Abstract
The mechanisms by which exosomes (nano-vesicular messengers of cells) are taken up by recipient cells are poorly understood. We hypothesized that histones associated with these nano-particles are the ligands which facilitate their interaction with cell surface syndecan-4 (SDC4) to mediate their uptake. We show that the incubation with fetuin-A (exosome-associated proteins) and histones mediates the uptake of exosomes that are normally not endocytosed. Similarly, hydroxyapatite nanoparticles incubated with fetuin-A and histones (FNH) are internalized by tumor cells, while nanoparticles incubated with fetuin-A alone (FN) are not. The uptake of exosomes and FNH, both of which move to the perinuclear region of the cell, is attenuated in SDC4-knockdown cells. Data show that FNH can compete with exosomes for uptake and that both use SDC4 as uptake receptors.
Keywords: Exosomes, Histones, Hydroxyapatite, nanoparticles, syndecan-4
Introduction
Exosomes are classified as members of extracellular vesicles with diameters in the range of 30–100 nm, which originate from luminal membranes of multi-vesicular bodies-MVB, secreted from a number of normal and transformed or tumorigenic cells upon fusion with cell membranes [1]. Whereas the current purification strategies may not strictly distinguish exosomes from micro-vesicles (100–1000 nm in diameter) [2], some of the exosomal associated proteins such as CD63, CD9 [3] and histones [4], are associated mainly with exosomes and not micro-vesicles and could be used as exosomal markers. It is becoming clear that the secretion and endocytic uptake of cellular exosomes represent significant cellular mechanisms for growth, motility and invasive capacity of tumor cells both in vitro and in vivo [5–7]. More broadly, exosomes shuttle biomolecules such as, miRNA and tRNA among cells in an autocrine, paracrine or endocrine fashion to affect the normal and pathophysiology of the recipient cells [8–10]. Cellular mechanisms that regulate secretion and endocytic uptake of exosomes are poorly understood. Recent studies indicate that exosomal secretion is promoted by invadopodia thereby influencing invasive and motile properties of tumor cells [11]. Secretion of these nano-vesicles is usually preceded by a rise in [Ca2+] and involves docking factors such as Rab11 and Rab-27A [11, 12].
The uptake mechanisms of secreted exosomes by recipient cells are just as important and warrant intense investigation. A number of studies have proposed, based on strong experimental evidence, that uptake is primarily mediated by heparan sulfate proteoglycans [13, 14] even though the ligand(s) on exosomes that interact with the heparan sulfate proteoglycans to mediate uptake is yet to be defined. A recent report suggested that fibronectin on exosomes is the ligand that interacts with cell surface heparan sulfate proteoglycans to mediate uptake of the exosomes [15]. We previously proposed that histones/fetuin-A were the exosomal ligands that interact with cell surface heparan sulfate proteoglycans. These two proteins were abundantly associated with a class of exosomes that were easily taken up by tumor cells [14]. This observation, however, did not rule out the other exosome-associated proteins such as CD63 and other tetraspanins [16]. To directly show that histones and or fetuin-A (exosome-associated proteins) are the ligands for the exosomal uptake, we questioned whether they would be sufficient to promote the uptake of nano-particles devoid of any other protein except the duo. We chose hydroxyapatite nanoparticles that have similar dimensions as cellular exosomes (~ < 200 nm) for this proof of concept experiment. Hydroxyapatite has a high affinity for fetuin-A [17], which in turn associates tightly with histones [14]. Thus, the disposition of fetuin-A/histones on the nanoparticles would be more or less similar to their disposition on cellular exosomes [14].
The interaction of histones with heparan sulfate proteoglycan-SDC4 is not without merit. It has been demonstrated that basic proteins such as histones bind to heparan sulfate proteoglycans by electrostatic interaction [18, 19]. The expression of the cell surface heparan sulfate proteoglycans syndecan-1 (SDC1) and syndecan 4 (SDC4) is increased or upregulated in more aggressive and metastatic tumor cells [20–22] and the knockdown of these receptors attenuate metastatic potential of tumor cells [22]. A number of studies have shown that there is significant cross-talk via exosomes from tumor cells to stromal cells and vice-versa to mediate processes such as cellular motility and invasion that are relevant in metastasis [23–26].
The data presented herein demonstrate that histones on exosomes and hydroxyapatite nanoparticles are the ligands that mediate the endocytic uptake of these nanoparticles via cell surface SDC4 uptake receptors.
Materials and methods:
Materials:
Fetuin-A, histone type II and hydroxyapatite nanoparticles (Cat. # 677418–5G) were purchased from Sigma-Aldrich (St. Louis, MO). Fetuin-A was further purified as described [27]. All the other reagents unless otherwise specified were purchased from Sigma-Aldrich.
Cells:
BT-549, MDA-MB-231 and LN229 were purchased from ATCC (Manassas, VA). The prostate cancer cell lines PC3 and DU145 were a kind gift from Dr. Zhenbang Chen (MMC). BT-549 was stably transfected with the GFP-CD63 expression plasmid as described [28] to yield the cell line BT-CD63, the source of GFP-CD63 exosomes. The cells were maintained in either DMEM/F12 or Iscove’s modified Dulbeco Medium (IMDM) containing 10% fetal bovine serum.
Uptake of GFP-CD63 exosomes by breast tumor cells
BT-CD63 cells were grown in 150 cm2 culture flasks until ~80% confluent. The cells were then washed in PBS and detached using 2 mM EDTA and washed X2 in serum free medium (SFM). The cells (~1 × 109 cells) were suspended in 1 ml of SFM containing either BSA (2 mg/ml) or purified fetuin-A (2 mg/ml) in siliconized Eppendorf tubes. The tubes were incubated at 38°C with end on end rotation for 1 h. Cells were then pelleted at 700 x g for 5 min and the supernatant centrifuged at 3,000 x g for 15 min to pellet dead cells and debris. Exosomes were then isolated by differential centrifugation as previously described [28]. To determine uptake, exosomes isolated in the presence of BSA (BSA-GFP-CD63 Exos) and in the presence of fetuin-A (FetA-GFP-CD63 Exos) after purification were added to BT-549 cells (1 × 105 cells/chamber) in 8-chambered glass slides (20 μg/chamber) in SFM at 37°C in CO2- humidified incubator and incubated for 2 h. Purified exosomes isolated in the presence of BSA were incubated with fetuin-A (2 mg/ml): histones (100 μg/ml) for 2 h followed by differential centrifugation as described [28], and added (20 μg/chamber) to BT-549 in 8-chambered slides (1 × 105 cells/chamber) and incubated for 2 h at 37°C in humidified CO2 incubator. The cells were washed with warm SFM and fixed with 4% formalin for 15 min and uptake examined under a confocal microscope (Nikon, A1R). Mean arbitrary units of fluorescence ± SD were quantified using Nikon Elements Advanced Research Software (NEARS).
Hydroxyapatite-nanoparticles uptake assay
Whereas our earlier studies had suggested that histones were the ligands on exosomes that mediated their uptake via heparan sulfate proteoglycans [14], exosomes have other proteins on their surfaces that have the potential to interact with heparan sulfate proteoglycans [15]. We therefore questioned whether immobilization of histones on nanoparticles similar in size to exosomes (< 200 nm) was sufficient to promote their uptake by tumor cells. We settled on hydroxyapatite-nanoparticles (< 200 nm). Hydroxyapatite nanoparticles have a strong affinity for fetuin-A [17] which in turn associates tightly with histones [14]. We suspended 20 mg of hydroxyapatite-nanoparticles and 20 mg of purified fetuin-A without (FN) or with 1 mg of histone type II (FNH) in 10 ml of Hanks Buffered saline and sonicated the mixtures in 15 ml centrifuge tubes. The mixtures were incubated overnight at 4°C with end on end rotation. The nanoparticles were then centrifuged at 700 x g to pellet large aggregates. Typically, the supernatant (1 ml) which consisted of the nanoparticles was further centrifuged at 5,000 x g for 5 min and the pellet re-suspended in 1 ml of complete medium and 100 μl added to the wells of 96-well microtiter plates to form a lawn of either FN or FNH nanoparticles. The cells to be assayed were then added on these particles at 1000 cells/well. After an incubation period ranging from 24 h to 48 h, the cells were photographed under an inverted microscope and the percentage of area cleared of nanoparticles determined using NEARS. In some cases, purified fetuin-A was labeled with rhodamine isothiocyanate as described [29] prior to incubating with either hydroxyapatite-nanoparticles (FN) or both nanoparticles and histones (FNH).
Knockdown of SDC4 and Rab-27A in the glioblastoma cell line LN229.
The cells were cultured in 6-well plates until a density of ~2 × 105 cells per well in 2 ml of DMEM/F12, then transfected with scrambled control or human Rab-27A or SDC4 shRNA in Lenti-GFP using DNAfectin™ Plus, as per the manufacture’s protocol (Applied Biological Materials Inc., Richmond, BC., Canada). The cells were selected in complete medium containing 2.5 μg/mL of puromycin for 4 weeks. Puromycin resistant and GFP positive cells were further isolated using fluorescence activated sorting (FACS) and thereafter maintained in selection medium. Knockdown was validated by western blot assays.
Uptake of rhodamine labeled exosomes and FNH nanoparticles by LN229 sh-scrambled controls, SDC4 and Rab-27A knockdown sub-clones.
Exosomes were isolated and purified from BT-549 cells in the presence of fetuin-A as described [28]. The exosomes were labeled with rhodamine isothiocyanate as described [29]. The labeled exosomes (in the void volume) were separated from unreacted rhodamine isothiocyanate using 5 ml HiTrap™ desalting columns (GE Healthcare Bio-Sciences, Uppsala, Sweden). The exosomes were further centrifuged at 200,000 x g for 2 h and the pellet dissolved in HBSS to give a final concentration of ~100 μg/ml. SCR-controls, SDC4-KD or Rab-27A-KD LN229 cells were seeded in 8-chambered glass slides (1 × 105 cells/chamber) and after an overnight incubation at 37°C, labeled exosomes (2 μg/chamber) were added to the cells and incubated for 2 h. The cells were washed with warm SFM and fixed in 4% formalin in PBS. The chambers were removed and a drop of anti-fade with Dapi added to the slides, cover-slipped and examined under a confocal microscope (Nikon, A1R). FNH nanoparticles labeled with rhodamine as described above were also added (20 μg/chamber, to SCR-control and SDC4-KD cells (1 × 105 cells/chamber)) in 8-chambered slides, incubated for 2 h and processed as described for exosomes.
Heparin and uptake of FNH nanoparticles
We and others previously demonstrated that heparin (0–40 μg/ml) attenuated the uptake of labeled exosomes [14]. To demonstrate that FNH nanoparticles were also taken up via similar mechanism, FNH uptake assay was repeated in the absence or presence of heparin (0–40 μg/ml).
To determine whether FNH nanoparticles attenuate the exosomal mediated motility and invasion in tumor cells
Motility and invasion are some of the key physiological mechanisms that are orchestrated by exosomes [30, 31]. Boyden chamber motility and invasion assays were done as previously described [32]. Briefly PC3 or DU145 prostate cancer cells (25,000 cells/chamber) were added to the upper chambers in SFM without (controls), and with FN nanoparticles (100 μg/chamber) or FNH (100 μg/chamber). The lower chambers contained 500 μl of complete medium containing 10% fetal bovine serum. After an overnight incubation (10 h) the cells migrated to the underside of the polycarbonate filters were fixed, stained with crystal violet and quantified as described [32].
Uptake of GFP-CD63 labeled exosomes in the presence of rhodamine labeled FN or FNH nanoparticles.
In order to directly show that FNH nanoparticles can compromise the uptake of exosomes, MDA-MB-231 breast carcinoma cells were plated in triplicates in 8-chambered glass slides (3 slides) as described above. Briefly the cells (2 × 105 cells/chamber) were allowed to attach overnight in complete medium. Medium was then replaced with warm SFM. To each of the chambers with cells, GFP-CD63 exosomes purified as described above and suspended in SFM were added to a final concentration of 20 ng/μl. To the upper four and lower four chambers of each slide, increasing concentrations of rhodamine labeled FN or FNH nanoparticles (2.5–20 ng/μl) in SFM were added as shown. The volume in each chamber was maintained at 200 μl. The proteins bound to the nano-particles (prepared as described above) were quantified by Bradford and used to estimate the amounts of particles added to each chamber. After 1 h of incubation at 37°C, uptake of exosomes (green channel) and nanoparticles (red channel) by the cells was monitored by confocal microscopy (Nikon A1R) and quantified using NEARS software as described above.
Results:
Uptake of GFP-CD63 labeled exosomes by BT-549 cells
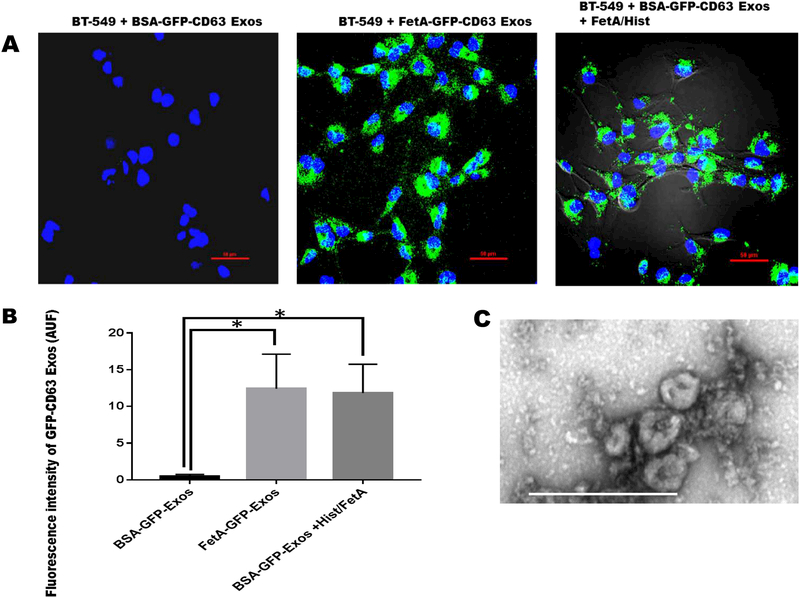
We previously demonstrated that only exosomes that were released by cells in the presence of fetuin-A had the capacity to mediate cellular adhesion in recipient cells while those released in the presence of BSA (control) lacked this capacity [33]. Further studies using Fluorescence-Activated Vesicle Sorting (FAVS) revealed that exosomes released or secreted in the presence of fetuin-A were decorated with histones and fetuin-A on their surfaces while those secreted in the absence had none [14]. The present study was therefore conducted to provide direct evidence that the presence of histones/fetuin-A on exosomes is sufficient to drive their endocytic uptake. In three separate experiments represented by Figure 1, we demonstrated that exosomes released from BT-CD63 cells in the presence of BSA (in SFM) named BSA-GFP-CD63 Exos, could not be internalized by naïve BT-549 cells (Fig. 1A and 1B). The same concentration of exosomes secreted in the presence of fetuin-A (FetA-GFP-CD63Exos) were easily taken up by BT-549 cells after 2 h of incubation (Fig. 1A and 1B). Interestingly, when the BSA-GFP-CD63 Exos were incubated with Fetuin-A: histone H2A at a molar ratio of 5:1 for 2 h and then re-purified, the resulting exosomes now loaded with fetuin-A and histones were endocytosed by BT-549 cells (Fig. 1A and 1B). Transmission electron micrograph (TEM) of exosomes released from BT-CD63 is represented by Fig. 1C.
Figure 1. Uptake of GFP-CD63 labeled exosomes by BT-549 breast carcinoma cells.
BT-539 cells were seeded in 8-chambered glass slides (1 × 105 cells/chamber), and incubated for 24 h in complete medium and which the medium was replaced with SFM. Purified exosomes (100 μg/ml) secreted from BT-CD63 cells in the presence of BSA (negative control) or fetuin-A (positive control) in SFM were incubated with the BT-549 cells (2 μg/chamber) to monitor uptake. Exosomes secreted from BT-CD63 in the presence of BSA were also incubated with fetuin-A/histone H2A and re-purified (100 μg/ml) and finally incubated with BT-549 cells (2 μg/chamber) for uptake studies (Fig. 1A; scale bar = 50 μm). Mean arbitrary units of fluorescence ± SD were quantified by NEARS for each of the three measurements (Fig. 1B *P < 0.05; N = 6). Transmission electron micrograph of the purified exosomes obtained as described (14) is represented by Fig. 1C (scale bar is 500 nm).
Endocytic uptake of nanoparticles coated with fetuin-A and histones (FNH) by tumor cells
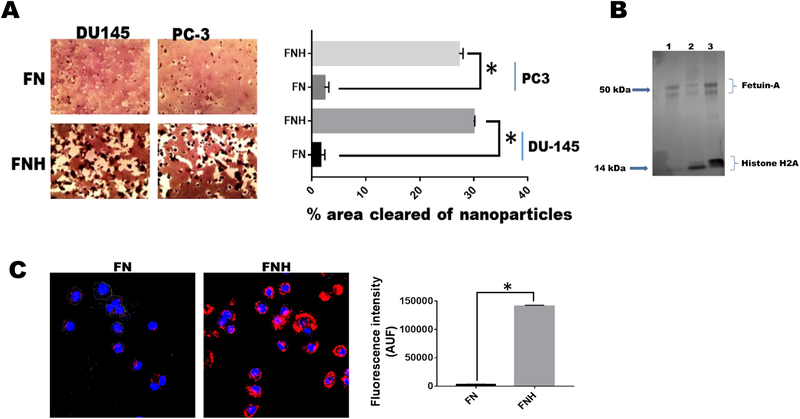
In order to directly implicate histones and fetuin-A in the uptake mechanisms of hydroxyapatite-nanoparticles, we reasoned that fetuin-A which has a high affinity for hydroxyapatite [17] and also associates with positively charged histones [14] could theoretically load histones on hydroxyapatitenanoparticles much the same way that it loads histones on exosomes. When fetuin-A was mixed with the nanoparticles resulting in FN to which cells were added in the wells of 96-well microtiter plates, the uptake of the particles by PC3 and DU145 cells was negligible (Fig. 2A). However, a mixture of the nanoparticles with fetuin-A: histones at a molar ratio of 5:1, resulting in FNH significantly improved the uptake over a 48 h period by both cell lines and the cells can be seen engorged with the nanoparticles (Fig. 2A). FN and FNH were boiled in Laemmli sample buffer and proteins released resolved in SDS-PAGE which was fixed and stained with colloidal-Coomassie blue. Only added fetuin-A and histone H2A were detected in the gel as expected (Fig. 2B). The FN and FNH uptake studies have been repeated over 10 times using different cell lines and similar results obtained. To demonstrate that the nanoparticles were in fact internalized and not merely concentrated on the cell surfaces, we demonstrated using confocal microscopy that only rhodamine labeled FNH was internalized to a significant extent and resided in the perinuclear region of the cells (Fig. 2C). Labeled FN was hardly internalized (Fig. 2C), meaning that histones are the main drivers of internalization as proposed.
Figure 2. Histones mediate the uptake of hydroxyapatite nanoparticles by tumor cells.
Hydroxyapatite-nanoparticles (2 mg/ml) were incubated with either 2 mg/ml of fetuin-A (FN) or fetuin-A (2 mg/ml) and histones (100 μg/ml) (FNH) in 10 ml of HBSS for 24 h at 4°C. The particles were centrifuged at 700 x g for 5 min to pellet large aggregated particles. The nanoparticles (colloidal) that remained suspended (9 ml) were washed 3X with HBSS, each time pelleted by centrifugation (5,000 x g for 5 min). The final pellets were suspended in 9 ml of complete medium and added to 96-well microtiter plates (100 μl/well) and the tumor cells (1,000 cells/well) added to the lawn of nanoparticles. After 48 h of incubation (37°C in humidified CO2 incubator), the cells were photographed and the % of areas cleared of nanoparticles quantified using NEARS (Panel A; *P < 0.001; N = 6). In panel B, the nanoparticles after the final wash in HBSS, were boiled in Laemmli sample buffer and resolved in NUPAGE and stained with colloidal Coomassie blue (lane 1-FN; lanes 2 and 3-FNH). In panel C, the fetuin-A was labeled with rhodamine isothiocyanate prior to incubation with hydroxyapatite nanoparticles (FN) or the nanoparticles and histones (FNH). The uptake of the nanoparticles (AUF) monitored using A1R confocal microscope and quantified by NEARS (*P < 0.001; N = 6).
Heparan sulfate proteoglycan-SDC4 is a major uptake receptor for exosomes and FNH nanoparticles
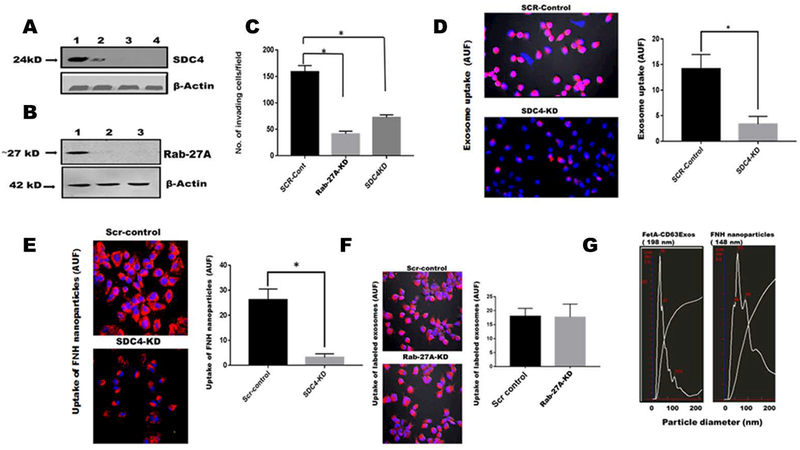
Positively charged proteins such as histones have been shown to interact with heparan sulfate proteoglycans mainly by electrostatic interaction, the latter having a net negative charge [18, 19]. Having demonstrated that heparin and heparitinase III attenuate the uptake of exosomes as well as exosomal mediated adhesion [14], we decided to knock-down one of the members of the family, SDC4, that is normally upregulated in more metastatic tumors. It was knocked down in LN229, a highly aggressive glioblastoma cell line. We also knocked down Rab-27A in the same cell line. Knockdown of SDC4 significantly attenuated the invasive capacity of LN229 cells (Fig. 3A and 3C). The results obtained here were in agreement with other studies that showed abrogation of motility and invasion in tumor cells where SDC4 was knocked-down or out. Knock-down of Rab-27A also attenuated invasion (Fig. 3B and 3C). The uptake of both rhodamine-labeled exosomes and FNH nanoparticles were significantly reduced in SDC4 knock-down cells compared to control cells transfected with the scrambled vectors (Figs. 3D and 3E). As control, we also examined the uptake of exosomes by Scr-control and Rab-27A knockdown cells. As shown in Fig. 3F, uptake was not attenuated in Rab-27A knockdown cells, indicating that Rab-27A which abrogates exosomal release, does not interfere with extracellular uptake of the particles. Similar data was shown for FNH nanoparticles (data not shown). Lastly, we determined by Nanosight™ that exosomes and FNH nanoparticles are of comparable sizes (< 200 nm) (Fig. 3G).
Figure 3. Invasion and uptake of labeled exosomes and FNH nanoparticles by LN229 are attenuated in SDC4-knocked down cells.
Panel A, lane 1 (SCR-control); lane 2 (SDC4-KD-clone 2; lane 3 (SDC4-KD-clone 3 and lane 4 (SDC4-KD-clone 4). Panel B, lane 1 (SCR-control); lane 2 (Rab-27A-KD-clone 2); lane 3 (Rab-27A-KD-clone 3). In panel C, the invasion potentials of control cells transfected with scrambled shRNA (SCR-control) as well as clone 3 of Rab-27A-KD and SDC4-KD were assayed using Boyden chambers (Mean ± SD *P < 0.05; N = 7) In panel D, exosomes isolated and purified from BT-549 cells were labeled with rhodamine isothiocyanate and uptake of both SCR-control and SDC4-KD clone 3 cells determined (Nikon A1R) and AUF quantified by NEARS (Mean + SD, *P < 0.05; N = 6). Panel E, FNH nanoparticles were similarly labeled with rhodamine and uptake by SCR-controls and SDC4-KD cells determined as described for panel D. Panel F, uptake of rhodamine labeled FNH nanoparticles by SCR-controls and Rab-27A-KD cells was determined as described for panel D. Panel G, represents the particle size distribution of BT-CD63 exosomes and FNH nanoparticles as determined by NanoSight™.
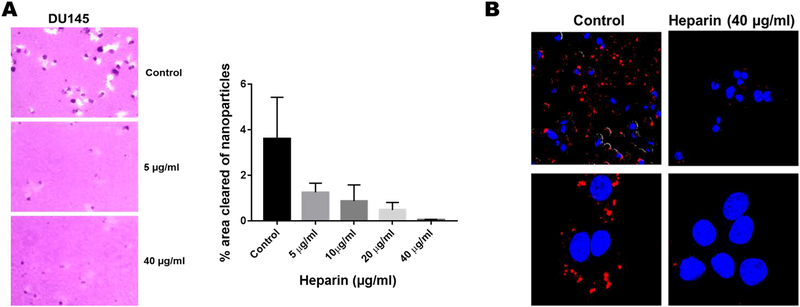
Heparin attenuates the endocytic uptake of FNH nanoparticles by tumor cells
Knowing that heparin abrogates the uptake of exosomes [14], we next asked whether it would also influence the uptake of FNH nanoparticles. Clearly, heparin at doses > 20 μg/ml was able to attenuate the uptake of FNH nanoparticles by DU145 prostate cancer cells (Fig. 4A). Uptake of rhodamine labeled FNH nanoparticles which was also attenuated by heparin confirmed that the nanoparticles were internalized and not merely concentrated on the cell surface (Fig. 4B).
Figure 4. Heparin inhibits the receptor mediated uptake of FNH nanoparticles.
In panel A, the areas cleared of nanoparticles in the absence or presence of heparin were quantified by NEARS. In panel B, the uptake of rhodamine labeled FNH nanoparticles by DU145 prostate cancer cells was determined by confocal microscopy (Nikon A1R). Lower panels represent zoomed images of the upper panels.
FNH nanoparticles and exosomes are endocytosed and move to the perinuclear regions of the cells
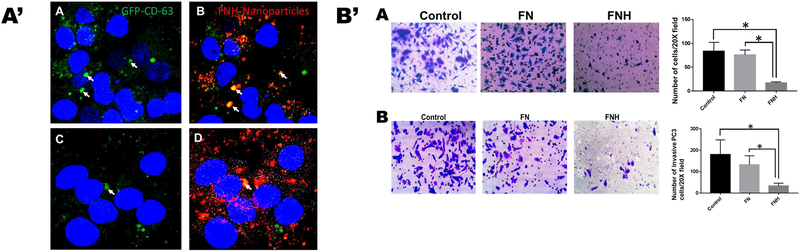
Having demonstrated that exosomes and FNH are internalized via the same pathway (SDC4), we next wanted to determine whether the nanoparticles move to the same compartments of the cell as exosomes and if so whether they can compete with exosomes for uptake and thereby abrogate the signature roles of the latter (motility and invasion) in tumor cells. As can be seen in Fig. 5A’, the exosomes (panels A and C) move to the perinuclear region of the cells, the same area to which FNH nanoparticles also move and some can be seen co-localized (arrows) with exosomes in the merged panels (Fig. 5A’ panels B and D respectively). Whereas FN nanoparticles in the upper wells of the Boyden chambers did not affect either motility or invasion of prostate cancer cells, PC3, the FNH nanoparticles significantly attenuated motility and invasion of these cells (Fig. 5B’; panels A and B).
Figure 5. FNH nanoparticles and GFP-CD63 exosomes move to the perinuclear compartments of the cell and attenuate both motility and invasion of prostate cancer tumor cells.
In A’, GFP-CD63 exosomes (4 μg/chamber) and rhodamine labeled FNH nanoparticles (4 μg/chamber) were added to DU145 prostate cancer cells in 8-chambered glass slides. The green channel, panels A and C represent uptake of GFP-CD63 exosomes while the merged panels B and D show both GFP-CD63 exosomes and FNH nanoparticles which indicates that some of the exosomes were co-localized with FNH nanoparticles (arrows). In B’, the motility (panel A) and invasion (panel B) assays were done using Boyden Chambers as described. Briefly, the cells (DU145) were added to the upper chambers in SFM without (control) or with nanoparticles FN or FNH (100 μg/chamber). The lower chambers contained 500 μl of complete medium (10% fetal bovine serum). * P < 0.05, N = 6; one way ANOVA.
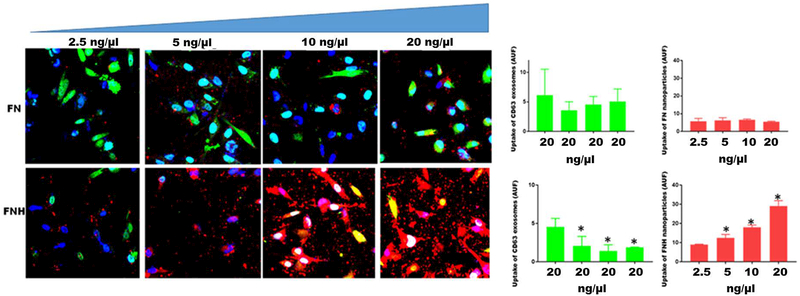
FNH nanoparticles competitively inhibit the uptake of exosomes by breast tumor cells.
Having demonstrated that FNH and exosomes share the same uptake route, we next questioned whether FNH particles compromise the exosomal uptake by the cells. The data show that as concentration of FNH in the chambers increased, the uptake of labeled exosomes by the cells decreased accordingly (Fig. 6). Interestingly, labeled FN particles (controls) that were not effectively internalized by the cells did not affect the uptake of exosomes even at the highest concentration of 20 ng/μl. The data support the notion that addition of FNH but not FN nanoparticles to the upper chambers of Boyden Chambers in motility and invasion assays attenuates the exosomal mediated processes.
Figure 6. FNH nanoparticles competitively inhibits the uptake of exosomes by breast carcinoma cells.
MDA-MB-231 breast carcinoma cells were plated (2 × 105 cells/chamber) in triplicates in 8-chambered glass slides. After an overnight attachment, complete medium in the chambers was replaced with SFM. GFP-CD63 labeled exosomes were added to each chamber (20 ng/μl) of three separate slides containing cells. To the upper four chambers of each slide, increasing concentrations of FN nanoparticles (2.5–20 ng/μl) in SFM were added. To the lower four chambers, increasing concentrations of FNH nanoparticles (2.5–20 ng/μl) in SFM were added. After 1 h of incubation at 37°C, the chambers were removed and the cells fixed in 4% formalin and a drop of slow-fade with Dapi added and cover-slipped. Uptake of exosomes (green channel) and nanoparticles (red channel) by the cells was monitored by confocal microscopy (Nikon A1R) and arbitrary units of fluorescence quantified using NEARS software as described in Materials and Methods. The bars represent means ± SD (*P < 0.05; N = 3; one way ANOVA)
Discussion
We hereby present evidence that extracellular histones mediate the uptake of exosomes and FNH nanoparticles by tumor cells through SDC4 mediated endocytosis. Mechanisms that govern both the release/secretion as well as uptake of exosomes are poorly understood [11, 34]. Various exosomal uptake mechanisms have been suggested ranging from phagocytosis [35] to receptor mediated endocytosis involving clathrin and micropinocytosis [36]. The present analyses were conducted to define the exosomal ligands that drive their uptake via cell surface heparan sulfate proteoglycan receptors.
The uptake mechanism of exosomes via heparan sulfate proteoglycans was initially suggested by Christianson et al. [13]. Since then other studies including ours have confirmed the involvement of heparan sulfate proteoglycans in the uptake of exosomes which is attenuated by heparin [14, 37]. However, the ligand(s) on exosomes that interact(s) with the heparan sulfate proteoglycans is yet to be defined. Functionally, exosomes have been shown to promote cellular adhesion, motility and invasion [28, 38]. Initial studies suggested that integrins and extracellular matrix ligands such as fibronectin on exosomes were responsible for the exosomal mediated cellular adhesion [39, 40]. We previously demonstrated that exosomes isolated and purified from breast carcinoma cells in SFM, failed to promote adhesion in the absence of complete medium (~10% FBS). On the other hand, a similar concentration of exosomes (~100 μg/ml) isolated and purified in the presence of fetuin-A, mediated adhesion in recipient cells in SFM [33]. Interestingly, the breast carcinoma cells readily adhered to plastic in SFM in the presence of unpurified or purified Pedersen fetuin-A (~0.5–2 mg/ml) [33]. At the molecular level, we reported that exosomes isolated in the presence of fetuin-A, contain all the proteins found in exosomes isolated in the absence of fetuin-A plus a compendium of histones, plasminogen and fetuin-A [33]. This suggested to us that either fetuin-A, plasminogen or histones or a combination of the three proteins were responsible for the adhesion promoting properties of exosomes. Further studies suggested that for the exosomes to mediate adhesion, it was necessary for the recipient cells to endocytose them [14]. The present data demonstrate that fetuin-A alone is incapable of driving the uptake of hydroxyapatite-nanoparticles that use the same uptake route as the exosomes. However, histones at concentrations ranging from 10–100 μg/ml on either hydroxyapatite nanoparticles or exosomes isolated in SFM containing BSA was sufficient to mediate uptake. Fetuin-A which has affinity for both exosomes and hydroxyapatite nanoparticles, most likely anchors the histones on these particles. On exosomes, fetuin-A could bind to annexins associated with these nano-vesicles [41]. Fetuin-A binds to annexins with Kdiss in the micro-molar range [42]. Plasminogen may also interact with exosomal annexins [43]. The data presented clearly show that exosomes isolated in the presence of BSA in SFM (control) are not internalized by recipient cells even though they may interact with heparan sulfate proteoglycans on the cell surface via their resident fibronectins [15]. The fact that BSA exosomes were not internalized suggested that their adhesion, motility and invasion promoting signals are passed from cell to cell and released inside recipient cells upon uptake. Therefore endocytic uptake of exosomes is essential for the signaling events mediated by them.
Given that hydroxyapatite-nanoparticles occur naturally in serum and extracellular space [44, 45] and can associate with fetuin-A/histones in this medium, there is a high likelihood that cells that express SDC4 such as endothelial cells and smooth muscle cells actively uptake these particles from blood [46, 47], based on the present data. It is established that one of the functional roles of fetuin-A is to inhibit ectopic calcification [48]. In circumstances where serum fetuin-A is low as in chronic kidney disease patients, increased calcification occasioned by the rapid uptake of the nanoparticles is expected [49]. Therefore the histone-SDC4 mediated uptake of exosomes and the nanoparticles has the potential to not only impact cancer metastasis but also cardiovascular disease which ranks as the top cause of mortality worldwide [50]. The present data suggest that hydroxyapatite nanoparticles that occur naturally in plasma, can compete with exosomes for uptake into tumor and cardiovascular cells to modulate processes that are mediated by these nano-vesicles.
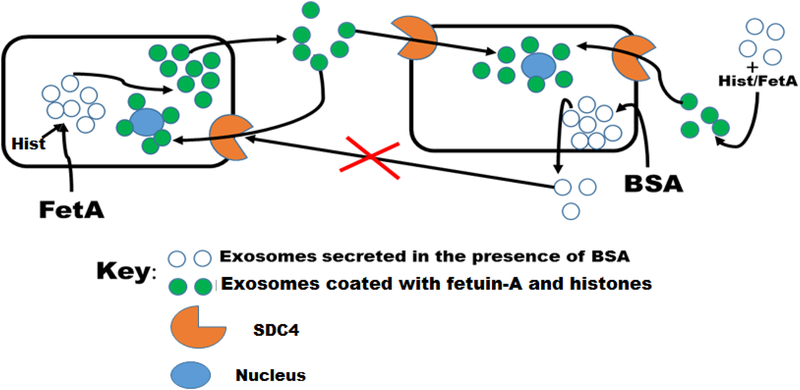
Based on the data presented, we believe that once fetuin-A enters the tumor cells, it loads a compendium of histones on the exosomes represented by empty dots, according to the model represented by Fig. 7. These are then converted to histone laden exosomes (represented by green dots; Fig. 7) and released from the cell. These exosomes (green dots) interact with SDC4 on the cell surface via histones and are consequently internalized. In the absence of fetuin-A but presence of BSA, exosomes that lack fetuin-A and histones (Fig. 7, empty dots) are secreted. These exosomes cannot be internalized and hence cannot transmit their signals to the recipient cells. Breast carcinoma cells and other tumor cells that do not synthesize and secrete fetuin-A, typically do not adhere nor move and invade in SFM that lack fetuin-A in vitro. Of note, is the ability of histone/fetuin-A to convert the so called BSA exosomes (Fig. 7, empty dots) to ‘uptake competent’ exosomes (Fig. 7, green dots). The significance of this is that in vivo, where you are likely to have fetuin-A and histones in the extracellular milieu, secreted exosomes are easily internalized upon their interaction with the histones.
Figure 7. Model depicting the uptake of exosome secreted in the absence or presence of fetuin-A.
In the presence of fetuin-A, the exosomes secreted are easily taken up by other tumor cells or by the secreting cells (autocrine). Exosomes secreted in the absence of fetuin-A but presence of BSA, lack the capacity to enter the cells via syndecan 4 (SDC4) receptors. Uptake incompetent exosomes secreted in the absence of fetuin-A can be incubated with a mixture of fetuin-A and histones and after further purification are now competent to enter the cells via SDC4.
In summary, we have demonstrated that histones associate with exosomes or hydroxyapatite nanoparticles via fetuin-A in the extracellular environment to efficiently mediate the uptake of these nanoparticles by tumor cells which express SDC4 on their surfaces.
Acknowledgments
This work was supported by NIH-U54MD007586–01, MeTRC-5U54MD007593–10 and U54CA163069
References
- 1.Syn N, Wang L, Sethi G, Thiery JP & Goh BC (2016) Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance, Trends in pharmacological sciences. 37, 606–617. [DOI] [PubMed] [Google Scholar]
- 2.Raposo G & Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends, The Journal of cell biology. 200, 373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khushman M, Bhardwaj A, Patel GK, Laurini JA, Roveda K, Tan MC, Patton MC, Singh S, Taylor W & Singh AP (2017) Exosomal Markers (CD63 and CD9) Expression Pattern Using Immunohistochemistry in Resected Malignant and Nonmalignant Pancreatic Specimens, Pancreas. 46, 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muhsin-Sharafaldine MR, Saunderson SC, Dunn AC, Faed JM, Kleffmann T & McLellan AD (2016) Procoagulant and immunogenic properties of melanoma exosomes, microvesicles and apoptotic vesicles, Oncotarget. 7, 56279–56294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr., Carter BS, Krichevsky AM & Breakefield XO (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers, Nature cell biology. 10, 1470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh R, Pochampally R, Watabe K, Lu Z & Mo YY (2014) Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer, Molecular cancer. 13, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arita T, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Ogino S, Fujita Y, Hiramoto H, Hamada J, Shoda K, Kosuga T, Fujiwara H, Okamoto K & Otsuji E (2016) Tumor exosome-mediated promotion of adhesion to mesothelial cells in gastric cancer cells, Oncotarget. 7, 56855–56863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratajczak MZ & Ratajczak J (2017) Extracellular Microvesicles as Game Changers in Better Understanding the Complexity of Cellular Interactions-From Bench to Clinical Applications, The American journal of the medical sciences. 354, 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guay C & Regazzi R (2017) Exosomes as new players in metabolic organ cross-talk, Diabetes, obesity & metabolism. 19 Suppl 1, 137–146. [DOI] [PubMed] [Google Scholar]
- 10.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Perez Lanzon M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N & Pegtel DM (2015) Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species, Stem cell research & therapy. 6, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ & Weaver AM (2013) Exosome secretion is enhanced by invadopodia and drives invasive behavior, Cell reports. 5, 1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savina A, Fader CM, Damiani MT & Colombo MI (2005) Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner, Traffic. 6, 131–43. [DOI] [PubMed] [Google Scholar]
- 13.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP & Belting M (2013) Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity, Proceedings of the National Academy of Sciences of the United States of America. 110, 17380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nangami G, Koumangoye R, Shawn Goodwin J, Sakwe AM, Marshall D, Higginbotham J & Ochieng J (2014) Fetuin-A associates with histones intracellularly and shuttles them to exosomes to promote focal adhesion assembly resulting in rapid adhesion and spreading in breast carcinoma cells, Experimental cell research. 328, 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EE & Sanderson RD (2016) Fibronectin on the Surface of Myeloma Cell-derived Exosomes Mediates Exosome-Cell Interactions, The Journal of biological chemistry. 291, 1652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW & Thery C (2014) Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles, Journal of extracellular vesicles. 3, 26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J, Baumann MJ & McCabe LR (2005) Adsorption of serum fetuin to hydroxylapatite does not contribute to osteoblast phenotype modifications, Journal of biomedical materials research Part A. 73, 39–47. [DOI] [PubMed] [Google Scholar]
- 18.Henriquez JP, Casar JC, Fuentealba L, Carey DJ & Brandan E (2002) Extracellular matrix histone H1 binds to perlecan, is present in regenerating skeletal muscle and stimulates myoblast proliferation, Journal of cell science. 115, 2041–51. [DOI] [PubMed] [Google Scholar]
- 19.Meneghetti MC, Hughes AJ, Rudd TR, Nader HB, Powell AK, Yates EA & Lima MA (2015) Heparan sulfate and heparin interactions with proteins, Journal of the Royal Society, Interface. 12, 0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erdem M, Erdem S, Sanli O, Sak H, Kilicaslan I, Sahin F & Telci D (2014) Up-regulation of TGM2 with ITGB1 and SDC4 is important in the development and metastasis of renal cell carcinoma, Urologic oncology. 32, 25 e13–20. [DOI] [PubMed] [Google Scholar]
- 21.Na KY, Bacchini P, Bertoni F, Kim YW & Park YK (2012) Syndecan-4 and fibronectin in osteosarcoma, Pathology. 44, 325–30. [DOI] [PubMed] [Google Scholar]
- 22.Ridgway LD, Wetzel MD & Marchetti D (2010) Modulation of GEF-H1 induced signaling by heparanase in brain metastatic melanoma cells, Journal of cellular biochemistry. 111, 1299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu H, Yang H, Zhang X & Xu W (2016) The emerging roles of exosomes in tumor-stroma interaction, Journal of cancer research and clinical oncology. 142, 1897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu CM, Hsieh CL, Shen CN, Lin CC, Shigemura K & Sung SY (2016) Exosomes from the tumor microenvironment as reciprocal regulators that enhance prostate cancer progression, International journal of urology: official journal of the Japanese Urological Association. 23, 734–44. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Zhang X, Wang J, Li M, Cao C, Tan J, Ma D & Gao Q (2017) TGFbeta1 in fibroblasts-derived exosomes promotes epithelial-mesenchymal transition of ovarian cancer cells, Oncotarget. 8, 96035–96047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman RM (2013) Stromal-cell and cancer-cell exosomes leading the metastatic exodus for the promised niche, Breast cancer research: BCR. 15, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakwe AM, Koumangoye R, Goodwin SJ & Ochieng J (2010) Fetuin-A ({alpha}2HS-glycoprotein) is a major serum adhesive protein that mediates growth signaling in breast tumor cells, The Journal of biological chemistry. 285, 41827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koumangoye RB, Sakwe AM, Goodwin JS, Patel T & Ochieng J (2011) Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading, PloS one. 6, e24234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furtak V, Hatcher F & Ochieng J (2001) Galectin-3 mediates the endocytosis of beta-1 integrins by breast carcinoma cells, Biochemical and biophysical research communications. 289, 845–50. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Liu DR, Li GG, Wang HH, Li XW, Zhang W, Wu YL & Chen L (2015) CD97 promotes gastric cancer cell proliferation and invasion through exosome-mediated MAPK signaling pathway, World journal of gastroenterology. 21, 6215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enriquez VA, Cleys ER, Da Silveira JC, Spillman MA, Winger QA & Bouma GJ (2015) High LIN28A Expressing Ovarian Cancer Cells Secrete Exosomes That Induce Invasion and Migration in HEK293 Cells, BioMed research international. 2015, 701390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nangami GN, Sakwe AM, Izban MG, Rana T, Lammers PE, Thomas P, Chen Z & Ochieng J (2016) Fetuin-A (alpha 2HS glycoprotein) modulates growth, motility, invasion, and senescence in high-grade astrocytomas, Cancer medicine. 5, 3532–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson K, Koumangoye R, Thompson P, Sakwe AM, Patel T, Pratap S & Ochieng J (2012) Fetuin-A triggers the secretion of a novel set of exosomes in detached tumor cells that mediate their adhesion and spreading, FEBS letters. 586, 3458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulcahy LA, Pink RC & Carter DR (2014) Routes and mechanisms of extracellular vesicle uptake, Journal of extracellular vesicles. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q & Sui SF (2010) Cellular internalization of exosomes occurs through phagocytosis, Traffic. 11, 675–87. [DOI] [PubMed] [Google Scholar]
- 36.Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH & Xiao ZD (2014) Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery, The Journal of biological chemistry. 289, 22258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L & Brigstock DR (2016) Integrins and heparan sulfate proteoglycans on hepatic stellate cells (HSC) are novel receptors for HSC-derived exosomes, FEBS letters. 590, 4263–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD, McConnell RE, Tyska MJ & Coffey RJ (2011) Amphiregulin exosomes increase cancer cell invasion, Current biology: CB. 21, 779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD & Hallett MB (2004) Adhesion and signaling by B cell-derived exosomes: the role of integrins, FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 18, 977–9. [DOI] [PubMed] [Google Scholar]
- 40.Rieu S, Geminard C, Rabesandratana H, Sainte-Marie J & Vidal M (2000) Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha4beta1, European journal of biochemistry. 267, 583–90. [DOI] [PubMed] [Google Scholar]
- 41.Schey KL, Luther JM & Rose KL (2015) Proteomics characterization of exosome cargo, Methods. 87, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kundranda MN, Ray S, Saria M, Friedman D, Matrisian LM, Lukyanov P & Ochieng J (2004) Annexins expressed on the cell surface serve as receptors for adhesion to immobilized fetuin-A, Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1693, 111–123. [DOI] [PubMed] [Google Scholar]
- 43.Kwon M, MacLeod TJ, Zhang Y & Waisman DM (2005) S100A10, annexin A2, and annexin a2 heterotetramer as candidate plasminogen receptors, Frontiers in bioscience: a journal and virtual library. 10, 300–25. [DOI] [PubMed] [Google Scholar]
- 44.Richards JM, Kunitake J, Hunt HB, Wnorowski AN, Lin DW, Boskey AL, Donnelly E, Estroff LA & Butcher JT (2018) Crystallinity of hydroxyapatite drives myofibroblastic activation and calcification in aortic valves, Acta biomaterialia. 71, 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heiss A, DuChesne A, Denecke B, Grotzinger J, Yamamoto K, Renne T & Jahnen-Dechent W (2003) Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles, The Journal of biological chemistry. 278, 13333–41. [DOI] [PubMed] [Google Scholar]
- 46.Cizmeci-Smith G, Langan E, Youkey J, Showalter LJ & Carey DJ (1997) Syndecan-4 is a primary-response gene induced by basic fibroblast growth factor and arterial injury in vascular smooth muscle cells, Arteriosclerosis, thrombosis, and vascular biology. 17, 172–80. [DOI] [PubMed] [Google Scholar]
- 47.Hunter LW, Charlesworth JE, Yu S, Lieske JC & Miller VM (2014) Calcifying nanoparticles promote mineralization in vascular smooth muscle cells: implications for atherosclerosis, International journal of nanomedicine. 9, 2689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T & Jahnen-Dechent W (2003) The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification, J Clin Invest. 112, 357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viegas CSB, Santos L, Macedo AL, Matos AA, Silva AP, Neves PL, Staes A, Gevaert K, Morais R, Vermeer C, Schurgers L & Simes DC (2018) Chronic Kidney Disease Circulating Calciprotein Particles and Extracellular Vesicles Promote Vascular Calcification: A Role for GRP (Gla-Rich Protein), Arteriosclerosis, thrombosis, and vascular biology. 38, 575–587. [DOI] [PubMed] [Google Scholar]
- 50.Saeed A, Dabhadkar K, Virani SS, Jones PH, Ballantyne CM & Nambi V (2018) Cardiovascular Disease Prevention: Training Opportunities, the Challenges, and Future Directions, Current atherosclerosis reports. 20, 35. [DOI] [PubMed] [Google Scholar]