Abstract
Exercise training provides physiological benefits for both improving athletic performance and maintaining good health. Different exercise training modalities and strategies exist. Two common exercise strategies are high-intensity interval training (HIIT) and moderate-intensity continuous exercise training (MCT). HIIT was first used early in the 20th century and popularized later that century for improving performance of Olympic athletes. The primary premise underlying HIIT is that, compared to energy expenditure-matched MCT, a greater amount of work is performed at a higher intensity during a single exercise session which is achieved by alternating high-intensity exercise intervals with low-intensity exercise or rest intervals. Emerging research suggests that this same training method can provide beneficial effects for patients with a chronic disease and should be included in the comprehensive medical management plan. Accordingly, a major consideration in developing an individual exercise prescription for a patient with a chronic disease is the selection of an appropriate exercise strategy. In order to maximize exercise training benefits, this strategy should be tailored to the individual's need. The focus of this paper is to provide a brief summary of the current literature regarding the use of HIIT to enhance the functional capacity of individuals with cardiovascular, pulmonary, and diabetes diseases.
Keywords: Cardiovascular disease, Diabetes, Low-intensity exercise interval training, Medical management plan, Oxygen consumption, Pulmonary disease
1. Introduction
High-intensity interval training (HIIT) became popular for training athletes during the early 1950s when Emil Zátopek, an Olympic champion long-distance runner, won the 1952 Helsinki Olympic 10,000 m race after utilizing HIIT.1, 2 HIIT utilizes repeated short to long bouts of relatively high-intensity exercise alternated with recovery periods of either low-intensity exercise or rest.3 As described by this broad definition, this review highlights present research literature for various forms of HIIT in comparison to traditional moderate-intensity continuous exercise training (MCT).
The basic premise underlying HIIT is that a greater volume of higher intensity exercise is accumulated during a single exercise session compared to energy expenditure-matched steady state MCT.1, 4 Cardiovascular fitness improvements are reported with HIIT, and these improvements are similar or superior to steady state MCT performed by healthy adults. Reindell and Roskamm5 described the ability of HIIT to enhance both anaerobic and aerobic fitness to higher levels when HIIT alternates periods of high-intensity exercise greater than 75% maximal oxygen consumption (VO2max) with accompanying low-to-moderate-intensity recovery periods performed at 40%–50% of VO2max.1 In the past several decades, scientists have had renewed interest to better understand the use of HIIT as part of the medical management plan for individuals with a chronic disease. The focus of this paper is to briefly summarize the current literature pertaining to the use of HIIT to enhance functional capacity of individuals with cardiovascular, pulmonary, and type 2 diabetes (T2D) diseases. These diseases are highlighted because they were the first to have widely adopted exercise rehabilitation programs.
2. HIIT
HIIT encompasses exercise prescriptions that are tailored to individual needs and can be used in most any exercise setting. This ability to adapt makes HIIT a valuable tool in the exercise programming of patients with a chronic disease.2, 6 Beforediscussing the use of HIIT in patients with chronic diseases, a brief description of HIIT programming in healthy individuals ispresented. Using healthy individuals, Midgley et al.7 reported that the high-intensity components of HIIT resulted in greater training improvements in maximal aerobic capacity compared to the improvements elicited by MCT. The precise mechanism responsible for this effect is not well understood, but various physiological pathways exist that might explain this adaptation. One proposed mechanism is that HIIT increases aerobic capacity and thus delays the onset of exhaustion. This enhanced aerobic capacity slows the depletion of anaerobic fuel stores prolonging time to exhaustion.7 In healthy trained subjects, Billat et al.8 compared intermittent running (30 s at VO2max alternated with 30 s at 50%VO2max) to continuous, strenuous running. The high-intensity components of intermittent running provided a greater exercise training stimulus than continuous running and are potentially responsible for the greater VO2max improvements that are correlated with oxygen consumption found after exercise training.
Exercise intensity for both high-intensity interval (referred to as the work interval) and low-intensity exercise interval (referred to as the recovery interval) is measured by any of the following methods: percentage heart rate maximum (%HRmax), percentage heart rate reserve (%HRR), percentage VO2max, percentage VO2 reserve (%VO2R), rating of perceived exertion (RPE), metabolic equivalence, or competition pace. These measures are used to develop the work to recovery ratio. A typical ratio is 1 min of high-intensity exercise followed by 1 min of low-intensity exercise (ratio of 1:1) (refer to Table 1 for other examples of HIIT programming).
Table 1.
Examples of high-intensity interval training.
| Population | Work to recovery ratio | High-intensity | Low-intensity | Number of cycles |
|---|---|---|---|---|
| Sedentary | 2:1 | 30 s each: push-ups, squats, butt kicks, triceps dips, side lunges, jumping jacks, sit-ups | 15 s recovery between each activity; 1 min between each cycle | 3 (1 cycle = 30 s per exercise alternated with 15 s recovery) |
| Recreationally trained | 2:1 | 20 s each: squat jacks, push-ups with oblique knee (alternating), star jumps, mountain climbers, thigh slap jumps, burpees, high knees, jumping lunges | 20 s between every other activity; 1 min between each cycle | 4 (1 cycle = 2 exercises (20 s each) alternated with 20 s recovery) |
| Running (sprint) | 1:9 | 30 s maximal effort sprint (9+ on 1–10 RPE scale) | 4.5 min low-intensity jog (4–5 on 1–10 RPE scale) | 4 |
| Swimming | 1:1 | 50 m sprint—freestyle (8+ on 1–10 RPE scale) | 50 m slow—breast stroke (4–5 on 1–10 RPE scale) | 6 |
| Soccer | 1:6 fartleks | Runner at the back of a 6-person line sprint to the front of the line (9+ on 1–10 RPE scale) | Low-intensity jog (4–5 on 1–10 RPE scale) until you become the runner at the back of the line | 30 min total |
| Basketball | Shuttle runs | Sprint from baseline to given point on court (near free throw line, top of near 3 point arch, mid court, top of far 3 point arch, far free throw line, far baseline) and back | 15 s rest between each distance; 1 min between each cycle | 5 |
Abbreviation: RPE = rating of perceived exertion.
3. Chronic disease management
A cycle of deconditioning is started when an individual with a chronic disease becomes less physically active. In turn, this deconditioning leads to a loss of functional capacity and subsequent further reductions in the ability to perform both exercise and activities of daily living. If this cycle of deconditioning is not stopped, the consequences of poor long-term health and suboptimal quality of life are greatly increased. In order to stop this downward cycle, individuals with a chronic disease should receive counseling regarding the safety, effectiveness, and proper use of physical activity and prescribed exercise to enhance health.9, 10, 11, 12 Considerable evidence exists regarding the use of exercise training strategies as part of the medical management plan for patients with a chronic disease that demonstrate significant improvements in exercise tolerance and quality of life.9, 11 In the past several decades, much attention has been directed toward primary and secondary disease prevention/treatment by developing the role of physical activity and exercise to improve health and physical fitness. From a secondary disease prevention/treatment perspective, the initial goals for incorporating exercise in rehabilitation programs are to reverse the physical deconditioning resulting from sedentary behavior, optimize physical functioning by exercise programming, and enhance overall health and well-being.10
3.1. Cardiovascular
Of the many chronic diseases, cardiovascular disease is the most studied regarding the potential advantages for using HIIT protocols. Guiraud et al.6 and Cornish et al.13 reviewed HIIT and cardiac rehabilitation literature and highlighted the overall consensus for HIIT's ability to improve peak oxygen consumption (VO2peak).6 These reports emphasized that individuals using HIIT methods achieved greater positive changes in cardiovascular risk factors than did MCT. Because VO2peak is a strong predictor of morbidity and mortality, clinicians are interested in the mechanisms associated with how HIIT affects these functional changes. Although the mechanisms for these changes are not well understood, some scientists have suggested that the rest periods, or the lower intensity exercise intervals, make it possible for cardiac patients to complete short exercise periods at higher intensity, which provides a greater exercise stimulus to the heart than what is possible when completing MCT.14
Using a randomized controlled trial (RCT), Rognmo et al.15 placed heart disease patients either into HIIT or MCT groups for 10 weeks to evaluate VO2peak. The HIIT group performed 4 sets of 4 min high-intensity exercise intervals at 80%–90% of their VO2peak accompanied with low-intensity exercise intervals at 50%–60% VO2peak for a total time of 33 min. The MCT group exercised for 41 continuous min at 50%–60% of their VO2peak. Total training volume was equal for the 2 groups as calculated by percentage of VO2peak. Compared to baseline, within-group analyses showed that VO2peak significantly increased in both groups. Furthermore, between-group analyses indicate that the HIIT group's 17.9%VO2peak improvement was significantly greater than the 7.9% improvement found for the MCT group (Fig. 1). When VO2peak was corrected for the number of training sessions attended, the HIIT group displayed a significant increase of 0.63% per session compared to the 0.29% per session increase found for the MCT group. An important safety note is that none of the patients in either HIIT or MCT suffered cardiac events during the training program. To summarize, in this study HIIT elicited greater aerobic capacity adaptations in cardiac patients compared to MCT without increasing medical risk.15
Fig. 1.
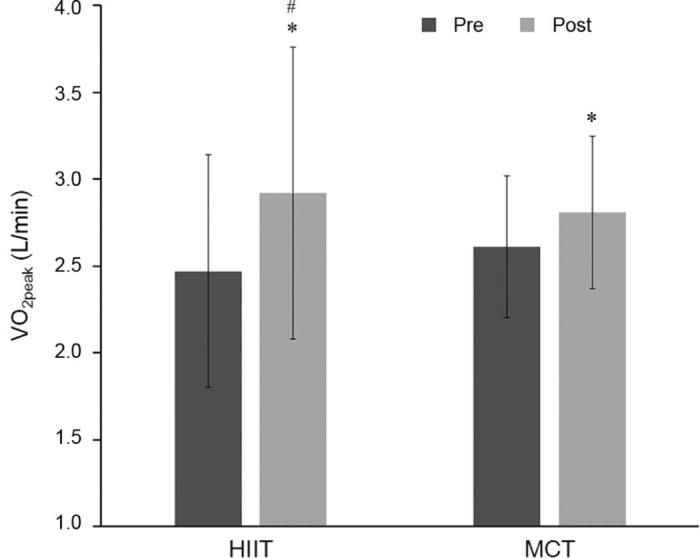
Average VO2peak of individuals before and after high-intensity interval training (HIIT) and moderate-intensity continuous exercise training (MCT) (mean ± SD). *p < 0.05, post significantly different from pre (within group); #p < 0.05, the increment change of 17.9% increase in the HIIT group is significantly larger compared to the increment change of 7.9% increase in the MCT group.15 Adapted with permission.
Wisløff et al.14 employed an RCT research design for post-infarction and heart failure patients who completed a 12-week training program evaluating VO2peak adaptations in HIIT, MCT, and a control group. Four work intervals (each 4 min in length) completed at 90%–95% heart rate peak (HRpeak) were accompanied by 3 min of low-intensity exercise at 50%–70% HRpeak. Total training time for the HIIT group was 38 min while the MCT group exercised for 47 consecutive min at 70%–75% HRpeak. Both HIIT and MCT groups' total amount of work was kept isocaloric. The HIIT group had a 46% increase in VO2peak compared to 14% increase for the MCT group. Reversal of left ventricular (LV) remodeling was found only in the HIIT group. LV remodeling for this study was represented by lower levels of pro-brain natriuretic peptide which is a marker of heart failure severity. As a result of reversal of LV remodeling, the HIIT group significantly improved ejection fraction, stroke volume, and ventricular relaxation, which suggest an overall increased myocardial contractile function.14
Freyssin et al.16 evaluated heart failure patients after 8 weeks of HIIT and MCT multidisciplinary cardiac rehabilitation programming. HIIT consisted of 12 repetitions of 30 s cycling alternated with 60 s of complete rest. This series was repeated 2 more times per training session. For the first 4 weeks of the intervention, the work interval utilized an exercise intensity that was 50% of subjects' maximal power. During the second 4 weeks, the work interval was 80% of maximal power. The MCT group completed 45 min of either cycle ergometer or treadmill work at an HR corresponding to their first ventilatory threshold. The HIIT group showed a significant increase in VO2peak and VO2 at ventilatory threshold compared to no change for the MCT group. No cardiac events throughout the intervention period were reported. Again, HIIT was found both safe and more effective than MCT for improving aerobic capacity in heart failure patients.16
Various studies have evaluated other cardiovascular conditions to include coronary artery bypass surgery,17 percutaneous coronary intervention with stent implantation,18 and myocardial infarction.19 The overriding finding of all studies is that both HIIT and MCT improved outcome measurements compared to a no exercise training control group. An interesting finding was that studies incorporating a comparison control group doing no exercise training report significantly greater number of cardiac events in the control group compared to the HIIT and MCT groups (e.g., cardiac events included chest pain, acute myocardial infarction, and unscheduled recatheterization). Present data suggest that HIIT can provide an effective means for improving functional capacity and endothelial function while decreasing C-reactive protein blood levels.18 Finally, Moholdt et al.20 pooled data from 4 RCTs involving heart disease patients utilizing different exercise intensity HIIT protocols and found that the higher the exercise intensity of the work interval, the better the improvement in aerobic capacity (Fig. 2).
Fig. 2.
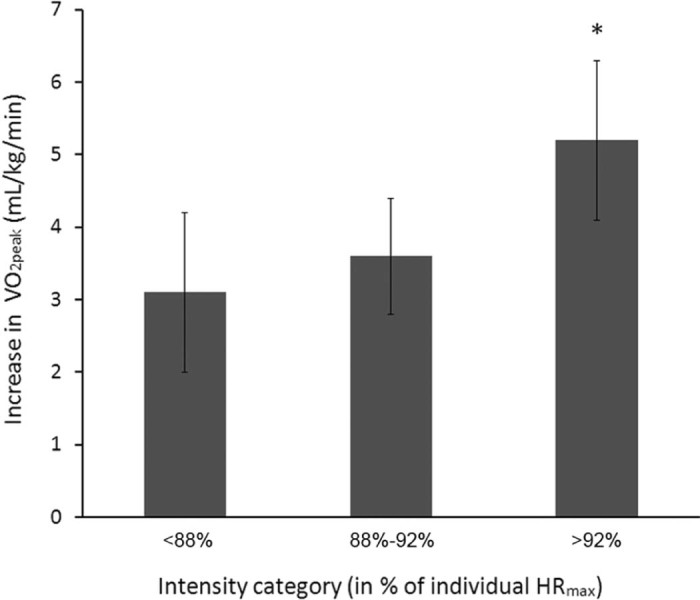
Increase in VO2peak according to exercise intensity categories (mean ± SD). Percentages are exercise intensity in the last 2 min of each 4-min interval, relative to individual maximal heart rate (HRmax). *p < 0.05, compared with the other 2 groups.20 Adapted with permission.
3.2. Pulmonary
HIIT has been evaluated in chronic obstructive pulmonary disease (COPD) patients.21, 22 The overall findings from these studies using patients with COPD indicate HIIT is at least as equally effective as MCT in producing beneficial physiological change.22 Additionally, subjects in the HIIT group reported greater reductions in leg discomfort and dyspnea.22 Usually, patients with moderate to severe COPD are unlikely to sustain high-intensity exercise for long durations without symptoms such as dyspnea causing them to stop exercise sooner compared to healthy individuals.
Vogiatzis et al.23 utilized an RCT with parallel 2-group design and randomly assigned patients with moderate to severe COPD to either the HIIT or MCT groups. Both groups completed 40-min exercise sessions twice a week for 12 weeks. HIIT and MCT groups had significantly improved exercise tolerance, quality of life scores, and reduced minute ventilation and breathlessness at a given exercise level. The magnitude of improvement following the exercise training did not differ between HIIT and MCT. Thus, COPD patients participating in HIIT or MCT can achieve substantial physiological improvements that are similar in magnitude.23
In a follow-up investigation, Vogiatzis et al.24 evaluated skeletal muscle morphologic and biochemical changes in patients with advanced COPD. Again, an RCT with parallel 2-group design was utilized to evaluate COPD patients randomly assigned to either HIIT or MCT. The HIIT protocol was similar to that protocol used in their earlier work.23 Both HIIT and MCT groups completed 45-min sessions of cycle ergometer exercise 3 times per week for 10 weeks. HIIT mean exercise training intensity for the 10-week training program was 124% ± 15% of baseline peak work rate, while MCT mean exercise training intensity was 75% ± 5% of baseline peak work rate. Total amount of work performed during the 10-week training period did not differ between groups. Muscle biopsy analysis of the vastus lateralis for both groups revealed no changes after exercise training for Type I and Type IIa fiber distribution, whereas the Type IIb fiber distribution was reduced within groups (Fig. 3). Fiber type cross sectional area (CSA) of Type I and Type IIa fibers was increased in both groups, but CSA of Type IIb fibers increased only in the MCT group (Fig. 4). The magnitude of change for all fiber type distribution and Type I and Type IIa CSA did not differ between groups. Both groups had significant increases in capillary-to-fiber ratio, peak work rate, and improved lactate threshold, but again, no differences between the HIIT and MCT groups. These results indicate that both HIIT and MCT are able to effectively induce peripheral muscle adaptations. However, HIIT was associated with fewer negative training symptoms with significantly less reported ratings of dyspnea and leg discomfort.24
Fig. 3.
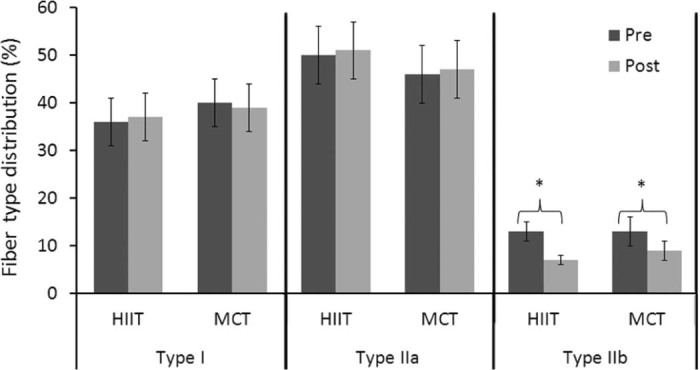
Fiber type distribution (%) of the vastus lateralis muscle before and after HIIT and MCT training (mean ± SEM). HIIT = high-intensity interval training; MCT = moderate-intensity continuous exercise training. *p < 0.05, post significantly different from pre.24 Adapted with permission.
Fig. 4.
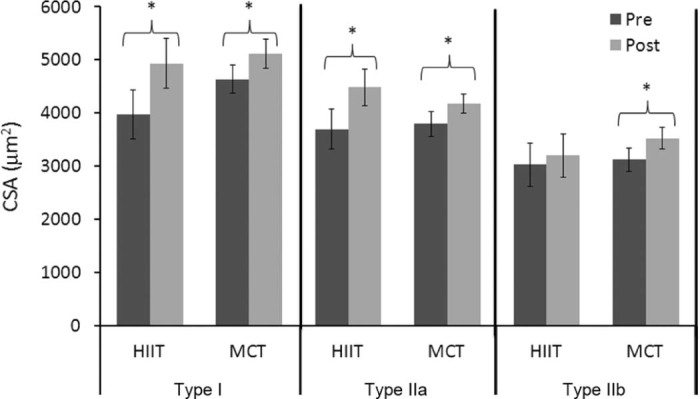
Cross sectional area (CSA) (µm2) of the vastus lateralis muscle before and after HIIT and MCT training (mean ± SEM). HIIT = high-intensity interval training; MCT = moderate-intensity continuous exercise training. *p < 0.05, post significantly different from pre.24 Adapted with permission.
Arnardóttir et al.25 compared the effects of HIIT and MCT on peak work rate using a 16-week exercise program RCT placing 60 moderate to severe COPD patients into either HIIT or MCT groups. Target exercise training intensity for HIIT was ≥80% of baseline peak exercise capacity for 3 min accompanied with 3 min at 30%–40% baseline peak exercise capacity. Target training intensity for MCT was ≥65% baseline peak exercise capacity. Exercise training included resistance training that incorporated upper body, lower body, and abdominal exercises. Total exercise time for both groups was 39 min per session. After exercise intervention, VO2peak, peak exhaled carbon dioxide, and peak exercise capacity increased significantly within groups but did not differ between groups.25Interestingly, when comparing HIIT to MCT at identical work rates, oxygen consumption, carbon dioxide exhaled, and ventilation rates were significantly decreased compared to baseline values for the HIIT group only. Both HIIT and MCT groups had similar improvements in quality of life, submaximal dyspnea, dyspnea during daily activities, functional capacity, and measures of mental health. These results demonstrated that HIIT and MCT are both effective for improving cardiopulmonary function peak exercise capacity, as well as quality of life measures in moderate to severe COPD patients. Because only the HIIT group had significant decreases in minute ventilation, oxygen consumption, and carbon dioxide expired during submaximal exercise, HIIT provided greater functional benefits when completing submaximal work. These patients likely enhanced subjects' ability to complete activities of daily living more easily than their MCT counterparts.25
3.3. Diabetes
As global rates of physical inactivity and metabolic syndrome reach an all-time high, so has interest in applying HIIT exercise protocols for patients with T2D. The most commonly cited barrier to engaging in regular exercise, regardless of gender, age, ethnicity, or health status, is a “lack of time”.26 Utilizing HIIT as part of the medical management plan for T2D patients proved promising for overcoming time as a barrier. Two recent publications provide support for using HIIT to gain improvements in glucose control, glycated hemoglobin A1c (HbA1c) control, and cardiorespiratory fitness in patients with T2D.12, 27 Few studies have been completed that directly compare HIIT to MCT in patients with T2D. Although the results of these studies are promising, they are limited by short duration interventions and small sample sizes.12 This field has great potential for future research to further the safety and efficacy of using HIIT in patients with T2D.
Karstoft et al.28 evaluated a 4-month trial of free-living interval-walking exercise program in T2D. This study compared a non-exercise control, continuous-walking, and interval-walking in an RCT of patients with T2D. Peak energy expenditure rate (PEER) was obtained from subjects' VO2peak measurement. Training was performed 5 days per week for 60 min. The MCT group walked at 55% PEER while the HIIT group alternated 3 min fast walking above 70% PEER with 3 min of slow walking below the 70% target rate. Intensity was monitored by both tri-axial accelerometry and HR monitors. Overall, the HIIT group showed greater beneficial changes in VO2max, body mass, adiposity, and glycemic control when compared to the MCT group. Additionally, the non-exercise control group worsened their glycemic control in regard to mean glycemia and fasting insulin levels. This study demonstrated that continuous walking was better able to attenuate the deterioration in glycemia as seen by the control group, and interval walking displayed superior effects for improving fitness, body composition, and glycemic control in patients with T2D.28
Terada et al.29 randomized patients with T2D to either HIIT or MCT. All subjects exercised 5 days a week for 12 weeks. HIIT and MCT groups were matched for exercise duration, exercise frequency, exercise volume, and mean relative exercise intensity (VO2R). The MCT group exercised at 40% VO2R, and the HIIT group performed 1 min intervals at 100% VO2R alternated with 3 min recovery intervals at 20% VO2R. One day a week, the HIIT group performed the MCT protocol instead of HIIT.29 After 12 weeks of exercise training, no significant changes in either group for feeling states or self-efficacy were found.29 Total percentage body fat, percentage leg fat, and subcutaneous fat width were significantly reduced in both exercise groups; no group provided better results than the other. Only the HIIT group elicited significant decreases in percentage trunk fat and increased peak power output. Glycated HbA1c did not change from baseline for either group, perhaps because initial baseline HbA1c was low. Although this study had a small sample size, the results are promising and demonstrated that a 12-week HIIT program is just as feasible and equally effective for positively altering body fat and increasing peak power output in patients with T2D.29
Terada et al.30 in 2016 utilized a randomized, controlled, crossover design to compare acute glycemic responses to HIIT or MCT in patients with T2D. The effects of each type of exercise protocol were evaluated in both fasted and postprandial conditions. The HIIT protocol included 1 min high-intensity exercise at 100% VO2peak alternated with 3 min at 40% VO2peak for a total of 60 min. The mean calculated relative intensity for the HIIT protocol was 55% VO2peak. The MCT group exercised for 60 min at 55% VO2peak. Energy expenditure between the 2 exercise protocols was not significantly different. The HIIT group reduced nocturnal and fasting glycemia on the day following exercise to a greater extent than the MCT group. Furthermore, their results suggest that performing HIIT in a fasted-state may be the most advantageous exercise strategy for glycemic control as it significantly lowered 24-h mean glucose, fasting glucose, overall postprandial glycemic increment, glycemic variability, and time spent in hyperglycemia.30
4. Conclusion
Considerable evidence is accumulating regarding the use of HIIT strategies for patients with a chronic disease. For example patients with cardiovascular disease demonstrate improved functional capacity and quality of life without increasing medical risk. In addition, HIIT was shown to significantly increase LV ejection fraction with associated reductions in LV end-diastolic volume and LV end-systolic volume when compared to an MCT group completing the same amount of total work. Fewer studies have been completed evaluating HIIT protocols in patients with pulmonary disease, and even fewer studies for patients with T2D. HIIT is at least as effective as MCT for improving functional capacity and quality of life measures in patients with pulmonary disease. In addition, HIIT may have added advantages by producing peripheral muscle changes resulting in fewer negative training symptoms such as less reported ratings of dyspnea and leg discomfort. Considering patients with T2D, HIIT programming is just as effective as MCT in positively altering percentage body fat and increasing peak power output. HIIT should always be considered inconjunction with, or as a supplement to, MCT in the medical management plan for patients with a chronic disease, and those individuals who are not able to tolerate high-intensity continuous exercise. Patient protocol preference is also an important consideration; as the patient's choice usually impacts adherence to the intervention. In the future, studies using larger participant size are necessary to better understand which HIIT protocols are most effective for optimal exercise responses and exercise training adaptations in patients with other chronic diseases. Using HIIT protocols with individuals having a chronic disease will always provide medical concern for patient safety. Literature reported in this brief review highlights the growing scientific evidence that HIIT presents little risk for stable patients when the prescribed exercise protocols are followed.9, 10, 11, 12
Authors' contributions
All authors were involved in the development of this manuscript. As the corresponding author, JLD oversaw the complete manuscript development. LMR performed the literature review and wrote much of this paper. RRP tabulated the information from the literature review, wrote the high-intensity interval section, and developed tables and figures. All authors have read and approved the final version of this manuscript and agree with the order of presentation of the authors.
Competing interests
None of the authors declare competing financial interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Billat L.V. Interval training for performance: a scientific and empirical practice. Special recommendations for middle- and long-distance running. Part I: aerobic interval training. Sports Med. 2001;31:13–31. doi: 10.2165/00007256-200131010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Gibala M.J., Little J.P., Macdonald M.J., Hawley J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590:1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchheit M., Laursen P.B. High-intensity interval training, solutions to the programming puzzle: part I: cardiopulmonary emphasis. Sports Med. 2013;43:313–338. doi: 10.1007/s40279-013-0029-x. [DOI] [PubMed] [Google Scholar]
- 4.Christensen E.H., Hedman R., Saltin B. Intermittent and continuous running. (A further contribution to the physiology of intermittent work) Acta Physiol Scand. 1960;50:269–286. doi: 10.1111/j.1748-1716.1960.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 5.Reindell H., Roskamm H. Ein Beitrag zu den physiologischen Grundlagen des Intervall training unter besonderer Berücksichtigung des Kreilaufes. 1959;7:1–8. [in Germany] [Google Scholar]
- 6.Guiraud T., Nigam A., Gremeaux V., Meyer P., Juneau M., Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med. 2012;42:587–605. doi: 10.2165/11631910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Midgley A.W., McNaughton L.R., Carroll S. Physiological determinants of time to exhaustion during intermittent treadmill running at vV·O2max. Int J Sports Med. 2007;28:273–280. doi: 10.1055/s-2006-924336. [DOI] [PubMed] [Google Scholar]
- 8.Billat V.L., Slawinski J., Bocquet V., Demarle A., Lafitte L., Chassaing P. Intermittent runs at the velocity associated with maximal oxygen uptake enables subjects to remain at maximal oxygen uptake for a longer time than intense but submaximal runs. Eur J Appl Physiol. 2000;81:188–196. doi: 10.1007/s004210050029. [DOI] [PubMed] [Google Scholar]
- 9.American College of Sports Medicine, Durstine J.L., Moore G., Painter P., Roberts S. 4th ed. Human Kinetics; Champaign, IL: 2016. ACSM's exercise management for persons with chronic diseases and disabilities. [Google Scholar]
- 10.Durstine J.L., Painter P., Franklin B.A., Morgan D., Pitetti K.H., Roberts S.O. Physical activity for the chronically ill and disabled. Sports Med. 2000;30:207–219. doi: 10.2165/00007256-200030030-00005. [DOI] [PubMed] [Google Scholar]
- 11.Durstine J., Gordon B., Wang Z., Luo X. Chronic disease and the link to physical activity. J Sport Health Sci. 2013;2:3–11. [Google Scholar]
- 12.Francois M.E., Little J.P. Effectiveness and safety of high-intensity interval training in patients with type 2 diabetes. Diabetes Spectr. 2015;28:39–44. doi: 10.2337/diaspect.28.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornish A.K., Broadbent S., Cheema B.S. Interval training for patients with coronary artery disease: a systematic review. Eur J Appl Physiol. 2011;111:579–589. doi: 10.1007/s00421-010-1682-5. [DOI] [PubMed] [Google Scholar]
- 14.Wisløff U., Støylen A., Loennechen J.P., Bruvold M., Rognmo Ø., Haram P.M. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 15.Rognmo Ø., Hetland E., Helgerud J., Hoff J., Slørdahl S.A. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11:216–222. doi: 10.1097/01.hjr.0000131677.96762.0c. [DOI] [PubMed] [Google Scholar]
- 16.Freyssin C., Verkindt C., Prieur F., Benaich P., Maunier S., Blanc P. Cardiac rehabilitation in chronic heart failure: effect of an 8-week, high-intensity interval training versus continuous training. Arch Phys Med Rehabil. 2012;93:1359–1364. doi: 10.1016/j.apmr.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Moholdt T.T., Amundsen B.H., Rustad L.A., Wahba A., Løvø K.T., Gullikstad L.R. Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: a randomized study of cardiovascular effects and quality of life. Am Heart J. 2009;158:1031–1037. doi: 10.1016/j.ahj.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Munk P.S., Staal E.M., Butt N., Isaksen K., Larsen A.I. High-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation: a randomized controlled trial evaluating the relationship to endothelial function and inflammation. Am Heart J. 2009;158:734–741. doi: 10.1016/j.ahj.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Moholdt T., Aamot I.L., Granøien I., Gjerde L., Myklebust G., Walderhaug L. Aerobic interval training increases peak oxygen uptake more than usual care exercise training in myocardial infarction patients: a randomized controlled study. Clin Rehabil. 2012;26:33–44. doi: 10.1177/0269215511405229. [DOI] [PubMed] [Google Scholar]
- 20.Moholdt T., Madssen E., Rognmo Ø., Aamot I.L. The higher the better? Interval training intensity in coronary heart disease. J Sci Med Sport. 2014;17:506–510. doi: 10.1016/j.jsams.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Beauchamp M.K., Nonoyama M., Goldstein R.S., Hill K., Dolmage T.E., Mathur S. Interval versus continuous training in individuals with chronic obstructive pulmonary disease—a systematic review. Thorax. 2010;65:157–164. doi: 10.1136/thx.2009.123000. [DOI] [PubMed] [Google Scholar]
- 22.Kortianou E.A., Nasis I.G., Spetsioti S.T., Daskalakis A.M., Vogiatzis I. Effectiveness of interval exercise training in patients with COPD. Cardiopulm Phys Ther J. 2010;21:12–19. [PMC free article] [PubMed] [Google Scholar]
- 23.Vogiatzis I., Nanas S., Roussos C. Interval training as an alternative modality to continuous exercise in patients with COPD. Eur Respir J. 2002;20:12–19. doi: 10.1183/09031936.02.01152001. [DOI] [PubMed] [Google Scholar]
- 24.Vogiatzis I., Terzis G., Nanas S., Stratakos G., Simoes D.C., Georgiadou O. Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest. 2005;128:3838–3845. doi: 10.1378/chest.128.6.3838. [DOI] [PubMed] [Google Scholar]
- 25.Arnardóttir R.H., Boman G., Larsson K., Hedenström H., Emtner M. Interval training compared with continuous training in patients with COPD. Respir Med. 2007;101:1196–1204. doi: 10.1016/j.rmed.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Trost S.G., Owen N., Bauman A.E., Sallis J.F., Brown W. Correlates of adults' participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Bird S.R., Hawley J.A. Exercise and type 2 diabetes: new prescription for an old problem. Maturitas. 2012;72:311–316. doi: 10.1016/j.maturitas.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Karstoft K., Winding K., Knudsen S.H., Nielsen J.S., Thomsen C., Pedersen B.K. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2013;36:228–236. doi: 10.2337/dc12-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terada T., Friesen A., Chahal B.S., Bell G.J., McCargar L.J., Boulé N.G. Feasibility and preliminary efficacy of high intensity interval training in type 2 diabetes. Diabetes Res Clin Pract. 2013;99:120–129. doi: 10.1016/j.diabres.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Terada T., Wilson B., Myette-Cote E., Kuzik N., Bell G., McCargar L. Targeting specific interstitial glycemic parameters with high-intensity interval exercise and fasted-state exercise in type 2 diabetes. Metabolism. 2016;65:599–608. doi: 10.1016/j.metabol.2016.01.003. [DOI] [PubMed] [Google Scholar]


