10.1080/0167482X.2016.1252910 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Leicht A, Slatcher A, Kraeuter AK, Ketheesan S, Larkins S, & Sarnyai Z (2017). Cortisol awakening response and acute stress reactivity in first nations people. Scientific Reports, 7, 41760. doi: 10.1038/srep41760 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, & Sarnyai Z (2015). “More than skin deep”: Stress neurobiology and mental health consequences of racial discrimination. Stress (Amsterdam, Netherlands), 18(1), 1–10. doi: 10.3109/10253890.2014.989204 [doi] [DOI] [PubMed] [Google Scholar]
- Bessinger RC, McMurray RG, & Hackney AC (2002). Substrate utilization and hormonal responses to moderate intensity exercise during pregnancy and after delivery. American Journal of Obstetrics and Gynecology, 186(4), 757–764. doi:a122093 [pii] [DOI] [PubMed] [Google Scholar]
- Busse D, Yim IS, Campos B, & Marshburn CK (2017). Discrimination and the HPA axis: Current evidence and future directions. Journal of Behavioral Medicine, 40(4), 539–552. doi: 10.1007/s10865-017-9830-6 [doi] [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Capron LE, Ramchandani PG, & Glover V (2018). Maternal prenatal stress and placental gene expression of NR3C1 and HSD11B2: The effects of maternal ethnicity. Psychoneuroendocrinology, 87, 166–172. doi:S0306-4530(17)30260-3 [pii] [DOI] [PubMed] [Google Scholar]
- Chae DH, Clouston S, Martz CD, Hatzenbuehler ML, Cooper HLF, Turpin R, … Kramer MR (2018). Area racism and birth outcomes among blacks in the united states. Social Science & Medicine (1982), 199, 49–55. doi:S0277-9536(17)30242-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Iams JD, Porter K, & Glaser R (2012). Epstein-barr virus reactivation during pregnancy and postpartum: Effects of race and racial discrimination. Brain, Behavior, and Immunity, 26(8), 1280–1287. doi: 10.1016/j.bbi.2012.08.006 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Mitchell AM, Gillespie SL, & Palettas M (2016). Serum brain-derived neurotrophic factor (BDNF) across pregnancy and postpartum: Associations with race, depressive symptoms, and low birth weight. Psychoneuroendocrinology, 74, 69–76. doi:S0306-4530(16)30612-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, & Porter K (2014). Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine, 70(2), 134–140. doi: 10.1016/j.cyto.2014.06.018 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, & Turner RB (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences of the United States of America, 109(16), 5995–5999. doi: 10.1073/pnas.1118355109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kessler R, & Underwood Gordon L (1995). Measuring stress: A guide for health and social scientists. New York, NY: Oxford University Press. [Google Scholar]
- Cole SW (2008). Social regulation of leukocyte homeostasis: The role of glucocorticoid sensitivity. Brain, Behavior, and Immunity, 22(7), 1049–1055. doi: 10.1016/j.bbi.2008.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Mendoza SP, & Capitanio JP (2009). Social stress desensitizes lymphocytes to regulation by endogenous glucocorticoids: Insights from in vivo cell trafficking dynamics in rhesus macaques. Psychosomatic Medicine, 71(6), 591–597. doi: 10.1097/PSY.0b013e3181aa95a9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colen CG, Ramey DM, Cooksey EC, & Williams DR (2018). Racial disparities in health among nonpoor african americans and hispanics: The role of acute and chronic discrimination. Social Science & Medicine (1982), 199, 167–180. doi:S0277-9536(17)30290-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JW Jr, David RJ, Symons R, Handler A, Wall SN, & Dwyer L (2000). Low-income african-american mothers’ perception of exposure to racial discrimination and infant birth weight. Epidemiology (Cambridge, Mass.), 11(3), 337–339. [DOI] [PubMed] [Google Scholar]
- Cook RD, & Weisberg S (1982). Residuals and influence in regression. New York, NY: Chapman and Hall. [Google Scholar]
- Corwin EJ, Guo Y, Pajer K, Lowe N, McCarthy D, Schmiege S, … Stafford B (2013). Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology, 38(9), 1786–1796. doi: 10.1016/j.psyneuen.2013.02.015 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Malarkey WB, Neri E, & McEwen BS (2012). Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: A tale of three hormones--curt richter award winner. Psychoneuroendocrinology, 37(9), 1345–1368. doi: 10.1016/j.psyneuen.2012.05.008 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, Stein M, McEwen BS, & Spencer RL (1994). Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain, Behavior, and Immunity, 8(1), 66–79. doi:S0889-1591(84)71006-3 [pii] [DOI] [PubMed] [Google Scholar]
- Francis B, Klebanoff M, & Oza-Frank R (2017). Racial discrimination and perinatal sleep quality. Sleep Health, 3(4), 300–305. doi:S2352-7218(17)30104-3 [pii] [DOI] [PubMed] [Google Scholar]
- Geronimus AT (1992). The weathering hypothesis and the health of african-american women and infants: Evidence and speculations. Ethnicity & Disease, 2(3), 207–221. [PubMed] [Google Scholar]
- Geronimus AT (1996). Black/white differences in the relationship of maternal age to birthweight: A population-based test of the weathering hypothesis. Social Science & Medicine (1982), 42(4), 589–597. [DOI] [PubMed] [Google Scholar]
- Gillespie SL, Christian LM, Alston AD, & Salsberry PJ (2017). Childhood stress and birth timing among african american women: Cortisol as biological mediator. Psychoneuroendocrinology, 84, 32–41. doi:S0306-4530(17)30075-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie SL, Porter K, & Christian LM (2016). Adaptation of the inflammatory immune response across pregnancy and postpartum in black and white women. Journal of Reproductive Immunology, 114, 27–31. doi:S0165-0378(15)30088-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giurgescu C (2009). Are maternal cortisol levels related to preterm birth? Journal of Obstetric, Gynecologic, and Neonatal Nursing : JOGNN/NAACOG, 38(4), 377–390. doi: 10.1111/j.1552-6909.2009.01034.x [DOI] [PubMed] [Google Scholar]
- Giurgescu C, Engeland CG, Templin TN, Zenk SN, Koenig MD, & Garfield L (2016). Racial discrimination predicts greater systemic inflammation in pregnant african american women. Applied Nursing Research : ANR, 32, 98–103. doi:S0897-1897(16)30030-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, … Jones RL (2012). Macrophages infiltrate the human and rat decidua during term and preterm labor: Evidence that decidual inflammation precedes labor. Biology of Reproduction, 86(2), 39. doi: 10.1095/biolreprod.111.095505 [doi] [DOI] [PubMed] [Google Scholar]
- Herrera-Munoz A, Fernandez-Alonso AM, Fischer-Suarez N, Chedraui P, & Perez-Lopez FR (2017). Maternal serum cytokine levels in pregnancies complicated with threatened preterm labour. Gynecological Endocrinology: The Official Journal of the International Society of Gynecological Endocrinology, 33(5), 408–412. doi: 10.1080/09513590.2017.1284786 [doi] [DOI] [PubMed] [Google Scholar]
- Hoffman MC, Mazzoni SE, Wagner BD, Laudenslager ML, & Ross RG (2016). Measures of maternal stress and mood in relation to preterm birth. Obstetrics and Gynecology, 127(3), 545–552. doi: 10.1097/AOG.0000000000001287 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho MR, Slavich GM, Tylova-Stein H, Wolkowitz OM, & Burke HM (2013) Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biological Psychology, 93(1), 150–158. doi: 10.1016/j.biopsycho.2013.01.018 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ER, Stowe ZN, Newport DJ, Kelley ME, Pace TW, Cubells JF, & Binder EB (2012). Regulation of mRNA expression encoding chaperone and co-chaperone proteins of the glucocorticoid receptor in peripheral blood: Association with depressive symptoms during pregnancy. Psychological Medicine, 42(5), 943–956. doi: 10.1017/S0033291711002121 [doi] [DOI] [PubMed] [Google Scholar]
- Korous KM, Causadias JM, & Casper DM (2017). Racial discrimination and cortisol output: A meta-analysis. Social Science & Medicine (1982), 193, 90–100. doi:S0277-9536(17)30586-5 [pii] [DOI] [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, & Barbeau EM (2005). Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Social Science & Medicine (1982), 61(7), 1576–1596. doi: 10.1016/j.socscimed.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Lee DB, Peckins MK, Heinze JE, Miller AL, Assari S, & Zimmerman MA (2018). Psychological pathways from racial discrimination to cortisol in african american males and females. Journal of Behavioral Medicine, 41(2), 208–220. doi: 10.1007/s10865-017-9887-2 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA (1988). A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association, 83(404), 1198–1202. doi: 10.1080/01621459.1988.10478722 [DOI] [Google Scholar]
- Lucas T, Wegner R, Pierce J, Lumley MA, Laurent HK, & Granger DA (2017). Perceived discrimination, racial identity, and multisystem stress response to social evaluative threat among african american men and women. Psychosomatic Medicine, 79(3), 293–305. doi: 10.1097/PSY.0000000000000406 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, & Rucker DD (2002). On the practice of dichotomization of quantitative variables. Psychological Methods, 7(1), 19–40. [DOI] [PubMed] [Google Scholar]
- Mancuso RA, Schetter CD, Rini CM, Roesch SC, & Hobel CJ (2004). Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosomatic Medicine, 66(5), 762–769. doi: 10.1097/01.psy.0000138284.70670.d5 [doi] [DOI] [PubMed] [Google Scholar]
- Manuck TA, Esplin MS, Biggio J, Bukowski R, Parry S, Zhang H, … Eunice Kennedy Shriver National Institute of Child Health and Human Development Genomics and Proteomics Network for Preterm Birth Research. (2015). The phenotype of spontaneous preterm birth: Application of a clinical phenotyping tool. American Journal of Obstetrics and Gynecology, 212(4), 487.e1–487.e11. doi: 10.1016/j.ajog.2015.02.010 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranville JC, Baxter SS, Torres JM, & Di Rienzo A (2013). Inter-ethnic differences in lymphocyte sensitivity to glucocorticoids reflect variation in transcriptional response. The Pharmacogenomics Journal, 13(2), 121–129. doi: 10.1038/tpj.2011.55 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, & Mathews TJ (2017). Births: Final data for 2015. National Vital Statistics Reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 66(1), 1. [PubMed] [Google Scholar]
- Matthews TJ, MacDorman MF, & Thoma ME (2015). Infant mortality statistics from the 2013 period linked birth/infant death data set. National Vital Statistics Reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 64(9), 1–30. [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133(1), 25–45. doi: 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, & Ritchey AK (2002). Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology, 21(6), 531–541. [DOI] [PubMed] [Google Scholar]
- Miller GE, Gaudin A, Zysk E, & Chen E (2009). Parental support and cytokine activity in childhood asthma: The role of glucocorticoid sensitivity. The Journal of Allergy and Clinical Immunology, 123(4), 824–830. doi: 10.1016/j.jaci.2008.12.019 [DOI] [PubMed] [Google Scholar]
- Miller R, Stalder T, Jarczok M, Almeida DM, Badrick E, Bartels M, … Kirschbaum C (2016). The CIRCORT database: Reference ranges and seasonal changes in diurnal salivary cortisol derived from a meta-dataset comprised of 15 field studies. Psychoneuroendocrinology, 73, 16–23. doi:S0306-4530(16)30423-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina TH, Raikkonen K, Riley SC, Norman JE, & Reynolds RM (2015). Maternal distress associates with placental genes regulating fetal glucocorticoid exposure and IGF2: Role of obesity and sex. Psychoneuroendocrinology, 59, 112–122. doi: 10.1016/j.psyneuen.2015.05.004 [doi] [DOI] [PubMed] [Google Scholar]
- Powell ND, Bailey MT, Mays JW, Stiner-Jones LM, Hanke ML, Padgett DA, & Sheridan JF (2009). Repeated social defeat activates dendritic cells and enhances toll-like receptor dependent cytokine secretion. Brain, Behavior, and Immunity, 23(2), 225–231. doi: 10.1016/j.bbi.2008.09.010 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KM, David RJ, & Collins JW Jr. (2011). African american women’s exposure to interpersonal racial discrimination in public settings and preterm birth: The effect of coping behaviors. Ethnicity & Disease, 21(3), 370–376. [PubMed] [Google Scholar]
- Reynolds RM, Pesonen AK, O’Reilly JR, Tuovinen S, Lahti M, Kajantie E, … Raikkonen K (2015). Maternal depressive symptoms throughout pregnancy are associated with increased placental glucocorticoid sensitivity. Psychological Medicine, , 1–8. doi:S003329171400316X [pii] [DOI] [PubMed] [Google Scholar]
- Romero R, Dey SK, & Fisher SJ (2014). Preterm labor: One syndrome, many causes. Science (New York, N.Y.), 345(6198), 760–765. doi: 10.1126/science.1251816 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz RL, Shah MK, Lewis ML, & Theall KP (2014). Perceived access to health services and provider information and adverse birth outcomes: Findings from LaPRAMS, 2007–2008. Southern Medical Journal, 107(3), 137–143. doi: 10.1097/SMJ.0000000000000065 [doi] [DOI] [PubMed] [Google Scholar]
- Sedov ID, Cameron EE, Madigan S, & Tomfohr-Madsen LM (2017). Sleep quality during pregnancy: A meta-analysis. Sleep Medicine Reviews, doi:S1087-0792(17)30029-1 [pii] [DOI] [PubMed] [Google Scholar]
- Simon CD, Adam EK, Holl JL, Wolfe KA, Grobman WA, & Borders AE (2016). Prenatal stress and the cortisol awakening response in african-american and caucasian women in the third trimester of pregnancy. Maternal and Child Health Journal, 20(10), 2142–2149. doi: 10.1007/s10995-016-2060-7 [doi] [DOI] [PubMed] [Google Scholar]
- Smith R, & Nicholson RC (2007). Corticotrophin releasing hormone and the timing of birth. Frontiers in Bioscience : A Journal and Virtual Library, 12, 912–918. doi:2113 [pii] [DOI] [PubMed] [Google Scholar]
- Staneva A, Bogossian F, Pritchard M, & Wittkowski A (2015). The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: A systematic review. Women and Birth : Journal of the Australian College of Midwives, 28(3), 179–193. doi: 10.1016/j.wombi.2015.02.003 [doi] [DOI] [PubMed] [Google Scholar]
- Thomson SP, McMahon LJ, & Nugent CA (1980). Endogenous cortisol: A regulator of the number of lymphocytes in peripheral blood. Clinical Immunology and Immunopathology, 17(4), 506–514. [DOI] [PubMed] [Google Scholar]
- Tsubouchi H, Shimoya K, Hayashi S, Toda M, Morimoto K, & Murata Y (2006). Effect of coffee intake on blood flow and maternal stress during the third trimester of pregnancy. International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics, 92(1), 19–22. doi:S0020-7292(05)00502-3 [pii] [DOI] [PubMed] [Google Scholar]
- Walsh CP, Ewing LJ, Cleary JL, Vaisleib AD, Farrell CH, Wright AGC, … Marsland AL (2017). Development of glucocorticoid resistance over one year among mothers of children newly diagnosed with cancer. Brain, Behavior, and Immunity, doi:S0889-1591(17)30552-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2000). Obesity: Preventing and managing the global epidemic. report of a WHO consultation. World Health Organization Technical Report Series, 894, i–xii, 1–253. [PubMed] [Google Scholar]


