Abstract
Models of infection have shaped our understanding of programmed memory T cell differentiation, yet whether these models apply to memory programming in the context of transplantation has yet to be defined. Previous work has identified differences in the response of antigen-specific CD8+ T cells to cognate antigen based on the environment in which the antigen is presented. Thus, we hypothesized that programming of antigen specific CD8+ T cells responding to graft and pathogen may be dissimilar. Here we find that antigen-specific CD8+ T cells primed by a skin graft contract faster than those primed by gammaherpesvirus (gHV), yet are able to expand more rapidly upon rechallenge. Moreover, graft-primed antigen-specific CD8+ T cells exhibited higher frequencies of cells secreting IL-2 and demonstrate lower expression of KLRG-1, which are qualities suggestive of increased recall potential. Additionally, the expression of CD127 at a memory time point suggests graft-elicited CD8+ antigen specific T cells are maintained in a less terminally-differentiated state compared to gHV-elicited CD8+ antigen specific T cells, despite fewer cells being present at that time point. Taken together, our findings suggest that the surface marker expression and functional profiles of T cells depends on the priming conditions and may be used to predict immunologic risk following transplantation after traditional allosensitization or heterologous immune priming.
INTRODUCTION
Models of infection have shaped understanding of programmed memory T cell differentiation, and were used in the initial characterization of the phenotype and functionality of CD8+ cells responding to cognate antigen. At the peak of a pathogen-stimulated immune response, the majority of antigen-specific CD8+ T cells expand and terminally differentiate to serve the functional purpose of clearing infection, though a small subset of antigen-specific T cells fated to a different developmental potential have the capacity to survive and generate this memory phenotype (1). These memory precursor cells (MPEC) express higher levels of CD127 (IL-7Rα) and lower levels of killer cell lectin-like receptor G-1 (KLRG-1) than their short-lived effector (SLEC) counterparts (2, 3). SLECs uniformly down-regulate CD127 and up-regulate KLRG-1, confirming an inverse relationship between these two surface markers (4). Additionally, these distinct subsets of CD8+ T cells have been defined by their cytokine profile, with interleukin (IL)-2 production being described as a selective property of long-lived antigen-specific CD8+ T cells (4, 5). Therefore, surface marker expression and cytokine profiles have helped to distinguish T cells destined to die during contraction from those that persist and provide long-term immunologic memory, indicating that initial antigenic encounter imprints a developmental program onto CD8+ T cells that persists for the life of that cell (6).
While these phenotypic and functional characteristics have been used to define the fate of CD8+ T cells through contraction and predict the presence of functional memory in viral models, whether these canonical descriptions hold true in models of transplantation is not known. Following infection, presence of immunologic memory is critically important for host protection upon re-encounter with a pathogen, while on the other hand, in transplantation, immunologic memory can pose a significant barrier to allograft tolerance (7). While “traditional” sensitization from previous allograft, pregnancy, or transfusion limits subsequent donor tolerance, so too can microbial-elicited T cell memory (8–10). In this process, known as heterologous immunity, pathogen-elicited memory T cells are cross-reactive with alloantigens and can precipitate and accelerate allograft rejection. Additionally, bystander T cell activation during an immune challenge can also provide an armamentarium of alloreactive cells. Thus, T cells elicited via different stimuli pose a threat to graft survival, but the question remains whether individual stimuli may elicit distinct differentiation programs that pose differential barriers to graft survival and/or tolerance.
We have previously observed that exposure to rapamycin paradoxically increases the quantity and quality of antigen-specific CD8+ T cell response when the antigen is presented in the context of a viral infection, but fails to do so when the antigen is presented in the context of a transplant, even when an identical model antigen is used (11). These results highlight differences in CD8+ T cell differentiation programs based on the environment in which the antigen is presented. The programming differences between these two groups have not been defined, and whether the memory fate of graft-elicited T cells can be predicted based on knowledge gleaned from viral models has yet to be determined. Because most examples of pathogen-elicited, allo cross-reactive T cell responses have been described in EBV (12–15), we chose gammaherpesvirus68 (gHV), the murine homolog of EBV, to investigate this problem and sought to determine whether transplantation results in the differentiation of KLRG-1hi CD127lo SLEC and KLRG-1lo CD127hi MPEC populations, and to define the functional characteristics of these subsets in the context of transplantation as compared to infection. We find that the expression of surface markers and cytokines in CD8+ T cells responding to skin grafts differs from those responding to an infection, and may help predict the strength of subsequent recall development.
MATERIALS AND METHODS
Mice
C57BL/6 (H-2b) mice were obtained from the National Cancer Institute (Frederick, MD). OT-I (16) and OT-II (17) transgenic mice were purchased from Taconic Farms (Germantown, NY) and bred to Thy1.1+ background at Emory University. mOVA mice (C57BL/6 background, H-2b) (18) were a generous gift from Dr. Marc Jenkins (University of Minnesota, Minneapolis, MN). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committee of Emory University (protocol number: DAR-2002050-092815GN). All surgery was performed under general anesthesia with maximum efforts made to minimize suffering. All animals were housed in specific pathogen-free animal facilities at Emory University.
T cell adoptive transfers
OT-I Thy1.1+ TCR transgenic T cells were harvested from spleen and mLN of naïve animals. Flow cytometry was used to determine the frequency of OT-I T cells prior to adoptive transfer by staining with anti-Vα2 (used by both TCRs) and anti-CD8 (BD Pharmingen, San Diego, CA). Cells were counted with a Nexcelom Cellometer Auto T4 (Nexcelom Bioscience, Lawrence, MA), and 106 WT OT-I T cells with our without the same number of OT-II T cells were transferred into naive C57BL/6 mice i.v. 24 to 48 hours prior to infection or transplantation.
Infections
Mice were infected with 105 PFU gHV68-OVA i.p. on day 0 as has been previously described (19). The virus was a gift of Dr. Sam Speck (Emory University, Atlanta, GA). For Listeria monocytogenes infections, mice were infected with 104 CFU LM-OVA i.p. on day 0 (20).
Skin grafting
Full thickness tail and ear skins were transplanted onto the dorsal thorax of recipient mice and secured with adhesive bandages as previously described (21). Grafts with less than 10% viable tissue remaining were scored as rejected. Where indicated, mice were treated on days 0, 2, 4, and 6 with 500 μg CTLA-4 Ig (abatacept, Bristol Myers-Squibb), 250ug anti-CD154 (clone MR-1, BioXCell, West Lebanon, NJ), and 250 μg anti-CD127 (clone A7R34, BioXCell.)
Secondary T cell effector generation
Mice footpad immunizations have been previously described (22). In brief, SIINFEKL stock [OVA257-264] (GenScript) was diluted in PBS and combined with Incomplete Freund’s Adjuvant (LifeTechnologies) to create an emulsion (final peptide concentration 0.1 mM). 50 μl of the prepared emulsion was injected subcutaneously into each hind foot (100 μl/mouse; 10 μg peptide/mouse) at four weeks post priming. Five days subsequent to footpad injection, popliteal lymph nodes were harvested for analysis.
Flow cytometric analyses
Splenocytes were stained according to the manufacturer’s guidelines with CD4-BUV395, CD8-BV786, CD44-APC-Cy7, KLRG-1-V450, IL-2-PE (BD Pharmingen), LIVE/DEAD Fixable Aqua Dead Cell Stain (ThermoFisher Scientific), and CD-127-PE-Cy7, CD3-BV711, Thy1.1-A700, IFN-γ-PE/Dazzle594 (BioLegend). Flow cytometry was performed using a BD LSR Fortessa Cell Analyzer (BD Biosciences, San Jose, CA). Data collected were analyzed using FlowJo Software (Tree Star, San Carlos, CA) and analyzed with Prism 6 software (GraphPad Software Inc).
Intracellular cytokine staining
Cells were cultured for 4 hours in 96-well round-bottomed plates at a concentration of 1-2.5 × 106 cells/well in 0.2 mL of complete medium and restimulated ex vivo with 1 μg/mL PMA (Sigma Life Sciences) and 1 μg/mL Ionomycin (Sigma Life Sciences) where indicated, in the presence of 1 μg/mL Brefeldin A (BD Biosciences) for 4 hours. The Fix/Perm intracellular staining kit (BD Pharmingen) was used to detect IL-2 (BD Biosciences), TNF (BioLegend), and IFN-γ (BD Biosciences), according to manufacturer’s instructions.
Statistical analyses
Groups were compared by nonparametric t-test or ANOVA (GraphPad Prism Software, GraphPad, La Jolla, CA). Survival curves were compared by log-rank test.
RESULTS
Graft-elicited CD8+ T cell responses exhibit more rapid contraction as compared to gHV-elicited CD8+ T cell responses
To interrogate the differences T cell programming between graft- and pathogen-elicited antigen specific CD8+ T cell responses, monoclonal TCR-transgenic Thy 1.1+ CD8+ T cells specific for chicken ovalbumin (OVA) were adoptively transferred into naïve C57BL/6 (B6) mice. Twenty-four hours later, mice were either infected with OVA-expressing gammaherpesvirus-68 (gHV-OVA) or received an OVA-expressing skin graft (OVA-SG). Gammaherpesvirus 68 was selected in this model given its functional similarity to and genetic homology with human Epstein Barr Virus (EBV). EBV has been shown to elicit T cells that exert allo-HLA cross reactivity (9, 15, 23). Thus, EBV in transplant poses potential risk for rejection by heterologous immunity. To evaluate subsequent differences in the kinetics of the immune response, frequencies and numbers of OVA-specific Thy 1.1+ CD8+ T cells were determined at the peak of the immune response (day 9-10), during contraction (day 16), and at day 30 (Fig. 1A). Spleens were harvested at 9, 16, and 30 days post-infection. The frequency of Thy1.1+ CD8+ antigen-specific T cells harvested from the spleens of mice that were transplanted was significantly lower than the frequency of Thy1.1+ CD8+ antigen specific T cells from the mice that were infected with gHV at all time points (day 9 p <0.05, day 16 p<0.05, day 30 p<0.0001) (Fig. 1B,C). The number of Thy1.1+ CD8+ antigen-specific T cells harvested from the spleens of mice that received a skin graft was also significantly lower than the number of Thy 1.1+ CD8+ antigen specific T cells harvested from the mice that were infected with gHV-OVA at all time points (p<0.01 at Days 9, 16, and 30 post-challenge (Figure 1D).
Figure 1. Magnitude and kinetics of antigen specific T cell responses following skin grafting or gHV infection.
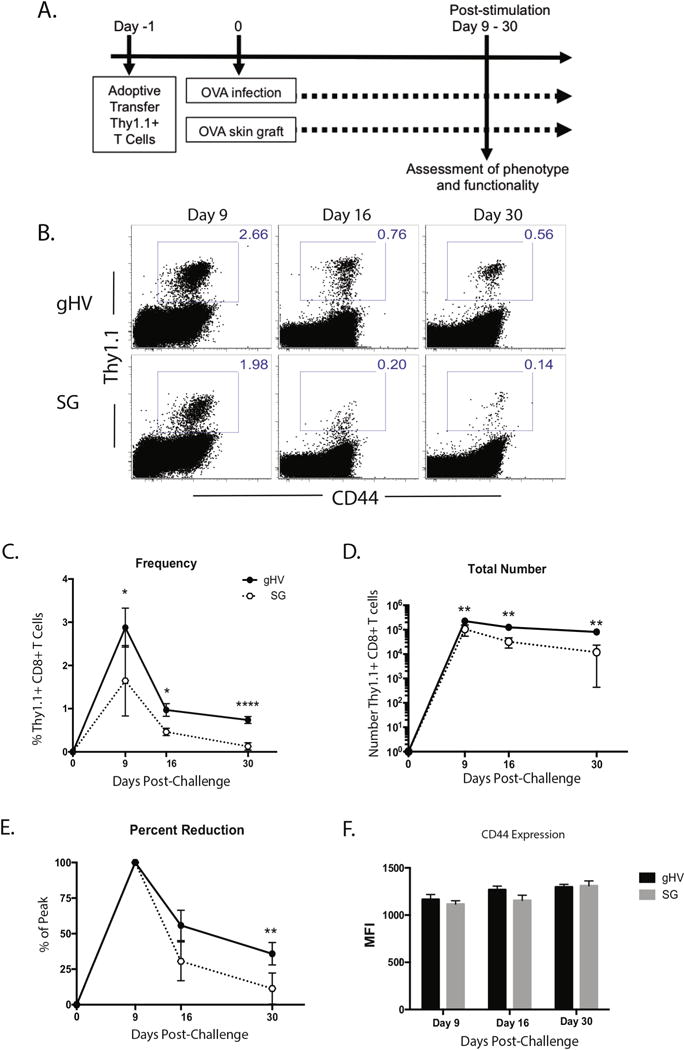
A) 106 WT CD45.2+ OVA-specific monoclonal TCR-transgenic Thy1.1+ CD8+ T cells were transferred into naïve CD45.1+ C57BL/6 mice at day -1. Twenty-four hours later these animals were infected with 105 PFU gHV-OVA or received two OVA-expressing full thickness dorsal skin grafts. Spleens were harvested and processed at multiple time points from day 9 onward and cells were collected for surface and intracellular cytokine analyses. B) Representative flow cytometric staining of Thy1.1+ CD8+ antigen-specific T cells at days 9, 16, and 30. C) The frequency of Thy 1.1+ CD8+ antigen-specific T cells harvested after gHV and skin graft priming was different at days 9, 16, and 30. D) The total number of Thy1.1+ CD8+ antigen-specific T cells was different at day 9, 16, 30. E) Peak numbers were characterized as mean number of Thy 1.1+ CD8+ antigen-specific T cells harvested at day 9 after gHV and skin graft priming. The percent reduction in Thy 1.1+ CD8+ antigen-specific T cell number from day 9 to day 16 was not statistically different, but was statistically different at day 30. F) CD44 expression among Thy 1.1+ CD8+ antigen-specific T cells stimulated by graft and pathogen is similar across all time points. Representative of 3 independent experiments with 5-10 mice/group. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns = not significant.
In order to assess the relative amount of contraction in each of the immune responses, the difference in the numbers of Thy1.1+ CD8+ antigen specific T cells present from day 9 (peak) to day 16 was calculated, and no differences were detected. However, the percent reduction in Thy1.1+ CD8+ antigen specific T cell number between day 9 and day 30 was statistically greater for graft-elicited T cells relative to gHV-elicited T cells (Figure 1E). Specifically, there was a nearly 89% reduction in Thy1.1+ CD8+ antigen specific T cell number on day 30 relative to the peak on day 9 after skin graft priming, as compared to an approximate 64% reduction in Thy1.1+ CD8+ antigen specific T cell number on day 30 relative to the peak on day 9 after infectious priming. These differences in frequency, number, and percent reduction from peak could not be explained by a difference in activation given that the expression of CD44 remained similar between the two groups (Figure 1F).
Skin-graft-elicited CD8+ antigen-specific T cells express lower levels of KLRG-1 than those generated following infection
Previous models of infection have described memory precursor effector cell (MPEC) phenotype as low KLRG-1 expression and high CD127 expression. Based on the reduced degree of contraction after infection as compared to skin grafting, one would expect a higher frequency of MPECs after infection during the effector phase of the response. In order to test this, the effector phase of the response was examined and surface marker expression studied by flow cytometry. At day 9, KLRG-1 expression in skin graft-primed Thy 1.1+ CD8+ antigen-specific T cells was significantly lower than that in infection-primed Thy 1.1+ CD8+ antigen-specific T cells. These differences were also observed at 16 and 30 days post-infection (Figure 2A–C). Conversely, there was no difference in CD127 expression at day 9, though day 30 expression of CD127 in skin graft-primed Thy 1.1+ CD8+ antigen-specific T cells was significantly higher than that of gHV-OVA-primed cells (Figure 2D). In order to exclude the possibility that these differences in KLRG-1 expression were specific to gHV, another infection was used as a comparator. After adoptively transferring Thy1.1+ CD8+ antigen specific T cells into B6 mice, cells were introduced to the cognate OVA antigen expressed by either Listeria monocytogenes (LM) or a skin graft. Similar to what was previously observed with gHV, skin graft-primed Thy 1.1+ CD8+ antigen-specific T cells showed lower KLRG-1 expression than LM-primed Thy 1.1+ CD8+ antigen-specific T cells (Fig. 2E–F).
Figure 2. Skin-graft-elicited Thy1.1+ CD8+ cells can be distinguished from those elicited by gHV infection based on KLRG-1 expression.
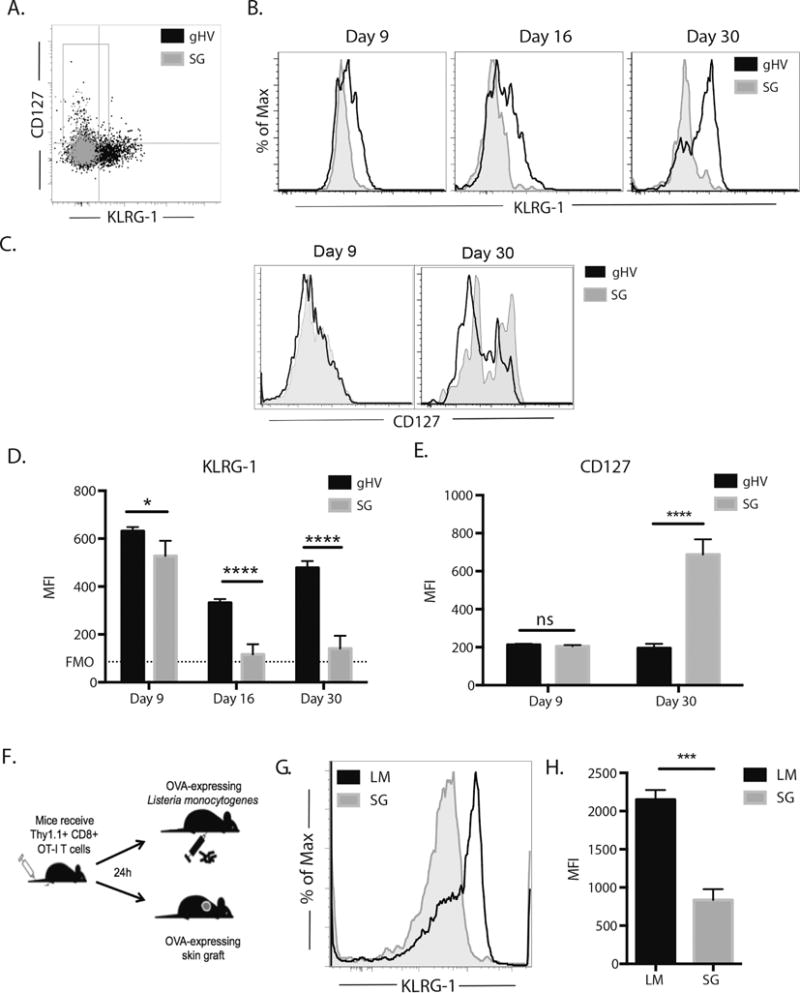
A) Representative flow cytometric staining of Thy1.1+ CD8+ antigen-specific T cells indicating the increased expression of KLRG-1 expression in gHV-primed Thy1.1+ CD8+ antigen-specific T cells as compared to similar skin graft-primed cells at day 9. B) Histogram representation of the increased expression of KLRG-1 in gHV-primed Thy1.1+ CD8+ antigen-specific T cells as compared to skin graft -primed cells at day 9, 16, and 30. C) Histogram representation of the increased expression of CD127 in gHV-primed Thy1.1+ CD8+ antigen-specific T cells as compared to skin graft -primed cells at days 9 and 30. D, Based on the gMFI, gHV-primed Thy1.1+ CD8+ antigen- specific T cells show increased expression of KLRG-1 as compared to skin graft -primed cells at day 9, day 16, and day 30. E), Based on gMFI, there is no difference in expression in CD127 at day 9 between gHV- and skin graft -primed antigen-specific Thy1.1+ CD8+ T cells, however skin graft -primed Thy1.1+ CD8+ antigen-specific T cells show increased expression of CD127 at memory. F) Histogram representation of increased KLRG-1 expression on Listeria monocytogenes (LM)-primed Thy1.1+ CD8+ antigen-specific T cells as compared to skin graft -primed cells at day 10. G) Graphical depiction of KLRG-1 expression by gMFI showing increased expression of KLRG-1 in LM-primed Thy1.1+ CD8+ antigen-specific T cells as compared to similar skin graft-primed cells at day. Representative of 3 independent experiments with 5-10 mice/group. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns = not significant.
Cytokine profiles of skin graft- versus gHV-elicited antigen-specific CD8+ T cells differ at the peak of the response
Given the phenotypic differences of Thy 1.1+ CD8+ antigen-specific cells primed in different environments, cytokine profile was assessed to determine whether the functionality of these cells mirrored the phenotypic profile as would be predicted based on viral models. After adoptively transferring antigen specific CD8+ T cells and priming with either gHV-expressing OVA or skin graft-expressing OVA, Thy1.1+ CD8+ antigen specific cells were harvested from the spleen at day 9 post-infection. After four hours of in vitro stimulation using PMA and ionomycin, cytokine production was evaluated. Skin graft-elicited Thy1.1+ CD8+ antigen-specific T cells produced more IL-2 and less IFN-γ than the gHV-elicited Thy1.1+ CD8+ antigen-specific T cells (Fig. 3A–B). Thus, the cytokine profile of graft-elicited cells was consistent with that of KLRG-1lo cells (MPECs), which exhibit enhanced IL-2 expression as compared to KLRG-1hi cells (SLECs).
Figure 3. Cytokine profiles of skin graft- versus gHV-elicited antigen-specific Thy1.1+ CD8+ T cells are different at the peak of the response.
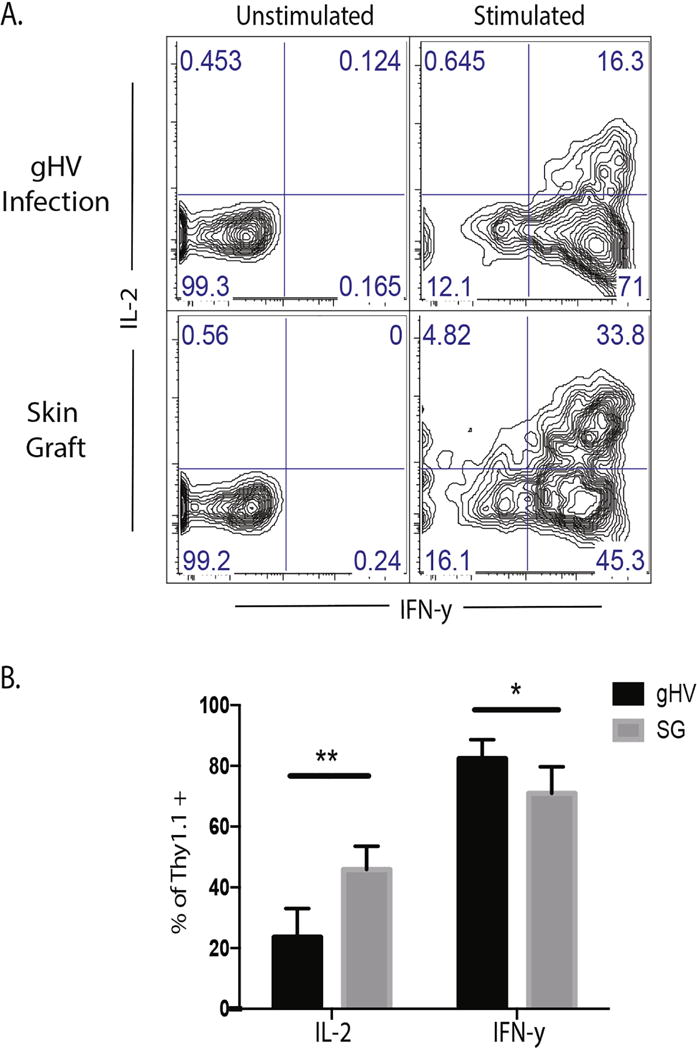
A) Splenocytes were harvested at day 9 and stimulated with PMA/Ionomycin. The frequency of IFN-γ and IL-2 producing cells was determined by flow cytometric staining. B) The results from figure 4A were quantitated. Skin graft-elicited antigen-specific Thy1.1+ CD8+ T cells show increased frequency of IL-2 producing cells and decreased frequency of IFNγ producing cells at day 9. Representative of 3 independent experiments with 5-10 mice/group. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns = not significant.
The context in which antigen is presented may affect recall potential of Thy1.1+ CD8+ antigen specific T cells
While the reduced frequency and number and accelerated contraction of Thy1.1+ CD8+ antigen specific T cells primed by skin graft suggested a potentially less robust antigen-specific memory T cell population, the phenotypic and functional characteristics of antigen-specific CD8+ T cells generated by a graft were consistent with the classically-defined MPEC phenotype (2, 3, 6). To interrogate recall potential of cells primed in different environments, cells were primed with either gHV-OVA or SG-OVA as previously described. Four weeks after priming, mesenteric lymph nodes were harvested to assess the memory status of Thy1.1+ CD8+ antigen specific T cells primed under these different conditions. A second cohort of mice received footpad immunization with SIINFEKL (OVA257-264) to generate secondary effectors. Draining popliteal nodes were harvested five days after rechallenge (Figure 4A).
Figure 4. The context in which antigen is presented may affect recall potential of Thy1.1+ CD8+ antigen specific T cells.
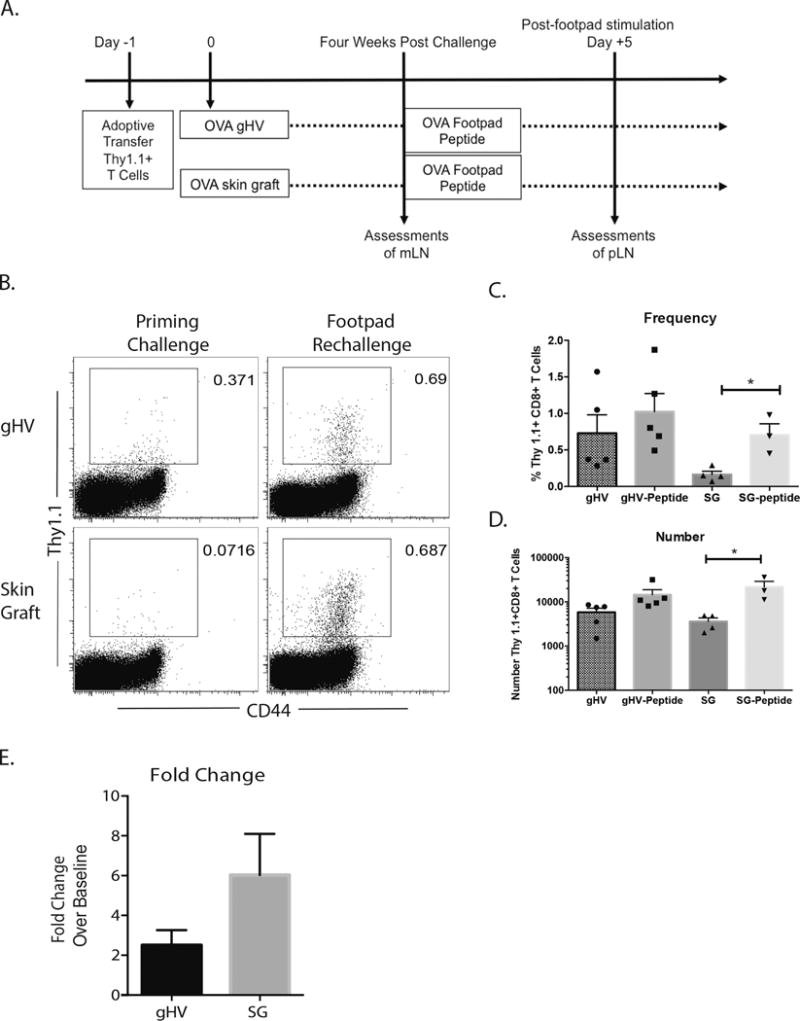
A) Experimental design for generation of secondary effectors and assessments. OVA-specific monoclonal TCR-transgenic Thy1.1+ T cells were transferred into C57BL/6 mice at day -1. The cells were then exposed OVA antigen in vivo by OVA expressing gHV or skin grafts at day zero. Mesenteric lymph nodes were harvested four weeks after primary challenge for baseline assessments and draining popliteal lymph nodes were harvested 5 days post footpad rechallenge. B) Representative flow cytometric staining of Thy1.1+ CD8+ antigen-specific T cells at four weeks post priming and five days after rechallenge. C) The frequency of Thy 1.1+ CD8+ antigen-specific T cells harvested after gHV and skin graft priming was not statistically different between priming environments. D) The number of Thy 1.1+ CD8+ antigen-specific T cells harvested after gHV and skin graft priming was not statistically different between priming environments. E) The fold change in number from average numbers of Thy 1.1+ CD8+ antigen-specific T cells was not statistically different, but there appears to be a trend toward a greater fold change in the number of Thy 1.1+ CD8+ antigen-specific T cells primed by skin graft. Representative of 3 independent experiments with 5-10 mice/group. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns = not significant.
Thy1.1+ CD8+ antigen specific T cells primed under both conditions showed a trend toward expansion after recall stimulus as evidenced by an increase in frequency and number of Thy1.1+ CD8+ antigen specific T cells, however, under these conditions only cells primed via a skin graft reached statistical significance (p<0.05) (Figure 4B–D). In comparing the fold change of Thy1.1+ CD8+antigen specific T cells numbers found after rechallenge to the average number of Thy1.1+ CD8+antigen T cells found four weeks after priming, the number of Thy1.1+ CD8+antigen specific T cells primed by skin graft and rechallenged with footpad peptide increased by 6.03 fold, while the number of Thy1.1+ CD8+antigen specific T cells primed by gHV and rechallenged with footpad peptide increased by only 2.5 fold. These results suggest that skin graft-elicited memory T cells are capable of more robust recall responses as compared to pathogen-elicited memory T cells.
CD127 blockade differentially impacts graft survival in recipients possessing graft vs. gHV-primed CD8+ memory T cells
Based on the observation that donor-reactive CD8+ memory T cells elicited via a prior transplant exhibited a more MPEC-like phenotype with greater expression of CD127, we posited that graft-elicited CD8+ memory T cell population may be more reliant on CD127-mediated signals for survival and/or recall responses, and therefore more susceptible to CD127 blockade. To test this, recipients containing either graft- or gHV-elicited CD8+ T cells were rechallenged with an OVA-expressing skin graft and treated with costimulation blockade (CTLA-4 Ig and anti-CD154 (MR-1)) along with anti-CD127. While animals containing gHV-primed CD8+ T cells rapidly rejected their skin grafts with a median survival time of 16 days, the majority of recipients possessing graft-elicited CD8+ memory T cells maintained their grafts for the duration of the observation period (45 days). These data suggest that graft-elicited memory CD8+ T cell populations may be more reliant on IL-7-mediated signals in order to persist and carry out secondary recall responses as compared to gHV-elicited CD8+ memory T cells.
DISCUSSION
We have identified a difference in the response of Thy1.1+ CD8+ antigen-specific T cells to cognate antigen based on the environment in which the antigen is presented. Antigen specific CD8+ T cells, when primed by a skin graft, contract faster than antigen specific CD8+ T cells primed by gHV, yet expand more rapidly upon restimulation. This increased recall potential is consistent with their KLRG-1lo CD127hi phenotype (as originally defined in viral models (4)), and higher frequencies of IL-2-secreting cells. Taken together, this study suggests that KLRG-1/CD127 surface marker expression and IL-2 production depend on the priming conditions, and reveal that the T cell differentiation program induced in the context of transplantation is distinct from those induced in the context of exposure to a viral pathogen.
What are the mechanisms mediating this effect? It is possible that differences in the degree of antigen exposure underlie the observed phenotypic and functional differences, because in previously published work, limiting the amount and duration of antigen exposure during priming resulted in reduced expression of KLRG-1 (24). However, our data do not support this possibility, because it is established that the clearance of Listeria monocytogenes and gammaherpesvirs-68 is different, as antigen clearance after inoculation with Listeria monocytogenes in low dose (105 CFU) is 5 days (25), while completion of antigen clearance and establishment of latency for acute gamma herpesvirus is around 15 days (26). In comparing the response of Thy1.1+ CD8+ antigen-specific T cells to gammaherpesvirus-68, Listeria monocytogenes, and skin graft, we demonstrate that in the face of variable antigen clearance, the phenotypic expression of KLRG-1 on the surface of pathogen-elicited Thy1.1+ CD8+ antigen-specific T cells remains similar to one another, and yet divergent from the expression seen on skin graft-elicited Thy1.1+ CD8+ antigen-specific T cells. Thus, the data presented here do not support the conclusion that variability in the degree of antigen exposure underlies the observed differences in KLRG-1 expression.
Alternatively, the difference in KLRG-1 expression may relate to a difference in the inflammatory milieu associated with infection versus grafting. Data have emerged regarding the role of inflammatory molecules in regulating CD8+ T cell potential (27). For example, IL-12 has been shown to influence the expression of KLRG-1 (28, 29), and type I interferons and IL-6 have potent immunomodulatory effects that can potentiate allograft rejection even after graft tolerance (30). Thus, differences in inflammatory environment associated with infection versus grafting are likely to influence T cell differentiation programs. It is also important to note that differences in memory T cell precursor frequency could impact the relative resistance of the population to CD127 blockade. Nonetheless, our data demonstrate that differential priming conditions result in different frequencies of graft-specific memory T cells and that these differences in numbers of graft-reactive CD8+ T cells could impact susceptibility to CD127 blockade at the population level.
Importantly, identifying distinguishing characteristics of T cells primed by graft versus infection may enable the definition of cellular recall potential within a memory repertoire and ultimately help to stratify risk of rejection. Memory T cells can be a barrier to successful transplantation, but which memory cells pose the greatest risk to the establishment of tolerance remains to be fully elucidated (7). We have observed that while accelerated contraction kinetics resulted in skin graft-primed Thy1.1+ CD8+ T cells forming a smaller memory pool, the surface marker and cytokine expression profile of these cells at the peak of the response bear functional and phenotypic markers of a long lived memory precursors rather than short lived effectors. Specifically, increased expression of CD127 on skin graft-elicited CD8+ T cells may suggest they are less terminally differentiated and better able to serve as secondary effectors on rechallenge relative to pathogen-primed Thy1.1+CD8+ T cells (despite fewer numbers). In support of this, subsequent evaluation of recall potential by peptide rechallenge revealed a trend toward increased expansion by skin graft-primed Thy1.1+CD8+ antigen specific T cells. These data further support the idea that surface marker expression and cytokine profile may be able to predict recall potential during transplantation. Moreover, this finding also suggests that “traditional” sensitization may pose a greater barrier to transplant tolerance than heterologous immunity because it may generate cells that are less differentiated and therefore have better recall potential.
While it is uncertain whether the differences in KLRG-1 expression on human CD8+ T cells would predict recall potential as it pertains to graft rejection, KLRG-1 expression as a marker of terminal differentiation and senescence has been described in humans (31). Emerging data in both non-human primates and humans have shown that presence of memory T cells with less evidence of senescence (i.e., those expressing high levels of CD28 and CD127 and secreting IL-2) may predict propensity toward belatacept (costimulation)-resistant rejection (32, 33). These findings are in line with in our murine model, where we describe lower expression of KLRG-1 at the peak of the response and higher expression of CD127 at memory time points on CD8+ antigen specific T cells that may have a more robust recall potential. Additionally, it is possible, even likely, that maintained expression of KLRG-1 and decreased expression of CD127 are due to continued antigen recognition, as gHV goes latent between 20- 30 days (7). However, the skin grafts are not rejected until about day 20 either, suggesting that antigen should similarly be present in those animals at least at the day 16 timepoint, where we do see differences in KLRG-1 expression (Figure 1C). Moreover, we also observed differences in the expression of KLRG-1 (Figure 1E–F) between graft-elicited cells and those elicited by Listeria, an infection in which the antigen is known to be cleared around day 4-5 post-infection(8).
Most importantly, more granular understanding of the cell subsets that are most capable of precipitating transplant rejection will facilitate development of immunosuppression to more specifically target these subsets. Our data indicate that at four weeks post priming, the antigen specific CD8+ T cells that are associated with the greatest potential for recall are those expressing high levels of CD127, and that blocking this receptor may be one way to overcome a graft-elicited memory barrier. Indeed, the addition of anti-CD127 to costimulation blockade-based immunosuppression in murine models has been previously been shown to prevent the development of allograft rejection and lead to indefinite survival (34), but further investigations will be needed to determine the utility in non-human primates and humans.
One limitation of the study presented here is that while it would be ideal to be able to compare antigen-specific cell responses in the lymph nodes between the graft- and pathogen-primed mice, we were limited to analysis of the spleen because of differences in the compartmentalization of the immune response to the pathogen and graft priming. The response to gHV is not primarily in the lymph nodes; instead it is localized to the spleen. Technical limitations have precluded us from analyzing antigen-specific cells in all secondary lymphoid organs in the organism in order to make this comparison, however, the fact that the numbers are not different at the peak of the response (Figure 1, day 7) leads us to believe that it is not simply that we are missing a large fraction of the antigen-specific cells in one group but not the other.
In sum, our data suggest that by understanding how phenotypic differences in memory CD8+ T cells impacts functionality during recall, it may be possible to characterize those cells that put patients at increased risk for allograft rejection, and may allow for more personalized immunosuppression following transplantation. Further, increased understanding of the cellular and molecular pathways by which stimulation history affects memory differentiation may lead to the identification of novel targets for therapeutic intervention in order to improve outcomes in patients at increased immunologic risk following transplantation. Importantly, our study suggests that patients whose donor-reactive memory T cells were elicited via prior alloimmunization history may be more susceptible to combined belatacept and CD127 blockade as compared to those patients whose donor-reactive memory T cells were elicited via prior infection. To be able to make this determination in the future, identifying and tracking of donor-reactive memory T cells will be necessary.
Figure 5. CD127 blockade differentially impacts graft survival in recipients possessing graft vs. gHV-primed CD8+ memory T cells.
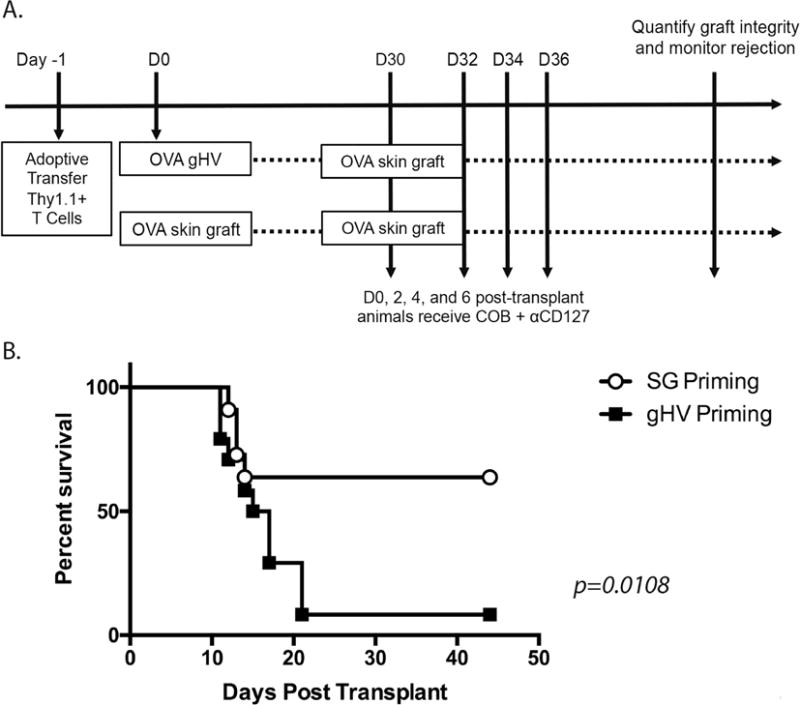
A) 106 WT CD45.2+ OVA-specific monoclonal TCR-transgenic Thy1.1+ CD8+ T cells were transferred into naïve CD45.1+ C57BL/6 mice at day -1. Twenty-four hours later these animals were infected with 105 PFU gHV-OVA or received an OVA-expressing full thickness dorsal skin grafts. At day 30 post-grafting or post-infection, all animals were rechallenged with an OVA-expressing skin graft. Animals were treated on days 0, 2, 4, and 6 post-challenge with CTLA-4 Ig and anti-CD154 costimulation blockade along with 250 ug/dose of anti-CD127 as described in materials and methods and were monitored for graft survival. While animals possessing gHV-primed T cells rejected their grafts with an MST of 16 days (n=24), the majority of animals possessing SG-primed donor-reactive T cells (n=13) went on to accept their allografts (MST undefined, p=0.0139, 3 independent experiments were performed).
HIGHLIGHTS.
Antigen-specific CD8+ T cells primed by a skin graft contract faster than those primed by gammaherpesvirus (gHV).
Graft-primed antigen-specific CD8+ T cells exhibited higher frequencies of cells secreting IL-2 and demonstrate lower expression of KLRG-1, suggestive of increased recall potential.
Expression of CD127 at a memory time point suggests graft-elicited CD8+ antigen specific T cells are maintained in a less terminally-differentiated state compared to gHV-elicited CD8+ antigen specific T cells.
Surface marker expression and functional profiles of T cells depends on the priming conditions.
Acknowledgments
This work was supported by National Institutes of Health NIAID Grants AI073707 and AI104699 (to M.L.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci U S A. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 4.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keppler SJ, Theil K, Vucikuja S, Aichele P. Effector T-cell differentiation during viral and bacterial infections: Role of direct IL-12 signals for cell fate decision of CD8(+) T cells. Eur J Immunol. 2009;39:1774–1783. doi: 10.1002/eji.200839093. [DOI] [PubMed] [Google Scholar]
- 6.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, Sachs DH, Allan J, Madsen JC, Kawai T, Cosimi AB, Benichou G. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011;3:86ra51. doi: 10.1126/scitranslmed.3002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735–739. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 9.Amir AL, D’Orsogna LJ, Roelen DL, Loenen MM van, Hagedoorn RS, Boer R de, Hoorn MA van der, Kester MG, Doxiadis II, Falkenburg JH, Claas FH, Heemskerk MH. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115:3146–3157. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]
- 10.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP. Heterologous immunity provides a potent barrier to transplantation tolerance. Journal of Clinical Investigation. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP, Ford ML. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol. 2010;185:2004–2008. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archbold JK, Macdonald WA, Miles JJ, Brennan RM, Kjer-Nielsen L, McCluskey J, Burrows SR, Rossjohn J. Alloreactivity between Disparate Cognate and Allogeneic pMHC-I Complexes Is the Result of Highly Focused, Peptide-dependent Structural Mimicry. Journal of Biological Chemistry. 2006;281:34324–34332. doi: 10.1074/jbc.M606755200. [DOI] [PubMed] [Google Scholar]
- 13.Burrows SR, Khanna R, Burrows JM, Moss DJ. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: implications for graft-versus-host disease. The Journal of experimental medicine. 1994;179:1155–1161. doi: 10.1084/jem.179.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.R BS, L SS, Rajiv K, M BJ, Maureen R, James M, J MD. Cross- reactive memory T cells for Epstein‐ Barr virus augment the alloresponse to common human leukocyte antigens: degenerate recognition of major histocompatibility complex‐ bound peptide by T cells and its role in alloreactivity. European Journal of Immunology. 1997;27:1726–1736. doi: 10.1002/eji.1830270720. [DOI] [PubMed] [Google Scholar]
- 15.Burrows SR, Silins SL, Moss DJ, Khanna R, Misko IS, Argaet VP. T-Cell Receptor Repertoire for a Viral Epitope in Humans Is Diversified by Tolerance to a Background Major Histocompatibility Complex Antigen. Journal of Experimental Medicine. 1995;182:1703–1715. doi: 10.1084/jem.182.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 17.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunology and cell biology. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 18.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 19.Weck KE, Barkon ML, Yoo LI, Speck SH, Virgin HI. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J Virol. 1996;70:6775–6780. doi: 10.1128/jvi.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen H, Slifka MK, Matloubian M, Jensen ER, Ahmed R, Miller JF. Recombinant Listeria-Monocytogenes as a Live Vaccine Vehicle for the Induction of Protective Antiviral Cell-Mediated-Immunity. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krummey SM, Martinez RJ, Andargachew R, Liu D, Wagener M, Kohlmeier JE, Evavold BD, Larsen CP, Ford ML. Low-Affinity Memory CD8+ T Cells Mediate Robust Heterologous Immunity. J Immunol. 2016;196:2838–2846. doi: 10.4049/jimmunol.1500639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barton E, Mandal P, Speck SH. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu Rev Immunol. 2011;29:351–397. doi: 10.1146/annurev-immunol-072710-081639. [DOI] [PubMed] [Google Scholar]
- 24.Floyd TL, Koehn BH, Kitchens WH, Robertson JM, Cheeseman JA, Stempora L, Larsen CP, Ford ML. Limiting the amount and duration of antigen exposure during priming increases memory T cell requirement for costimulation during recall. Journal of immunology (Baltimore, Md: 1950) 2011;186:2033–2041. doi: 10.4049/jimmunol.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 26.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 28.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol. 2012;12:459–471. doi: 10.1038/nri3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henson SM, Akbar AN. KLRG1–more than a marker for T cell senescence. Age (Dordr) 2009;31:285–291. doi: 10.1007/s11357-009-9100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortes-Cerisuelo M, Laurie SJ, Mathews DV, Winterberg PD, Larsen CP, Adams AB, Ford ML. Increased Pre-Transplant Frequency of CD28+CD4+TEM Predicts Belatacept-Resistant Rejection in Human Renal Transplant Recipients. Am J Transplant. 2017;17(9):2350–2362. doi: 10.1111/ajt.14350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathews DV, Wakwe WC, Kim SC, Lowe MC, Breeden C, Roberts ME, Farris AB, Strobert EA, Jenkins JB, Larsen CP, Ford ML, Townsend R, Adams AB. Belatacept Resistant Rejection is Associated with CD28+ Memory CD8 T cells. Am J Transplant. 2017;17(9):2285–2299. doi: 10.1111/ajt.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathews D, Dong Y, Kim S, Breeden C, Adams A. Costimulation Independent Acute Rejection Requires CD127 Signaling. Am J Transplant. 2017;17(supplement 3) [Google Scholar]


