Abstract
The pulp and pericarp of mangosteen (Garcinia mangostana) fruit are popular food, beverage and health products whereby 60% of the fruit consist of the pericarp. The major metabolite in the previously neglected or less economically significant part of the fruit, the pericarp, is the prenylated xanthone α-mangostin. This highly bioactive secondary metabolite is typically isolated using solvent extraction methods that involve large volumes of halogenated solvents either via direct or indirect extraction. In this study, we compared the quantities of α-mangostin extracted using three different extraction methods based on the environmentally friendly solvents methanol and ethyl acetate. The three solvent extractions methods used were direct extractions from methanol (DM) and ethyl acetate (DEA) as well as indirect extraction of ethyl acetate obtained via solvent partitioning from an initial methanol extract (IEA). Our results showed that direct extraction afforded similar and higher quantities of α-mangostin than indirect extraction (DM: 318 mg; DEA: 305 mg; IEA: 209 mg per 5 g total dried pericarp). Therefore, we suggest that the commonly used method of indirect solvent extraction using halogenated solvents for the isolation of α-mangostin is replaced by single solvent direct extraction using the environmentally friendly solvents methanol or ethyl acetate.
Introduction
Garcinia mangostana L. (Guttiferae/Clusiaceae) commonly known as mangosteen is a tropical economic plant native to Southeast Asia that is believed to have originated from Peninsular Malaysia. It is planted in Southeast Asia and other tropical regions namely India, Sri Lanka, Northern Australia and tropical America [1] and can be differentiated (according to their respective localities) by careful examination of the leaves via Fourier transform-infrared coupled with chemometric analysis [2]. Its economic value arises from its perception as a superfruit [3] that is dubbed the “queen of fruits” in Southeast Asia [4]. Its fruit predominantly comprises an inedible dark purple or red pericarp (> 60%) that encases an edible succulent pulp [5]. The pericarp has been used as a natural dye and has recently shown potential as a dye-based sensitiser in solar cells [6]. Mangosteen seeds are recalcitrant due to its low temperature intolerance and sensitivity toward desiccation [7]. The latter part of the fruit is popularly consumed directly/freshly or as processed food products such as syrup, canned drinks and cubes, marmalade as well as health-related dietary supplements [8]. More recently, the pericarp that in the past was typically discarded as waste has gained ever increasing attention for its purported health-related properties thereby creating a secondary economic demand for it. This trend has been bolstered by the long-standing use of the pericarp in traditional medicine and the growing scientific studies on its potent biological activities. In traditional medicine, the extract of mangosteen pericarp has been used to treat a variety of ailments such as fever, diarrhoea, dysentery, menstrual cramps, urinary tract infections and many other conditions [9, 10]. Recent studies on the pericarp extracts of mangosteen have shown inhibition and modulation towards, for example, advanced glycation, α-glucosidase activity, immune response and hyperglycaemia performed in cell-free and cell-based studies including whole animals and humans [11–13]. Therefore, the increasing economic value of this plant has resulted in its commercial cultivation especially in Southeast Asia for the regional and international markets [14, 15].The pericarp of G. mangostana is saliently characterised by the abundant presence of prenylated xanthones of which α-mangostin is the major constituent. α-Mangostin has been reported to be stable under normal and stress conditions [16, 17]. This metabolite is highly bioactive that has been attributed with multiple in vitro biological activities ranging from the inhibition of pathogen- to metabolic-related activities such as antimicrobial [18], cytotoxic [19], antitumour [20], anti-inflammatory [21], antimalarial [22], antiviral [23], antimycobacterial [24], antioxidant [25], antiglucosidase [12] and antileptospiral [26] activities. The in vivo antihyperglycaemic, anti-inflammatory, antioxidant and antitumour activities of α-mangostin applied as pure compound or as part of a food/health product prepared from the pericarp has been reported (including studies in humans) [12, 27–30].
The preparative-scale abundance of α-mangostin in the pericarp has facilitated the feasibility of these in vivo studies. Its high abundance and wide range of in vitro and in vivo biological activities show some parallels with curcumin, the diarylheptanoid that is highly abundant in turmeric [31,32]. The reported in vitro and in vivo bioactivities of α-mangostin necessitate the preparation of preparative quantities for further functional studies as well as commercial health-related applications. The known low bioavailability of α-mangostin (30) has been improved via incorporation into carriers such as cotton seed oil [33, 34] and poly(N-isopropylacrilamide)-co-2VP [35]. It has been shown in vivo that α-mangostin is conjugated during Phase II metabolism into mono- and diglucuronide forms [34] which would increase water solubility for excretion. The intraperitoneal injection of α-mangostin was found to be a better pharmacokinetic alternative to oral administration with a higher Cmax (maximum serum concentration after treatment) value of 7.5 μM in mice [36].
Preparation of derivatives of α-mangostin via chemical and enzymatic syntheses or biotransformations, will expand the repertoire of structures available for biological and functional studies. The chemical derivatisation of α-mangostin followed by the assessment of biological activities has been reported in several studies [37–39]. Chemoenzymatic syntheses of α-mangostin glycosides have performed using a glycosyl transferase from Bacillus lichemiformis [40] and amyloglucosidase from Aspergillus niger [41]. Biotransformations of α-mangostin have afforded several non-glycosylated and glycosylated derivatives [42, 43]. Several unique sulfur-based derivatives of α-mangostin have been obtained via biotransformations using the fungi (Colletotrichum gloeosporioides (EYL131) and Neosartorya spathulata (EYR042)) that have yet to be synthesised chemically. Many of these chemically prepared or biotranformed derivatives have shown stronger biological activities than α-mangostin such as towards Mycobacterium tuberculosis H37Ra [44]. Therefore, the importance of α-mangostin and its prepared derivatives justifies improving the isolation of α-mangostin from the pericarp of mangosteen.
Isolation of α-mangostin from the pericarp predominantly involves solvent extraction methods with long processing times and the use of large volumes of organic solvents despite the recent application of some alternative methods such as dynamic ultrasonic-assisted extraction [45], supercritical fluid extraction [46] and aqueous micellar biphasic system extraction [47]. The most commonly reported preparation of G. mangostana pericarp extract employs conventional solvent extraction by soaking or distillation (viz. Soxhlet-based). Solvent extraction of Garcinia mangostana for the isolation of α-mangostin is performed either directly (without solvent partitioning) or indirectly using solvent partitioning. The former approach involves direct contact of the plant material in a single solvent, e.g. chloroform [12] or methanol [48] or sequentially in multiple solvents [49] whereas the latter typically involves indirect extraction via successive solvent partitioning. Indirect extraction is initially carried out using a polar alcohol solvent usually methanol or ethanol in direct contact with the plant material following by sequential liquid-liquid extraction with less polar solvents. A common workflow for indirect extraction would be the sequential solvent partitioning of the initial methanol or ethanol extract beginning with the least polar solvent, for example ethanol → hexane → chloroform → n-butanol [50] or methanol → dichloromethane → ethyl acetate → n-butanol [25]. For simpler solvent partitioning, only a single solvent, e.g. ethyl acetate [51] or chloroform [52], is employed for liquid-liquid extraction with the initial alcohol fraction.
Although direct and indirect solvent extractions have been applied to the isolation of α-mangostin from the pericarp of G. mangostana, a comparison of α-mangostin yields between both approaches has yet to be reported. Additionally, in studies reporting both extraction approaches, many continue to use halogenated solvents such as chloroform or dichloromethane which restrict their use in food, herbal and pharmaceutical applications due to toxicity [53]. Therefore, there is a need to pursue the use of green solvents that are more environmentally friendly and less toxic such as ethanol, methanol or ethyl acetate [54, 55] that are commonly used for the isolation of α-mangostin.
Although the DM extract showed the lowest concentration of α-mangostin (53.8% w/w) compared to the DEA (70.7% w/w) and IEA (66.9% w/w) extracts, it afforded the highest amount of extract. This corresponded to both the DM and DEA extracts showing the higher quantities of α-mangostin per total amount of dried pericarp used in this study (DM: 305.4 mg; DEA: 280.1 mg; IEA: 244.2 mg). Therefore, single-solvent direct extraction using the methanol or ethyl acetate is a better option than indirect extraction based on solvent partitioning for the extraction of α-mangostin. Furthermore, methanol or ethyl acetate are green solvents that can replace the halogenated solvents commonly used in many studies reporting the extraction and isolation of α-mangostin.
Materials and methods
Chemicals and reagents
The solvents used were of HPLC or analytical grades, and were purchased from Merck. Silica gel 60 PF254 containing gypsum 107749 was used for radial chromatography with a thickness of 4 mm. α-Mangostin was isolated in a single step from the directly obtained ethyl acetate extract (500 mg) using radial chromatography with stepwise n-hexane/ethyl acetate elution (9:1 to 5:5). The purity of the targeted α-mangostin containing fractions was evaluated by analytical TLC (Rf = 0.8 hexane:ethyl acetate = 2:8) and high-performance liquid chromatography (HPLC) (described below). Identification of α-mangostin was carried out using direct-infusion electrospray ionisation mass spectrometry (DI-ESI-MS; Waters Single Quadrupole Detector), and both 1-D proton and carbon NMR spectroscopy (Bruker/AscendTM 700 MHz with cryoprobe).
Preparation and extraction of plant materials
Mature mangosteen (G. mangostana Linn.) fruits were collected from May to June at an orchard on private land (with consent of the owner; name included in the Acknowledgments section) in Felda Kampung Sertik (3.4826350, 102.0233610), Karak, Pahang, Malaysia. A herbarium voucher specimen UKMb40416 was prepared and deposited at the Herbarium of the Faculty of Science and Technology, Universiti Kebangsaan Malaysia. Dried G. mangostana pericarp (5 g) was weighed and then cut into small pieces. This material was soaked in 60 ml of either methanol or ethyl acetate (done in triplicate). The samples were then stirred at room temperature for 3 days and filtered using Whatman No. 1 filter paper. For the methanol extract, water (40 ml) was added to the filtrates followed by two steps of successive solvent partitioning with ethyl acetate. The combined ethyl acetate fractions were dried, weighed and assigned as the indirect ethyl acetate (IEA) extract (IEA). Mangosteen pericarp soaked directly and separately in ethyl acetate and methanol. The obtained extracts were dried, weighted and designated as the direct ethyl acetate (DEA) and methanol (DM) extracts. Finally, both the directly and indirectly obtained extracts (IEA, DEA, DM) were dissolved in acetonitrile (0.1 mg/ml) and filtered using 0.45 μm membrane filters for HPLC analysis.
Instrumentation and HPLC conditions
The HPLC system (Agilent, series 1100, USA) used consisted of a quaternary pump, autosampler, solvent degasser, and diode array detector (DAD). The quantification wavelength of α-mangostin and samples were set at 254 nm and 330 nm. The chromatographic separation was performed at ambient temperature using an Atlantis T3 C18 column (2.1 mm x 150 mm, 100Å, 3 μm) was attached to a C18 guard column. The HPLC analysis was performed using a previously reported method [54] that was slightly modified to the following conditions: 70% acetonitrile and 0.1% (v/v) acetic acid diluted in ultra-pure water was delivered at the flow rate of 0.2 mL/min and run for 30 minutes. The sample injection volume was adjusted to 0.5 μl (0.5 μg). All solutions of mobile phase were freshly prepared, vacuum filtered through a 0.45 μm membrane and degassed by sonication for 20 min prior to use.
Preparation of standard and calibration curve
A HPLC standard stock solution of α-mangostin (1 mg/mL) was prepared by dissolving the carefully weighed standard in an appropriate volume of acetonitrile and filtered using a 0.45 μm syringe filter and stored at 4°C until use. Serial dilution was performed thereafter to prepare (10, 50, 250,500 and 1000 μg/mL) for a calibration curve. Each standard solution was injected in triplicate (10 μL per injection) to enable further statistical analysis. The calibration curve was obtained by plotting the mean peak area versus the concentration of standard α-mangostin (μg/mL). Each calibration point was carried out in triplicate. Linearity (coefficient of determination, R2) of the calibration curve was determined by regression analysis. For intra-day intermediate precision, measurement repeatability was assessed by analysing the triplicate injections of the α-mangostin standard solution at five different concentrations (10, 50, 250, 500 and 1000 μg/mL) that were done within the same day. For inter-day intermediate precision, the same procedure was repeated on two different days. The precision was estimated from the relative standard deviation (RSD) and presented in term of % RSD of the peak area (n = 3) of the standard mixture. The limit of detection (LOD) and limit of quantification (LOQ) were calculated through the slope and standard deviation method [56] using the following formula:
LOD = (3.3 x δ)/S, and LOQ = (10 x δ)/S, where: δ is the standard deviation of the Y-intercept of the linear regression equations and S is the slope of the linear regression equations.
Results and discussion
Standard α-mangostin was isolated from the directly obtained EA extract as a yellow compound that showed a [M-H]- base peak at m/z = 409 in the DI-ESI-MS (negative mode). The signals shown in the 1D 1H-NMR and 13C-NMR spectra (S2–S4 Figs) were consistent with values reported for α-mangostin in the literature [57].
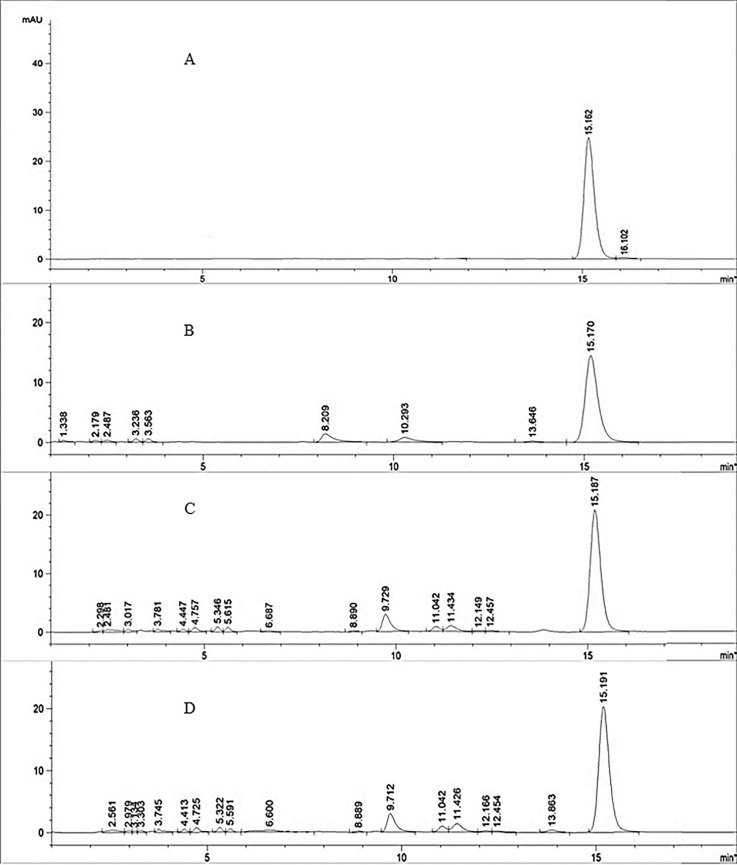
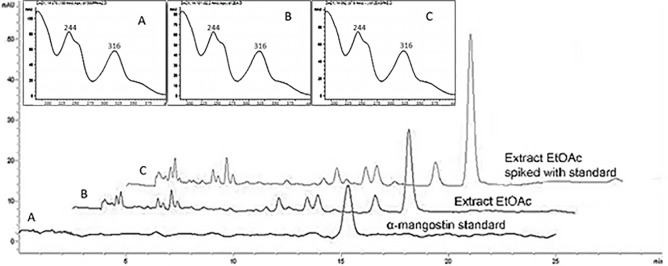
Based on HPLC profiles (Fig 1), the major peak in the chromatograms of all the extracts showed the same retention time as the reference α-mangostin standard that eluted at 15.1 minutes. The assignment of the major peak as α-mangostin was supported via spiking of the DEA extract with α-mangostin (0.5 mg/mL). The spiked chromatogram showed an increase in the intensity of the major peak. Additionally, the UV-Vis spectra of the major peak in the directly EA obtained extract before and after spiking showed the same profile as the α-mangostin standard (Fig 2).
Fig 1.
Chromatograms of the (A) α-mangostin standard, (B) MeOH direct extract (DM) (C) EA indirect extract (IEA)s (D) EA direct extract (DEA).
Fig 2.
Chromatogram and UV Spectra of (A) α-mangostin standard, (B) α-mangostin peak in EA direct extract (DEA), and (C) DEA extract spiked with α-mangostin (0.5 mg/ml).
The amount of α-mangostin from triplicate injections exhibited linearity over the evaluated range (S1 Data). The linear equations of the LOD and LOQ were y = 1205.5x -15.34 with LOD and LOQ of 61.9 ng and 187.7 ng, respectively. Linearity of the HPLC method was presented in terms of regression coefficient (R2) and was > 0.999 for the α-mangostin standard (S1 Fig). The % RSD of the peak area between intraday and interday precision was 0.36% which indicated good reproducibility. Likewise, the student’s t-test analysis showed no significant difference between intraday and interday precision, P > 0.01 (Table 1).
Table 1. Precision analysis of the HPLC method.
| α-mangostin | Wavelength (nm) | P value (One-way ANOVA) | |
|---|---|---|---|
| 254 | 330 | ||
| aIntraday data | 0.87 ± 0.74 | 0.29 ± 0.27 | 0.45 |
| aInterday data | 0.51 ± 0.13 | 0.55 ± 0.49 | 1.00 |
| bP value (Student’s t-test) | 0.26 | 0.18 | 0.17 |
aThe relative standard deviation of the peak area was calculated in the intraday and interday data, and the results are presented as average ± SD (n = 3).
bThe P value was calculated by two tests: The One-way ANOVA to study the effect of the wavelength on the precision, and the Student’s t-test to study the difference between the intraday and interday data. Differences are considered significant at P < 0.01.
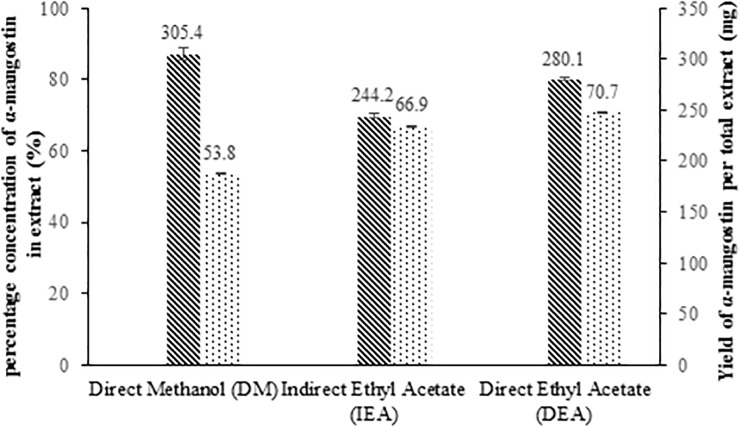
Analysis of the number of peaks in the chromatograms of the different extracts (Fig 1) showed that more metabolites were present in both the DEA and IEA extracts (directly and indirectly) compared to the DM extract. However, the amount of DM extract obtained from the total dried pericarp used (5 g) was much higher (568.2 mg) than the DEA (396.0 mg) and IEA (365.0 mg) extracts (Table 2). This may be due to the higher abundance of non-xanthone compounds such as oligomeric polyphenolics in the DM extract that are non-/weakly UV active [58] compared to the DEA and IEA extracts. The percentage (% w/w) of α-mangostin content determined from the standard curve in the extracts (e) and dried pericarp (dp), were: DEA (70.7% e; 6.1% dp), IEA (66.9% e; 4.9% dp) and DM (53.8% e; 5.6% dp). Despite the differences in masses obtained for all the extracts, the amount of α-mangostin (mg) per total dried pericarp used (5 g) was similar in the DM (305.4 ± 5.8 mg) and DEA (280.1 ± 1.3 mg) extracts but was much lower in the IEA extract (244.2 ± 2.0 mg) (Fig 3) (S2 Data).
Table 2. Quantitative analysis of α-mangostin content using different extraction methods.
| Extracts | |||
|---|---|---|---|
| DM | IEA | DEA | |
| Total dried pericarp used (g) | 5.0 | 5.0 | 5.0 |
| Quantity of extract weighed (mg) | 568.2 ± 83.7 | 365.0 ± 88.2 | 396.0 ± 48.9 |
| Concentration of α-mangostin in extract (w/w) | 56.2 ± 8.9 | 49.1 ± 13.6 | 60.6 ± 7.8 |
| Yield of α-mangostin in total extract (mg) | 305.4 ± 5.8 | 244.2 ± 2.0 | 280.1 ± 1.3 |
DM = Direct Methanol extract, IEA = Indirect Ethyl Acetate extract, DEA = Direct Ethyl Acetate extract
Fig 3. Comparison between concentration of α-mangostin in extract (%) and the yield of α-mangostin in total DM, IEA and DEA extracts (mg) in 5 g of dried pericarp.
In the literature, the extraction and isolation of α-mangostin from G. mangostana was mainly carried out using indirect extraction via solvent partitioning commonly involving chloroform or dichloromethane as exemplified by some recent reports [11, 52]. The use of direct extraction was less common particularly so with multiple solvents which is far more time-consuming. Although extraction based on halogenated solvents afforded good yields of α-mangostin [53, 59], our results indicate that comparable yields can be achieved with direct extraction using a single solvent with the more environmentally friendly and less toxic solvents methanol or ethyl acetate. Extraction using these green solvents would be more compliant with the needs of the pharmaceutical and food industries.
Although the DM extract showed the lowest concentration of α-mangostin (53.8% w/w) compared to the DEA (70.7% w/w) and IEA (66.9% w/w) extracts, it afforded the highest amount of extract. This corresponded to both the DM and DEA extracts showing the higher quantities of α-mangostin per total amount of dried pericarp used in this study (DM: 305.4 mg; DEA: 280.1 mg; IEA: 244.2 mg). Therefore, single-solvent direct extraction using the methanol or ethyl acetate is a better option than indirect extraction based on solvent partitioning for the extraction of α-mangostin. Furthermore, methanol or ethyl acetate are green solvents that can replace the halogenated solvents commonly used in many studies reporting the extraction and isolation of α-mangostin.
Supporting information
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors wish to thank Abdul Rashid Haji Taib for access to his orchard for sample collection as well Chye Gin Chung, Nike Dewi Utami, Amirul Husna Sudin, Nur-Adibah Mohd Suhaimi and Norsuhadah Sujangin for their assistance with some of the laboratory work. The authors also extend their appreciation to the Centre for Advanced Materials and Renewable Resources and the Institute of Systems Biology, Universiti Kebangsaan Malaysia, and the Malaysia Genome Institute, Ministry of Science, Technology and Innovation Malaysia (MOSTI) for technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Ministry of Higher Education under Grant FRGS/1/2014/ST01/UKM/01/1. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.Sosef M, Hong L, Prawirohatmodjo S. Plant resources of South-East Asia. No. 5 (3): Timber trees: lesser-known timbers. 1998.
- 2.Samsir SAj, Bunawan H, Yen CC, Noor NM (2016) Dataset of Fourier transform-infrared coupled with chemometric analysis used to distinguish accessions of Garcinia mangostana L. in Peninsular Malaysia. Data in Brief 8:1–5. 10.1016/j.dib.2016.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross PM. Superfruits:Top 20 fruits packed with nutrients and phytochemicals, best ways to eat fruits for maximum nutrition, and 75 simple and delicious recipes for overall wellness. New York: McGraw-Hill; 2009. [Google Scholar]
- 4.Fairchild D. The mangosteen: “Queen of Fruits” now almost confined to Malayan archipelago, but can be acclimated in many parts of tropics—Experiments in America—Desirability of Widespread Cultivation. Journal of Heredity. 1915;6(8): 339–347. [Google Scholar]
- 5.Chen Y, Huang B, Huang M, Cai B. On the preparation and characterization of activated carbon from mangosteen shell. Journal of the Taiwan Institute of Chemical Engineers. 2011;42(5): 837–842. [Google Scholar]
- 6.Ismail M, Ludin NA, Hamid NH, Ibrahim MA, Zulfakar MS, Mohamed NM, et al. , editors. Characterizations of natural dye from Garcinia mangostana with graphene oxide (GO) as sensitizer in dye-sensitizer solar cells AIP Conference Proceedings; 2017: AIP Publishing; 10.1063/1.4976594 [DOI] [Google Scholar]
- 7.Normah M, Ramiya SD, Gintangga M. Desiccation sensitivity of recalcitrant seeds—A study on tropical fruit species. Seed Science Research. 1997;7(2): 179–84. [Google Scholar]
- 8.Ho C-T, Shahidi F, Shi J. Asian functional foods: CRC Press; 2005. [Google Scholar]
- 9.Burkill IH. A dictionary of the economic products of the Malay Peninsula. 2015. [Google Scholar]
- 10.Perry LM, Metzger J. Medicinal plants of east and southeast Asia: Attributed properties and uses: MIT press; 1980. [Google Scholar]
- 11.Abdallah HM, El-Bassossy HM, Mohamed GA, El-Halawany AM, Alshali KZ, Banjar ZM. Mangostanaxanthones III and IV: Advanced glycation end-product inhibitors from the pericarp of Garcinia mangostana. Journal of Natural Medicines. 2017;71(1):216–226. 10.1007/s11418-016-1051-8 [DOI] [PubMed] [Google Scholar]
- 12.Ryu HW, Cho JK, Curtis-Long MJ, Yuk HJ, Kim YS, Jung S, et al. α-Glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from Garcinia mangostana. Phytochemistry. 2011;72(17): 2148–2154. 10.1016/j.phytochem.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 13.Jantan I, Harun NH, Septama AW, Murad S, Mesaik M. Inhibition of chemiluminescence and chemotactic activity of phagocytes in vitro by the extracts of selected medicinal plants. Journal of Natural Medicines. 2011;65(2): 400–405. 10.1007/s11418-010-0492-8 [DOI] [PubMed] [Google Scholar]
- 14.Ashraf MA, Othman R, Ishak CF. Soils of Malaysia: CRC Press; 2017. [Google Scholar]
- 15.Win HE. Analysis of Tropical Fruits in Thailand 2017 [cited 2018]. Available from: http://ap.fftc.agnet.org/ap_db.php?id=818.
- 16.Yodhnu S, Sirikatitham A, Wattanapiromsakul C. Validation of LC for the determination of α-mangostin in mangosteen peel extract: a tool for quality assessment of Garcinia mangostana L. Journal of Chromatographic Science. 2009;47(3): 185–189. [DOI] [PubMed] [Google Scholar]
- 17.Pothitirat W, Pithayanukul P, Chomnawang MT, Supabphol R, Gritsanapan W. Biological and chemical stability of mangosteen fruit rind extract. Functional Plant Science and Biotechnology. 2009;3(1): 16–18. [Google Scholar]
- 18.Sakagami Y, Iinuma M, Piyasena K, Dharmaratne H. Antibacterial activity of α-mangostin against vancomycin resistant Enterococci (VRE) and synergism with antibiotics. Phytomedicine. 2005;12(3): 203–208. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto K, Akao Y, Yi H, Ohguchi K, Ito T, Tanaka T, et al. Preferential target is mitochondria in α-mangostin-induced apoptosis in human leukemia HL60 cells. Bioorganic & medicinal chemistry. 2004;12(22):5799–5806. [DOI] [PubMed] [Google Scholar]
- 20.Li G, Thomas SP, Johnson J. Polyphenols from the mangosteen (Garcinia mangostana) fruit for breast and prostate cancer. Frontiers in pharmacology. 2013;4:80 10.3389/fphar.2013.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L-G, Yang L-L, Wang C-C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food and Chemical Toxicology. 2008;46(2): 688–693. 10.1016/j.fct.2007.09.096 [DOI] [PubMed] [Google Scholar]
- 22.Chaijaroenkul W, Na-Bangchang K. The in vitro antimalarial interaction of 9-hydroxycalabaxanthone and α-mangostin with mefloquine/artesunate. Acta Parasitologica. 2014;60(1): 105–111. 10.1515/ap-2015-0013 [DOI] [PubMed] [Google Scholar]
- 23.Tarasuk M, Songprakhon P, Chimma P, Sratongno P, Na-Bangchang K, Yenchitsomanus P-t. Alpha-mangostin inhibits both dengue virus production and cytokine/chemokine expression. Virus Research. 2017;240:180–189. 10.1016/j.virusres.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 24.Suksamrarn S, Suwannapoch N, Phakhodee W, Thanuhiranlert J, Ratananukul P, Chimnoi N, et al. Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chemical and Pharmaceutical Bulletin. 2003;51(7): 857–859. [DOI] [PubMed] [Google Scholar]
- 25.Jung H-A, Su B-N, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). Journal of Agricultural and Food Chemistry. 2006;54(6): 2077–2082. 10.1021/jf052649z [DOI] [PubMed] [Google Scholar]
- 26.Kondo M, Zhang L, Ji H, Kou Y, Ou B. Bioavailability and antioxidant effects of a xanthone-rich mangosteen (Garcinia mangostana) product in humans. Journal of Agricultural and Food Chemistry. 2009;57(19): 8788–8792. 10.1021/jf901012f [DOI] [PubMed] [Google Scholar]
- 27.Seesom W, Jaratrungtawee A, Suksamrarn S, Mekseepralard C, Ratananukul P, Sukhumsirichart W. Antileptospiral activity of xanthones from Garcinia mangostana and synergy of gamma-mangostin with penicillin G. BMC Complementary and Alternative Medicine. 2013;13(1): 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutierrez-Orozco F, Failla ML. Biological activities and bioavailability of mangosteen xanthones: a critical review of the current evidence. Nutrients. 2013;5(8): 3163–3183. 10.3390/nu5083163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JJ, Petiwala SM, Syed DN, Rasmussen JT, Adhami VM, Siddiqui IA, et al. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis. 2011;33(2): 413–419. 10.1093/carcin/bgr291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li G, Petiwala SM, Pierce DR, Nonn L, Johnson JJ. Selective modulation of endoplasmic reticulum stress markers in prostate cancer cells by a standardized mangosteen fruit extract. PloS one. 2013;8(12): e81572 10.1371/journal.pone.0081572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberti A, Riethmuller E, Beni S. Characterization of diarylheptanoids: An emerging class of bioactive natural products. Journal of Pharmaceutical and Biomedical Analysis. 2018. January 5;147: 13–34. 10.1016/j.jpba.2017.08.051 . Epub 2017/09/30. eng. [DOI] [PubMed] [Google Scholar]
- 32.Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. British Journal of Pharmacology. 2017. June;174(11): 1325–1348. 10.1111/bph.13621 . Pubmed Central PMCID: PMC5429333. Epub 2016/10/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Brunner I, Han A-R, Hamburger M, Kinghorn AD, Frye R, et al. Pharmacokinetics of α-mangostin in rats after intravenous and oral application. Molecular Nutrition & Food Research. 2011;55(S1): S67–S74. [DOI] [PubMed] [Google Scholar]
- 34.Ramaiya A, Li G, M Petiwala S, J Johnson J. Single dose oral pharmacokinetic profile of α-mangostin in mice. Current Drug Targets. 2012;13(14): 1698–1704. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad M, Yamin BM, Lazim AM. A study on dispersion and characterisation of α-mangostin loaded pH sensitive microgel systems. Chemistry Central Journal. 2013;7(1): 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li G, Petiwala SM, Nonn L, Johnson JJ. Inhibition of CHOP accentuates the apoptotic effect of α-mangostin from the mangosteen fruit (Garcinia mangostana) in 22Rv1 prostate cancer cells. Biochemical and Biophysical Research Communications. 2014;453(1): 75–80. 10.1016/j.bbrc.2014.09.054 [DOI] [PubMed] [Google Scholar]
- 37.Fei X, Jo M, Lee B, Han S-B, Lee K, Jung J-K, et al. Synthesis of xanthone derivatives based on α-mangostin and their biological evaluation for anti-cancer agents. Bioorganic & Medicinal Chemistry Letters. 2014;24(9): 2062–2065. [DOI] [PubMed] [Google Scholar]
- 38.Lin S, Sin WLW, Koh J-J, Lim F, Wang L, Cao D, et al. Semisynthesis and biological evaluation of xanthone amphiphilics as selective, highly potent antifungal agents to combat fungal resistance. Journal of Medicinal Chemistry. 2017;60(24): 10135–10150. 10.1021/acs.jmedchem.7b01348 [DOI] [PubMed] [Google Scholar]
- 39.Narasimhan S, Maheshwaran S, Abu-Yousef IA, Majdalawieh AF, Rethavathi J, Das PE, et al. Anti-bacterial and anti-fungal activity of xanthones obtained via semi-synthetic modification of α-mangostin from Garcinia mangostana. Molecules. 2017;22(2): 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le TT, Pandey RP, Gurung RB, Dhakal D, Sohng JK. Efficient enzymatic systems for synthesis of novel α-mangostin glycosides exhibiting antibacterial activity against Gram-positive bacteria. Applied Microbiology and Biotechnology. 2014;98(20): 8527–8538. 10.1007/s00253-014-5947-5 [DOI] [PubMed] [Google Scholar]
- 41.Zarena A, Sankar KU. Synthesis of α− mangostin-D-glucoside in supercritical carbon dioxide media. Journal of Food Science and Technology. 2015;52(10): 6547–6555. 10.1007/s13197-014-1705-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arunrattiyakorn P, Suwannasai N, Aree T, Kanokmedhakul S, Ito H, Kanzaki H. Biotransformation of α-mangostin by Colletotrichum sp. MT02 and Phomopsis euphorbiae K12. Journal of Molecular Catalysis B: Enzymatic. 2014;102: 174–179. [Google Scholar]
- 43.He L, Zhu C, Yuan Y, Xu Z, Qiu S-x. Specific glycosylated metabolites of α-mangostin by Cunninghamella blakesleana. Phytochemistry Letters. 2014;9: 175–178. [Google Scholar]
- 44.Arunrattiyakorn P, Suksamrarn S, Suwannasai N, Kanzaki H. Microbial metabolism of α-mangostin isolated from Garcinia mangostana L. Phytochemistry. 2011;72(8): 730–734. 10.1016/j.phytochem.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Liu C, Qi Y, Li Y, Li S. Dynamic ultrasonic-assisted extraction coupled with paralleled counter-current chromatography for continuous extraction and online isolation of xanthenones from Garcinia mangostana. Separation and Purification Technology. 2015;144: 215–222. [Google Scholar]
- 46.Chhouk K, Quitain AT, Pag-asa DG, Maridable JB, Sasaki M, Shimoyama Y, et al. Supercritical carbon dioxide-mediated hydrothermal extraction of bioactive compounds from Garcinia mangostana pericarp. The Journal of Supercritical Fluids. 2016;110: 167–175. [Google Scholar]
- 47.Tan GYT, Zimmermann W, Lee K-H, Lan JC-W, Yim HS, Ng HS. Recovery of mangostins from Garcinia mangostana peels with an aqueous micellar biphasic system. Food and Bioproducts Processing. 2017;102: 233–240. [Google Scholar]
- 48.Mohamed GA, Ibrahim SR, Shaaban MI, Ross SA. Mangostanaxanthones I and II, new xanthones from the pericarp of Garcinia mangostana. Fitoterapia. 2014;98: 215–221. 10.1016/j.fitote.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 49.Ee G, Daud S, Taufiq-Yap Y, Ismail N, Rahmani M. Xanthones from Garcinia mangostana (Guttiferae). Natural Product Research. 2006;20(12): 1067–1073. 10.1080/14786410500463114 [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, Liu J-P, Lu D, Li P-Y, Zhang L-X. A new antioxidant xanthone from the pericarp of Garcinia mangostana Linn. Natural Product Research. 2010;24(17): 1664–1670. 10.1080/14786419.2010.499539 [DOI] [PubMed] [Google Scholar]
- 51.Zhou X, He L, Wu X, Zhong Y, Zhang J, Wang Y, et al. Two new xanthones from the pericarp of Garcinia mangostana. Natural Product Research. 2015;29(1): 19–23. 10.1080/14786419.2014.927873 [DOI] [PubMed] [Google Scholar]
- 52.Mohamed GA, Al-Abd AM, El-Halawany AM, Abdallah HM, Ibrahim SR. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. Journal of Ethnopharmacology. 2017;198: 302–312. 10.1016/j.jep.2017.01.030 [DOI] [PubMed] [Google Scholar]
- 53.Pothitirat W, Chomnawang MT, Gritsanapan W. Anti-acne-inducing bacterial activity of mangosteen fruit rind extracts. Medical Principles and Practice. 2010;19(4): 281–286. 10.1159/000312714 [DOI] [PubMed] [Google Scholar]
- 54.Abdalrahim F, Khalid M, Mohammad J, Zhari I, Amin M. Quantification of ά-, β-and γ-mangostin in Garcinia mangostana fruit rind extracts by a reverse phase high performance liquid chromatography. Journal of Medicinal Plants Research. 2012;6: 4526–4534. [Google Scholar]
- 55.Capello C, Fischer U, Hungerbühler K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chemistry. 2007;9(9): 927–934. [Google Scholar]
- 56.Guideline IHT, editor Validation of analytical procedures: text and methodology Q2 (R1). International Conference on Harmonization, Geneva, Switzerland; 2005.
- 57.Mahabusarakam W, Wiriyachitra P, Phongpaichit S. Antimicrobial activities of chemical constituents from Garcinia mangostana Linn. Journal of the Science Society of Thailand. 1986;12(4): 239–242. [Google Scholar]
- 58.Eng Kiat Loo A, Huang D. Assay-guided fractionation study of α-amylase inhibitors from Garcinia mangostana pericarp. Journal of Agricultural and Food Chemistry. 2007;55(24): 9805–9810. 10.1021/jf071500f [DOI] [PubMed] [Google Scholar]
- 59.Bundeesomchok K, Filly A, Rakotomanomana N, Panichayupakaranant P, Chemat F. Extraction of α-mangostin from Garcinia mangostana L. using alternative solvents: Computational predictive and experimental studies. LWT-Food Science and Technology. 2016;65: 297–303. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.