Summary
Background:
Pneumocystis jirovecii can cause severe potentially life-threatening pneumonia (PCP) in kidney transplant patients. Prophylaxis of patients against PCP in this setting is usually performed during six months after transpantation
Objectives:
To describe the molecular epidemiology of a cluster of PCP in renal transplant recipients in Brazil.
Methods:
Renal transplant patients who developed PCP between May and December 2011 had their formalin fixed paraffin embedded (FFPE) lung biopsy samples analyzed. Pneumocystis jirovecii 23S mitochondrial large subunit of ribosomal RNA (23S mtLSU-rRNA), 26S rRNA, and dihydropteroate synthase (DHPS) genes were amplified by polymerase chain reaction (PCR), sequenced, and analyzed for genetic variation.
Results:
During the study period, 17 patients developed PCP (only 4 infections were documented within the first year after transplantation) and 6 (35.3%) died. Thirty FFPE samples from 11 patients, including 1 external control HIV-infected patient, had fungal DNA successfully extracted for further amplification and sequencing for all three genes. A total of 5 genotypes were identified among the 10 infected patients. Of note, 4 patients were infected by more than one genotype and 7 patients were infected by the same genotype.
Conclusions:
DNA extracted from FFPE samples can be used for genotyping; this approach allowed us to demonstrate that multiple P. jirovecii strains were responsible for this cluster, and one genotype was found infecting 7 patients. The knowledge of the causative agents of PCP may help to develop new initiatives for control and prevention of PCP among patients undergoing renal transplant and improve routine PCP prophylaxis
Keywords: Pneumocystis, pneumocistosis, genotyping of Pneumocystis jirovecii, kidney transplantation
Introduction
Pneumocystis jirovecii can cause severe potentially life-threatening pneumonia (PCP) in immunocompromised patients, especially those with HIV/AIDS and those who have undergone solid organ transplantation.1,2 Kidney transplant patients appear to be at especially high risk, primarily within the first 6 month after transplantation, with outbreaks being reported over the past ten years from multiple transplant centers throughout the world, especially in Europe, Japan, and Australia.3–10 Of note, to date, there have been no reports of PCP outbreaks or clusters in renal transplant patients in Brazil.
PCP in transplant recipients can be associated with mortality rates up to 50%, highlighting the need for early diagnosis and timely initiation of anti-Pneumocystis therapy.8,11 The reason for the relatively recent increase in frequency of outbreaks is unclear; potential explanations include the introduction of specific P. jirovecii strains into the hospital environment, changes in immunosuppressive regimens, or other factors affecting patient susceptibility.8,11,12
Since Pneumocystis cannot be cultured, molecular typing methods have been developed, and have primarily included single nucleotide polymorphisms, multi-locus sequence typing (MSLT), restriction fragment length polymorphism (RFLP), and analysis of tandem repeats. Multiple studies using such typing methods have shown that outbreaks of PCP are often caused by one or a limited number of strains of Pneumocystis.3–5,9,10,13–17
Few Pneumocystis typing studies have been carried out using samples derived from FFPE tissues,18 in part because it is difficult to obtain good-quality DNA from such tissues due to potential cross-linking of DNA, the presence of inhibitors, and other factors19. This study reports on the molecular epidemiology of a cluster of PCP cases in renal transplant patients that occurred in a single medical center in the city of São Paulo, Brazil; most cases developed more than 6 months after transplantation, the period traditionally considered highest risk for development of PCP.
Patients and methods
Study design, setting, and ethics
This was a cross-sectional retrospective study enrolling all kidney transplant recipients admitted at a renal transplantation hospital (in São Paulo, Brazil) who developed an episode of PCP between May 2011 and December 2011, in whom lung tissue samples were available for analysis. The Hospital do Rim (Kidney Hospital) of Federal University of São Paulo (UNIFESP) is a unique institution focusing on the care of renal disease and transplantation in Brazil. It’s a quaternary teaching public health care institution that serves as a national and international reference center for kidney transplantation, performing an average of 900 kidney transplants per year.
This study was approved by the local ethics committee (protocol no. CEP 1414/09). Informed consent from patients was waived due to the retrospective character of this study.
Casuistic
In this series, cases were included only when formalin fixed paraffin embedded (FFPE) lung biopsy samples were available from patients with histological confirmation of PCP by conventional microscopy of slides stained with silver stain. Molecular methods and immunofluorescence tests for PCP are not available in routine diagnosis of our institution. To maximize our ability to detect strain variation, only tissue samples for which P. jirovecii DNA could be amplified by all three primer sets selected for molecular typing (23S mtLSU-rRNA, DHPS and 26S-rRNA genes) were included in the analysis. For comparison, we also included one HIV-infected patient with PCP from a neighboring hospital.
DNA extraction and PCR
Five-micrometer serial sections from FFPE tissue specimens were used for DNA extraction using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, with some modifications based on a prior report.18 The total DNA yield and purity were measured by analyzing the absorbance at 260, 280 and 230 nm, and the DNA amount was adjusted to 40 ng/µL for PCR protocols. Polymerase chain reaction (PCR) was carried out using the PCR Mastermix Kit (Promega, Madison, WI, USA). Primers for the following genes were used (sequences are provided in Supplementary Table 1): human beta-globin gene19 and pan-fungal internal transcribed spacers (ITS) (primers ITS1 and ITS4/ITS3 and ITS4)20 as positive controls for quality of extracted DNA, and 23S mtLSU-rRNA,21 DHPS22 and 26S-rRNA23 genes as specific targets for molecular typing of P. jirovecii.
A total volume of 25 µL was used for each reaction, and PCR was run on a Proflex PCR System (Applied Biosystems, Inc., Foster City, CA, USA). For quality controls of DNA amplification, each PCR set included one reaction containing DNA from P. jirovecii as positive control, and one reaction with DNA from Aspergillus spp., Mucorales fungi, or Cryptococcus spp., as well as ultrapure water as negative controls.
Procedures for DNA sequencing and analysis
DNA sequencing was performed as previously described.24 For analysis of 23S mtLSU rRNA, 26S rRNA and DHPS genes of P. jirovecii, the forward and reverse primers for the target genes analyzed were used, and the Big Dye Terminator Reaction kit v3.1 (Applied Biosystems, Inc.), according to the manufacturer’s instructions. Samples were run on an automated ABI 3130 genetic analyzer (Applied Biosystems, Inc.)
DNA sequence assembly and editing was performed using Sequencher 4.1.4 (Gene Codes Corporation, Ann Arbor, MI, USA) and Phred/Phrap with the sequence editor Consed.25–27 The consensus sequences were aligned and compared with sequences deposited in public genomic databases: GenBank (Nucleotide Basic Local Alignment Search Tool, NCBI at http://www.ncbi.nlm.nih.gov/,) and CBS database (http://www.cbs.knaw.nl/). The accurate identification of P. jirovecii was ensured by an e-value of less than 10−5, and a maximum identity of ≥98%. (GenBank accession numbers: 23S mtLSU-rRNA and 26S: KJ634811- 40 and 26S: KT272415 – 44 respectively).
Sequences for each gene were aligned and trimmed with MAFFT v.728 using default settings. To assess genetic variability of the P. jirovecii sequences, genotype analyses were first carried out using the 26S, DHPS or 23S mtLSU gene sequences separately. After that, 26S and 23S mtLSU sequences were concatenated and subjected to sequence polymorphism analysis using DnaSP version 5.10.29 A genotype was defined as a unique combination of SNPs along a sequence, i.e., each different sequence in an alignment. The analysis was based on the number of genotypes (G), variable sites, genotype or haplotype diversity (Hd), and nucleotide diversity (Pi).30 For the concatenated data (26S + mt23S), only the 30 sequences generated in this study were used (n = 11 patients), as there were no nucleotides sequences derived from well-characterized P. jirovecii for both loci (26S + mt23S) available in public databases. The genotype network file (Roehl data file) was created using DnaSP v.5.10, considering gaps. The network was generated by the median-joining method31 using Network v4.6 software (http://www.fluxus-engineering.com/).
Genetic relationships among Pneumocystis sequences were investigated by phylogenetic analysis of concatenated 26S and mt23S using neighbor-joining, maximum likelihood, and maximum parsimony methods. Phylogenetic trees were constructed in MEGA7.32 Considering the Bayesian information criterion (BIC) and Akaike information criterion (AIC),33 the Tamura 3-parameter model (T92 model)34 was found to be the best evolutionary model, and the robustness of branches was assessed by bootstrap analysis of 1,000 replicates.35
Results
During the seven-month period of study, 17 patients among a total of 6,196 kidney transplant recipients assisted at our institution developed PCP. During the cluster period we found an incidence rate of 0.27 cases per 100 kidney transplants — while along the 10 years-period previously to this cluster only 7 cases of PCP had been diagnosed among 7,063 kidney transplant recipients attending our division, representing 0.099 cases per 100 kidney transplants. Among the 17 initially selected patients, 16 had received their organ from deceased donors, including two kidney-pancreas transplant recipients, and one from a living donor. Table 1 summarizes clinical characteristics and treatment of the 17 cases of PCP. Nine patients (53%) were male, and the mean age was 40 years (range 24–63). The median time to onset of lung disease after transplantation was 30 months (4–75 months). The most common comorbidity among these patients was diabetes mellitus, in 7 (41.2%) cases. Induction therapy had been performed at the time of transplantation in 14 cases, including rabbit anti-thymocyte globulin in 5/14 cases, and anti-interleukin 2 receptor (basiliximab) in 9/14.
Table 1.
Clinical characteristics of 17 renal transplant patients at the time of diagnosis of Pneumocystis pneumonia (PCP).
| Variable | N (%) or median (range) |
|---|---|
| Male/female | 9 / 8 (53%/47%) |
| Median age, years (range) | 40 (24-63) |
| Kidney transplant type | |
| Living donor | 1 (6%) |
| Deceased donor | 14 (82.3%) |
| Kidney-pancreas | 2 (11.7%) |
| Second transplant | 3 (17.6%) |
| Opportunistic infections and other co-morbidities | |
| Cytomegalovirus within 6 months before the diagnosis of PCP | 3 (17.6%) |
| Systemic lupus erythematosus (SLE) | 2 (11.8%) |
| Diabetes mellitus | 7 (41.2%) |
| Change or modulation of immunosuppression within 6 months before the diagnosis of PCP | 4 (23.5%) |
| Rejection within 6 months before diagnosis of PCP | 5 (29.4%) |
| Immunosuppressive drugs | |
| Calcineurin inhibitor | 13 (76.4%) |
| Corticosteroid | 17 (100%) |
| Azathioprine | 3 (17.6%) |
| Mycophenolate preparation | 13 (76.4%) |
| Sirolimus | 1 (5.9%) |
| Median time, months, from transplant to diagnosis of PCP | 30 (4-75) |
| Mean duration, days, of respiratory symptoms | 16 (1-86) |
| Cough | 15 (88.2%) |
| Dyspnea | 16 (94.1%) |
| Fever | 13 (76.5%) |
| Diagnosis | |
| Direct detection by toluidine blue O staining in bronchoalveolar lavage | 0 (0%) |
| Transbronchial biopsy | 9 (52.9%) |
| Open lung biopsy | 8 (47.1%) |
| ICU admission | 16 (94.1%) |
| Intubation | 12 (70.6%) |
| Death | 6 (35.3%) |
ICU = intensive care unit.
Only one case (6%) of PCP occurred within the first six months after transplantation involving a patient without prophylaxis with trimethoprim/sulfamethoxazole (TMP/SMX) since he was allergic to this medication. Another three cases of PCP (17.6%) occurred between 6 and 12 months and the others were documented after 1 year of transplantation. In our institution, prophylaxis with TMP/SMX is routinely performed along the first six months after transplantation. After this period, prophylaxis is used only in patients who presented with acute cellular rejection and received treatment with pulse therapy containing steroids in high doses or with therapy containing antithymocyte antibodies. In addition, we do not routinely evaluate lymphocyte counts (TCD4 and TCD8) at our patients along their follow up period after transplantation, even in the investigations of opportunistic diseases.
The mean time between the onset of symptoms and the hospital admission for diagnostic investigation was 16 days (range 1–86 days). No patient was receiving trimethoprim-sulfamethoxazole prophylaxis at the time they developed PCP. At the time of diagnosis of PCP, all patients were receiving a combination of immunosuppressive drugs including prednisone (17/17, 100%), calcineurin inhibitors (13/17, 76.4%) and azathioprine (3/17, 17.6%) or mycophenolic acid (13/17, 76.4%). Treatment of organ rejection was reported in 5 (29.4%) patients, and changes in immunosuppression regimens within six months before the diagnosis of PCP was seen in 4 (23.5%) cases.
All 17 PCP cases were diagnosed by lung biopsy and conventional histopathology (Grocott Methenamine Silver stain) since immunofluorescence tests and PCR for PCP diagnosis were not available in our institution at that time. In our institution, there were no cases of pneumocystosis diagnosed by any other method besides pulmonary biopsy in that period.
All patients with PCP were treated with standard doses of intravenous trimethoprim-sulfamethoxazole. A reduction or withdrawal of immunosuppressive drugs was necessary for all episodes of PCP after diagnosis. Six (35.3%) patients died during their hospitalization for PCP.
At the time we initiated our investigation, a total of 47 FFPE lung samples from 17 patients were available for analysis. Due to limitations of the method, DNA amplification was only possible with 30 to 47 FFPE. Consequently, for genotyping analysis, only 30 FFPE samples related to 11 patients were further evaluated.
From the 30 samples collected from 11 patients, a single amplicon of each gene with approximately 381 bp (26S), 290 bp (mt23S) and 186 bp (DHPS) was seen by agarose gel electrophoresis for all 30 samples. The complete alignment included the 30 sequences generated in this study. The DHPS sequence analysis was not informative concerning strain variation, as no nucleotide variability was found (Table 2). Thus, sequences of concatenated 26S and mt23S were aligned, which were 594 bp long, including 587 invariable sites, and 7 variable parsimony-informative sites (1.17%) (Table 2).
Table 2.
Parameters and statistical values from the different phylogenetic and genotypic analyses
| Locus/Region | Isolates (n) | Best Model | N. of sites |
C | V | Pi | S | π | H | Hd |
|---|---|---|---|---|---|---|---|---|---|---|
| DHPS | 30 | JC | 185 | 185 | 0 | 0 | 0 | 0 | 1 | 0.00 |
| 26S | 30 | JC | 355 | 350 | 5 | 5 | 0 | 0.0052 | 2 | 0.37 |
| 23S rRNA mtLSU | 30 | T92 | 239 | 237 | 2 | 2 | 0 | 0.0034 | 3 | 0.47 |
| 26S +23S rRNA mtLSU | 30 | T92 | 594 | 587 | 7 | 7 | 0 | 0.0045 | 5 | 0.69 |
DHPS: dihydropteroate synthase; 26S: 26S large subunit ribosomal RNA; 23S rRNA mtLSU: mitochondrial large subunitribosomal RNA; JC: Jukes-Cantor; T92: Tamura-3 parameter; C: conserved characters; V: variable characters; Pi: Parsimony-informative characters; S: singletons; π: nucleotide diversity; H: haplotype/genotype number; Hd: haplotype/genotype diversity.
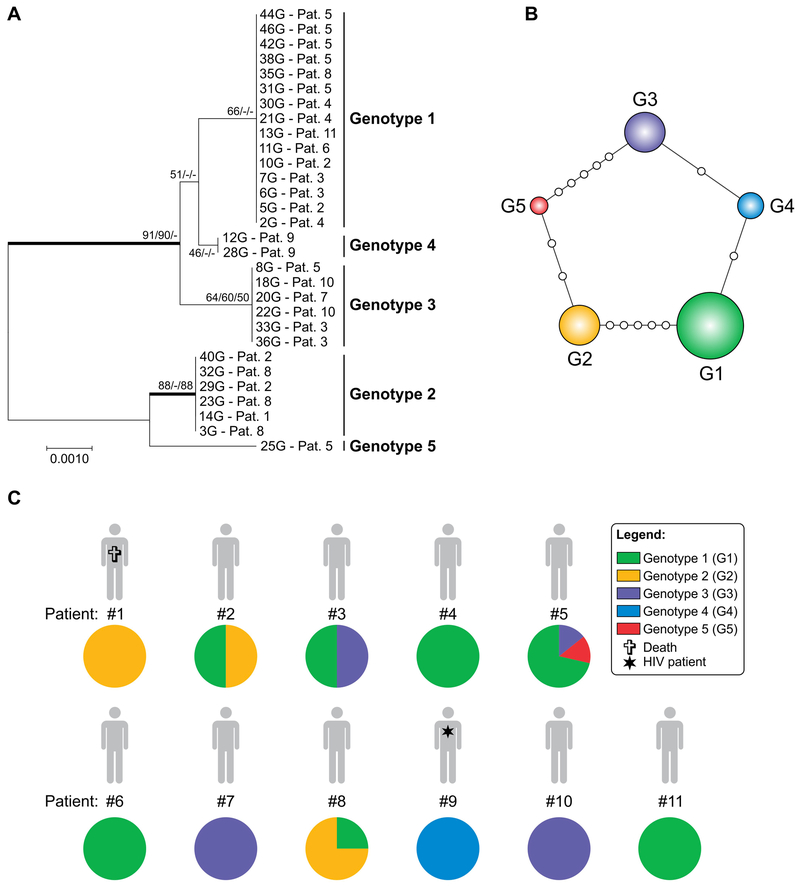
A phylogenetic tree was constructed using neighbor-joining (model T92) with 1,000 bootstrap replications (Figure 1A). The 30 operational taxonomic units were distributed into 5 main P. jirovecii clusters (genotypes 1–5). Remarkably, different FFPE samples recovered from the same patient were often in different clusters, revealing a substantial level of co-infection (4 patients, 36.3%) with at least two genotypes. When evolutionary relationships among strains were evaluated using haplotype networks, to visualize differences and diversity among P. jirovecii sequences, the 5 genotypes above were recognized (G1-G5, Hd = 0.69; Figure 1B). The majority of sequences were assigned to G1 (7 out 11 patients; Figure 1C). The more samples per patient that were analyzed, the more diversity was seen. In the patient #5, for whom 7 samples were analyzed, 3 unique genotypes were identified (G1, G3, and G5). The patient #9 (HIV-positive) presented a different genotype when compared to the renal transplant patients, belonging to a distinct group (G4) as can be observed in Figure 1 (A-C). A detailed analysis of the genotypes by patient and sample is provided in a supplementary table (S2 Table).
Figure 1.
Genetic diversity of Pneumocystis jirovecii pneumonia during a cluster in renal transplant recipients (RTRs). A, Phylogenetic relationships, as inferred from a neighbour‐joining analysis (model T92) of the concatenated nuclear 26S and the mitochondrial 23S mtLSU sequences from 28 samples obtained from 10 RTRs and 2 samples from one (1) HIV‐infected patient (pt. 9). The numbers close to the branches represent indices of support (NJ/ML/MP) based on 1000 bootstrap replications. The branches with bootstrap support higher than 80% are indicated in bold. NJ, neighbour‐joining; ML, maximum likelihood; MP, maximum parsimony. B, Median‐joining haplotype/genotype network of P. jirovecii samples, covering all genotypes (G_1–G_5) found in this study. The size of the circumference is proportional to the genotype frequency in the dataset. Mutational steps are represented by white dots. C, The genotypes are colour coded, and their frequencies per patient are shown. Coinfection with at least two different P. jirovecii genotypes was found in four of the eleven patients evaluated. Further information about isolate source can be found in the Table S2
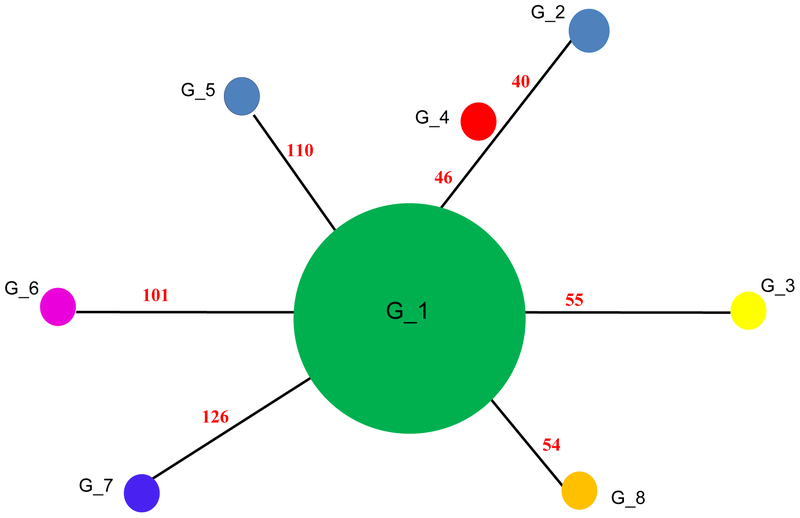
As mentioned, the DHPS sequence analysis was not informative concerning strain variation. However, when compared to other sequences deposited in GenBank, all 30 sequences belonged to one group, which was named as G_11, with a 100% identity to the following sequences: U66282; U66279; AJ586567; JX101868; AF139132. Although no nucleotide variability has been found among the samples from our study, the haplotype analysis revealed other seven genotypes (G-2-G_8) comprising distinct nucleotide sequences from GenBank: G_2 (U66281); G_3 (U66280); G_4 (U66278); G_5 (GU479993); G_6 (GU479992); G_7 (GU479994); G_8 (AM404075) (Figure 2).
Figure 2.
Comparative analysis of DHPS gene of Pneumocystis jirovecii pneumonia during a cluster in renal transplant recipients (RTRs). Median‐joining haplotype/genotype network of P. jirovecii samples, covering all genotypes (G_1 to G_8) found in this study and in GenBank. The size of the circumference is proportional to the genotype frequency in the dataset. G_1: comprises all the 30 samples of the study and the following sequences deposited on GenBank: U66282; U66279; AJ586567; JX101868; AF139132. But it also (G_1) showed some minor differences compared to the following genotypes: G_2: U66281; G_3: U66280; G_4: U66278; G_5: GU479993; G_6: GU479992; G_7: GU479994; G_8: AM404075 (sequences from GenBank)
Discussion
This is the first report of a cluster of PCP documented by molecular typing of samples obtained from renal transplant recipients from South America. Three P. jirovecii genes from 30 of 47 FFPE lung biopsy samples (60%), all obtained from renal transplant patients with PCP and one external control HIV patient, were successfully amplified. By using this methodology we were able to demonstrate that multiple P. jirovecii strains were responsible for this cluster. Thus, despite some limitations, FFPE samples can be used for molecular typing of P jirovecii when other clinical specimens, such as BAL fluid or induced sputum samples, are unavailable. While there are potential limitations of the method associated with deterioration of DNA and presence of PCR inhibitors, FFPE samples have additional advantages, including long-term storage, histopathological evaluation of the affected tissue, and identification of other potential pathologic processes It is important to highlight that bronchoscopy and lung biopsies of the present patients were requested by their medical assistants to investigate other causes of pneumonia usually documented in the late period after kidney transplantation. In addition, as mentioned before, immunofluorescence tests and PCR for PCP are not available in our institution.
The present cluster of PCP cases was caused by multiple strains, suggesting that different environmental sources of infection could be involved. It is worth mentioning that the molecular typing we used may be more sensitive in identifying intra-species variation, in contrast to other reports where most of strains causing clusters of PCP were identical. In this scenario, Rostved et al. (2013) described three unique Pneumocystis genotypes shared among renal transplanted and liver transplanted patients presenting three distinct clusters of PCP, two of which overlapped temporally 9.
Our methodology may, however, have impacted our findings. In many cases of PCP, multiple P. jirovecii strains can be identified in one patient.23,36,38 Many typing methods that rely on PCR will, however, often only identify the predominant strain unless amplified products are subcloned prior to Sanger sequencing, or next generation sequencing is utilized. By testing multiple tissue samples from the same patient, we potentially were more successful in documenting genetic variability as a consequence of infection with multiple strains of P. jirovecii, especially if there is uneven distribution of the strains within the lung. As shown in the current study, the more FFPE samples we tested, the greater the level of genetic diversity we identified (e.g., patient #5) 23,36,38.
However, despite the fact that multiple genotypes were documented in 4 patients, by checking carefully our data, we were able to document the predominance of some genotypes infecting different patients. As an example, genotype G1 was found infecting 7 out 10 patients enrolled in our casuistic, and genotype G3 was found in 4 patients.
MLST-based genotyping is the gold standard for characterization of strains (especially because Pneumocystis spp. are not able to grow in culture media), providing an excellent discriminatory power. However, several studies have shown small number of mutations in the DHPS gene, characterizing its low discriminatory power 39,40,41,42.
One important clinical observation in this study was the late occurrence of PCP in 76% of patients (14/17), a time during which the risk of PCP has previously been considered low. At the time we documented the cluster of PCP, prophylaxis with trimethoprim/sulfamethoxazole (TMP/SMX) was usually prescribed for only six months after transplantation. It means that all patients with PCP were not under TMP/SMX prophylaxis at the time they developed infection.
Several other recent studies have reported an increase in the incidence of PCP more than one year after transplantation, suggesting that the risk period for PCP in renal transplant patients may need to be re-assessed, and the time for antifungal prophylaxis in this group of patients should be reevaluated, especially for high risk patients 43–45.
Our hospital is a reference transplant center in Brazil and performs organ transplantation for patients from different states and regions. Although most of our patients were from the state of São Paulo, where our institution is located, there were many patients in the PCP cluster period who came from neighboring states. As these patients were already sick at the time they came to the hospital, it is more likely than their infection were community acquired. This scenario could help us to explain the 4 different genotypes we found infecting our patients. Otherwise, we may not exclude the possibility that some of them could have been colonized at any time during their medical assistance in our hospital setting, including the outpatient clinic where they collect routine laboratory exams. That possibility would explain the finding of a single genotyping (G-1) infecting 7 out 10 patients of the cluster
A limitation of the study includes problems related to molecular tool used, as well as its interpretation. Currently, Multilocus sequence typing (MLST) has become the most used method and was applied in several cluster or outbreak analyses. However, highly discriminant methods such as ITS sequencing with subcloning of the PCR products and multitarget SSCP (single-strand conformation polymorphism analysis) are still used in many others studies. Molecular methods of Pneumocystis genotyping are under construction and they should be analyzed with caution 39,40,41,42,46.
Finally, it is worth noting that crude mortality rates of patients with PCP in the current study was 35%. This finding highlights that PCP continues to be a potentially life-threatening complication of renal transplantation. It is critical to better understand the epidemiology and risk factors of patients who develop PCP more than 6 months after transplantation in order to optimize strategies for prevention of this infection in this setting of patients 7,11,42,43,44.
Conclusion
This is the first report of PCP cluster confirmed by molecular method in renal transplant patients that occurred in a single medical center in São Paulo, Brazil. Herein, DNA extracted from FFPE samples was successfully used for genotyping agents of PCP. By checking several DNA samples from the same patient we found a high diversity of P. jirovecii genotypes causing lung infection, suggesting that PCP may be caused by simultaneous inhalation of multiple genotypes. Finally, we found a single genotype causing infection in 7 out of 10 patients, suggesting a common source of infection. The knowledge of the causative agents of PCP may help to develop new initiatives of control and prevention of PCP among patients submitted to renal transplant and improve routine PCP prophylaxis, especially among those under higher risk to develop PCP.
Supplementary Material
Acknowledgements:
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant number: 2013/01280–7) and Fundação Oswaldo Ramos, São Paulo, SP, Brazil for financial support. This research was supported in part by the Intramural Research Program of the NIH Clinical Center. We would like to thank Elaine Cristina Francisco and Marcel V. Soldá who helped us with sequencing studies.
References
- 1.Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA 2009;301(24):2578–85. [DOI] [PubMed] [Google Scholar]
- 2.Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol 2007;5(4):298–308. [DOI] [PubMed] [Google Scholar]
- 3.de Boer MG, Bruijnesteijn van Coppenraet LE, Gaasbeek A, Berger SP, Gelinck LB, van Houwelingen HC, et al. An outbreak of Pneumocystis jiroveci pneumonia with 1 predominant genotype among renal transplant recipients: interhuman transmission or a common environmental source? Clin Infect Dis 2007;44(9):1143–9. [DOI] [PubMed] [Google Scholar]
- 4.Le Gal S, Damiani C, Rouillé A, Grall A, Tréguer L, Virmaux M, et al. A cluster of Pneumocystis infections among renal transplant recipients: molecular evidence of colonized patients as potential infectious sources of Pneumocystis jirovecii. Clin Infect Dis 2012;54(7):e62–71. [DOI] [PubMed] [Google Scholar]
- 5.Sassi M, Ripamonti C, Mueller NJ, Yazaki H, Kutty G, Ma L, et al. Outbreaks of Pneumocystis pneumonia in 2 renal transplant centers linked to a single strain of Pneumocystis: implications for transmission and virulence. Clin Infect Dis 2012;54(10):1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas S, Vivancos R, Corless C, Wood G, Beeching NJ, Beadsworth MB. Increasing frequency of Pneumocystis jirovecii pneumonia in renal transplant recipients in the United Kingdom: clonal variability, clusters, and geographic location. Clin Infect Dis 2011;53(3):307–8. [DOI] [PubMed] [Google Scholar]
- 7.Chapman JR, Marriott DJ, Chen SC, MacDonald PS. Post-transplant Pneumocystis jirovecii pneumonia-a re-emerged public health problem? Kidney Int 2013;84(2):240–3. [DOI] [PubMed] [Google Scholar]
- 8.de Boer MG, de Fijter JW, Kroon FP. Outbreaks and clustering of Pneumocystis pneumonia in kidney transplant recipients: a systematic review. Med Mycol 2011;49(7):673–80. [DOI] [PubMed] [Google Scholar]
- 9.Rostved AA, Sassi M, Kurtzhals JA, Sørensen SS, Rasmussen A, Ross C, et al. Outbreak of pneumocystis pneumonia in renal and liver transplant patients caused by genotypically distinct strains of Pneumocystis jirovecii. Transplantation 2013;96(9):834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazaki H, Goto N, Uchida K, Kobayashi T, Gatanaga H, Oka S. Outbreak of Pneumocystis jirovecii pneumonia in renal transplant recipients: P. jirovecii is contagious to the susceptible host. Transplantation 2009;88(3):380–5. [DOI] [PubMed] [Google Scholar]
- 11.Eitner F, Hauser IA, Rettkowski O, Rath T, Lopau K, Pliquett RU, et al. Risk factors for Pneumocystis jiroveci pneumonia (PcP) in renal transplant recipients. Nephrol Dial Transplant 2011;26(6):2013–7. [DOI] [PubMed] [Google Scholar]
- 12.Martin SI, Fishman JA; AST Infectious Diseases Community of Practice. Pneumocystis pneumonia in solid organ transplantation. Am J Transplant 2013;13 Suppl 4:272–9. [DOI] [PubMed] [Google Scholar]
- 13.Curran T, McCaughey C, Coyle PV. Pneumocystis jirovecii multilocus genotyping profiles in Northern Ireland. J Med Microbiol 2013;62(Pt 8):1170–4. [DOI] [PubMed] [Google Scholar]
- 14.Gianella S, Haeberli L, Joos B, Ledergerber B, Wüthrich RP, Weber R, et al. Molecular evidence of interhuman transmission in an outbreak of Pneumocystis jirovecii pneumonia among renal transplant recipients. Transpl Infect Dis 2010;12(1):1–10. [DOI] [PubMed] [Google Scholar]
- 15.Goto N, Oka S. Pneumocystis jirovecii pneumonia in kidney transplantation. Transpl Infect Dis 2011;13(6):551–8. [DOI] [PubMed] [Google Scholar]
- 16.Hauser P, Rabodonirina M, Nevez G. Pneumocystis jirovecii genotypes involved in pneumocystis pneumonia outbreaks among renal transplant recipients. Clin Infect Dis 2013;56(1):165–6. [DOI] [PubMed] [Google Scholar]
- 17.Ripamonti C, Orenstein A, Kutty G, Huang L, Schuhegger R, Sing A, et al. Restriction fragment length polymorphism typing demonstrates substantial diversity among Pneumocystis jirovecii isolates. J Infect Dis 2009;200(10):1616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bialek R, Ibricevic A, Aepinus C, Najvar LK, Fothergill AW, Knobloch, et al. Detection of Paracoccidioides brasiliensis in tissue samples by a nested PCR assay. J Clin Microbiol 2000;38(8):2940–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattás GJ, Soares-Vieira JA. Cytochrome P450–2E1 and glutathione S-transferase mu polymorphisms among Caucasians and mulattoes from Brazil. Occup Med (Lond) 2000;50(7):508–11. [DOI] [PubMed] [Google Scholar]
- 20.White TJ, Bruns TD, Lee SB, Taylor JW. Amplification and Direct Sequencing of Fungal Ribosomal Rna Genes for Phylogenetics In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds. PCR Protocols: A Guide to Methods and Applications. San Diego: Academic Press, Inc; 1990. p. 315–22. [Google Scholar]
- 21.Durand-Joly I, Chabé M, Soula F, Delhaes L, Camus D, Dei-Cas E. Molecular diagnosis of Pneumocystis pneumonia. FEMS Immunol Med Microbiol 2005;45(3):405–10. [DOI] [PubMed] [Google Scholar]
- 22.Costa MC, Gaspar J, Mansinho K, Esteves F, Antunes F, Matos O. Detection of Pneumocystis jirovecii diihydropteroato synthase polymorphisms in patients with Pneumocystis pneumonia. Scand.J.Infect.Dis 2005; 37:766–771. [DOI] [PubMed] [Google Scholar]
- 23.Hauser PM, Francioli P, Bille J, Telenti A, Blanc DS. Typing of Pneumocystis carinii f. sp. hominis by single-strand conformation polymorphism of four genomic regions. J Clin Microbiol 1997;35(12):3086–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merseguel KB, Nishikaku AS, Rodrigues AM, Padovan AC, e Ferreira RC, de Azevedo Melo AS, et al. Genetic diversity of medically important and emerging Candida species causing invasive infection. BMC Infect Dis 2015;15:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res 1998;8(3):186–94. [PubMed] [Google Scholar]
- 26.Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 1998;8(3):175–85. [DOI] [PubMed] [Google Scholar]
- 27.Gordon D, Abajian C, Green P. Consed: a graphical tool for sequence finishing. Genome Res 1998;8(3):195–202. [DOI] [PubMed] [Google Scholar]
- 28.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 2013;30(4):772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009;25(11):1451–2. [DOI] [PubMed] [Google Scholar]
- 30.Nei M Molecular Evolutionary Genetics New York: Columbia University Press; 1987. [Google Scholar]
- 31.Bandelt HJ, Forster P, Röhl A. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 1999;16(1):37–48. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol 2016;33(7):1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akaike H A new look at the statistical model identification. IEEE Transactions on Automatic Control, 1974;19(6):716–22. Available from: http://bayes.acs.unt.edu:8083/BayesContent/class/Jon/MiscDocs/Akaike_1974.pdf Accessed in 2017 (Apr 3). [Google Scholar]
- 34.Tamura K Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol Biol Evol 1992;9(4):678–87. [DOI] [PubMed] [Google Scholar]
- 35.Felsenstein J Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1974;39(4):783–91. Available from: https://www.jstor.org/stable/2408678?seq=1#page_scan_tab_contents Accessed in 2017 (Apr 3). [DOI] [PubMed] [Google Scholar]
- 36.Ma L, Kutty G, Jia Q, Imamichi H, Huang L, Atzori C, et al. Analysis of variation in tandem repeats in the intron of the major surface glycoprotein expression site of the human form of Pneumocystis carinii. J Infect Dis 2002;186(11):1647–54. [DOI] [PubMed] [Google Scholar]
- 37.Steinau M, Patel SS, Unger ER. Efficient DNA extraction for HPV genotyping in formalin-fixed, paraffin-embedded tissues. J Mol Diagn 2011;13(4):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helweg-Larsen J, Lundgren B, Lundgren JD. Heterogeneity and compartmentalization of Pneumocystis carinii f. sp. hominis genotypes in autopsy lungs. J Clin Microbiol 2001;39(10):3789–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olga Matos & Francisco Esteves. Pneumocystis jirovecii multilocus gene sequencing: findings and implications. Future Microbiol 2010; 5(8), 1257–1267. [DOI] [PubMed] [Google Scholar]
- 40.Wissmann G, Alvarez-Martinez MJ, Meshnick SR, Dihel AR, Prolla JC. Absence of dihydropteroate synthase mutations in Pneumocystis jiroveci from Brazilian AIDS patients. J Eukaryot Microbiol 2006; 53: 305–7. [DOI] [PubMed] [Google Scholar]
- 41.Jessica Beser; Leigh Dini; Silvia Botero-Kleiven; Margareta Krabbe; Johan LindhPer Hagblom. Absence of dihydropteroate synthase gene mutations in Pneumocystis jirovecii isolated from Swedish patients. Medical Mycology 2010; 50(3): 320–323. [DOI] [PubMed] [Google Scholar]
- 42.Tark Kim; Sang-Oh Lee; Hyo-Lim Hong; Ju Young Lee; Sung-Han Kim; Sang-Ho Choi; Mi-Na Kim; Yang Soo Kim; Jun Hee Woo; and Heungsup Sung. Clinical characteristics of hospital-onset Pneumocystis pneumonia and genotypes of Pneumocystis jirovecii in a single tertiary centre in Korea. BMC Infectious Diseases 2015; 15:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulpuru S, Knoll G, Weir C, Desjardins M, Johnson D, Gorn I, et al. Pneumocystis pneumonia outbreak among renal transplant recipients at a North American transplant center: Risk factors and implications for infection control. Am J Infect Control 2016; 44(4):425–31. [DOI] [PubMed] [Google Scholar]
- 44.Brakemeier S, Dürr M, Bachmann F, Schmidt D, Gaedeke J, Budde K. Risk Evaluation and Outcome of Pneumocystis jirovecii Pneumonia in Kidney Transplant Patients. Transplant Proc 2016;48(9):2924–30. [DOI] [PubMed] [Google Scholar]
- 45.Jairam A, Dassi M, Chandola P, Lall M, Mukherjee D, Hooda AK. Pneumocystis jiroveci outbreak in a renal transplant center: Lessons learnt. Indian J Nephrol 2014;24(5):276–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alanio A, Desnos-Ollivier M, Garcia-Hermoso D, Bretagne S. Investigating Clinical Issues by Genotyping of Medically Important Fungi: Why and How?. Clin Microbiol Rev 2017. July;30(3):671–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.