Abstract
Objectives
African American (AA) emerging adults may become more vulnerable to the consequences of racial discrimination (discrimination) as many begin to occupy racially mixed contexts. Little is known, however, about whether the effect of discrimination on cortisol concentration varies by neighborhood racial composition. We evaluated whether the percentage of White neighbors qualified the association between discrimination and overall cortisol concentration.
Methods
We used self-report data from the Flint Adolescent Study and block-level census data linked to the participant’s home address. Our sample consisted of 241 AA emerging adults (56.8% Female; 19-22 year olds). We used multilevel regression analyses to evaluate whether the percentage of White neighbors modified the association between discrimination and overall cortisol concentration.
Results
Discrimination experienced in the past year, but not chronic discrimination was linked to lower cortisol concentrations among AA emerging adults living in neighborhoods with a high concentration of White neighbors. Specifically, past year discrimination was negatively associated among AAs residing in neighborhoods with 46.9% of White residents or higher.
Conclusions
Our results lay the foundation for future research on racial health disparities by suggesting that contextual factors such as neighborhood racial composition can shape the influence race-based discrimination has on health.
Keywords: racial discrimination, cortisol, stress, neighborhood, health disparities
Since Arnett’s (2000) seminal conception of emerging adulthood, a proliferation of research has documented racial discrimination (discrimination) as a distinctive feature of this life stage for African Americans (AAs; Arnett & Brody, 2008). As 90% of AA emerging adults report discrimination encounters in a given year (Mouzon, Taylor, Woodward, & Chatters, 2017), researchers have theorized that AA emerging adults are especially vulnerable to discrimination experiences as they begin to occupy racially mixed or predominantly White contexts (Arnett & Brody, 2008). Scholars have also reported that exposure to discrimination during this life stage is associated with stress-related alterations in the neuroendocrine response such as a decreased cortisol awakening response and flatter diurnal cortisol slopes (Adam et al., 2015; Zeiders, Hoyt, & Adam, 2014). While scholars have suggested the significance of neighborhood racial composition in the experience of discrimination in relation to other social contexts such as school (e.g., Seaton & Yip, 2009), the modifying role of neighborhood racial composition in the discrimination-cortisol link is limited. To address this gap, we assessed whether residing in predominantly White neighborhoods more strongly attenuated the effect of discrimination on overall cortisol concentrations among AA emerging adults.
Discrimination is a chronic stressor that is ubiquitous in the lives of AA emerging adults (Lee, Neblett, & Jackson, 2015). From the perspective of Clark and colleagues’(1999) biopsychosocial model of racism and health, exposure to discrimination is thought to set in motion a cascade of stress-related physiological responses (e.g., elevated blood pressure) that can, if chronically occurring, accelerate health deterioration (Mays, Cochran, & Barnes, 2007). Researchers identified the hypothalamic-pituitary-adrenal (HPA) axis and its end-product cortisol as a key pathway by which discrimination contributes to poorer health in AAs (Adam et al., 2015). The HPA axis, which activates in response to acute social stressors such as hearing a racial epithet, leads to the production of cortisol (Dickerson & Kemeny, 2004). When the HPA axis is repeatedly activated, as is usually the case for chronic stressors such as discrimination, researchers have reported attenuations in waking cortisol and flatter diurnal cortisol slopes (Adam et al., 2015; Doane et al., 2013). Scholars have attributed these findings to the attenuation hypothesis (Susman, 2006), which asserts that the HPA axis adapts to chronic stress by downregulating cortisol secretion to limit the body’s exposure to cortisol.
Studies with repeated measures of cortisol, spanning only a few hours, have also reported direct and indirect effects between discrimination and overall cortisol concentration (Kaholokula et al., 2012; Lee et al., 2017). For instance, in one study, anxiety symptoms mediated the association between discrimination and cortisol concentration (Lee et al., 2017). Although researchers have adopted several measures to estimate cortisol concentration (e.g., repeated measurements spanning several hours to an entire day), total cortisol concentration can be approximated by calculating the area under the curve with respect to ground with cortisol assessments spanning a few hours (AUCg; see Pruessner et al., 2003 for review). Moreover, controlling for the start time of saliva sampling (see Lee et al., 2017) in multivariate analyses can account for the normative diurnal declines in cortisol that occurs throughout the day.
Although researchers have documented the association between discrimination and HPA dysregulation (e.g., Busse, Yim, Campos, & Marshburn, 2017), only a few scholars have adopted the multi-level intergenerational model (Gee & Payne-Sturges, 2004) to explain how neighborhood racial composition can considerably invoke greater levels of discrimination-related stress (e.g., English, Lambert, Evans, & Zonderman, 2014). That is, scholars have aptly noted that residential factors (e.g., percentage of White neighbors) may lead to greater discrimination exposure, which, in turn, may routinely invoke the physiological stress response and adversely influence health. Researchers have documented, for example, that living in predominantly White neighborhoods is stressful for AAs as they need to contend with racialized messages such as stereotyped behavior and expectations (Stewart, Baumer, Brunson, & Simons, 2009). Furthermore, researchers have suggested that Whites who feel threatened by increasing racial diversity in the US may be more likely to harbor racial biases towards racial minority groups (Craig & Richeson, 2014), which can motivate discriminatory actions (Pager, 2008). Scholars have documented positive associations between discrimination and the percentage of White neighbors among African Americans (English et al., 2014). Consequently, occupying racially diverse or predominantly White contexts may lead to more frequency to discrimination exposure and discrimination-related distress (English et al., 2014), which may result in the attenuation of overall cortisol as suggested by the attenuation hypothesis (Susman, 2006).
Current study
To advance our understanding of the link between discrimination and overall levels of cortisol, our study entailed two aims. The first aim was to assess whether discrimination exposure during the past year and the percentage of White neighbors influenced circulating levels of cortisol. Guided by the biopsychosocial model of racism and health (Clark et al., 1999), we hypothesized that discrimination and percentage of White neighbors would predict lower overall levels of cortisol for their African-American neighbors. Our second aim was to examine whether a higher percentage of White neighbors amplified the association between discrimination exposure and overall cortisol concentration among African-American residents. As researchers suggest a stronger association between discrimination and psychological well-being in neighborhoods with a greater concentration of White residents (e.g., English et al., 2014), we hypothesized that higher percentages of White neighbors would exacerbate the negative effects of discrimination exposure on cortisol levels for AA residents. Lastly, given the chronicity of discrimination exposure, we explored whether average discrimination experience in the past three years, in conjunction with the percentage of White neighbors, influenced cortisol concentration for AAs.
Method
Participants
Participants included 241 AA emerging adults from an ongoing longitudinal study of school-dropout and alcohol/substance use (Zimmerman & Schmeelk-Cone, 2003). To capture youth most vulnerable to high school drop-out (i.e., the goal of the parent study), 9th graders with a grade-point average of 3.0 or below during 8th grade were recruited from the four main public high schools in Flint, Michigan. In addition, participants diagnosed by the schools with a developmental or emotional disability were excluded from the parent study. Participants for this study included those assessed at the seventh wave of the study (2001-2002) which occurred during emerging adulthood (Mage = 22.07, SD = 0.67) and (1) had complete cortisol data; (2) had geo-coded neighborhood-level data; and (3) self-reported as AA. Of note, because the U.S. Census reports data decennially (i.e., 1990, 2000), the 2000 census data was analyzed as it is the most proximal to wave 7. We excluded 6 participants who did meet these three inclusion criteria, but were pregnant or had salivary blood protein contamination levels greater than or equal to 3 mg/DL. With exception to a trend-level difference in cortisol level (i.e., t(241) = −1.74, p = .08), differences were not observed between the study sample and the 6 excluded participants across the study variables.
Study Context
Study participants were recruited from Flint, MI. Following the closure of General Motors manufacturing plants, Flint experienced a 35% decrease in the population, and increases in poverty, unemployment, and crime. Flint has been consistently ranked as having the highest poverty rate among all other Michigan cities (US Census, 2001). In spite of these challenges, Flint has a longstanding history of community activism that is culturally tailored to target racism, enhance education, and address community-based public health concerns (Young, 2013).
Procedure
We received approval from the University of Michigan Institutional Review Board (UMIRB #H03-0001309). Trained interviewers conducted 60 minute, face-to-face interviews with participants in the participants’ home or in the community setting (e.g., public library). Following the interview, participants were self-administered a paper-and-pencil questionnaire that included questions about demographics, racial discrimination experiences, health status, and alcohol and other drug use. Participants were compensated for their time.
Measures
Cortisol
Saliva samples for cortisol assay were collected at three designated time-points during wave 7 interviews. Saliva was only collected from participants who reported not eating, drinking, or using tobacco in the previous hour. Following consent procedures, participants were instructed to rinse their mouth with water. For each saliva collection, participants collected saliva in their mouth for one minute and then expectorated through a straw into a cryotube. Participants were interviewed between 9:50 AM and 6:49 PM and the first saliva sample was taken approximately 10 minutes following consent. The second saliva sample was taken approximately 22 minutes after the first saliva sample. The third and final saliva sample was taken approximately 30 minutes after the second saliva sample. Immediately following collection, the saliva samples were placed on ice and refrigerated, then transported to a −80 degree Fahrenheit freezer for storage.
Cortisol was assessed by a high sensitivity salivary cortisol enzyme immunoassay (Salimetrics, State College, PA). Saliva samples were thawed and centrifuged at 3,000 rpm for 15 minutes prior to assay, and the assay followed standard enzyme immunoassay procedures as described by Klimes-Dougan and colleagues (2001). Intra-assay coefficients of variability ranged from 3.88% to 7.12%, while the inter-assay coefficient of variability ranged from 6.69% to 6.88%. The lower limit of sensitivity of this assay is .007 ug/DL (Schmeelk-Cone, Zimmerman, & Abelson, 2003).
To enhance the interpretability of the repeated salivary cortisol measurements, we calculated the area under the curve with respect to ground (AUCg). This allowed us to assess total circulating levels of cortisol at the first (M = .24 ug/dL, SD = .25), second (M = .20 ug/dL, SD = .24), and third (M = .19 ug/dL, SD = .22) sampling times (Pruesser et al., 2003; Fekedulegn et al., 2007). AUCg values were log-transformed to produce more acceptable residual diagnostics in the regression models reported here.
Racial Discrimination (Discrimination)
We used the 18-item Daily Life Experience scale (DLE; Harrell, Merchant, & Young, 1997) to assess participants’ experiences of perceived interpersonal discrimination during Waves 5 (1998-1999; Mage = 20.05), 6 (1999-2000; Mage = 21.07), and 7 (2000-2001; Mage = 22.07). The DLE assesses the frequency of race-related hassles experienced in the past year on a 6-point Likert-type scale ranging from 0 (never happened to me) to 5 (once a week or more). Items were averaged to capture the participants’ overall discrimination experience during the previous year (αwave5 = .94, αwave6 = .95, αwave7 = .96). In addition, discrimination scores across waves 5 to 7 were averaged to calculate a composite discrimination score. Researchers have demonstrated that the DLE is a reliable and valid measure of racial discrimination for AA emerging adults (e.g., Neblett & Carter, 2012).
Percentage of White Neighbors
To obtain the percentage of White neighbors within a neighborhood block, participants’ addresses were geocoded at the block level using the 2000 census information. In our sample, 232 participants resided in 94 different neighborhood blocks with an average of 5 participants per block. Of note, a strong inverse association between percentage Black and percentage White neighbors (r = −.99) suggests that most neighborhoods consisted of mostly White or mostly Black residents.
Covariates
Participants self-reported their sex at Wave 1. The socioeconomic status (SES) of participants was assessed using two indicators. First, participants reported their level of educational attainment (i.e., no high school degree, high school degree/GED, 1-4 years of college) at wave 7. Second, participants reported their financial situation (i.e., difficulty affording basic necessities) on a 3-point Likert-type scale ranging from 0 (never) to 2 (a lot) at wave 7. The frequency of depressive symptoms at wave 7 was measured using 6-items from the Brief Symptom Inventory (BSI: Derogatis & Spencer, 1982). Participants responded on a Likert-type scale ranging from 0 (never) to 4 (very often) and participants’ scores were averaged (α = .89). We also assessed participants’ lifetime prevalence of chronic illnesses (i.e., asthma, chronic bronchitis/emphysema, diabetes, or high blood pressure/hypertension; coded as “yes” or “no” if ever diagnosed) and the number of doctor visits in the past year due to an illness. Lastly, the start time of saliva collection was included as a control variable to take into account the diurnal decline in cortisol. Specifically, the start time of saliva collection was converted into a decimal value reflecting (e.g., 6:00 AM = 0.25; 12:00 PM = 0.50).
Analytic Approach
Preliminary analyses included descriptive statistics and unadjusted analyses between all study variables. Linear mixed effects models were used to estimate adjusted associations between each predictor and log AUCg, while controlling for residual within and between neighborhood correlations. The key predictor of interest was perceived discrimination within the past year, while the percentage of white residents was included as a level 2 predictor. We additionally adjusted for covariates at level 1 and 2. At level 1, we controlled for participants’ sex, SES, depressive symptoms, start time of saliva collection, lifetime prevalence of chronic health illnesses, number of doctor visits in the past year due to an illness, and the frequency of discrimination at waves 5 and 6. At level 2, we adjusted for the neighborhood poverty rate (i.e., percentage of neighbors with income-to-poverty ratio below 1.5). Next, a cross-level interaction between discrimination and the percentage of White neighbors was examined and the regions of significance plot was examined to probe significant interactions. Lastly, the aforementioned main effects and the cross-level interaction were re-estimated using a composite discrimination score that spanned three years. All models included a random intercept by block group to adjust for residual within-neighborhood correlation. Standard diagnostics were applied to the fitted models (e.g. checking normality of residuals; collinearity) and no threats to the veracity of the inference were identified.
Results
Descriptive statistics and bivariate correlations are presented in Table 1. Notably, the percentage of White neighbors was associated with lower levels of overall cortisol concentration in AA emerging adults, but not depressive symptoms. In addition, discrimination experience was associated with depressive symptoms, but not overall cortisol concentration.
Table 1.
Descriptive Statistics and Intercorrelations of Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | M (SD) or % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Female | – | 56.8% Females | |||||||||||||
| 2. High School Diploma | .07 | – | 45.7% Yes | ||||||||||||
| 3. College | .09 | −.62* | – | 31.3% Yes | |||||||||||
| 4. Financial situation | .05 | .06 | −.04 | – | 0.41 (0.58) | ||||||||||
| 5. Chronic illness | .05 | .02 | −.09 | .05 | – | 0.19 (0.46) | |||||||||
| 6. Doctor visits | .28* | .03 | .06 | .10 | .20* | – | 0.93 (1.31) | ||||||||
| 7. Depressive Symptoms | .13* | .04 | −.11 | .23* | .09 | .16* | – | 0.71 (0.69) | |||||||
| 8. Discrimination (Wave 5) | −.05 | −.04 | .03 | .16* | .08 | .10 | .21* | – | 0.81 (0.82) | ||||||
| 9. Discrimination (Wave 6) | −.13* | −.09 | .01 | .10 | .02 | .04 | .25* | .40* | – | 0.76 (0.84) | |||||
| 10. Discrminiation (Wave 7) | −.23* | −.03 | −.02 | .03 | .14* | .12 | .19* | .37* | .45* | – | 0.77 (0.86) | ||||
| 11. Neighborhood Poverty Rate | .12 | .06 | −.10 | .03 | −.01 | −.03 | .02 | −.09 | −.02 | −.17* | – | 0.39 (0.15) | |||
| 12. Percentage of White Neighbors | .01 | −.08 | .05 | .05 | .00 | −.01 | .07 | −.04 | −.07 | −.03 | −.33* | – | 0.17 (0.24) | ||
| 13. Saliva Collection Start Time | .09 | −.01 | .06 | −.14* | −.01 | .00 | .03 | .02 | .10 | .05 | −.03 | .05 | – | 1:32 PM (4.5 hours) | |
| 14. Overall Cortisol Level (AUCg) | −.24* | .13* | −.17* | .07 | .06 | −.02 | −.12 | −.08 | −.01 | .03 | −.04 | −.17* | −.17* | – | 11.01 (11.91) |
Note.
p < .05.
Past Year Discrimination on Cortisol Concentration
The adjusted models are shown in Table 2. In the model including only main effects, discrimination was not associated with overall levels of cortisol, whereas a negative association was observed between percentage of White neighbors and overall cortisol level (b = −.72). In the subsequent model, we found a cross level interaction between discrimination and percentage of White neighbors, with greater percentage White corresponding to a stronger negative association between discrimination and cortisol (see Figure 1). Specifically, we found that discrimination and cortisol concentration was negatively associated among African Americans (n = 24) who resided in neighborhoods with percentages of White residents equal to or exceeding 46.9%. The residual within-neighborhood ICC for the proportion of variance in cortisol at the neighborhood level was approximately 2%, suggesting the majority of the residual variance in cortisol was due to between person differences.
Table 2.
Fixed and Random Effects for Main Effects-Only and Full Model
| Main Effects Model | Full Model | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| b | s.e. | p -value | 95% CI | b | s.e. | p -value | 95% CI | |
| Intercept | 3.69 | .40 | .00 | 2.90, 4.48 | 3.76 | 0.41 | <.01 | 2.95, 4.57 |
| Individual level factors (Level 1) | ||||||||
| Female | −0.53 | 0.13 | <.01 | −0.78, −0.28 | −0.55 | 0.13 | <.01 | −0.80, −0.30 |
| High school diploma | 0.07 | 0.14 | .48 | −0.21, 0.35 | 0.08 | 0.14 | .58 | −0.20, 0.36 |
| College | −0.22 | 0.17 | .17 | −0.52, 0.09 | −0.19 | 0.16 | .23 | −0.49, 0.11 |
| Financial situation | 0.15 | 0.10 | .13 | −0.04, 0.35 | 0.15 | 0.10 | .13 | −0.04, 0.34 |
| Chronic illness | 0.16 | 0.12 | .21 | −0.09, 0.40 | 0.15 | 0.12 | .22 | −0.09, 0.39 |
| Doctor visits | 0.00 | 0.05 | .94 | −0.09, 0.08 | 0.01 | 0.04 | .89 | −0.08, 0.09 |
| Depressive Symptoms | −0.02 | 0.09 | .81 | −0.19, 0.14 | −0.06 | 0.09 | .47 | −0.23, 0.11 |
| Discrimination (Wave 5) | −0.11 | 0.08 | .17 | −0.25, 0.03 | −0.12 | 0.07 | .09 | −0.27, 0.02 |
| Discrimination (Wave 6) | 0.01 | 0.08 | .92 | −0.14, 0.16 | −0.01 | 0.08 | .99 | −0.16, 0.15 |
| Discrminiation (Wave 7) | −0.03 | 0.07 | .65 | −0.18, 0.11 | 0.06 | 0.09 | .50 | −0.11, 0.24 |
| Saliva Collection Start Time | −1.98 | 0.63 | <.01 | −3.21, −0.74 | −1.87 | 0.63 | <.01 | −3.10, −0.64 |
| Neighborhood level factors (Level 2) | ||||||||
| Percentage of White Neighbors | −0.72 | 0.25 | .01 | −1.22, −0.22 | −0.21 | 0.35 | .55 | −0.91, 0.49 |
| Neighborhood Poverty Rate | −0.57 | 0.42 | .17 | −1.39, 0.24 | −0.60 | 0.41 | .15 | −1.41, 0.22 |
| Cross-level interaction | ||||||||
| Percent white X discrimination | – | – | – | – | −0.61 | 0.30 | .04 | −1.19, −0.02 |
|
| ||||||||
| Variance components | Estimate | s.e. | 95% CI | Estimate | s.e. | 95% CI | ||
|
| ||||||||
| Between neighborhood variance | 0.02 | 0.04 | – | 4.53 × 10⁴, 0.85 | 0.02 | 0.03 | – | 1.92 × 104, 1.33 |
| Within neighborhood variance | 0.61 | 0.07 | – | 0.49, 0.77 | 0.61 | 0.07 | – | 0.49, 0.76 |
Notes. s.e. = standard error. CI = confidence interval. No high school diploma reference group.
Figure 1.
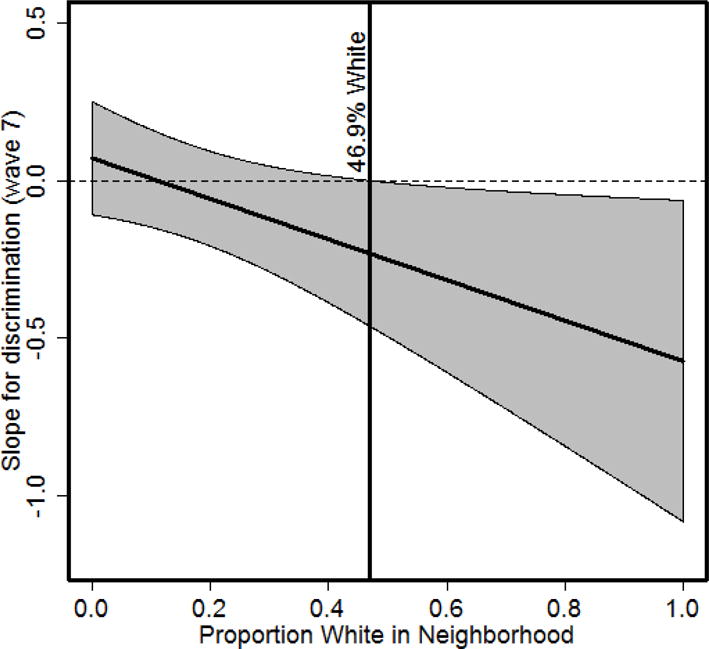
The conditional effects of racial discrimination on cortisol across the proportion of White neighbors. The association between discrimination (Wave 7) and cortisol is significant when the proportion of White neighborhoods is at or above 46.9%.
Sensitivity analyses were conducted to ascertain the validity of the association between discrimination and cortisol concentration across the percentage of White neighbors. First, discrimination in waves 5 and 6 did not interact with the percentage of White neighbors in predicting cortisol concentration. Second, with exception to financial situations (b = .81, p = .01) and sex (b = .70, p = .02), no other study variable, including the cross-level interaction, predicted saliva collection start time.
Average Discrimination Experiences across Three Years on Cortisol Concentration
As shown in Table 3, average discrimination experience within the past three years was unassociated with overall levels of cortisol. Further, an interaction between discrimination (i.e., past three years) and the percentage of White neighbors was not observed (see Table 3).
Table 3.
Fixed and Random Effects for Main Effects-Only and Full Model (Discrimination in the Past Three Years)
| Main Effects Model | Full Model | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| b | s.e. | p -value | 95% CI | b | s.e. | p -value | 95% CI | |
| Intercept | 3.80 | .37 | < .01 | 3.07, 4.52 | 3.93 | .38 | <.01 | 3.18, 4.68 |
| Individual level factors (Level 1) | ||||||||
| Female | −.45 | .11 | < .01 | −0.67, −0.23 | −.45 | .11 | < .01 | −0.67, −0.22 |
| High school diploma | .03 | .13 | .81 | −0.22, 0.28 | .02 | .13 | .86 | −0.23, 0.28 |
| College | −.23 | .14 | .10 | −0.52, 0.05 | −.24 | .14 | .10 | −0.51, 0.04 |
| Financial situation | .11 | .09 | .23 | −0.07, 0.29 | .11 | .09 | .23 | −0.07, 0.29 |
| Chronic illness | .12 | .11 | .29 | −0.10, 0.34 | .11 | .11 | .32 | −0.11, 0.33 |
| Doctor visits | −.01 | .04 | .89 | −0.09, 0.08 | −.01 | .04 | .92 | −0.08, 0.08 |
| Depressive Symptoms | −.04 | .08 | .62 | −0.19, 0.12 | −.05 | .08 | .56 | −0.20, 0.11 |
| Chronic Discrimination (Waves 5 to 8) | −.11 | .09 | .21 | −0.27, 0.06 | −.07 | .11 | .48 | −0.28, 0.13 |
| Saliva Collection Start Time | −2.19 | .57 | < .01 | −3.31, −1.07 | −2.18 | .57 | < .01 | −3.30, −1.06 |
| Neighborhood level factors (Level 2) | ||||||||
| Percentage of White Neighbors | −.80 | .23 | < .01 | −1.26, −.34 | −.65 | .36 | .07 | −1.35, 0.04 |
| Neighborhood Poverty Rate | −.53 | .39 | .17 | −1.29, 0.24 | −.07 | .11 | .48 | −0.28, 0.13 |
| Cross-level interaction | ||||||||
| Percent white X discrimination | – | – | – | – | −.21 | .39 | .58 | −0.98, 0.54 |
|
| ||||||||
| Variance components | Estimate | s.e. | 95% CI | Estimate | s.e. | 95% CI | ||
|
| ||||||||
| Between neighborhood variance | .02 | .03 | – | 0.001, 0.35 | 0.02 | 0.03 | – | 0.001, 0.35 |
| Within neighborhood variance | .57 | .06 | – | 0.46, 0.71 | 0.58 | 0.06 | – | 0.47, 0.71 |
Notes. s.e. = standard error. CI = confidence interval. No high school diploma reference group.
Discussion
Our study highlights the significance of understanding the social conditions under which discrimination influences cortisol concentrations in AA emerging adults. Consistent with the multi-level intergenerational framework (Gee & Payne-Sturges, 2004), our results suggest that discrimination exposure in the past year attenuates overall levels of cortisol for AA emerging adults residing in neighborhoods with a higher percentage of White residents. Several lines of evidence suggest that racial biases and stereotypes (e.g., Blacks are dangerous) may function to make living in predominantly White neighborhoods stressful for AAs (Lane et al., 2007; Olsson et al., 2005). White residents who feel threatened by the upsurge in racial diversification in the US population may hold resentment towards racial minority groups, which can motivate discriminatory actions (Pager, 2008). Brondolo and colleagues (2012) have also suggested that residential segregation can foster interracial anxieties, making racism-related stressors especially damaging in racially mixed or predominantly White contexts. Our results offer preliminary evidence that neighborhoods with a higher concentration of White neighbors exacerbate the attenuation of cortisol concentration among AAs within the context of discrimination.
While a few researchers have documented the long-term effect of discrimination on cortisol functioning (e.g., Adam et al., 2015), recent discrimination experiences may also have an especially potent effect on cortisol. To date, longitudinal research of discrimination and cortisol functioning have documented that discrimination at an earlier developmental period (e.g., youth) shapes cortisol functioning at a later developmental period (i.e., adulthood). It is plausible, however, that within a single developmental period, proximal experiences of discrimination (e.g., 1 year ago) more strongly influence cortisol concentration than distal experiences (e.g., 3 years). Additionally, adolescence, but not emerging adulthood, has been classified as a sensitive period in which discrimination dysregulates cortisol functioning in adulthood (Adam et al., 2015). It is also plausible that racism-related stress more strongly implicates the hippocampus as emerging adults age (Lupien, Ewen, Gunnar, & Heim, 2009). Hippocampal impairment, a biomarker of stress during emerging adulthood and adulthood, has been linked with cortisol dysregulation during stress (Lupien et al., 2009). Existing evidence, and findings from this study, suggest that proximal discrimination experiences, and discrimination experiences during sensitive periods, may shape cortisol concentration among AAs.
Contrary to what we originally conceived, residing in a neighborhood with a higher concentration of White residents was uncorrelated with discrimination experience. Therefore, our findings suggest that certain social contexts such as neighborhood racial composition may not necessarily produce discrimination-induced physiological stress responses through increased discrimination exposure. We attribute this finding to several explanations. First, discrimination may indirectly influence cortisol functioning through psychological mediators such as anxiety symptoms (Lee et al., 2017). Second, the vast majority of neighborhoods in Flint, Michigan, consist of low-income, AA households (>75%). Only 10% of our sample (n = 24) resided in neighborhoods with a higher proportion of White neighbors (> 46.9%), suggesting that our sample does not reflect samples used in other studies connecting neighborhood-level factors to discrimination experience (e.g., English et al. 2014) and cortisol functioning (Adam et al., 2015). In a study by Adam and colleagues (2015), for example, associations between discrimination and cortisol outcomes may not have been altered by contextual factors because their sample consisted of Black and White Americans. Living in Flint, one of the most racially segregated cities in the U.S. (U.S. Census, 2000), may damage health by increasing exposure to interpersonal (e.g., anti-Black sentiments in nearby White neighborhoods) and institutional discrimination (e.g., harsher policing in Black neighborhoods; Kramer & Hogue, 2009).
Although percentage of White neighbors was associated with a stronger attenuation in cortisol concentration among AAs, we did not observe this effect for depressive symptoms. This may be attributed to the demographic and socioeconomic characteristics that are unique to Flint. Unlike other racially segregated cities (e.g., Chicago, Baltimore), AAs residing in racially isolated and integrated neighborhoods in Flint reported comparable levels of family socioeconomic status, depressive symptoms, and anxiety symptoms (Hurd, Stoddard, & Zimmerman, 2013). As exposure to structural disadvantage and financial strain is ubiquitous for AAs residing in all areas of Flint (Hurd et al., 2013), it is possible that neighborhood racial composition may not uniquely influence stress and depressive symptoms.
Our study strengthens the biopsychosocial model of racism and health (Clark et al., 1999) and the Attenuation Hypothesis (Susman, 2006). Our results are preliminary and suggests that discrimination can downregulate the HPA axis leading to an attenuation of overall cortisol levels. This may occur in order to limit the body’s exposure to cortisol (Susman, 2006). Importantly, at higher percentages of white neighbors, discrimination was linked to attenuated levels of overall cortisol (i.e., hyposecretion) in AA emerging adults - a pattern of cortisol release commonly observed in individuals exposed to chronic and severe stress (Miller et al., 2007) such as domestic violence (Griffin, Resick, & Yehuda, 2005). Several researchers have documented the health risks associated with attenuated cortisol levels including heart disease, infectious diseases, and mental health problems (Heim, Ehlert, & Hellhammer, 2000; Miller et al., 2007; Pruessner et al., 2013; Shirtcliff, Peres, Dismukes, Lee, & Phan, 2014). These findings suggest that cortisol may be a crucial pathway by which discrimination influences health. Our results highlight discrimination and neighborhood racial composition as key factors in understanding the psychophysiological mechanisms underlying racial health disparities.
The findings from this study must be considered with its limitations. First, although we account for the start time of saliva collection, we did not have the data to account for the participant’s wake times in our analyses. This issue notwithstanding, our study provides compelling initial evidence that neighborhood context can affect cortisol concentration. Second, as AUCg were calculated within an hour rather than an entire day, our cortisol assessments are an approximation, rather than a definitive value, of cortisol concentration. Nevertheless, this is likely to reduce diurnal variation in our concentration measures making it more difficult to find effects. Given our somewhat small sample it is likely that the effects of neighborhood and discrimination are robust and probably even larger than we found in our study. Third, although effortful engagement on tasks (i.e., interviewing) have been linked with increases in cortisol, attenuated cortisol levels in our study may be a result of maladaptive disengagement from the interview (D’angiulli et al., 2012). Yet, the interview is somewhat familiar to respondents because they have participated in the very similar voluntary interview annually over several years (i.e., this was not a particularly new experience). Fourth, due to documented instabilities in the level of cortisol across days (Doane et al., 2015), one day of cortisol may not generalize to the participant’s global experience. This may be one reason that we did not find effects for the average discrimination experience over three years, but did find such effects for more proximal experiences. Fifth, although researchers have conceived of racial discrimination as a multidimensional construct (e.g., institutional, interpersonal), our measure of discrimination was limited to interpersonal race-related encounters (Williams & Mohammed, 2013). Future studies can advance our understanding of discrimination and cortisol by examining the unique effects of discrimination at the interpersonal, institutional, and cultural level (Williams & Mohammed, 2013). Sixth, start time of cortisol was associated with financial situation and sex, indicating that females and financially strained participants were more likely to be interviewed later in the day. Although this may contribute to bias, we account for these associations by controlling for sex, collection time, and financial situation in our analyses. Lastly, AA emerging adults, like most emerging adults, occupy various social contexts such as higher education and employment (Arnett & Brody, 2008). Little is known, to date, about how racial composition across multiple contexts shape the experience and psychophysiological consequences of discrimination. In one study, for instance, Seaton and Yip (2009) found that school diversity, but not neighborhood diversity, was associated with individual and cultural race-related stressors. Neighborhood racial composition, nonetheless, has been implicated as an important contextual variable that can shape the consequence of discrimination experiences (e.g., cultural racism) even after accounting for other contextual factors (e.g., school diversity; Seaton & Yip, 2009). It is, thus, plausible that racial composition across multiple contexts may shape the physiological consequences of discrimination.
Despite these limitations, our study contributes to the research on racial health disparities in several important ways. By adopting the multi-level intergenerational framework (Gee & Payne-Sturges, 2004), our study provides preliminary evidence that neighborhood racial composition may modify the influence of discrimination on cortisol levels. Our findings are especially relevant to AA emerging adults as they are more likely to traverse beyond their immediate social world into racially diverse or predominantly White contexts where they are at greater risk for experiencing discrimination. Our study lays the foundation for future research to investigate the underlying mechanisms linking discrimination to health across multiracial contexts.
Acknowledgments
This research was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD) (T32 HD 79350-2) for the first author (D.B.L.). The fourth author (M.K.P.) was supported by a grant from the NICHD (2T32 HD007109-36).
References
- Adam EK. Emotion-cortisol transactions occur over multiple time scales in development: Implications for research on emotion and the development of emotional disorders. Monographs of the Society for Research in Child Development. 2012;77(2):17–27. doi: 10.1111/j.1540-5834.2012.00657.x. [DOI] [Google Scholar]
- Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, Eccles JS. Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: A 20-year prospective study. Psychoneuroendocrinology. 2015;62:279–291. doi: 10.1016/j.psyneuen.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiyer SM, Heinze JE, Miller AL, Stoddard SA, Zimmerman MA. Exposure to violence predicting cortisol response during adolescence and early adulthood: Understanding moderating factors. Journal of Youth and Adolescence. 2014;43(7):1066–1079. doi: 10.1007/s10964-014-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist. 2000;55(5):469–480. doi: 10.1037//0003-066X.55.5.469. [DOI] [PubMed] [Google Scholar]
- Arnett JJ, Brody GH. A fraught passage: The identity challenges of African American emerging adults. Human Development. 2008;51:291–293. doi: 10.1159/000170891. [DOI] [Google Scholar]
- Brondolo E, Libretti M, Rivera L, Walsemann KM. Racism and social capital: The implications for social and physical well‐being. Journal of Social Issues. 2012;68(2):358–384. doi: 10.1111/j.1540-4560.2012.01752.x. [DOI] [Google Scholar]
- Busse D, Yim IS, Campos B, Marshburn CK. Discrimination and the HPA axis: current evidence and future directions. Journal of Behavioral Medicine. 2017;40(4):539–552. doi: 10.1007/s10865-017-9830-6. [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist. 1999;54(10):805–816. doi: 10.1037/0003-066X.54.10.805. [DOI] [PubMed] [Google Scholar]
- Craig MA, Richeson JA. More diverse yet less tolerant? How the increasingly diverse racial landscape affects White Americans’ racial attitudes. Personality and Social Psychology Bulletin. 2014;40(6):750–761. doi: 10.1177/0146167214524993. [DOI] [PubMed] [Google Scholar]
- D’Angiulli A, Weinberg J, Oberlander TF, Grunau RE, Hertzman C, Maggi S. Frontal EEG/ERP correlates of attentional processes, cortisol and motivational states in adolescents from lower and higher socioeconomic status. Frontiers in Human Neuroscience. 2012;6:1–16. doi: 10.3389/fnhum.2012.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR, Spencer PM. Brief Symptom Inventory: Administration, scoring, and procedure manual. Baltimor, MD: Clinical Psychometric Research; 1982. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doane LD, Chen FR, Sladek MR, Van Lenten SA, Granger DA. Latent trait cortisol (LTC) levels: Reliability, validity, and stability. Psychoneuroendocrinology. 2015;55:21–35. doi: 10.1016/j.psyneuen.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Doane LD, Mineka S, Zinbarg RE, Craske M, Griffith JW, Adam EK. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Development and Psychopathology. 2013;25(3):629–642. doi: 10.1017/S0954579413000060. [DOI] [PubMed] [Google Scholar]
- English D, Lambert SF, Evans MK, Zonderman AB. Neighborhood racial composition, racial discrimination, and depressive symptoms in African Americans. American Journal of Community Psychology. 2014;54(3–4):219–228. doi: 10.1007/s10464-014-9666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, Miller DB. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosomatic Medicine. 2007;69(7):651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gee GC, Payne-Sturges DC. Environmental health disparities: A framework integrating psychosocial and environmental concepts. Environmental Health Perspectives. 2004;112(17):1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MG, Resick PA, Yehuda R. Enhanced cortisol suppression following dexamethasone administration in domestic violence survivors. American Journal of Psychiatry. 2005;162(6):1192–1199. doi: 10.1176/appi.ajp.162.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell SP, Merchant MA, Young SA. Psychometric properties of the racism and life experiences scales (RaLES) Unpublished Manuscript 1997 [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-relatd boldily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/S0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hunt MO, Wise LA, Jipguep M, Cozier YC, Rosenberg L. Neighborhood racial composition and perceptions of racial discrimination: Evidence from the Black Women’s Health Study. Social Psychology Quarterly. 2007;70(3):272–289. doi: 10.1177/019027250707000306. [DOI] [Google Scholar]
- Kaholokula JKA, Grandinetti A, Keller S, Nacapoy AH, Mau MK. Association between perceived racism and physiological stress indices in Native Hawaiians. Journal of Behavioral Medicine. 2012;35(1):27–37. doi: 10.1007/s10865-011-9330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes–Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13(3):695–719. doi: 10.1017/S0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kramer MR, Hogue CR. Is segregation bad for your health? Epidemiologic reviews. 2009;31(1):178–194. doi: 10.1093/epirev/mxp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane KL, Stanton-Chapman T, Phillips A. Teacher and parent expectations of preschoolers’ behavior: Social skills necessary for success. Topics in Early Childhood Special Education. 2007;27(2):86–97. doi: 10.1177/02711214070270020401. [DOI] [Google Scholar]
- Lee DB, Neblett EW, Jackson V. The role of optimism and religious involvement in the association between race-related stress and anxiety symptomatology. Journal of Black Psychology. 2015;41(3):221–246. doi: 10.1177/0095798414522297. [DOI] [Google Scholar]
- Lee DB, Peckins MK, Heinze JE, Miller AL, Assari S, Zimmerman MA. Psychological pathways from racial discrimination to cortisol in African American males and females. Journal of Behavioral Medicine. 2017;41(2):1–13. doi: 10.1007/s10865-017-9887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annual Review of Psychology. 2007;58:201–225. doi: 10.1146/annurev.psych.57.102904.190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the Hypothalamic-Pituitary-Adrenocortical axis in humans. Psychological Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mouzon DM, Taylor RJ, Woodward AT, Chatters LM. Everyday racial discrimination, everyday non-racial Discrimination, and physical health among African-Americans. Journal of Ethnic & Cultural Diversity in Social Work. 2017;26(1–2):68–80. doi: 10.1080/15313204.2016.1187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neblett EW, Carter SE. The protective role of racial identity and Africentric worldview in the association between racial discrimination and blood pressure. Psychosomatic Medicine. 2012;74(5):509–516. doi: 10.1097/PSY.0b013e3182583a50. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ebert JP, Banaji MR, Phelps EA. The role of social groups in the persistence of learned fear. Science. 2005;309(5735):785–787. doi: 10.1126/science.1113551. [DOI] [PubMed] [Google Scholar]
- Pager D. The dynamics of discrimination. In: Lin AC, Harris DR, editors. The colors of poverty: Why racial and ethnic disparities persist. New York: Russell Sage Foundation; 2008. pp. 21–51. [Google Scholar]
- Pruessner M, Béchard-Evans L, Boekestyn L, Iyer SN, Pruessner JC, Malla AK. Attenuated cortisol response to acute psychosocial stress in individuals at ultra-high risk for psychosis. Schizophrenia Research. 2013;146(1–3):79–86. doi: 10.1016/j.schres.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Salimetrics, LLC. [Internet] Saliva Collection and Handling Advice. 2015 https://www.salimetrics.com/assets/documents/Saliva_Collection_Handbook.pdf.
- Schmeelk-Cone KH, Zimmerman MA, Abelson JL. The buffering effects of active coping on the relationship between SES and cortisol among African American young adults. Behavioral Medicine. 2003;29(2):85–94. doi: 10.1080/08964280309596061. [DOI] [PubMed] [Google Scholar]
- Seaton EK, Yip T. School and neighborhood contexts, perceptions of racial discrimination, and psychological well-being among African American adolescents. Journal of Youth and Adolescence. 2009;38(2):153–163. doi: 10.1007/s10964-008-9356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers RM, Caldwell CH, Schmeelk-Cone KH, Zimmerman MA. Racial identity, racial discrimination, perceived stress, and psychological distress among African American young adults. Journal of Health and Social Behavior. 2003;44(3):302–317. [PubMed] [Google Scholar]
- Shirtcliff EA, Peres JC, Dismukes AR, Lee Y, Phan JM. Hormones: commentary. Riding the physiological roller coaster: adaptive significance of cortisol stress reactivity to social contexts. Journal of Personality Disorders. 2014;28(1):40–51. doi: 10.1521/pedi.2014.28.1.40. [DOI] [PubMed] [Google Scholar]
- Stewart EA, Baumer EP, Brunson RK, Simons RL. Neighborhood racial context and perceptions of police‐based racial discrimination among black youth. Criminology. 2009;47(3):847–887. doi: 10.1111/j.1745-9125.2009.00159.x. [DOI] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30(3):376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- U.S. Census. Census 2000 Summary File 1. Washington: Author; 2001. Available at: http://factfinder.census.gov/, accessed on April 20, 2005. [Google Scholar]
- Williams DR, Mohammed SA. Racism and health I: Pathways and scientific evidence. The American Behavioral Scientist. 2013;57:1152–1173. doi: 10.1177/0002764213487340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young G. Teardown: Memoir of a Vanishing City. Univ of California Press; 2013. [Google Scholar]
- Zeiders KH, Hoyt LT, Adam EK. Associations between self-reported discrimination and diurnal cortisol rhythms among young adults: The moderating role of racial-ethnic minority status. Psychoneuroendocrinology. 2014;50(1):280–288. doi: 10.1016/j.psyneuen.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MA, Schmeelk-Cone KH. A longitudinal analysis of adolescent substance use and school motivation among African American youth. Journal of Research on Adolescence. 2003;13(2):185–210. doi: 10.1111/1532-7795.1302003. [DOI] [Google Scholar]


