Abstract
Stem cell therapy for Parkinson’s disease requires effective production of dopaminergic neurons. In this issue of Cell Stem Cell, Sacchetti et al. (2009) report an unexpected role of liver X receptors and their ligand, oxysterols, in regulating dopaminergic neurogenesis.
Midbrain dopaminergic neurons are important for coordinating voluntary movements and for regulating diverse cognitive functions. Degenerative loss of dopaminergic neurons is a hallmark of Parkinson’s disease (PD). Pioneering works indicated initial promise of transplanting fetal neural tissues to replace lost dopaminergic neurons and ameliorate certain pathological symptoms of PD patients (Lindvall et al., 1990). Such attempts fueled hopes to use better-defined stem cell-derived dopaminergic neurons in treating PD and to circumvent logistic and ethical issues associated with fetal human tissue transplantation. Dopaminergic neurons have since been successfully generated from embryonic stem cells (ESCs) and tested in preclinical animal models (Gale and Li, 2008; Kim and de Vellis, 2009; McKay, 2004). The beneficial outcome, however, is limited and variable, in part because of inefficient ex vivo differentiation of dopaminergic neurons from ESCs. Therefore, a better understanding of extrinsic and intrinsic mechanisms underlying dopaminergic neurogenesis during midbrain development should illuminate how to further enhance directed dopaminergic differentiation from ESCs. In this issue of Cell Stem Cell, Sacchetti et al. (2009) report a critical role of liver X receptors (LXRs) in ventral midbrain neurogenesis. Moreover, oxysterols, natural agonists of LXRs, enhance dopaminergic neurogenesis from human ESCs.
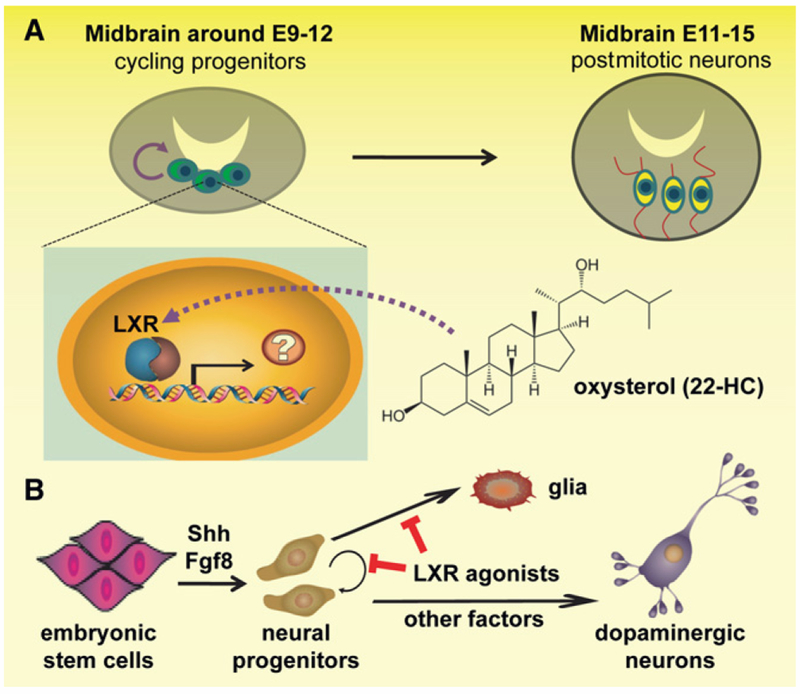
The generation of specific neuronal subtypes and glia in the developing brain is subjected to precise temporal and spatial control involving coordinated interaction between extrinsic inductive signals and intrinsic genetic programs. Previous studies have revealed Shh and Fgf8 as two key inductive signals for establishing the ventral midbrain (VM) identity (Gale and Li, 2008). VM neural progenitors upregulate a battery of genes, including Wnt1, Lmx1a, Ngn2, Pitx3I, and Nurr1, and undergo differentiation to become postmitotic dopaminergic neurons expressing tyrosine hydroxylase (TH). The maturation and survival of dopaminergic neurons are then regulated by neurotrophins GDNF and BDNF. LXRs are nuclear receptors, best known as intracellular sensors for various sterols in multiple adult organs, and they regulate gene transcription in response to oxysterols, oxidized derivatives of cholesterol (Kalaany and Mangelsdorf, 2006). In the nervous system, LXRs were recently implicated in preventing degeneration of dopaminergic neurons in adult mice through maintaining proper lipid homeostasis (Kim et al., 2008). By characterizing dopaminergic neurogenesis in LXR null mice and in culture, Sacchetti et al. (2009) presented a series of compelling findings that support a surprising developmental role for LXRs during midbrain dopaminergic neuron generation, via mechanisms other than lipid homeostatic regulation (Figure 1).
Figure 1. A Schematic Illustration of The role of Oxysterols in Promoting Dopaminergic Neurogenesis.
Dopaminergic neural progenitors undergo active cell-cycle progression in the ventral midbrain domain from the mouse embryonic stage E9 to E12 and then dopaminergic neuron fate determination and maturation from the stage E11 to E15 (A). Acting in the nuclei of the early progenitors in a presumably cell-autonomous manner, oxysterols (22-oxycholesterol depicted) activate LXRs and regulate target gene transcription to induce cell-cycle exit and dopamine neuron differentiation. Such effects of oxysterols are conserved during dopaminergic neurogenesis from human embryonic stem cells (B). Shh and Fgf8 induce neural progenitor cell competency for midbrain neuron specification. By inducing cell-cycle exit specifically toward neuronal fate, oxysterols then act in coordination with other factors and genetic programs to promote dopaminergic neuron differentiation from progenitor cells.
To probe potential roles of LXRs in midbrain development, the authors found that the spatiotemporal expression pattern of LXRs (LXRα and LXRβ) in the VM domain is highly correlated with dopaminergic neuron generation. Furthermore, LXRαβ null mice exhibit reduced expression of a specific subset of genes important for dompaminergic neurogenesis, including Limx1b, Wnt1, and Pitx3, and reduced numbers of TH+ or Nurr+ dopaminergic neurons at E11.5. In parallel culture experiments with primary mouse VM progenitors, both overexpression of LXRβ and application of exogenous LXR agonist oxysterols enhance dopaminergic neurogenesis, and these two manipulations exhibit additive effects. Importantly, the neurogenic enhancing effect of oxysterols is abolished in LXR-deficient progenitors, indicating specific action of oxysterols on LXRs to yield this outcome. Collectively, these experiments established that activation of LXRs is both required and sufficient to promote dopaminergic neurogenesis. Surprisingly, LXRs do not seem to directly bind to and regulate Wnt1, Lmx1b, or Ngn2, all of which are genes that exhibit altered expression in LXR null mice. To further explore the underlying mechanism responsible for the observed dopaminergic neurogenesis, the authors ruled out potential contributions stemming from cell death or lipid homeostatic dysregulation. Instead, combined Ki67- and BrdU-labeling analysis suggested that LXR deficiency causes delayed or impaired cell-cycle progression of proliferating progenitors at the G2/M phase, leading to progenitor accumulation and impairment of differentiation required for dopaminergic neurogenesis. Accordingly, the authors observed bidirectional expression level changes of cyclin-dependent kinase inhibitor p27Kip upon LXR activation or in LXR-deficient cultures, although the causal role of p27Kip remains to be determined.
Importantly, the authors proceeded to apply the new insights gained in their mouse studies to promote dopaminergic differentiation of human ESCs. Conventional protocols use PA6 stromal feeders supplemented with Shh, Fgf8, GDNF, and BDNF, and yet the efficiency of these conditions for dopaminergic neuron generation remains low (Gale and Li, 2008; Kim and de Vellis, 2009). Treatment with oxysterols during neuronal differentiation of human ESCs markedly increases the production of TH+ neurons, along with concurrent reduction in the numbers of astrocytes, neural progenitors, and proliferative cells (Figure 1). These TH+ neurons appear to have acquired many hallmarks of dopaminergic neurons, including depolarization-induced release of dopamine, and seem to be metabolically normal with respect to cellular lipid and cholesterol homeostasis. One challenge that must be overcome prior to wide application of stem cell-based cell replacement therapy is the ability to generate desired cell types in large quantities that are also of high quality and sufficient purity so as to minimize contamination of undesired cells, especially proliferating progenitors that may cause tumorigenesis after transplantation. Oxysterols enhance dopaminergic neuronal differentiation, promote cell-cycle exit, and decrease gliogenesis of stem cells and thus kill multiple birds with one stone.
The majority of identified nuclear receptors are known to be expressed in the embryonic and adult brain, and yet few have been investigated as to their potential roles in regulating neurogenesis and development, with the exception of retinoic acid receptor. The identification of novel functions of LXRs in neurogenesis, distinct from their classic roles in lipid homeostasis, opens doors for studying other nuclear receptors in neural development. This study also illustrates a prime example of how discoveries in basic developmental biology can be highly instructive for improving practical schemes of therapeutic stem cell applications. Several important issues, however, remain to be addressed by future studies. First, the mechanism responsible for the impact of oxysterol/LXRs on cell-cycle dynamics of dopaminergic neural progenitors remains unclear. Activation of LXRs has been shown to suppress key cell-cycle genes in other cell types (Vedin et al., 2009). Identifying direct targets of LXRs through Chip-on-chip or Chip-seq technologies would yield insights into this issue. Given that Shh and retinoic acid signaling components exhibit altered expression in acute response to oxysterols or in LXR null mice (Dwyer et al., 2007; Volle et al., 2007), these pathways represent attractive candidates as potential LXR targets, and as converging points that may couple oxysterol signaling to known genetic programs for dopaminergic neuron specification. Second, LXR-mediated effects appear to be highly specific for the midbrain region, for the dopaminergic neuron subtype, and for a subset of dopaminergic specification genes. How is such specificity achieved? How is oxysterol/LXR signaling initiated and regulated in different biological contexts? From the perspective of disease and stem cell-based therapeutic interventions, screening for more potent LXR agonists and inducers of LXR expression is warranted, given the observed additive effects of oxysterols and overexpression of LXRβ on dopaminergic neurogenesis. Additional work is also needed to understand whether defective oxysterol/LXR signaling might be involved in predisposing PD patients for the onset of symptoms. Furthermore, it will be interesting to assay how oxysterol-like molecules might be used to augment dopaminergic neurogenesis from other stem cell populations, such as adult neural stem cells and PD patient-specific induced pluripotent stem cells. Success in these endeavors may offer meaningful advances for both preclinical and translational applications, as well as drug testing and in vitro disease modeling.
REFERENCES
- Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, and Parhami F (2007). J. Biol. Chem 282, 8959–8968. [DOI] [PubMed] [Google Scholar]
- Gale E, and Li M (2008). Mol Brain 1, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaany NY, and Mangelsdorf DJ (2006). Annu. Rev. Physiol 68, 159–191. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Fan X, Gabbi C, Yakimchuk K, Parini P, Warner M, and Gustafsson JA (2008). Proc. Natl. Acad. Sci. USA 105, 2094–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SU, and de Vellis J (2009). J. Neurosci. Res 87, 2183–2200. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Brundin P, W idner H, Rehncrona S, Gustavii B, Frackowiak R, Leenders KL, Sawle G, Rothwell JC, Marsden CD, et al. (1990). Science 247, 574–577. [DOI] [PubMed] [Google Scholar]
- McKay RD (2004). Philos. Trans. R. Soc. Lond. B Biol. Sci 359, 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti P, Sousa KM, Hall AC, Liste I, Steffensen KR, Theofilopoulos S, Parish CL, Hazenberg C, Richter LÄ, Hovatta O, et al. (2009). Cell Stem Cell 5, this issue, 409–419. [DOI] [PubMed] [Google Scholar]
- Vedin LL, Lewandowski SA, Parini P, Gustafsson JA, and Steffensen KR (2009). Carcinogenesis 30, 575–579. [DOI] [PubMed] [Google Scholar]
- Volle DH, Mouzat K, Duggavathi R, Siddeek B, Dechelotte P, Sion B, Veyssiere G, Benahmed M, and Lobaccaro JM (2007). Mol. Endocrinol 21, 1014–1027. [DOI] [PubMed] [Google Scholar]