Abstract
In an elegant example of bench to bedside research, a hypothesis that cells in the outflow pathway actively regulate conventional outflow resistance was proposed in the 1990s and systematically pursued, exposing novel cellular and molecular mechanisms of IOP regulation. The critical discovery that pharmacological manipulation of the cytoskeleton of outflow pathway cells decreased outflow resistance placed a spotlight on the Rho kinase pathway that was known to regulate the cytoskeleton. Ultimately, a search for Rho kinase inhibitors led to the discovery of several molecules of therapeutic interest, leaving us today with two new ocular hypotensive agents approved for clinical use – ripasudil in Japan and netarsudil in the US. These represent members of the first new class of clinically useful ocular hypotensive agents since the US FDA approval of latanoprost in 1996.
The development of Rho kinase inhibitors as a class of medications to lower intraocular pressure in patients with glaucoma and ocular hypertension represents a triumph in translational research. Rho kinase inhibitors are effective alone or when combined with other known ocular hypotensive medications. They also offer the possibility of neuroprotective activity, a favorable impact on ocular blood flow and even an anti-fibrotic effect that may prove useful in conventional glaucoma surgery. Local adverse effects, however, including conjunctival hyperemia, subconjunctival hemorrhages and cornea verticillata, are common. Development of Rho kinase inhibitors targeted to the cells of the outflow pathway and the retina may allow these agents to have even greater clinical impact.
The objectives of this review are to describe the basic science underlying the development of Rho kinase inhibitors as a therapy to lower intraocular pressure and to summarize the results of the clinical studies reported to date. The neuroprotective and vasoactive properties of Rho kinase inhibitors as well as the antifibrotic properties of these agents are reviewed in the context of their possible role in the medical and surgical treatment of glaucoma.
Keywords: Glaucoma, ocular hypertension, intraocular pressure, Rho-associated protein kinase inhibitor, norepinephrine transport inhibitor, neuroprotection, trabecular meshwork, Schlemm’s canal, glaucoma surgery
I. Introduction
The glaucomas are a group of progressive optic neuropathies characterized by optic disc excavation and apoptotic loss of retinal ganglion cells with corresponding vision loss1. Although the underlying pathophysiologic mechanisms are multifactorial, intraocular pressure (IOP) is a continuous risk factor for the development and progression of the disease. The only therapeutic intervention that has been proven to be effective in slowing disease progression is IOP reduction.
IOP is the level of pressure in the eye at which the aqueous humor produced in the ciliary body and flowing into the posterior chamber of the eye is balanced by the aqueous humor leaving the eye through the conventional outflow pathway (trabecular meshwork, Schlemm’s canal, aqueous veins and collector channels) and unconventional outflow pathway (the uveoscleral and uveovortex pathways). IOP can be lowered by decreasing the rate of aqueous humor formation, decreasing the aqueous outflow resistance of the conventional outflow pathway, or by increasing aqueous outflow through the unconventional pathway2, 3.
IOP elevation, associated with primary open-angle glaucoma (POAG), is caused by an increased resistance to the outflow of aqueous humor from the eye4. Medical therapy for glaucoma started in 1875 with the discovery that pilocarpine lowers IOP5. Pilocarpine and other miotics such as carbachol and eserine stimulate contraction of the ciliary muscle, pulling on the trabecular meshwork and opening Schlemm’s canal, thereby decreasing outflow resistance and lowering IOP6–8. However, use of pilocarpine and other miotics to treat glaucoma is associated with significant adverse effects including spasm of accommodation in younger patients, accelerated development of cataract, iris and ciliary body cyst formation, and retinal detachment9–11. Epinephrine was introduced for lowering of IOP in the 1930s11 and was later replaced by dipivefrin; they are non-selective alpha-adrenergic agonists and also lower outflow resistance, although the mechanism is not well understood. Both are associated with the frequent development of local adverse events, including blepharoconjunctivitis12, 13. These various adverse effects limit the utility of these drug classes for long-term therapy.
Beta-adrenergic antagonists, particularly timolol, were introduced in the 1970s to lower the rate of aqueous humor secretion into the eye, thereby lowering IOP. These agents have been particularly successful, and they are well tolerated by most patients. Topical carbonic anhydrase inhibitors and alpha2-adrenergic agonists (brimonidine, apraclonidine) were later introduced and also lower the rate of flow of aqueous humor into the eye14–16.
The 1990s saw the introduction of latanoprost, the first of several clinically useful prostaglandin F2α analogues (PGAs), that substantially lower IOP (by about 30%) by acting on a second pathway through which aqueous humor can leave the eye20–23. Under normal circumstances in adults, the bulk of aqueous humor exits the eye through the conventional aqueous outflow pathway; however, a small fraction (15% or less on average, but variable throughout the day) flows through the unconventional outflow pathway 24–26. PGAs cause structural changes to the unconventional flow pathway through the ciliary muscle bundles, remodeling the extracellular matrix and generating open spaces in this region, thereby greatly increasing flow through this pathway and significantly decreasing IOP 27. Brimonidine may lower IOP, in part, by stimulating prostaglandin release thereby increasing unconventional outflow28, 29. PGAs are, for the most part, well tolerated by patients.
Although treatment modalities for lowering IOP include topical and systemic ocular hypotensive medical therapy as well as various laser and incisional surgical procedures, topical medical therapy is the most commonly utilized strategy and PGAs are the most commonly prescribed first-line agents30. For patients on medical therapy, clinical trial experience indicates that approximately 40–50% of patients require two or more medications to adequately lower IOP31, 32. Currently, beta-adrenergic antagonists, alpha2-adrenergic agonists, and topical carbonic anhydrase inhibitors are commonly used adjunctively for long-term glaucoma therapy in combination with prostaglandin analogs. When used adjunctively with PGAs, the additional mean diurnal IOP reduction achieved with each of these agents is approximately 1.5–3 mmHg33. Beta-adrenergic antagonists34 and alpha2-adrenergic agonists35 do not lower IOP during the nocturnal period if pressures are measured in the habitual position (i.e. supine during the nocturnal period).
There are only 4 classes of topical ocular hypotensive medical agents that are commonly used for long-term therapy: beta-adrenergic antagonists, carbonic anhydrase inhibitors, PGF2α analogs and alpha2-selective adrenergic agonists. Many patients are unable to tolerate one or more of these agents due to allergy, other adverse effects or contraindications. Even when all four agents are used in combination, IOP lowering can be insufficient36–38. Many patients require incisional surgery to achieve sufficiently low IOP to adequately stabilize their disease process. Such procedures are associated with a substantial risk of short and long-term complications that can lead to discomfort and vision loss39. There is a need, therefore, for additional and more effective medications for IOP lowering, particularly when added to prostaglandin analogs.
Laser trabeculoplasty reduces trabecular outflow resistance40 and various surgical procedures involve bypassing, incising or removing the trabecular meshwork41. It is notable, however, that none of the medications currently available to reduce IOP address the underlying cause of the elevated IOP that is commonly associated with glaucoma, namely increased outflow resistance4, 42. Agents with a novel mechanism of action directed at lowering this resistance would be expected to be clinically useful alone or adjunctively with other existing medical therapies. The ideal agent would be highly effective during both diurnal and nocturnal periods, easy to use (i.e. once daily dosing) and well tolerated with minimal adverse effects. Additionally, pharmacologic properties that support retinal function such as neuroprotective activity and improved ocular perfusion would be desirable.
In 1993, Dr. David Epstein organized the second Trabecular Meshwork Study Club (sponsored by the Glaucoma Research Foundation) and invited experts on aqueous outflow but also reached out to experts in other areas of physiology including Dr. Benjamin Geiger, whose expertise was cell biology. This meeting was seminal as Dr. Geiger there met Dr. Paul Kaufman, and they began a collaboration examining the role of cells in regulating aqueous humor outflow resistance. They were able to show that cytoskeletally-active agents such as latrunculin (that depolymerizes f-actin) and H7 (a protein kinase inhibitor that affects rho kinase) significantly decreased aqueous humor outflow resistance43–46. These studies began a focus on the role of cell mechanics in the aqueous humor outflow pathways and the role of Rho kinase in this process.
II. Rho kinases modulate the cytoskeleton
Rho and Rho kinases
The Rho family (RhoA, RhoB, RhoC) are small G-proteins that are active when bound to guanosine triphosphate (GTP) and inactive when bound to guanosine diphosphate (GDP). They are activated by a number of secreted cytokines including endothelin-1, thrombin, agiontension II, lysophophatidic acid, and transforming growth factor (TGF)-β, or via integrin activation47. They regulate cell morphology, polarity, proliferation, adhesion, motion, cytokinesis, and apoptosis along with smooth muscle contraction and neurite elongation48–50.
The effectors of the Rho family are the Rho kinases, ROCK1 and ROCK2. These two serine/threonine kinase isoforms are RhoGTP-binding proteins51. ROCK1 and ROCK2 contain an N-terminal kinase domain that phosphorylates target proteins, followed by a coiled-coil region with a Rho-binding domain and a domain with similarity in structure to pleckstrin, and then finally a cysteine-rich autoinhibitory domain toward the C terminus that limits kinase activity via intramolecular interactions (Fig. 1)49, 52. ROCK1 and ROCK2 have a similar structure with 65% overall homology, and 87% identity in the kinase domain, indicating that both isoforms can activate the same targets but allows for some differences in effect53. Their genes are located on chromosome 18 (18q11.1) and chromosome 2 (2p24), respectively52.
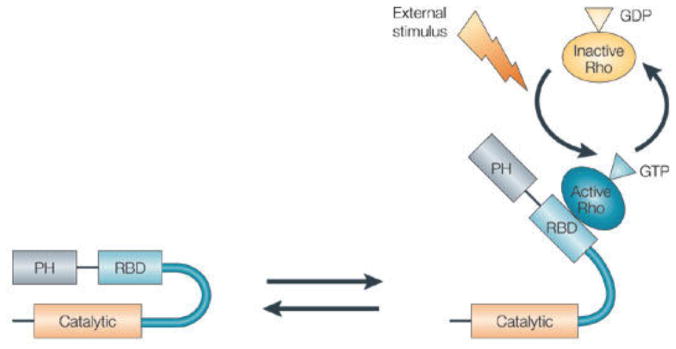
Figure 1.
Schematic of Rho kinase. Left: intramolecular interactions of the auto-inhibitory loop maintain the molecule in an inactive state; Right: Rho is activated when bound to GTP, thereby binding to the coil-coil region (RBD: Rho binding domain) and disrupting the negative regulatory interaction between the catalytic domain and the autoinhibitory C-terminal region, resulting in activation of the enzyme. PH, pleckstrin-homology domain51.
The activation mechanism of Rho kinase by Rho is shown in Fig. 1. Rho can bind to Rho kinase only when it in the active GTP-bound form. There are other independent activators for Rho kinase including arachidonic acid, phingosylphosphorylcholine and apoptosis51. Rho kinase phosphorylates a number of downstream target proteins. It acts to phosphorylate myosin light chain (MLC), stimulating myosin–actin interactions and promoting formation of stress fibers and focal adhesion complexes47, 51. Rho kinase also phosphorylates Lin-11/Isl-1/Mec-3 kinase (LIMK) that then inhibits cofilin-mediated actin-filament disassembly leading to an increase in actin filament density, rigidity and stability51, 54, 55. Other cytoskeletal effects of Rho kinase include depolymerization of intermediate filaments and modulation of microtubule dynamics and polarity51, 56. Through these actions, Rho kinase acts to increase the contractile state and stiffness of cells, particularly of the cell cortex, and regulates a variety of cell process, particularly those involving movement and smooth muscle contraction50, 52, 57.
Rho kinases inhibitors
Rho kinase inhibitors have a variety of effects. They can increase blood flow by causing vascular smooth muscle relaxation leading to vasodilation58. On the ocular surface, this can lead to conjunctival hyperemia59. Rho kinase inhibitors also have anti-tumor activity, acting to inhibit tumor cell invasion and metastasis, presumably by decreasing cell motility and cell division60. Rho kinase inhibitors prevent axonal degeneration and promote axon regeneration61, 62. Most known rho kinase inhibitors act on both ROCK1 and ROCK2. These include fasudil (HA-1077), approved for treatment of cerebral vasospasm in Japan and China63, and two rho kinase inhibitors currently approved for treatment of glaucoma: Ripasudil (K-115)64, a fluorinated analog of fasudil but with more potent and selective rho-kinase inhibitory activity65–67 that is approved for use in Japan and netarsudil (AR-13324), an amino-isoquinoline amide similar to but more potent than AR-12286 that is approved for use in the United States. Other rho kinase inhibitors that act on both ROCK1 and ROCK2 include Y-27632, H-1152, Wf-536, Y-39983, AMA-0076, GSK-269962A, SB-772077-B, SAR-407899, and RKI-144752, 68. Currently, the only ROCK2-specific inhibitor is KD-02552.
Interestingly, statins also inhibit Rho kinase69. Rho kinase activation requires intermediates involved in cholesterol synthesis, and the cholesterol-lowering activity of statins can interfere with this process49, 70. Statins have been shown to lower outflow resistance in post-mortem human eyes71. However, a large population-based study in the United Kingdom demonstrated that statin use was not independently associated with lower IOP after adjustment for beta-blocker use72.
III. Role of Rho kinase inhibitors in lowering aqueous outflow resistance
Kaufman and Bill showed in 1977 that cytochalasin B, an actin depolymerizing agent, reversibly decreased outflow resistance suggesting a possible role of the cytoskeleton in determining aqueous humor outflow resistance73. The decreased outflow resistance was attributed to increased density of pores in Schlemm’s canal cells along with breaks between cells74. While the pores in the inner wall endothelium are thought to be too large and numerous to generate significant flow resistance themselves75, a hydrodynamic interaction known as the “funneling” between these pores and the extracellular matrix in the juxtacanalicular connective tissue (JCT) makes inner wall pore density an important determinant of outflow resistance76. The streamlines on which aqueous humor passes through the JCT are forced to “funnel” or converge to enter the widely spaced pores in the inner wall endothelium of Schlemm’s canal, and this non-uniform flow significantly increases outflow resistance. Decreased Schlemm’s canal cell stiffness has been shown to be correlated with an increased number of these pores77 thereby decreasing aqueous outflow resistance.
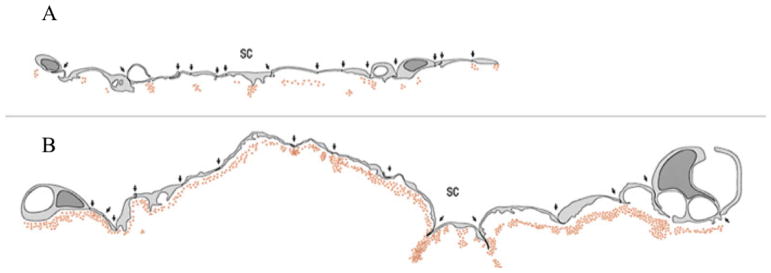
Further evidence that agents affecting the cytoskeleton can alter aqueous outflow resistance was provided by Kaufman and Geiger. The actin depolymerizing agents latrunculin-A and latrunculin-B were shown to increase outflow facility44, 45 and were associated with increased pore density in the inner wall endothelium along with separation of the inner wall endothelium from the JCT78. H-7 is a cytoskeletally-active isoquinoline sulfonamide derivative that blocks the phosphorylation activity of a variety of kinases including Rho kinase thereby inhibiting cell contractility and inducing general cellular relaxation79. H-7 acts to reversibly decrease outflow resistance43, 46, 80. Sabanay et al. used colloidal gold to show that H-7 alters flow pattern in the inner wall region consistent with loss of the funneling and decreased outflow resistance81, 82 (see Fig. 2).
Figure 2.
Schematic of inner wall endothelium of monkey eye perfused with colloidal gold. (A) shows a control eye with punctate distribution of colloidal gold; (B) shows an eye perfused with H-7 where the distribution of colloidal gold is much more uniform81.
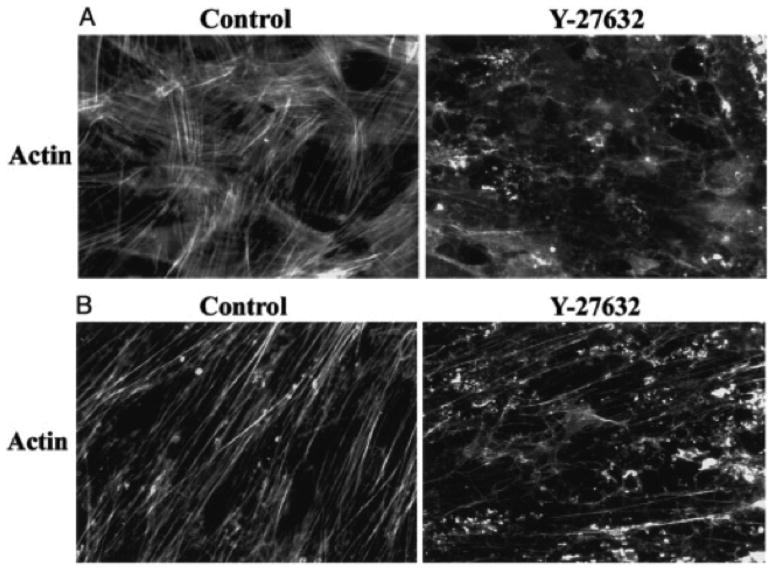
The first specific Rho kinase inhibitors to be investigated for their effects on outflow were Y-27632 and fasudil. These agents have significant effects on the cytoskeleton of both trabecular meshwork and Schlemm’s canal cells, decreasing the density of actin stress fibers (Figs. 3, 4). These agents significantly increased outflow facility in enucleated porcine eyes and live rabbits, respectively, while leaving the inner wall endothelium intact83–85. Other rho kinase inhibitors (AR-12286, netarsudil, H-1152, Y-39983, AMA-0076) were also found to significantly decrease outflow resistance in postmortem porcine eyes86 and IOP in living rabbits and monkeys87–91; maximum reductions in outflow resistance and IOP as much as 65% were achieved. No effect on unconventional outflow was seen88, 92. Perfusion of adenoviral vectors expressing dominant negative Rho-binding domain of Rho-kinase into post-mortem human eyes also decreased outflow resistance93.
Figure 3.
Y-27632 induces changes in the distribution of actin stress fibers in cultured Trabecular Meshwork (A) and Schlemm’s canal (B) cells. Magnification x40083.
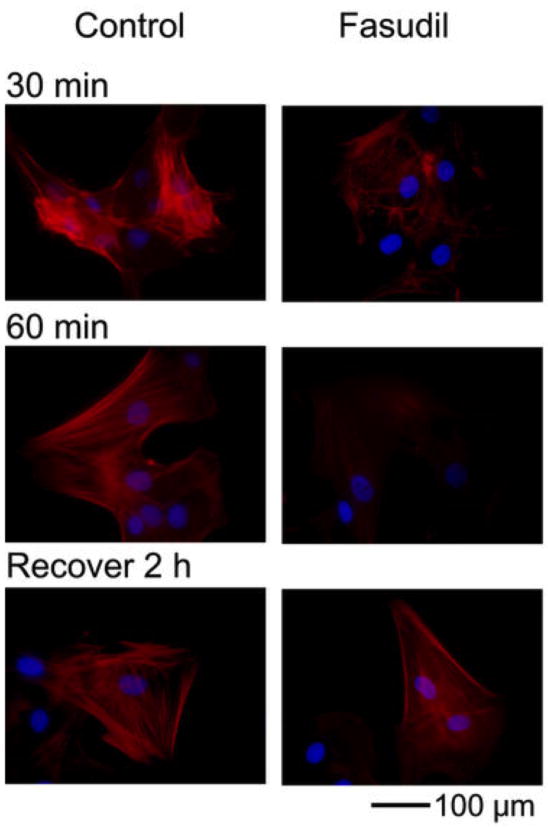
Figure 4.
Distribution of F-actin (red) incubated with fasudil as compared to buffered saline (control) for 30 and 60 min. Fasudil caused loss of actin stress fibers and bundles. Recovery was observed 2 h after the removal of fasudil. Scale bar: 100 μm66.
The involvement of the Rho kinase pathway was further established when it was shown that MLC kinase significantly decreased outflow resistance and had no effect on unconventional flow, and H-1152 decreased MLC phosphorylation in the trabecular meshwork of drug-perfused eyes86, 94. The downstream effectors of the Rho kinase pathway — MLC, LIM kinase and cofilin — are all expressed in human trabecular meshwork47. Trabecular meshwork cells have been shown to express both ROCK1 and ROCK248. Thus, the evidence of cytoskeletal regulation of outflow resistance is strong, as is the potential for altering this regulation using Rho kinase inhibitors.
A number of mechanisms have been proposed as to how modifying the cytoskeleton can alter outflow resistance. It has been proposed that Schlemm’s canal cell stiffness modulates aqueous outflow resistance by affecting the propensity of these cells to form pores77, 95, 96. Less stiff cells are able to form more pores, thus decreasing the funneling effect and decreasing outflow resistance. A related hypothesis is that changes in the Schlemm’s canal cell cytoskeleton lead to changes in focal adhesions thereby releasing the attachments between the Schlemm’s canal cells and the JCT, expanding the spaces in the JCT and decreasing the magnitude of the funneling effect47, 81–83, 97–99. Both of these hypotheses have strong experimental support.
It has also been suggested that Rho kinase inhibitors lower aqueous outflow resistance by relaxing smooth muscle cells in the trabecular meshwork100. However, pilocarpine and other miotics that decrease aqueous outflow resistance do so by contracting the ciliary muscle that then pulls on the trabecular meshwork, thereby opening Schlemm’s canal6–8; this is not consistent with a relaxation mechanism. Thieme et al100 proposed that miotics may also cause contraction of trabecular meshwork cells, and this could have an opposite effect on outflow resistance from that of ciliary muscle contraction. However, aceclidine preferentially contracts the trabecular meshwork as compared to pilocarpine and yet lowers IOP more than pilocarpine101. Notably, Camras et al found that the circumferential stiffness of the trabecular meshwork in post-mortem glaucomatous human eyes is significantly reduced as compared with normal eyes, and these glaucomatous eyes had increased outflow resistance102.
IV. Clinical studies of the ocular effects of Rho kinase inhibitors
Clinical trial results have been published in the peer-reviewed literature for only four Rho kinase inhibitors: SNJ-1656103, 104, AR-12286105–107, ripasudil37, 38, 108–112 and netarsudil113–116. All of these agents are mixed ROCK1 and ROCK2 inhibitors. Table 1 provides a summary of the phase 2 and phase 3 clinical trials.
Table 1.
Summary of Phase 2 and 3 Clinical Trials of Rho Kinase Inhibitors
| Study | Study Design | Duration of Treatment | Diagnosis and Baseline IOP Inclusion Range (mmHg) | Drugs (number of subjects) | Baseline IOP mmHg (SD) | Efficacy mmHg (SD) | Frequent Adverse Events | Comments | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| SNJ-1656 Phase 2 Inoue et al (2015)100 |
Multicenter RCT Double-masked Placebo control |
7 days | POAG (37%) OHT (63%) |
~22.5 | Change from baseline at trough | Change from baseline at peak | CH | ||||
|
|
|
|
|||||||||
| Placebo | −2.2 (1.9) | −1.5 (2.2) | Not reported | ||||||||
| 22≤ IOP ≤ 31 | SNJ-1656 0.03% (16) | −3.8 (2.7) | −5.0 (2.4) | 60% | |||||||
| SNJ-1656 0.05% (15) | −4.3 (2.3) | −4.4 (2.7) | 100% | ||||||||
| SNJ-1656 0.1% (18) | −4.0 (2.5) | −4.5 (1.9) | 83% | ||||||||
|
| |||||||||||
| AR-12286 Phase 2 Williams et al (2011)101 |
Multicenter RCT Double-masked Vehicle control |
3 consecutive 7-day dosing periods: QD AM QD PM BID |
OAG (58%) OHT (42%) |
Mean diurnal | Mean diurnal IOP reduction from baseline | CH | |||||
| QD AM Group | BID Group | ||||||||||
|
|
|
|
|
||||||||
| 24≤ IOP ≤ 36 | Vehicle (22) | 26.3 (2.47) | −1.9 | −2.4 | 9.1% | ||||||
| AR-12286 0.05% (22) | 26.0 (2.17) | −4.0 | −4.1 | 27.3% | |||||||
| AR-12286 0.1% (23) | 27.3 (3.18) | −5.0 | −4.4 | 39.1% | |||||||
| AR-12286 0.25% (22) | 26.9 (2.03) | −4.8 | −6.0 | 59.1% | |||||||
|
| |||||||||||
| Ripasudil Phase 2 Tanihara et al (2013)l105 |
Multicenter RCT Double-masked Placebo control |
8 weeks | POAG (41%) OHT (59%) |
9 AM | Change from baseline at trough | Change from baseline at peak | CH | ||||
|
|
|
|
|
||||||||
| Placebo (54) | 23.0 (2.1) | −2.2 | −2.5 | 13% | |||||||
| 21< IOP < 35 | Ripasudil 0.1% (53) | 23.3 (2.4) | −3.4 | −3.7 | 43% | ||||||
| Ripasudil 0.2% (54) | 23.2 (2.0) | −3.2 | −4.2 | 57% | |||||||
| Ripasudil 0.4% (49) | 23.2 (1.9) | −3.5 | −4.5 | 65% | |||||||
|
| |||||||||||
| Ripasudil Phase 3 Tanihara et al (2016)108 |
Multicenter Non-randomized open-label clinical trial |
1 year | POAG (65%) OHT (31%) XFG (4%) |
9 AM | Change from baseline at trough | Change from baseline at peak | All Treatments: CH
(75%) Blepharitis (21%) Allergic conjunctivitis (17%) |
||||
|
|
|
|
|||||||||
| Ripasudil 0.4% BID (173) | 19.3 (2.7) | −2.6 | −3.7 | ||||||||
| 15< IOP ≤ 35 | Ripasudil 0.4% BID + PGA (62) | 17.6 (2.0) | −1.4 | −2.4 | |||||||
| Ripasudil 0.4% BID + BB (60) | 18.2 (2.3) | −2.2 | −2.0 | ||||||||
| Ripasudil 0.4% BID + FC PGA and BB (59) | 17.6 (2.0) | −1.7 | −1.7 | ||||||||
|
| |||||||||||
| Additive effect of ripasudil with
timolol Phase 3 Tanihara et al (2015)107 |
Multicenter RCT Double-masked Placebo control |
8 weeks | POAG (47%) OHT (53%) IOP ≥ 18 on timolol |
On timolol 9 AM | Change from baseline at trough | Change from baseline at peak | CH | ||||
|
|
|
|
|
||||||||
| Placebo (104) | 19.7 (1.7) | −1.5 | −1.3 | 5.8% | |||||||
| Ripasudil 0.4% BID (104) | 19.9 (1.9) | −2.4 | −2.9 | 65.4% | |||||||
|
| |||||||||||
| Additive effect of ripasudil with
latanoprost Phase 3 Tanihara et al (2015)107 |
Multicenter RCT; Double-masked Placebo control |
8 weeks | POAG (61%) OHT (39%) IOP ≥ 18 on latanoprost |
On latanoprost 9 AM | Change from baseline at trough | Change from baseline at peak | CH | ||||
|
|
|
|
|
||||||||
| Placebo (103) | 19.6 (1.9) | −1.8 | −1.8 | 8.7% | |||||||
| Ripasudil 0.4% BID (102) | 20.1 (1.9) | −2.2 | −3.2 | 55.9% | |||||||
|
| |||||||||||
| Netarsudil Phase 3 Bacharach (2015)109 |
Multicenter RCT Double-masked |
28 days | POAG (60%) OHT (40%) |
Mean diurnal IOP | Change at trough | Change in mean diurnal IOP | CH | Netarsudil did not meet pre-determined criteria for non-inferiority to latanoprost. Eyes with baseline IOP ≤ 26 mm Hg did meet those criteria | |||
|
|
|
|
|
||||||||
| netarsudil 0.01% QD (74) | 25.8 | −5.4 | −5.5 | 52% | |||||||
| 24 ≤ IOP ≤ 36 | netarsudil 0.02% QD (72) | 25.6 | −5.9 | −5.7 | 57% | ||||||
| latanoprost 0.005% QD (77) | 25.5 | −7.6 | −6.8 | 16% increased lacrimation (5–7%) and CH (5–6%) also observed with netarsudil |
|||||||
|
| |||||||||||
| ROCKET-1 Netarsudil Phase 3 Serle et al (2017)112 |
Multicenter RCT Double-masked |
3 months | POAG (66%) OHT (34%) |
Mean diurnal IOP | Change from baseline (diurnal range) | CH | HEM | VER | Netarsudil did not meet the non-inferiority criteria. | ||
|
|
|
|
|||||||||
| 20<IOP < 27 at 8 AM and | netarsudil 0.02% QD (202) | 22.5 | −3.3 to −5.0 | 53% | 13% | 5% | |||||
| 17< IOP < 27 at 10 AM & 4 PM | timolol 0.5% BID (209) | 22.3 | −3.7 to −5.1 | 7% | 0.5% | 0% | |||||
|
| |||||||||||
| ROCKET-2 Netarsudil Phase3 Serle et al (2017)112 |
Multicenter RCT Double-masked |
3-month Interim data reported for the 12-month trial |
POAG (66%) OHT (34%) |
Mean diurnal IOP* | Change from baseline (diurnal
range)* *Primary efficacy population (maximum baseline IOP < 25 mmHg) |
CH | HEM | VER | Netarsudil QD & BID met non-inferiority criteria in the primary efficacy population | ||
|
|
|
|
|||||||||
| 20< IOP < 27* at 8 AM and | netarsudil 0.02% QD (251) | 21.4 | −3.3 to −4.6 | 50% | 15% | 9% | |||||
| 17< IOP < 27* at 10 AM and 4 PM | netarsudil 0.02% BID (254) | 21.5 | −4.1 to −5.4 | 59% | 17% | 15% | |||||
| timolol 0.5% BID (251) | 21.5 | −3.7 to −5.1 | 10% | 0% | 0.4% | ||||||
|
| |||||||||||
| Fixed Combination netarsudil and
latanoprost (PG324) Phase 2 Lewis et al (2016) 111 |
Multicenter RCT Double-masked |
28 days | POAG (56%) OHT (44%) |
Mean diurnal IOP | Mean diurnal IOP | CH | Fixed combination formulations superior to latanoprost and netarsudil | ||||
|
|
|
|
|||||||||
| 24≤ IOP < 36 at 8 AM and | latanoprost – netarsudil 0.01% QD (74) | 25.1 (2.3) | 17.3 (2.8) | 41% | |||||||
| IOP ≥ 21 at 10 AM and 4 PM | latanoprost – netarsudil 0.02% QD (73) | 25.1 (2.4) | 16.5 (2.6) | 40% | |||||||
| latanoprost QD (73) | 26.0 (2.8) | 18.4 (2.6) | 14% | ||||||||
| netarsudil 0.02% (78) | 25.4 (2.7) | 19.1 (3.2) | 40% | ||||||||
Abbreviations: IOP = intraocular pressure; SD = standard deviation; RCT = randomized clinical trial; POAG = primary open-angle glaucoma; OHT = ocular hypertension; QD = once daily; BID = twice daily; OAG = open-angle glaucoma; XFG = exfoliation glaucoma; PGA = prostaglandin analog; BB = beta-blocker; FC = fixed combination; CH = conjunctival hyperemia; HEM = conjunctival hemorrhage; VER = cornea verticillata
SNJ-1656 (previously known as Y-39983)
SNJ-1656, developed by Senju Pharmaceutical Co, was the first Rho kinase inhibitor studied in a clinical trial to lower IOP. It is thirty times more effective in inhibiting Rho kinase activity than Y-27632, and in animal studies, topical administration of SNJ-1656 resulted in large reductions in outflow resistance and IOP88, 117. The phase 1 study evaluated the ocular hypotensive efficacy and safety compared to the vehicle in healthy subjects after a single instillation and after 7 days of repeated (QD or BID) instillation. Peak IOP reduction was achieved at 4 hours after instillation, 3.0 ± 1.2 mm Hg with the highest concentration tested (0.1%). Conjunctival hyperemia was observed in all patients but resolved in most cases within 24 hours after a single instillation103.
The placebo-controlled phase 2 study evaluated various concentrations of SNJ-1656 (0.03% to 0.1%) for 7 days in patients with POAG and OHT. The relative IOP reduction compared to placebo from a baseline of about 22 mmHg was 3 – 3.5 mmHg at peak (2 hours after instillation of the morning dose) and 2 mmHg at trough (prior to instillation of the morning dose). Mild to moderate conjunctival hyperemia occurred in about 60% of subjects. One subject experienced hepatic dysfunction that resolved after discontinuation of treatment; however, no other details were reported104.
AR-12286
AR-12286 was developed by Aerie Pharmaceuticals by screening a collection of water soluble aminoisoquinoline amides to find those that were both stable and active in affecting the shape of trabecular meshwork cells118. A phase 1 study in normal subjects of AR-12286 0.5% for 8 days demonstrated significant IOP-lowering with an average maximum decrease of approximately 7 mmHg, however, there were frequent side effects including conjunctival hyperemia, ocular irritation, increased lacrimation, and blurred vision106. A larger, placebo-controlled randomized phase 2 clinical trial in patients with POAG or OHT evaluated AR-12286 at a lower maximum concentration (0.25%) over a 3-week study period showed a maximum average pressure reduction of approximately 4.5 mm Hg, as compared to placebo105. The most common side effect was conjunctival hyperemia, occurring in approximately 60% of patients. AR-12286 was abandoned by Aerie Pharmaceuticals, Inc. for use in glaucoma because netarsudil, also developed by Aerie Pharmaceuticals, was judged to have a longer duration of action119.
Ripasudil (K-115)
Ripasudil (Glanatec®) was approved in Japan for the treatment of glaucoma and OHT in September 2014. Ripasudil hydrochloride hydrate was originally discovered by D. Western Therapeutics Institute and developed for the treatment of glaucoma and OHT by Kowa Company, Ltd64. Phase 1 and phase 2 clinical trials as well as a 24-hour time course study established ripasudil 0.4% BID as a clinically useful concentration and dosing frequency for the treatment of glaucoma and OHT108–110. The 0.4% solution lowered IOP on average by 2–4.4 mmHg two hours after instillation in patients with glaucoma or OHT as compared with placebo and continued to deliver statistically significant pressure reduction for at least 7 hours. A non-comparative, one-year, open-label study reported IOP reduction from baseline of 2.6 mmHg at trough and 3.7 mmHg at peak in patients with POAG or OHT after 52 weeks of ripasudil monotherapy112. IOP reduction in the subgroup of patients with baseline IOP ≥ 21 mmHg was 3.8 mmHg at trough and 4.8 mmHg at peak.
The clinical trials demonstrated the dose-dependent and transient nature of conjunctival hyperemia associated with its use108–110. A study specifically designed to investigate the time-course of ripasudil-induced conjunctival hyperemia found peak intensity at 15 minutes after instillation and a gradual return to baseline at 120 minutes120. Another study found that retention was fair with 69% of subjects completing 12 months in the study. Conjunctival hyperemia (76%), blepharitis (21%), and allergic conjunctivitis (20%) were the most commonly reported adverse events attributed to ripasudil monotherapy. Most cases of allergic conjunctivitis had their onset after 12 weeks of therapy, explaining why this adverse reaction was not detected in the earlier clinical trials.
As ripasudil was primarily evaluated as an adjunctive agent for use in combination with commonly used first-line agents, the phase 3 trials were short-term (8-week), placebo-controlled randomized clinical trials designed to evaluate the additive IOP-lowering efficacy of ripasudil 0.4% BID with either timolol 0.5% BID or latanoprost 0.005% QD in patients with POAG or OHT111. Treatment with ripasudil resulted in a lower mean IOP in the timolol group. The additive effect was 0.9 mmHg at trough and 1.6 mmHg at peak. In the latanoprost group, there was no significant difference compared to placebo at trough; at peak, ripasudil resulted in an additional 1.4 mmHg reduction. Conjunctival hyperemia occurred in 65% of subjects in the timolol-ripasudil group and 56% of patients in the latanoprost-ripasudil group whereas the incidence was only 6% and 9% in the respective placebo groups.
Two retrospective studies36, 121 and one small, non-comparative prospective study with results reported after three37 and twelve38 months evaluated adjunctive treatment with ripasudil in Japanese patients already on maximum medical therapy. They demonstrated IOP reductions ranging from 2.6–3.1 mmHg or about 15–16% from baseline. In one of the retrospective studies, there was no significant additional IOP reduction in the subgroup of patients defined as having normal-tension glaucoma36. Two retrospective studies suggest it is safe to use ripasudil to lower IOP in eyes with ocular hypertensive eyes with uveitis122, 123. Study design limitations confound interpretation of the IOP-lowering efficacy results that were reported in these two studies.
Netarsudil (AR-13324)
Netarsudil (Rhopressa®), a Rho kinase inhibitor and norepinephrine transporter inhibitor, was developed by Aerie Pharmaceuticals as one of a class of amino-isoquinoline amide Rho kinase inhibitors. It was approved for use in the United States to treat glaucoma in late 2017. Netarsudil has a longer duration of action than AR-12286 67. It is different from other rho kinase inhibitors in that it not only lowers IOP in animals by lowering outflow resistance91, 124, but it has also been shown to decrease aqueous humor production in monkeys91, 124 and to decrease episcleral venous pressure in rabbits and humans125, 126. These latter mechanisms of IOP reduction have not been reported for other rho kinase inhibitors and may be related to the norepinephrine transporter inhibitory activity of netarsudil89.
A 28-day double-masked randomized clinical trial compared the ocular hypotensive efficacy of netarsudil 0.01% QD, netarsudil 0.02% QD and latanoprost 0.005% QD in patients with OHT or POAG with baseline IOP ≥24 mmHg and <36 mmHg after washout. Mean baseline IOP was about 25.5 mmHg113. IOP lowering observed on day 28 was similar to that on day 14 and was found to be 5.5, 5.7 and 6.8 mmHg in the netarsudil 0.01%, netarsudil 0.02% and latanoprost 0.005% groups, respectively. Neither concentration of netarsudil was as effective as latanoprost nor did they meet the non-inferiority criteria vs. latanoprost (upper 95% confidence interval for the difference in mean diurnal IOP within 1.5 mmHg). In the subgroup of patients with baseline IOP ≤ 26 mmHg, however, the ocular hypotensive efficacy of netarsudil 0.02% was statistically non-inferior to latanoprost. Netarsudil was therefore thought to be relatively more effective in patients with lower baseline IOP, possibly due to its ability to lower episcleral venous pressure. Conjunctival hyperemia, most commonly graded as mild, occurred in 52%, 57% and 15% of subjects in the netarsudil 0.01%, netarsudil 0.02% and latanoprost 0.005% groups, respectively. Increased lacrimation (6%), subconjunctival hemorrhage (5%) were also reported in the two netarsudil groups.
Two double-masked, randomized, parallel-group 3-month clinical trials compared netarsudil ophthalmic solution 0.02% QD (ROCKET-1 and ROCKET-2) or BID (ROCKET-2) to timolol maleate ophthalmic solution 0.5% BID in patients with POAG or OHT with baseline IOP >20 mmHg and <27 mmHg after washout116. The focus on patients with lower baseline IOPs was driven by the earlier results in the phase 2 clinical trial that compared netarsudil to latanoprost. In ROCKET-1, a post-hoc analysis of the subgroup of patients with maximum baseline IOP < 25 mmHg was reported along with the per-protocol outcomes for the entire study population. The pre-determined primary efficacy population in ROCKET-2 was the subgroup of patients with baseline IOP < 25 mmHg. Netarsudil was considered to be non-inferior to timolol if the upper limit of the 2-sided confidence intervals of the difference between groups (netarsudil – timolol) was within 1.5 mmHg at all time points and within 1.0 mmHg at the majority of time points. These non-inferiority criteria were met for netarsudil in both studies among the sub-group of subjects with maximum baseline IOP <25 mmHg. In the entire cohort in ROCKET-1, however, netarsudil did not meet the non-inferiority criteria.
Treatment with netarsudil resulted in discontinuation from the study due to adverse events in a substantial proportion of study subjects, 10–12% in the netarsudil QD group and 30% in the BID group and 1 – 2% in the timolol groups. Conjunctival hyperemia was reported in 50–53% of patients for netarsudil QD and 59% for netarsudil BID, compared to only 8–10% for timolol. Hyperemia incidence and severity remained stable through the 3-month study period. Conjunctival hemorrhage was reported in 13.3–15%, 17%, and 0% of patients in the netarsudil QD, netarsudil BID and timolol groups, respectively. The conjunctival hemorrhages have been described as small, peri-limbal “microhemorrhages”116. Cornea verticillata, seen primarily in the netarsudil groups with an onset of 2–13 weeks were reported in 9% and 15% of patients in the netarsudil QD and netarsudil BID groups, respectively, and < 1% of timolol patients. Verticillata appeared to be similar that that seen with the use of some systemic medications, most notably amiodarone127. This could potentially be of significance in patients with glaucoma who have reduced contrast sensitivity as a result of their underlying optic neuropathy. Visual acuity was not impacted by any of these adverse events, and resolution occurred after cessation of netarsudil.
To evaluate netarsudil as an adjunctive agent in combination with latanoprost, a 28-day randomized, controlled clinical trial evaluated the fixed combination of netarsudil (at concentrations of 0.01% and 0.02%) and latanoprost 0.005% dosed once-daily. The mean diurnal efficacy of the fixed combination formulated with a concentration of netarsudil 0.02% was statistically superior to each of its components alone by a margin of 2.6 mmHg vs. netarsudil and 1.9 mmHg vs. latanoprost (each agent was dosed once-daily)115. The fixed combination of 0.02% netarsudil and 0.005% latanoprost is known as Roclatan (PG324).
This formulation was subsequently evaluated in two large phase 3 clinical trials, the results of which have been released by Aerie Pharmaceuticals, Inc., but not published in the peer-reviewed literature at the time of this writing128, 129. Patients were randomized to (i) a fixed combination of latanoprost 0.005% and netarsudil 0.02% QD, (ii) latanoprost 0.005% QD or (iii) netarsudil 0.02% QD. Mean diurnal IOP in the fixed combination group was significantly lower vs. latanoprost (1.6 mmHg) and netarsudil (2.3 mmHg) after 12 months of treatment. The incidence of discontinuation due to adverse events was about 6–7% in the fixed combination and netarsudil groups by month 3 and about 20% by month 12 compared to about 2% in the latanoprost group at both time points. The types of adverse events that occurred and their frequencies are similar to those observed in previous studies with netarsudil (see Table 2).
Table 2.
Adverse events observed in the 12-month Mercury 1 phase 3 clinical trial of Roclatan and Rhopressa. Patients with known contraindications or hypersensitivity to latanoprost were excluded.124
| Adverse Event (≥5.0% in any group) | Netarsudil 0.02%/Latanoprost 0.005% q.d. (n=243) | Netarsudil 0.02% q.d. (n=243) | Latanoprost 0.005% q.d. (n=237) |
|---|---|---|---|
| Conjunctival Hyperemia | 150 (63.0%) | 125 (51.4%) | 52 (21.9%) |
| Conjunctival Hemorrhage | 31 (13.0%) | 44 (18.1%) | 3 (1.3%) |
| Cornea Verticillata | 42 (17.6%) | 33 (13.6%) | 0 |
| Eye Pruritus | 27 (11.3%) | 22 (9.1%) | 3 (1.3%) |
| Punctate Keratitis | 12 (5.0%) | 18 (7.4%) | 10 (4.2%) |
| Increased Lacrimation | 17 (7.1%) | 20 (8.2%) | 1 (0.4%) |
| Reduced Visual Acuity | 13 (5.5%) | 13 (5.3%) | 6 (2.5%) |
| Blurred Vision | 11 (4.6%) | 15 (6.2%) | 3 (1.3%) |
| Instillation Site Pain | 55 (23.1%) | 60 (24.7%) | 18 (7.6%) |
Other clinical trials
Fasudil was examined in 4 eyes of 4 patients with POAG and no light perception in the study eyes. Baseline IOP was 53.5 ± 3.4 mm Hg and the IOP reductions at 2–4 hours was 8–9 mmHg130. Clinical trials have been completed on AMA-0076 (NCT02136940), ATS-907 (NCT01520116), and INS-117548 (NCT00767793); however, no results have been published as yet in peer-reviewed literature.
Summary of clinical trials
None of the rho kinase inhibitors tested in these clinical trials proved themselves superior to commonly used first-line agents for lowering IOP. Where these new agents are likely to have their greatest utility is as adjunctive agents since their mechanism of action is thought primarily to be one of lowering of aqueous humor outflow resistance, and thus should be somewhat additive to the actions of other agents in clinical use that act either on aqueous inflow or unconventional outflow. Ripasudil’s additional IOP-lowering efficacy when added to timolol is similar to the additive IOP-lowering observed with brimonidine or dorzolamide; however, the incidence of conjunctival hyperemia was substantially higher with ripasudil.109, 131–133 Netarsudil lowers IOP approximately an additional 2 mmHg when added to a PGA. This is in the same range as has been observed with other agents that are commonly used adjunctively with PGAs; however, the incidence of adverse events is higher with netarsudil.33, 115
The fact that pre-clinical studies in animal models demonstrated greater IOP-lowering efficacy than was achieved in human clinical trials may relate to the higher concentration of drugs used in some of the animal studies134 compared to clinical trials113. It could also be a consequence of abnormalities in the outflow pathway in some patients with glaucoma or ocular hypertension that may not respond to Rho kinase inhibition.
Rho kinase inhibitors induce relaxation of vascular smooth muscle explaining the high incidence of conjunctival hyperemia and possibly subconjunctival hemorrhage in these studies. Punctate subconjunctival hemorrhages were previously reported in monkeys and rabbits treated with the rho kinase inhibitor, Y-3998388. The most frequently observed other adverse events included blepharitis, allergic conjunctivitis and cornea verticillata. It is notable that a large proportion of patients withdrew from clinical trials due to adverse events raising some questions about the ease with which these agents can be used in clinical practice. Despite that concern, there are no known a priori contraindications to the use of either ripasudil or netarsudil nor are there any known interactions with other medications.
V. Effects of rho kinase inhibitors on the retina
In some patients with glaucoma, worsening of the disease continues despite seemingly adequate IOP reduction suggesting IOP-independent mechanisms may play a major role in the disease process. For this reason, there is much interest in the development of neuroprotective strategies for glaucoma treatment. Rho kinase activity has been implicated in a variety of neurodegenerative disease processes and many studies have evaluated the possible neuroprotective activity of Rho kinase inhibitors135. There is also extensive evidence that points to the importance of ocular blood flow in glaucoma136, particularly in the context of POAG in patients with lower baseline IOP137. Since Rho kinase inhibitors are known to increase blood flow58, it has been proposed that they might slow progression of glaucomatous optic neuropathy by acting directly to increase perfusion of the retina and optic disc47.
Neuroprotection of retinal ganglion cells
In POAG, it is widely thought that the initial site of neuronal injury is the retinal ganglion cell axon at the level of the lamina cribrosa1. Rho kinase signaling is critical in axonal development, maintenance and regeneration138. Its role is exerted in part through its regulation of many elements of the axonal cytoskeleton, including actin, microtubules and intermediate filaments as well as through regulation of inflammation mediated by activation of NF-κB139. Central nervous system axons are limited in their ability to regenerate when injured due in part to the presence of growth inhibitors in their extracellular milieu. Rho inhibits these extracellular growth inhibitors140. In vitro studies demonstrate that the Y-27632 stimulates neurite growth and central nervous system axonal regeneration141.
Since microglia use Rho kinase signaling to regulate axonogenesis138, it is not surprising that Rho kinase inhibition results in enhanced axonal regeneration. This may indeed be an important component of a neuroprotective or neuro-regenerative strategy for glaucoma therapy; however, what remains to be determined is whether inhibition of this signaling pathway interferes with axonal targeting to the appropriate secondary neuron.
Neuroprotective activity of Rho kinase inhibitors has been demonstrated in the eye. Treatment with the fasudil at the time of iatrogenic retinal detachment in a pig model was associated with reduced photoreceptor degeneration and relative preservation of the rod-bipolar synapse142. Statins have been shown to have neuroprotective activity against glutamate-induced excitotoxicity, a property possibly linked to their inhibitory effect on Rho kinase143, 144. SNJ-1656 promotes regeneration of crushed axons of retinal ganglion cells into the optic nerve of adult cats145. Ripasudil has also been shown to have neuroprotective activity in rodent optic nerve crush injury models146, 147. In one of these studies, topical netarsudil enhanced retinal ganglion cell survival and axonal regeneration. Reduced phosphorylation of cofilin and LIM kinase, two downstream targets in the Rho kinase signaling pathway, was observed in retinal ganglion cells and optic nerve glial cells147.
Significantly elevated levels of RhoA have been found in the optic nerve head of glaucomatous eyes as compared with age-matched controls, supporting a possible role for Rho in glaucomatous neuropathy148. Further investigation, particularly human clinical trials, will be required to determine if these agents are therapeutically effective in neuroprotection in glaucoma beyond their IOP-lowering effect.
Ocular blood flow
Large, population-based studies suggest that lower ocular blood flow and perfusion pressure are associated with glaucoma prevalence and is a risk factor for the incident development of glaucoma and progression of the disease149, 150. Both the optic nerve head and retinal circulation are subject to autoregulation and some investigators have reported evidence of abnormal autoregulation in patients with POAG, particularly those with lower baseline IOP levels137. Strategies to improve retinal and optic nerve blood flow may therefore be beneficial in the treatment of glaucoma and mounting evidence suggests that the Rho kinase signaling cascade may be a therapeutic target.
SNJ-1656 in rabbits significantly increased optic nerve head blood flow after topical administration151. Studies in rabbits also showed that vasoconstriction and reductions in optic head blood flow caused by NG-nitro-L-arginine methyl ester (L-NAME), endothelin-1 (ET-1) or phenylephrine could be mitigated by topical application of fasudil (L-NAME, ET-1) or ripasudil (phenylephrine) with resultant reduction in optic disc cupping and retinal ganglion cell loss 151–153. Ohta et al153 also showed the effect of ripasudil did not correspond temporally to an observed reduction in IOP, suggesting the two processes are independent.
Ohta et al153 further demonstrated that ex vivo rabbit posterior ciliary artery fragments that were pre-contracted in a high-potassium medium relaxed with ripasudil treatment in a dose dependent manner. A similar study demonstrated the same phenomenon with the rho kinase inhibitors, Y-27632 and SNJ-1656154. Other studies in isolated human and bovine retinal arterioles suggest that adenosine-induced vasodilation is partially mediated by nitric oxide whereas rho kinase activation increases myogenic vascular tone and ET-1-induced vasoconstriction155.
As yet, no studies have reported on the effects of Rho kinase inhibitors on ocular blood flow in humans, and the results above suggest such studies may be warranted. Recent studies, however, have shown that extreme dips in nocturnal blood pressure are associated with the glaucoma,156, 157 and thus, even if Rho kinase inhibitors can effectively improve human ocular blood flow, it is unclear if this effect would be sufficient to overcome the deleterious effects of these extreme dips in blood pressure.
VI. Effects of Rho kinase inhibitors on conjunctival scarring after glaucoma surgery
Conventional glaucoma surgeries such as trabeculectomy and tube shunt surgery lower IOP by creating a direct pathway for aqueous humor between the anterior chamber and the subconjunctival space. The most common cause of surgical failure is formation of excessive subconjunctival fibrosis which prevents or limits the egress of aqueous humor158. TGF-β is an important cytokine involved in the regulation of post-surgical wound healing and scar formation in the setting of glaucoma surgery159.
In vitro studies of human Tenon’s fibroblasts demonstrated treatment with TGF-β resulted in rapid activation of a rho-mediated cascade that included cell contraction, cytoskeletal changes, the formation of focal cell adhesions and a subsequent but delayed myofibroblast trans-differentiation mediated by increased expression of α-smooth muscle actin (α-SMA). These responses were blocked by treatment with Y-27632160. In vitro studies with ripasudil showed similar results in human conjunctival fibroblasts with respect to inhibition of TGF-β mediated increased expression of α-SMA161. Subsequent studies in in vivo rabbit models of trabeculectomy demonstrated that Y-27632162 and AMA0526163 improved surgical outcomes.
During the course of observation of patients after trabeculectomy surgery, clinicians can often detect a pattern of gradual fibrosis and contraction of the filtering bleb. Intervention with a rho kinsase inhibitor could potentially serve a dual purpose in such patients in that the drug could be used to lower IOP and to slow or prevent further scar formation.
VII. Conclusions
Most patients with glaucoma or OHT require life-long medical treatment. Many patients with severe damage or low baseline IOP levels require very low target pressures to adequately stabilize their disease process. Despite the many available ocular hypotensive agents, IOP cannot be sufficiently controlled even with multiple-medication regimens in substantial numbers of patients, frequently necessitating incisional surgery with its inherent risks. Furthermore, even with very low IOPs, a small minority of patients experience worsening of their disease and progressive vision loss. These challenges point to the need for additional therapeutic options to lower IOP and to provide neuroprotection of retinal ganglion cells beyond IOP lowering.
Because their primary mechanism of action is different from other ocular hypotensive medications, in that they act to normalize outflow resistance, Rho kinase inhibitors were developed with the hope that in addition to their use in monotherapy, they could provide additional IOP reduction when used with other ocular hypotensive agents. Although these agents have been shown to be effective in lowering IOP, both as monotherapy and adjunctively with beta-blockers and prostaglandin analogs, their side effect profile raises serious concerns about the likelihood of their acceptance by patients.
It is tantalizing that laboratory evidence suggests Rho kinase inhibitors may have neuroprotective activity and might improve ocular blood flow. It would be ideal to have a drug that not only lowers IOP but also protects retinal ganglion cells from IOP-independent factors that contribute to disease progression.
The first generation Rho kinase inhibitors, despite their limitations, are therapeutically effective. More importantly, with the advent of Rho kinase inhibitors, a new door to therapy has been opened. It would be beneficial to develop next generation Rho kinase inhibitors that are targeted to the cells of the outflow pathway or to the retina so that local adverse effects can be minimized while maximizing their therapeutic effects. That might also allow the use of higher drug concentrations with greater pressure lowering and possibly neuroprotective or vasoactive potential.
Acknowledgments
Financial Support: Supported by an unrestricted grant from Research to Prevent Blindness, New York, NY (APT) and NIH EY019696 (MJ)
We gratefully acknowledge support from the National Institutes of Health grant R01 EY01969 (MJ).
Footnotes
Conflicts of Interest: APT: Consultant to Alcon, Bausch & Lomb, Lynntech, Inc., Par Pharmaceuticals, Sandoz, Watson Laboratories, Zeiss.
MJ: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–11. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson M, Erickson K. Mechanisms and routes of aqueous humor drainage. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology. Vol. 4. Philadelphia: WB Saunders Co; Glaucoma; 2000. chapter 193B. [Google Scholar]
- 3.Johnson M. What controls aqueous humour outflow resistance? Experimental Eye Research. 2006;82(4):545–57. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant WM. Clinical measurements of aqueous outflow. Archives of Ophthalmology. 1951;46:113–31. doi: 10.1001/archopht.1951.01700020119001. [DOI] [PubMed] [Google Scholar]
- 5.Grewe R. Zur Geschichte des Glaukoms. Klin Mbl Augenheik. 1986;188(2):167–9. doi: 10.1055/s-2008-1050606. [DOI] [PubMed] [Google Scholar]
- 6.Van Buskirk EM. Changes in the facility of aqueous outflow induced by lens depression and intraocular pressure in excised human eyes. American Journal of Ophthalmology. 1976;82:736–40. doi: 10.1016/0002-9394(76)90011-8. [DOI] [PubMed] [Google Scholar]
- 7.Van Buskirk EM. Anatomic correlates of changing aqueous outflow facility in excised human eyes. Investigative Ophthalmology and Visual Science. 1982;22:625–32. [PubMed] [Google Scholar]
- 8.Van Buskirk EM, Grant WM. Lens depression and aqueous outflow in enucleated primate eyes. American Journal of Ophthalmology. 1973;72:632–40. doi: 10.1016/0002-9394(73)90555-2. [DOI] [PubMed] [Google Scholar]
- 9.Van Buskirk EM. Hazards of medical glaucoma therapy in the cataract patient. Ophthalmology. 1982;89(3):238–41. doi: 10.1016/s0161-6420(82)34811-3. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman TJ, Wheeler TM. Side Effects and Ways to Avoid Them. Ophthalmology. 1982;89(1):76–80. doi: 10.1016/s0161-6420(82)34866-6. [DOI] [PubMed] [Google Scholar]
- 11.Epstein D. Chandler and Grant’s Glaucoma. 3. Philadelphia: Lea & Febiger; 1986. p. 539. [Google Scholar]
- 12.Newell F, Ernest J. Ophthalmology, Principles and concepts. 3. Saint Louis: C.V. Mosby Co; 1974. p. 529. [Google Scholar]
- 13.Wandel T, Spinak M. Toxicity of dipivalyl epinephrine. Ophthalmology. 1981;88(3):259–60. doi: 10.1016/s0161-6420(81)35042-8. [DOI] [PubMed] [Google Scholar]
- 14.Maren TH. The development of topical carbonic anhydrase inhibitors. J Glaucoma. 1995;4(1):49–62. [PubMed] [Google Scholar]
- 15.Group TM-CS. Long-term Glaucoma Treatment with MK-507, Dorzolamide, a Topical Carbonic Anhydrase Inhibitor. Journal of Glaucoma. 1995;4(1):6–10. [PubMed] [Google Scholar]
- 16.Maus TL, Nau C, Brubaker RF. Comparison of the early effects of brimonidine and apraclonidine as topical ocular hypotensive agents. Archives of Ophthalmology. 1999;117(5):586–91. doi: 10.1001/archopht.117.5.586. [DOI] [PubMed] [Google Scholar]
- 17.Brubaker RF. Flow of aqueous humor in humans [The Friedenwald Lecture] Investigative Ophthalmology & Visual Science. 1991;32(13):3145–66. [PubMed] [Google Scholar]
- 18.McCannel CA, Heinrich SR, Brubaker RF. Acetazolamide but not timolol lowers aqueous humor flow in sleeping humans. Graefes Arch Clin Exp Ophthalmol. 1992;230(6):518–20. doi: 10.1007/BF00181771. [DOI] [PubMed] [Google Scholar]
- 19.Wayman LL, Larsson L, Maus TL, Brubaker RF. Additive effect of dorzolamide on aqueous humor flow in patients receiving long-term treatment with timolol. Archives of Ophthalmology. 1998;116(11):1438–40. doi: 10.1001/archopht.116.11.1438. [DOI] [PubMed] [Google Scholar]
- 20.Camras CB, Bito LZ, Eakins KE. Reduction of intraocular pressure by prostaglandins applied topically to the eyes of conscious rabbits. Investigative Ophthalmology & Visual Science. 1977;16(12):1125–34. [PubMed] [Google Scholar]
- 21.Alm A, Villumsen J. PhXA34, a new potent ocular hypotensive drug. A study on dose-response relationship and on aqueous humor dynamics in healthy volunteers. Arch Ophthalmol. 1991;109(11):1564–8. doi: 10.1001/archopht.1991.01080110100045. [DOI] [PubMed] [Google Scholar]
- 22.Crawford K, Kaufman PL. Pilocarpine antagonizes prostaglandin F2 alpha-induced ocular hypotension in monkeys. Evidence for enhancement of Uveoscleral outflow by prostaglandin F2 alpha. Archives of Ophthamology. 1987;105(8):1112–6. doi: 10.1001/archopht.1987.01060080114039. [DOI] [PubMed] [Google Scholar]
- 23.Camras CB, Alm A, Watson P, Stjernschantz J. Latanoprost, a prostaglandin analog, for glaucoma therapy. Efficacy and safety after 1 year of treatment in 198 patients. Latanoprost Study Groups. Ophthalmology. 1996;103(11):1916–24. doi: 10.1016/s0161-6420(96)30407-7. [DOI] [PubMed] [Google Scholar]
- 24.Bill A, Phillips CI. Uveoscleral drainage of aqueous humor in human eyes. Experimental Eye Research. 1971;12:275–81. doi: 10.1016/0014-4835(71)90149-7. [DOI] [PubMed] [Google Scholar]
- 25.Johnson M, McLaren JW, Overby DR. Unconventional aqueous humor outflow: A review. Exp Eye Res. 2017;158:94–111. doi: 10.1016/j.exer.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nau CB, Malihi M, McLaren JW, et al. Circadian Variation of Aqueous Humor Dynamics in Older Healthy Adults. Invest Ophthalmol Vis Sci. 2013;54:7623–9. doi: 10.1167/iovs.12-12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lütjen-Drecoll E, Tamm E. Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2a. Experimental Eye Research. 1988;47(5):761–9. doi: 10.1016/0014-4835(88)90043-7. [DOI] [PubMed] [Google Scholar]
- 28.Toris CB, Camras CB, Yablonski ME. Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. American Journal of Ophthalmology. 1999;128(1):8–14. doi: 10.1016/s0002-9394(99)00076-8. [DOI] [PubMed] [Google Scholar]
- 29.Brubaker RF. Targeting Outflow Facility in Glaucoma Management. Survey of Ophthalmology. 2003;48(2):S17–S20. doi: 10.1016/s0039-6257(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 30.Trese MGJ, Lewis AW, Blachley TS, et al. Changing Initial Glaucoma Medical Therapy Increases Healthcare Resource Utilization. J Ocul Pharmacol Ther. 2017;33(8):591–7. doi: 10.1089/jop.2017.0051. [DOI] [PubMed] [Google Scholar]
- 31.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943–53. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 32.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–13. doi: 10.1001/archopht.120.6.701. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 33.Tanna AP, Rademaker AW, Stewart WC, Feldman RM. Meta-analysis of the efficacy and safety of alpha2-adrenergic agonists, beta-adrenergic antagonists, and topical carbonic anhydrase inhibitors with prostaglandin analogs. Arch Ophthalmol. 2010;128(7):825–33. doi: 10.1001/archophthalmol.2010.131. [DOI] [PubMed] [Google Scholar]
- 34.Liu JH, Medeiros FA, Slight JR, Weinreb RN. Comparing diurnal and nocturnal effects of brinzolamide and timolol on intraocular pressure in patients receiving latanoprost monotherapy. Ophthalmology. 2009;116(3):449–54. doi: 10.1016/j.ophtha.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 35.Liu JH, Medeiros FA, Slight JR, Weinreb RN. Diurnal and nocturnal effects of brimonidine monotherapy on intraocular pressure. Ophthalmology. 2010;117(11):2075–9. doi: 10.1016/j.ophtha.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 36.Sato S, Hirooka K, Nitta E, et al. Additive Intraocular Pressure Lowering Effects of the Rho Kinase Inhibitor, Ripasudil in Glaucoma Patients Not Able to Obtain Adequate Control After Other Maximal Tolerated Medical Therapy. Adv Ther. 2016;33(9):1628–34. doi: 10.1007/s12325-016-0389-3. [DOI] [PubMed] [Google Scholar]
- 37.Inazaki H, Kobayashi S, Anzai Y, et al. Efficacy of the Additional Use of Ripasudil, a Rho-Kinase Inhibitor, in Patients With Glaucoma Inadequately Controlled Under Maximum Medical Therapy. J Glaucoma. 2017;26(2):96–100. doi: 10.1097/IJG.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 38.Inazaki H, Kobayashi S, Anzai Y, et al. One-year efficacy of adjunctive use of Ripasudil, a rho-kinase inhibitor, in patients with glaucoma inadequately controlled with maximum medical therapy. Graefes Arch Clin Exp Ophthalmol. 2017;255(10):2009–15. doi: 10.1007/s00417-017-3727-5. [DOI] [PubMed] [Google Scholar]
- 39.Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–14e1. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gulati V, Fan S, Gardner BJ, et al. Mechanism of Action of Selective Laser Trabeculoplasty and Predictors of Response. Investigative Ophthalmology & Visual Science. 2017;58(3):1462–8. doi: 10.1167/iovs.16-20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bovee CE, Pasquale LR. Evolving Surgical Interventions in the Treatment of Glaucoma. Seminars in Ophthalmology. 2017;32(1):91–5. doi: 10.1080/08820538.2016.1228393. [DOI] [PubMed] [Google Scholar]
- 42.Kopczynski CC, Epstein DL. Emerging trabecular outflow drugs. J Ocul Pharmacol Ther. 2014;30(2–3):85–7. doi: 10.1089/jop.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian B, Kaufman P, Volberg T, et al. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch Ophthalmol. 1998;116:633–43. doi: 10.1001/archopht.116.5.633. [DOI] [PubMed] [Google Scholar]
- 44.Peterson JA, Tian B, Bershadsky AD, et al. Latrunculin-A increases outflow facility in the monkey. Invest Ophthalmol Vis Sci. 1999;40(5):931–41. [PubMed] [Google Scholar]
- 45.Peterson JA, Tian B, Geiger B, Kaufman PL. Effect of Latrunculin-B on Outflow Facility in Monkeys. Experimental Eye Research. 2000;70(3):307–13. doi: 10.1006/exer.1999.0797. [DOI] [PubMed] [Google Scholar]
- 46.Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41(3):619–23. [PubMed] [Google Scholar]
- 47.Inoue T, Tanihara H. Rho-associated kinase inhibitors: a novel glaucoma therapy. Prog Retin Eye Res. 2013;37:1–12. doi: 10.1016/j.preteyeres.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Liu X, Zhong Y. Rho/Rho-associated kinase pathway in glaucoma (Review) International Journal of Oncology. 2013;43(5):1357–67. doi: 10.3892/ijo.2013.2100. [DOI] [PubMed] [Google Scholar]
- 49.Wang SK, Chang RT. An emerging treatment option for glaucoma: Rho kinase inhibitors. Clinical Ophthalmology (Auckland, NZ) 2014;8:883–90. doi: 10.2147/OPTH.S41000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chircop M. Rho GTPases as regulators of mitosis and cytokinesis in mammalian cells. Small GTPases. 2014;5:e29770. doi: 10.4161/sgtp.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4(5):387–98. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- 52.Loirand G. Rho Kinases in Health and Disease: From Basic Science to Translational Research. Pharmacological Reviews. 2015;67(4):1074–95. doi: 10.1124/pr.115.010595. [DOI] [PubMed] [Google Scholar]
- 53.Rath N, Olson MF. Rho - associated kinases in tumorigenesis: re - considering ROCK inhibition for cancer therapy. EMBO reports. 2012;13(10):900–8. doi: 10.1038/embor.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang TY, DerMardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Current Opinion in Cell Biology. 2006;18(1):26–31. doi: 10.1016/j.ceb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 55.McCullough BR, Blanchoin L, Martiel J-L, De La Cruz EM. Cofilin Increases the Bending Flexibility of Actin Filaments: Implications for Severing and Cell Mechanics. Journal of molecular biology. 2008;381(3):550–8. doi: 10.1016/j.jmb.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67(9):545–54. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizuno Y, Isotani E, Huang J, et al. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. American Journal of Physiology - Cell Physiology. 2008;295(2):C358–C64. doi: 10.1152/ajpcell.90645.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao VP, Epstein DL. Rho GTPase/Rho kinase inhibition as a novel target for the treatment of glaucoma. Biodrugs. 2007;21(3):167–77. doi: 10.2165/00063030-200721030-00004. [DOI] [PubMed] [Google Scholar]
- 59.Tanihara H, Inoue T, Yamamoto T, et al. Phase 2 Randomized Clinical Study of a Rho Kinase Inhibitor, K-115, in Primary Open-Angle Glaucoma and Ocular Hypertension. American Journal of Ophthalmology. 2013;156(4):731–6e2. doi: 10.1016/j.ajo.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Wei L, Surma M, Shi S, et al. Novel Insights into the Roles of Rho Kinase in Cancer. Archivum Immunologiae et Therapiae Experimentalis. 2016;64:259–78. doi: 10.1007/s00005-015-0382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koch JC, Tonges L, Barski E, et al. ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS. Cell Death Dis. 2014;5:e1225. doi: 10.1038/cddis.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J, Gao H-y, Wang X-f. The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system. Neural Regeneration Research. 2015;10(11):1892–6. doi: 10.4103/1673-5374.170325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends in Pharmacological Sciences. 2011;32(3):167–73. doi: 10.1016/j.tips.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garnock-Jones KP. Ripasudil: first global approval. Drugs. 2014;74(18):2211–5. doi: 10.1007/s40265-014-0333-2. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto K, Maruyama K, Himori N, et al. The Novel Rho Kinase (ROCK) Inhibitor K-115: A New Candidate Drug for Neuroprotective Treatment in Glaucoma. Investigative Ophthalmology & Visual Science. 2014;55(11):7126–36. doi: 10.1167/iovs.13-13842. [DOI] [PubMed] [Google Scholar]
- 66.Kaneko Y, Ohta M, Inoue T, et al. Effects of K-115 (Ripasudil), a novel ROCK inhibitor, on trabecular meshwork and Schlemm’s canal endothelial cells. Sci Rep. 2016;6:19640. doi: 10.1038/srep19640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sturdivant JM, Royalty SM, Lin C-W, et al. Discovery of the ROCK inhibitor netarsudil for the treatment of open-angle glaucoma. Bioorganic & Medicinal Chemistry Letters. 2016;26(10):2475–80. doi: 10.1016/j.bmcl.2016.03.104. [DOI] [PubMed] [Google Scholar]
- 68.Strzelecka-Kiliszek A, Mebarek S, Roszkowska M, et al. Functions of Rho family of small GTPases and Rho-associated coiled-coil kinases in bone cells during differentiation and mineralization. Biochimica et Biophysica Acta (BBA) - General Subjects. 2017;1861(5 Part A):1009–23. doi: 10.1016/j.bbagen.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21(11):1712–9. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 70.Nohria A, Prsic A, Liu P-Y, et al. Statins inhibit Rho kinase activity in patients with atherosclerosis. Atherosclerosis. 2009;205(2):517–21. doi: 10.1016/j.atherosclerosis.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song J, Deng P-F, Stinnett SS, et al. Effects of Cholesterol-Lowering Statins on the Aqueous Humor Outflow Pathway. Investigative Ophthalmology & Visual Science. 2005;46(7):2424–32. doi: 10.1167/iovs.04-0776. [DOI] [PubMed] [Google Scholar]
- 72.Khawaja AP, Chan MP, Broadway DC, et al. Systemic medication and intraocular pressure in a British population: the EPIC-Norfolk Eye Study. Ophthalmology. 2014;121(8):1501–7. doi: 10.1016/j.ophtha.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaufman PL, Bill A, Bárány EH. Effect of cytochalasin B on conventional drainage of aqueous humor in the cynomolgus monkey: the ocular and cerebrospinal fluids. Exp Eye Res. 1977;25:411–4. doi: 10.1016/s0014-4835(77)80037-7. [DOI] [PubMed] [Google Scholar]
- 74.Svedbergh B, Lutjen-Drecoll E, Ober M, Kaufman PL. Cytochalasin B-induced structural changes in the anterior ocular segment of the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1978;17(8):718–34. [PubMed] [Google Scholar]
- 75.Bill A, Svedbergh B. Scanning electron microscopic studies of the trabecular meshwork and the canal of Schlemm — an attempt to localize the main resistance to outflow of aqueous humor in man. Acta Ophthamologica. 1972;50:295–320. doi: 10.1111/j.1755-3768.1972.tb05954.x. [DOI] [PubMed] [Google Scholar]
- 76.Johnson M, Shapiro A, Ethier CR, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Investigative Ophthalmology and Visual Science. 1992;33(5):1670–5. [PubMed] [Google Scholar]
- 77.Overby DR, Zhou EH, Vargas-Pinto R, et al. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. PNAS. 2014;111(38):13876–81. doi: 10.1073/pnas.1410602111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ethier CR, Read AT, Chan DW. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest Ophthalmol Vis Sci. 2006;47(5):1991–8. doi: 10.1167/iovs.05-0327. [DOI] [PubMed] [Google Scholar]
- 79.Tian B, Peterson JA, Gabelt BT, et al. The Actin Cytoskeleton and Aqueous Outflow. In: Gramer E, Grehn F, editors. Pathogenesis and Risk Factors of Glaucoma. Berlin, Heidelberg: Springer Berlin Heidelberg; 1999. [Google Scholar]
- 80.Tian B, Gabelt B, Peterson J, et al. H-7 increases trabecular facility and facility after ciliary muscle disinsertion in monkeys. Investigative Opthalmology & Visual Science. 1999;40(1):239–42. [PubMed] [Google Scholar]
- 81.Sabanay I, Gabelt BT, Tian B, et al. H-7 effects on the structure and fluid conductance of monkey trabecular meshwork. Arch Ophthalmol. 2000;118(7):955–62. [PubMed] [Google Scholar]
- 82.Overby D, Stamer D, Johnson M. The changing paradigm of outflow resistance generation: towards synergistics models of the JCT and inner wall endothelium. Experimental Eye Research. 2009;88(4):656–70. doi: 10.1016/j.exer.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42(5):1029–37. [PubMed] [Google Scholar]
- 84.Honjo M, Inatani M, Kido N, et al. EFfects of protein kinase inhibitor, ha1077, on intraocular pressure and outflow facility in rabbit eyes. Archives of Ophthalmology. 2001;119(8):1171–8. doi: 10.1001/archopht.119.8.1171. [DOI] [PubMed] [Google Scholar]
- 85.Honjo M, Tanihara H, Inatani M, et al. Effects of Rho-Associated Protein Kinase Inhibitor Y-27632 on Intraocular Pressure and Outflow Facility. Investigative Ophthalmology & Visual Science. 2001;42(1):137–44. [PubMed] [Google Scholar]
- 86.Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res. 2005;80(2):197–206. doi: 10.1016/j.exer.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 87.Nishio M, Fukunaga T, Sugimoto M, et al. The effect of the H-1152P, a potent Rho-associated coiled coil-formed protein kinase inhibitor, in rabbit normal and ocular hypertensive eyes. Curr Eye Res. 2009;34(4):282–6. doi: 10.1080/02713680902783763. [DOI] [PubMed] [Google Scholar]
- 88.Tokushige H, Inatani M, Nemoto S, et al. Effects of Topical Administration of Y-39983, a Selective Rho-Associated Protein Kinase Inhibitor, on Ocular Tissues in Rabbits and Monkeys. Investigative Ophthalmology & Visual Science. 2007;48(7):3216–22. doi: 10.1167/iovs.05-1617. [DOI] [PubMed] [Google Scholar]
- 89.Lin CW, Sherman B, Moore LA, et al. Discovery and Preclinical Development of Netarsudil, a Novel Ocular Hypotensive Agent for the Treatment of Glaucoma. J Ocul Pharmacol Ther. 2017 doi: 10.1089/jop.2017.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van de Velde S, Van Bergen T, Sijnave D, et al. AMA0076, a Novel, Locally Acting Rho Kinase Inhibitor, Potently Lowers Intraocular Pressure in New Zealand White Rabbits with Minimal Hyperemia. Investigative Ophthalmology & Visual Science. 2014;55(2):1006–16. doi: 10.1167/iovs.13-13157. [DOI] [PubMed] [Google Scholar]
- 91.Wang RF, Williamson JE, Kopczynski C, Serle JB. Effect of 0. 04% AR-13324, a ROCK, and norepinephrine transporter inhibitor, on aqueous humor dynamics in normotensive monkey eyes. J Glaucoma. 2015;24(1):51–4. doi: 10.1097/IJG.0b013e3182952213. [DOI] [PubMed] [Google Scholar]
- 92.Isobe T, Mizuno K, Kaneko Y, et al. Effects of K-115, a rho-kinase inhibitor, on aqueous humor dynamics in rabbits. Curr Eye Res. 2014;39(8):813–22. doi: 10.3109/02713683.2013.874444. [DOI] [PubMed] [Google Scholar]
- 93.Rao PV, Deng P, Maddala R, et al. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol Vis. 2005;11:288–97. [PubMed] [Google Scholar]
- 94.Honjo M, Inatani M, Kido N, et al. A myosin light chain kinase inhibitor, ML-9, lowers the intraocular pressure in rabbit eyes. Exp Eye Res. 2002;75(2):135–42. doi: 10.1006/exer.2002.2009. [DOI] [PubMed] [Google Scholar]
- 95.Zhou EH, Krishnan R, Stamer WD, et al. Mechanical responsiveness of the endothelial cell of Schlemm’s canal: scope, variability and its potential role in controlling aqueous humour outflow. Journal of the Royal Society Interface. 2012;9(71):1144–55. doi: 10.1098/rsif.2011.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stamer W, Braakman S, Zhou E, et al. Biomechanics of Schlemm’s canal endothelium and intraocular pressure reduction. Progress in Retinal and Eye Research. 2014;44:86–98. doi: 10.1016/j.preteyeres.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu Z, Overby DR, Scott PA, et al. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp Eye Res. 2008;86(2):271–81. doi: 10.1016/j.exer.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu M, Chen X, Wang N, et al. H-1152 effects on intraocular pressure and trabecular meshwork morphology of rat eyes. J Ocul Pharmacol Ther. 2008;24(4):373–9. doi: 10.1089/jop.2008.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ren R, Li G, Le TD, et al. Netarsudil Increases Outflow Facility in Human Eyes Through Multiple Mechanisms. Invest Ophthalmol Vis Sci. 2016;57(14):6197–209. doi: 10.1167/iovs.16-20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thieme H, Nuskovski M, Nass JU, et al. Mediation of calcium-independent contraction in trabecular meshwork through protein kinase C and rho-A. Investigative Ophthalmology & Visual Science. 2000;41(13):4240–6. [PubMed] [Google Scholar]
- 101.Lepple-Wienhues A, Stahl F, Wiederholt M. Differential smooth muscle-like contractile properties of trabecular meshwork and ciliary muscle. Experimental Eye Research. 1991;53(1):33–8. doi: 10.1016/0014-4835(91)90141-z. [DOI] [PubMed] [Google Scholar]
- 102.Camras LJ, Stamer WD, Epstein D, et al. Circumferential tensile stiffness of glaucomatous trabecular meshwork. Invest Ophthalmol Vis Sci. 2014;55(2):814–23. doi: 10.1167/iovs.13-13091. [DOI] [PubMed] [Google Scholar]
- 103.Tanihara H, Inatani M, Honjo M, et al. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch Ophthalmol. 2008;126(3):309–15. doi: 10.1001/archophthalmol.2007.76. [DOI] [PubMed] [Google Scholar]
- 104.Inoue T, Tanihara H, Tokushige H, Araie M. Efficacy and safety of SNJ-1656 in primary open-angle glaucoma or ocular hypertension. Acta Ophthalmol. 2015;93(5):e393–5. doi: 10.1111/aos.12641. [DOI] [PubMed] [Google Scholar]
- 105.Williams RD, Novack GD, van Haarlem T, et al. Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am J Ophthalmol. 2011;152(5):834–41. doi: 10.1016/j.ajo.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 106.Kopczynski C, Novack GD, Swearingen D, van Haarlem T. Ocular hypotensive efficacy, safety and systemic absorption of AR-12286 ophthalmic solution in normal volunteers. Br J Ophthalmol. 2013;97(5):567–72. doi: 10.1136/bjophthalmol-2012-302466. [DOI] [PubMed] [Google Scholar]
- 107.Skaat A, Jasien JV, Ritch R. Efficacy of Topically Administered Rho-Kinase Inhibitor AR-12286 in Patients With Exfoliation Syndrome and Ocular Hypertension or Glaucoma. J Glaucoma. 2016;25(9):e807–14. doi: 10.1097/IJG.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 108.Tanihara H, Inoue T, Yamamoto T, et al. Phase 1 clinical trials of a selective Rho kinase inhibitor, K-115. JAMA Ophthalmol. 2013;131(10):1288–95. doi: 10.1001/jamaophthalmol.2013.323. [DOI] [PubMed] [Google Scholar]
- 109.Tanihara H, Inoue T, Yamamoto T, et al. Phase 2 randomized clinical study of a Rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2013;156(4):731–6. doi: 10.1016/j.ajo.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 110.Tanihara H, Inoue T, Yamamoto T, et al. Intra-ocular pressure-lowering effects of a Rho kinase inhibitor, ripasudil (K-115), over 24 hours in primary open-angle glaucoma and ocular hypertension: a randomized, open-label, crossover study. Acta Ophthalmol. 2015;93(4):e254–60. doi: 10.1111/aos.12599. [DOI] [PubMed] [Google Scholar]
- 111.Tanihara H, Inoue T, Yamamoto T, et al. Additive Intraocular Pressure-Lowering Effects of the Rho Kinase Inhibitor Ripasudil (K-115) Combined With Timolol or Latanoprost: A Report of 2 Randomized Clinical Trials. JAMA Ophthalmol. 2015;133(7):755–61. doi: 10.1001/jamaophthalmol.2015.0525. [DOI] [PubMed] [Google Scholar]
- 112.Tanihara H, Inoue T, Yamamoto T, et al. One-year clinical evaluation of 0. 4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta Ophthalmol. 2016;94(1):e26–34. doi: 10.1111/aos.12829. [DOI] [PubMed] [Google Scholar]
- 113.Bacharach J, Dubiner HB, Levy B, et al. Double-masked, randomized, dose-response study of AR-13324 versus latanoprost in patients with elevated intraocular pressure. Ophthalmology. 2015;122(2):302–7. doi: 10.1016/j.ophtha.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 114.Levy B, Ramirez N, Novack GD, Kopczynski C. Ocular hypotensive safety and systemic absorption of AR-13324 ophthalmic solution in normal volunteers. Am J Ophthalmol. 2015;159(5):980–5e1. doi: 10.1016/j.ajo.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 115.Lewis RA, Levy B, Ramirez N, et al. Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol. 2016;100(3):339–44. doi: 10.1136/bjophthalmol-2015-306778. [DOI] [PubMed] [Google Scholar]
- 116.Serle JB, Katz LJ, McLaurin E, et al. Two Phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure. Am J Ophthalmol. 2017 doi: 10.1016/j.ajo.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 117.Whitlock NA, Harrison B, Mixon T, et al. Decreased intraocular pressure in mice following either pharmacological or genetic inhibition of ROCK. J Ocul Pharmacol Ther. 2009;25(3):187–94. doi: 10.1089/jop.2008.0142. [DOI] [PubMed] [Google Scholar]
- 118.deLong MA, Sturdivant JM, Royalty SM, et al. Discovery and in vitro SAR of AR-12286, a Potent Kinase Inhibitor for the Treatment of Glaucoma. Investigative Ophthalmology & Visual Science. 2009;50(13):4058. [Google Scholar]
- 119.Kopczynski C. In: Chief Scientific Officer. Tanna AP, editor. Aerie Pharmaceuticals, Inc; 2017. [Google Scholar]
- 120.Terao E, Nakakura S, Fujisawa Y, et al. Time Course of Conjunctival Hyperemia Induced by a Rho-kinase Inhibitor Anti-glaucoma Eye Drop: Ripasudil 0. 4. Curr Eye Res. 2017;42(5):738–42. doi: 10.1080/02713683.2016.1250276. [DOI] [PubMed] [Google Scholar]
- 121.Inoue K, Okayama R, Shiokawa M, et al. Efficacy and safety of adding ripasudil to existing treatment regimens for reducing intraocular pressure. Int Ophthalmol. 2017 doi: 10.1007/s10792-016-0427-9. [DOI] [PubMed] [Google Scholar]
- 122.Yamada H, Yoneda M, Inaguma S, et al. A Rho-Associated Kinase Inhibitor Protects Permeability in a Cell Culture Model of Ocular Disease, and Reduces Aqueous Flare in Anterior Uveitis. J Ocul Pharmacol Ther. 2017;33(3):176–85. doi: 10.1089/jop.2016.0085. [DOI] [PubMed] [Google Scholar]
- 123.Yasuda M, Takayama K, Kanda T, et al. Comparison of intraocular pressure-lowering effects of ripasudil hydrochloride hydrate for inflammatory and corticosteroid-induced ocular hypertension. PLoS One. 2017;12(10):e0185305. doi: 10.1371/journal.pone.0185305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li G, Mukherjee D, Navarro I, et al. Visualization of conventional outflow tissue responses to netarsudil in living mouse eyes. Eur J Pharmacol. 2016;787:20–31. doi: 10.1016/j.ejphar.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kiel JW, Kopczynski CC. Effect of AR-13324 on Episcleral Venous Pressure in Dutch Belted Rabbits. Journal of Ocular Pharmacology and Therapeutics. 2015;31(3):146–51. doi: 10.1089/jop.2014.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sit AJ, Kazemi A, McLaren JW, et al. The Effects of Netarsudil Ophthalmic Solution on Aqueous Humor Dynamics in Humans. Investigative Ophthalmology & Visual Science. 2017;58(8):2112. [Google Scholar]
- 127.Aerie Pharmaceuticals I. Aerie Pharmaceuticals, Inc; Administration USFaD, editor. Presentation Regarding Rhopressa to Dermatologic and Ophthalmic Drugs Advisory Committee of the United States Food and Drug Adminstration. 2017. [Google Scholar]
- 128.Aerie Pharmaceuticals I. Aerie Pharmaceuticals Roclatan™ Mercury 1 Phase 3 Topline Safety Results Conference Call [Google Scholar]
- 129.Aerie Pharmaceuticals I. Aerie Pharmaceuticals Roclatan™ Mercury Phase 3 Topline Efficacy Results Conference Call. 2017 [Google Scholar]
- 130.Pakravan M, Beni AN, Ghahari E, et al. The Ocular Hypotensive Efficacy of Topical Fasudil, a Rho-Associated Protein Kinase Inhibitor, in Patients With End-Stage Glaucoma. Am J Ther. 2017;24(6):e676–e80. doi: 10.1097/MJT.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 131.Boyle JE, Ghosh K, Gieser DK, Adamsons IA. A randomized trial comparing the dorzolamide-timolol combination given twice daily to monotherapy with timolol and dorzolamide. Ophthalmology. 1999;106(12 Suppl):10–6. [PubMed] [Google Scholar]
- 132.Sherwood MB, Craven ER, Chou C, et al. Twice-daily 0.2% brimonidine-0. 5% timolol fixed-combination therapy vs monotherapy with timolol or brimonidine in patients with glaucoma or ocular hypertension: a 12-month randomized trial. Arch Ophthalmol. 2006;124(9):1230–8. doi: 10.1001/archopht.124.9.1230. [DOI] [PubMed] [Google Scholar]
- 133.Craven ER, Walters TR, Williams R, et al. Brimonidine and timolol fixed-combination therapy versus monotherapy: a 3-month randomized trial in patients with glaucoma or ocular hypertension. J Ocul Pharmacol Ther. 2005;21(4):337–48. doi: 10.1089/jop.2005.21.337. [DOI] [PubMed] [Google Scholar]
- 134.Cheng-Wen L, Bryan S, AML, et al. Discovery and Preclinical Development of Netarsudil, a Novel Ocular Hypotensive Agent for the Treatment of Glaucoma. Journal of Ocular Pharmacology and Therapeutics. 2018;34(1–2):40–51. doi: 10.1089/jop.2017.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hensel N, Rademacher S, Claus P. Chatting with the neighbors: crosstalk between Rho-kinase (ROCK) and other signaling pathways for treatment of neurological disorders. Front Neurosci. 2015;9:198. doi: 10.3389/fnins.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Prada D, Harris A, Guidoboni G, et al. Autoregulation and neurovascular coupling in the optic nerve head. Surv Ophthalmol. 2016;61(2):164–86. doi: 10.1016/j.survophthal.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 137.Galassi F, Giambene B, Varriale R. Systemic vascular dysregulation and retrobulbar hemodynamics in normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2011;52(7):4467–71. doi: 10.1167/iovs.10-6710. [DOI] [PubMed] [Google Scholar]
- 138.Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol. 2010;2(2):a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Van de Velde S, De Groef L, Stalmans I, et al. Towards axonal regeneration and neuroprotection in glaucoma: Rho kinase inhibitors as promising therapeutics. Prog Neurobiol. 2015;131:105–19. doi: 10.1016/j.pneurobio.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 140.McKerracher L, Winton MJ. Nogo on the go. Neuron. 2002;36(3):345–8. doi: 10.1016/s0896-6273(02)01018-8. [DOI] [PubMed] [Google Scholar]
- 141.Dergham P, Ellezam B, Essagian C, et al. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22(15):6570–7. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Townes-Anderson E, Wang J, Halasz E, et al. Fasudil, a Clinically Used ROCK Inhibitor, Stabilizes Rod Photoreceptor Synapses after Retinal Detachment. Transl Vis Sci Technol. 2017;6(3):22. doi: 10.1167/tvst.6.3.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu X, Walker CL, Lu Q, et al. RhoA/Rho Kinase Mediates Neuronal Death Through Regulating cPLA2 Activation. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bosel J, Gandor F, Harms C, et al. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem. 2005;92(6):1386–98. doi: 10.1111/j.1471-4159.2004.02980.x. [DOI] [PubMed] [Google Scholar]
- 145.Sagawa H, Terasaki H, Nakamura M, et al. A novel ROCK inhibitor, Y-39983, promotes regeneration of crushed axons of retinal ganglion cells into the optic nerve of adult cats. Exp Neurol. 2007;205(1):230–40. doi: 10.1016/j.expneurol.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 146.Yamamoto K, Maruyama K, Himori N, et al. The novel Rho kinase (ROCK) inhibitor K-115: a new candidate drug for neuroprotective treatment in glaucoma. Invest Ophthalmol Vis Sci. 2014;55(11):7126–36. doi: 10.1167/iovs.13-13842. [DOI] [PubMed] [Google Scholar]
- 147.Shaw PX, Sang A, Wang Y, et al. Topical administration of a Rock/Net inhibitor promotes retinal ganglion cell survival and axon regeneration after optic nerve injury. Exp Eye Res. 2017;158:33–42. doi: 10.1016/j.exer.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goldhagen B, Proia AD, Epstein DL, Rao PV. Elevated levels of RhoA in the optic nerve head of human eyes with glaucoma. J Glaucoma. 2012;21(8):530–8. doi: 10.1097/IJG.0b013e318241b83c. [DOI] [PubMed] [Google Scholar]
- 149.Costa VP, Harris A, Anderson D, et al. Ocular perfusion pressure in glaucoma. Acta Ophthalmol. 2014;92(4):e252–66. doi: 10.1111/aos.12298. [DOI] [PubMed] [Google Scholar]
- 150.Moore D, Harris A, WuDunn D, et al. Dysfunctional regulation of ocular blood flow: A risk factor for glaucoma? Clinical Ophthalmology (Auckland, NZ) 2008;2(4):849–61. doi: 10.2147/opth.s2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tokushige H, Waki M, Takayama Y, Tanihara H. Effects of Y-39983, a selective Rho-associated protein kinase inhibitor, on blood flow in optic nerve head in rabbits and axonal regeneration of retinal ganglion cells in rats. Curr Eye Res. 2011;36(10):964–70. doi: 10.3109/02713683.2011.599106. [DOI] [PubMed] [Google Scholar]
- 152.Sugiyama T, Shibata M, Kajiura S, et al. Effects of fasudil, a Rho-associated protein kinase inhibitor, on optic nerve head blood flow in rabbits. Invest Ophthalmol Vis Sci. 2011;52(1):64–9. doi: 10.1167/iovs.10-5265. [DOI] [PubMed] [Google Scholar]
- 153.Ohta Y, Takaseki S, Yoshitomi T. Effects of ripasudil hydrochloride hydrate (K-115), a Rho-kinase inhibitor, on ocular blood flow and ciliary artery smooth muscle contraction in rabbits. Jpn J Ophthalmol. 2017;61(5):423–32. doi: 10.1007/s10384-017-0524-y. [DOI] [PubMed] [Google Scholar]
- 154.Watabe H, Abe S, Yoshitomi T. Effects of Rho-associated protein kinase inhibitors Y-27632 and Y-39983 on isolated rabbit ciliary arteries. Jpn J Ophthalmol. 2011;55(4):411–7. doi: 10.1007/s10384-011-0048-9. [DOI] [PubMed] [Google Scholar]
- 155.Hein TW, Rosa RH, Jr, Yuan Z, et al. Divergent roles of nitric oxide and rho kinase in vasomotor regulation of human retinal arterioles. Invest Ophthalmol Vis Sci. 2010;51(3):1583–90. doi: 10.1167/iovs.09-4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Melgarejo JD, Lee JH, Petitto M, et al. Glaucomatous Optic Neuropathy Associated with Nocturnal Dip in Blood Pressure: Findings from the Maracaibo Aging Study. Ophthalmology. 2018 doi: 10.1016/j.ophtha.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Choi J, Jeong J, Cho HS, Kook MS. Effect of nocturnal blood pressure reduction on circadian fluctuation of mean ocular perfusion pressure: a risk factor for normal tension glaucoma. Invest Ophthalmol Vis Sci. 2006;47(3):831–6. doi: 10.1167/iovs.05-1053. [DOI] [PubMed] [Google Scholar]
- 158.Skuta GL, Parrish RK., 2nd Wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987;32(3):149–70. doi: 10.1016/0039-6257(87)90091-9. [DOI] [PubMed] [Google Scholar]
- 159.Cordeiro MF, Reichel MB, Gay JA, et al. Transforming growth factor-beta1, -beta2, and -beta3 in vivo: effects on normal and mitomycin C-modulated conjunctival scarring. Invest Ophthalmol Vis Sci. 1999;40(9):1975–82. [PubMed] [Google Scholar]
- 160.Meyer-ter-Vehn T, Sieprath S, Katzenberger B, et al. Contractility as a prerequisite for TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci. 2006;47(11):4895–904. doi: 10.1167/iovs.06-0118. [DOI] [PubMed] [Google Scholar]
- 161.Futakuchi A, Inoue T, Fujimoto T, et al. The effects of ripasudil (K-115), a Rho kinase inhibitor, on activation of human conjunctival fibroblasts. Exp Eye Res. 2016;149:107–15. doi: 10.1016/j.exer.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 162.Honjo M, Tanihara H, Kameda T, et al. Potential role of Rho-associated protein kinase inhibitor Y-27632 in glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2007;48(12):5549–57. doi: 10.1167/iovs.07-0878. [DOI] [PubMed] [Google Scholar]
- 163.Van de Velde S, Van Bergen T, Vandewalle E, et al. Rho kinase inhibitor AMA0526 improves surgical outcome in a rabbit model of glaucoma filtration surgery. Prog Brain Res. 2015;220:283–97. doi: 10.1016/bs.pbr.2015.04.014. [DOI] [PubMed] [Google Scholar]