Abstract
Although the retrosplenial cortex (RSC) is necessary for the retrieval of remotely-acquired fear to a discrete auditory cue, it is not necessary for the retrieval of recently-acquired cued-fear memories. Thus, the RSC’s role in memory retrieval for discrete cues is time-dependent. The purpose of the current experiment was to identify the larger circuit involved in the retrieval of remotely-acquired auditory fear memories. One candidate circuit involves the RSC and secondary auditory cortex; the secondary auditory cortex is also necessary for the retrieval of remotely-acquired auditory fear memories (Sacco & Sachetti, 2010), and sends direct projections to the RSC. To test this possibility, we assessed retrieval of remote memory following functional disconnection of the RSC and secondary auditory cortex. Complete disconnection of these regions produced a larger impairment in fear expression to a remotely-acquired auditory cue compared to partial disconnection of these regions. These results are consistent with the notion that RSC and secondary auditory cortex form a functional circuit involved in the retrieval of remotely-acquired fear to a discrete auditory cue.
Keywords: retrosplenial, auditory cortex, remote memory, fear conditioning
Perhaps because fear memories form the basis of anxiety disorders such as post-traumatic stress disorder (PTSD), there has long been considerable interest in understanding the neural underpinnings of the acquisition, storage, and retrieval of these memories (e.g., Kim & Jung, 2006). Recently, the functional role of the retrosplenial cortex (RSC) has garnered increased attention, with several studies demonstrating a role for RSC in contextual and cue-specific fear memories (Corcoran et al., 2011; Cowansage et al., 2014; Keene & Bucci, 2008a, 2008b; Kwapis et al., 2014, 2015; Robinson et al., 2012). The RSC is well-suited to contribute to these learning and memory processes, being positioned at the interface between sensory cortical regions and the medial temporal lobe memory system (for reviews see Ranganath and Ritchey, 2012; Vann, Aggleton, & Maguire, 2009).
In the laboratory, fear memories are often studied using Pavlovian fear conditioning. In a “delay” conditioning procedure, rats are placed in a novel context and receive pairings of a brief visual or auditory stimulus that co-terminates with mild foot-shock (e.g., Maren et al., 1997). Subsequently, rats will freeze when returned to the context where conditioning occurred, and will also freeze when re-exposed to the conditioned stimulus (CS). There is now a significant body of research demonstrating that although the RSC contributes to contextual fear conditioning, it often is not necessary for the acquisition or retrieval of fear memories for discrete cues established with delay fear conditioning. For example, permanent lesions of the RSC made before training, or 1-day after training, impair memory for the context, but not the tone CS (Keene & Bucci, 2008a, Experiment 1). More selective manipulations have produced similar findings. For example, blocking protein synthesis in the RSC prior to conditioning also impairs context memory but not tone fear memories (see Kwapis et al., 2015, Experiment 3) and blockade of NMDA glutamate receptors at the time of retrieval does not impair expression of freezing to a delay conditioned auditory cue (Corcoran et al., 2011; see also Kwapis et al., 2014, 2015).
Most studies to date, however, have examined the contribution of the RSC to cue-specific fear memories only shortly after conditioning, typically 24 hours later (but see Corcoran et al., 2011). In a recent study from our lab, we assessed fear for “remotely” conditioned memories (Todd et al., 2016). In this study, lesions of the RSC were made 28-days after initial tone-shock pairings. Damage to the RSC was found to disrupt memory for both the context and the discrete auditory cue. The overall pattern of data therefore suggests the RSC has a time-independent role in contextual memories (see also Corcoran et al., 2011), but has a time-dependent role in memory for discrete cues. While the RSC is not necessary for the retrieval of recently-acquired auditory fear memories, it is necessary for the retrieval of remotely-acquired auditory fear memories. This time-dependent involvement of the RSC is not limited to auditory fear memories, but also includes memory retrieval of visual cues (Jiang et al., in press).
The purpose of the current experiment was to assess the larger circuit involved in the retrieval of remotely-acquired auditory fear memories. The RSC receives primarily ipsilateral projections from secondary auditory cortex (Todd et al., 2016; Vogt & Miller, 1983). Importantly, secondary auditory cortex is also necessary for the retrieval of remotely-acquired, but not recently-acquired fear memories for discrete auditory cues (Sacco & Sacchetti, 2010). Thus, a candidate circuit for the retrieval of remotely acquired auditory fear memories includes the RSC and secondary auditory cortex. To test this possibility, we assessed retrieval of remote memory following functional disconnection of the RSC and secondary auditory cortex. All rats first received tone-shock pairings; following a 28-day retention interval, rats were then assigned to one of three surgical groups. One group (Contra), received lesions of the RSC in one hemisphere and lesions of secondary auditory cortex in the opposite (i.e., contralateral) hemisphere, putatively disrupting direct communication between these regions in both hemispheres. A second group (Ipsi) received lesions of the RSC and secondary auditory cortex in the same (i.e., ipsilateral) hemisphere, thereby leaving direct communication between these regions intact in the other hemisphere. Finally, there was a sham-lesion group (Sham) that served as a baseline to assess the overall impact of damage to the RSC and secondary auditory cortex.
Given that both the RSC and secondary auditory cortex are necessary for the retrieval of remotely-acquired auditory fear memories (Sacco & Sachetti, 2010; Todd et al., 2016), there are at least two possible outcomes for this experiment. If, on the one hand, communication between these two regions is necessary for retrieval, then contralateral lesions would be expected to have the largest impact on memory retrieval since communication between these regions is fully disrupted. If, on the other hand, communication between these two regions is not necessary for retrieval, then it would be expected that contralateral and ipsilateral lesions would impair memory retrieval to the same degree, perhaps as a result of the sum damage to both regions.
Methods
Subjects
The subjects were 28 naïve male Long–Evans rats (~60 days old at start of training), obtained from Envigo Laboratories (Indianapolis, IN). Rats were housed individually and allowed at least 6 days to acclimate to the vivarium prior to surgery. Food and water was available ad libitum (Purina standard rat chow: Nestle Purina, St. Louis, MO). Throughout the study, rats were maintained on a 14:10 light-dark cycle and monitored and cared for in compliance with association for Assessment and Accreditation of laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Surgery
All surgery occurred 28 days following behavioral training. Rats were anesthetized with isoflurane gas (1.5–3% in oxygen) and placed in a Kopf stereotaxic apparatus. Ten rats received unilateral lesions of RSC and ventral secondary auditory cortex (AuV) in opposite hemispheres (Group Contra) and ten rats received lesions in the same hemisphere (Group Ipsi). RSC and AuV were targeted via the coordinates outlined in Table 1 and the location of each lesion (left or right hemisphere) was counterbalanced within each group. At each coordinate, an electrode was lowered and a 2.5 mA current was passed through the tip for 15 s at RSC lesion sites and 20 s at AuV lesion sites. Eight control rats received sham-lesions consisting of a craniotomy and shallow, non-puncturing burr holes to minimize damage to underlying cortex. For half of the sham-lesions the burr holes for the RSC and AuV were on the same hemisphere, and for the other half they were on the opposite hemisphere. Rats were allowed to recover for 10–12 days prior to the context and tone tests.
Table 1.
Stereotaxic coordinates for retrosplenial cortex (RSC) and secondary auditory cortex (AuV) lesions.
| Region | AP | ML | DV |
|---|---|---|---|
| RSC | |||
| −2.0 | ± 0.3 | −2.0 and −2.7 | |
| −3.5 | ± 0.4 | −2.0 and −2.7 | |
| −5.0 | ± 0.4 and ± 1.0 | −2.0 and −2.7 (medial site) and −2.0 (lateral site) | |
| −6.5 | ± 0.8 and ± 1.5 | −2.0 and −2.8 (medial site) and −3.4 (lateral site) | |
| −8.0 | ± 1.6 and ± 2.4 | −2.5 (medial site) and −3.1 (lateral site) | |
| −9.0 | ± 3.4 | −4.0 | |
| AuV | |||
| −4.0 | ± 6.0 | −6.0 at 16° angle | |
| −6.0 | ± 6.0 | −6.0 at 20° angle |
Note. All anterior-posterior (AP), medial-lateral (ML) and dorsal-ventral (DV) measurements are derived from bregma, midline, and skull surface, respectively (measurements are in mm).
Behavioral Apparatus
Two sets of four conditioning chambers served as the two contexts (counterbalanced). All chambers were of the same standard design (Med Associates, Inc., St. Albans, VT, ENV-007; 24 cm W × 30.5 cm L × 29 cm H) and each was housed in its own sound attenuation chamber (Med Associates, ENV-017M; 66 cm W × 56 cm L × 56 cm H) with an exhaust fan to provide airflow and background noise (~68 dB). Each chamber was outfitted with a food cup, recessed in the center of the front wall, and a retractable lever (Med Associates, ENV-112CM) positioned to the right of the food cup, which remained retracted throughout the experiment. Each chamber had a panel light (Med Associates, ENV-221M) mounted approximately 16 cm above the grid floor and centered over the food cup, and a house light (Med Associates, ENV-215M) mounted approximately 24 cm above the grid floor on the back wall of the chamber. Neither the food cup, lever, panel light, nor the house light were used in this experiment. A speaker (Med Associates, ENV-224AM) was located 20 cm above and to the right of the food cup and used to deliver a 1,500 Hz tone for 10 seconds (the conditioned stimulus, CS). Mild electrical current (1 mA, 1 s; unconditioned stimulus) was delivered using a Med Associates shock generator (ENV-414) connected to each chamber.
In one set of chambers, the sidewalls and ceiling were made of clear acrylic plastic and the front and rear walls were made of brushed aluminum. The grid floor was stainless steel rods (5 mm diameter) spaced 1.5 cm apart (center-to-center). In the second set of chambers the rods of the floor were staggered such that odd- and even-numbered rods were mounted in two separate planes, one 0.5 cm above the other. The staggered grid floor provided a distinct tactile feature. In these chambers, the ceiling and door were covered with laminated black and white checkerboard paper (1 cm squares) to provide distinct visual cues.
Because these two sets of chambers were located within the same room of the laboratory, in order to prevent diffusion of the olfactory cues, one olfactory cue was used for Context A sessions and a second olfactory cue was used for Context B sessions. During Context A sessions, 3 mL of Pine-Sol (Clorox, Co., Oakland, CA) was placed in the chamber tray below the grid floor, and for Context B sessions approximately 0.5 g of Vicks Vaporub (Proctor & Gamble, Cincinnati, OH) was placed along the chamber tray below the grid floor.
Both sets of chambers were illuminated with a 2.8 W bulb (with a red cover) mounted to the ceiling of the sound attenuating chamber. The apparatus was controlled by computer equipment located in an adjacent room. Surveillance cameras located inside the sound-attenuating chambers were used to monitor the rats’ behavior.
Behavioral Procedures
The training session consisted of three 10-second presentations of the tone co-terminating with the foot shock. The interval from shock to the next tone (intertrial interval, ITI) was 64 s. The first trial began 3 minutes after the rat was placed in the chamber. Following recovery from surgery, rats were then re-exposed to the original training chamber (Context A) for a single 20-min context test session during which no tones or shocks were presented. Twenty-four hours after the context test, a tone test session was carried out by placing the rats in Context B and presenting the tone 20 times (10 s each, 30 s ITI) beginning 3 min after the rat was placed in the chamber. Again, no shock was delivered during this test session.
Behavioral Observations
Freezing served as the index of conditioned fear and was operationally defined as total motor immobility except for breathing (Blanchard & Blanchard, 1969; Fanselow, 1980). On the training day, the incidence of freezing behavior was recorded during the 64 second period prior to the first trial (baseline freezing) and during the 64-s period following each trial (post-shock freezing). The rats’ behavior was scored every 8 s during the 64-s epochs and the mean percent freezing across the three post-shock epochs was calculated for each rat. For the context test session, each rat was scored every 8 s for the first 8 min and 32 s, yielding 64 observations for each rat (Maren et al., 1997). For the tone test session, freezing was recorded every 2 s during each 10-s presentation of the tone. For each rat, the data was used to calculate the average freezing during the context test session and the average freezing during the tone test session. The frequency of freezing behavior was converted to a percentage of total observations. A single primary observer, blind to treatment conditioning, scored all of the behavioral data while a second observer scored a subset of the data to assess objectivity. The observations from the two observers were highly correlated (r ≥ 0.9).
Lesion verification and analysis
After the behavioral procedures were completed rats were deeply anesthetized with an overdose of sodium pentobarbital and transcardially perfused with 0.9% saline for 2 min, followed by 10% buffered formalin for 6 min. Coronal brain sections (60 μm) were collected using a freezing microtome and were Nissl-stained using thionin. Using a compound microscope (Axioskop I, Zeiss, Inc.), we identified gross tissue damage as necrosis, missing tissue, or marked thinning of the cortex. Outlines of the lesions were drawn onto digital images adapted from Paxinos and Watson (2009) using PowerPoint at 6 levels along the rostro-caudal extent of the RSC (−1.8, −3.0, −4.2, −5.4, −6.6, and −7.8 mm from bregma). At each level, area measurements where then made with ImageJ, including the total area of the target region and the area of the target region that exhibited gross tissue damage. From these measurements, we report the average percentage of RSC that was damaged. In addition, we report the average percentage of sections across the rostro-caudal plane that exhibited RSC damage (out of ~23 sections collected for each rat), the average percentage of sections with damage outside the RSC, and the number of rats with damage to regions outside the RSC. For AuV, regions of gross tissue damage were similarly identified and lesions were drawn onto digital images along the rostro-caudal extent of the cortex (−3.6, −4.52, −5.4, −6.36 mm from bregma). Area measurements were made for each level as described above, and the average percentage of AuV damage was reported.
Results
Histology
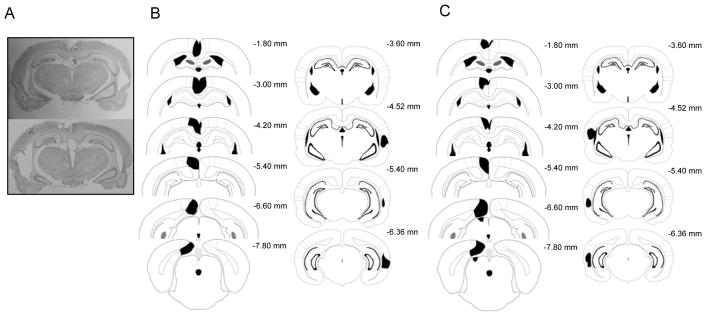
Three rats in the Contra group and one rat in the Ipsi group were excluded from the experimental analysis due to incorrect lesion placement. Outlines of representative Contra (n = 7) and Ipsi (n = 9) lesions are shown in Figure 1. In the Contra group, the average area of unilateral RSC damage on each section analyzed was 57.8% (SEM = 3.72), and the average area of unilateral AuV damage was 23.1% (SEM = 4.74). In the IPSI group, the average area of unilateral RSC damage on each section analyzed was 60.4% (SEM = 3.34), and the average area of unilateral AuV damage was 23.6% (SEM = 5.22). Damage to the RSC was present on 99.3% (SEM = 0.68) of sections collected from the Contra group and 100% (SEM = 0.00) of sections collected from the Ipsi group indicating that damage extended throughout the rostro-caudal extent of the RSC. Minor bilateral damage to the RSC was observed in both the lesion groups. The average area of RSC damage in the opposite hemisphere was 11.1% (SEM = 2.91) for the Contra group and 7.2% (SEM = 2.04) for the Ipsi group. Importantly, damage in the opposite hemisphere was restricted to the most anterior regions of the RSC where lesion sites were very close to the midline, and this damage was similar between the two lesion groups. There was also minor damage outside the RSC in all rats (e.g., anterior cingulate, visual cortex, motor cortex, cingulum bundle, forceps major - corpus callosum) and outside AuV in all rats (e.g., temporal association cortex, ectorhinal cortex, primary auditory cortex (Au1), dorsal cerebral white matter, perirhinal cortex). Unilateral damage to Au1 was observed in all seven rats of the Contra group and eight of the nine rats in the Ipsi group.
Figure 1.
A: Photomicrograph of a representative contralateral and ipsilateral lesion. B (Contralateral) and C (Ipsilateral): representative drawings of lesions at six levels along the rostro-caudal extent of the RSC (−1.8, −3.0, −4.2, −5.4, −6.6, and −7.8 mm posterior to bregma) and at four levels for AuV (−3.60, −4.52, −5.40, and −6.36 mm posterior to bregma). DS = dorsal subiculum, POS = postsubiculum, M2 = secondary motor cortex, RSCd = restrosplenial dysgranular cortex, RSCg = retrosplenial granular cortex, V2 = secondary visual cortex.
Behavior
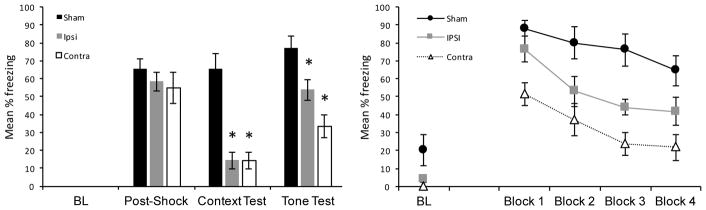
The results from the training session are presented in the left panel of Figure 2. There were no incidences of freezing during the baseline (“BL”) period of the training session. Furthe,r there was no difference between groups during the post-shock period of the training session, F(2, 21) < 1, p = .49. The results from the context test are also presented in the left panel of Figure 2 (“Context”). For the context test, a one-way ANOVA revealed a main effect of group, F(2, 21) = 22.33, p < .001. Post hoc analysis (Fisher’s least significant different) revealed that rats in the Ipsi group and rats in the Contra group froze significantly less than sham-lesioned rats (ps < .001). Groups Ipsi and Contra did not differ from each other, p = .98.
Figure 2.
Left panel: results of Experiment 1. “BL” = freezing during the baseline period (prior to light-shock pairings) in the training session, “Post-Shock” = freezing during the three post-shock periods of the training session, “Context Test” = freezing during the test session in Context A, “Tone” = freezing averaged over the entire tone test in Context B (20 tone presentations). Right panel: results from the Tone Test session plotted in 5-trial blocks. “BL”: freezing during the baseline period just prior to the presentation of the first tone stimulus. Sham = sham lesioned rats, Ipsi = lesions of RSC and AuV in the same hemisphere, Contra = lesions of the RSC and AuV is opposite hemispheres. Error bars represent ±1 SEM. *p < .05.
The results from the tone test session are presented in the left panel of Figure 2 (averaged over all 20 trials), and the right panel of Figure 2 (presented in 5-trial blocks). A one-way ANOVA on the mean of all 20 trials revealed a main effect of group, F(2, 21) = 11.69, p < .001. Post hoc analysis (Fisher’s least significant different) revealed that freezing in the Ipsi group was significantly lower than in the sham-lesion group, p = .01. Freezing in the Contra group was likewise lower than in sham-lesioned rats, p < .001, and importantly, the amount of freezing in the Contra group was significantly lower than in the Ipsi group, p = .03. Due to heterogeneity of variance, freezing during the baseline period of the tone test (“BL”) was analyzed with a non-parametric test (Kruskal-Wallis). Although freezing was numerically higher in Sham controls, the group effect was not significant (p = .071). It is especially noteworthy that groups Ipsi and Contra showed virtually identical freezing during the baseline period of the tone test session, but nonetheless showed reliably different levels of freezing when the tone was presented.
Discussion
The present study used a functional disconnection approach to test the hypothesis that communication between the RSC and secondary auditory cortex is necessary for the retrieval of remote auditory fear memories. Rats were fear conditioned by pairing a tone with footshock, and then 28 days later, received unilateral lesions of the RSC or AuV in either the same (Ipsi group) or opposite hemispheres (Contra group). In this way, contralateral lesions resulted in a complete functional disconnection of RSC and AuV, while ipsilateral lesions left connections intact in one hemisphere. We found that fear memory for the tone was dramatically reduced in the Contra group, but less so in the Ipsi group, while both lesion groups froze less than the Sham control group. This significant difference between the two lesion groups suggests that communication between RSC and AuV is necessary for retrieving remote cue-specific fear memories. Indeed, if communication was not essential, we would have expected to find comparable impairment between the two lesion groups, since they are equated for the total amount of structural damage to RSC and AuV. The finding that memory for the context was impaired similarly in the Contra and Ipsi lesion groups supports the notion that communication between RSC and AuV is specifically important for tone-specific fear memory.
These findings build on prior studies demonstrating that RSC and secondary sensory cortical regions have similar, time-dependent roles in cue-specific fear memory. Specifically, RSC is necessary for recalling remotely-acquired fear to a tone (Todd et al, 2016) or a light (Jiang et al., 2018) paired with shock, but not recently-acquired fear to a tone or to a light. Similarly, the secondary sensory cortices contribute in a modality-specific way to retrieving remote but not recent cue-specific fear memories (Sacco et al., 2010; Grosso et al., 2015). The present findings extend these data by indicating that communication between RSC and secondary sensory cortex is essential for remote fear memory. Although further studies are required to fully define the function of this circuit, it is tempting to consider that RSC, by virtue of its connections with multiple sensory cortical regions (Todd et al., 2016; Vogt & Miller, 1983; van Groen & Wyss, 1990, 1992, 1993), may function as a hub for the intersection of information across sensory modalities. For example, since real-world stimuli are typically composed of features from multiple sensory modalities, RSC might serve to integrate the sensory features of a stimulus in the service of long-term memory. Further, this may be a unique function of RSC, since other regions of association cortex do not appear to be involved in remote fear memory for specific stimuli (Sacco & Sachetti, 2010). Regardless, further research is required to fully characterize the functional circuit between RSC and secondary sensory cortical regions, and how this pathway interfaces with the amygdala (Cambiaghi et al., 2016), a region that is critical for both recent and remote cue-specific fear memories (Maren et al., 1996).
One potential caveat of the present study is that permanent electrolytic lesions were used to damage RSC and AuV. Thus, it is possible that damage to fibers of passage, rather than neurons in RSC and AuV themselves, may have contributed to the observed effects. However, this seems unlikely for several reasons. First, it has been shown previously that electrolytic and neurotoxic lesions of RSC have the same effect on remotely-acquired fear to a discrete auditory cue (Todd et al., 2016), while having no impact on recently-acquired cue-specific fear (Keene and Bucci, 2008b). Further, the observation that the Contra and Ipsi lesions had differential effects on fear memory suggests that damage to fibers of passage through RSC and/or AuV cannot explain the present findings. Indeed, the same amount of overall damage to RSC and AuV was present in both lesion groups, yet the effects of the Contra lesions were significantly greater than the Ipsi lesions. It also seems unlikely that the findings are due to general effects of the lesions on anxiety and/or locomotion. This is because rats in the two lesion groups exhibited a nearly identical reduction in contextual fear when tested in the training context, but responding to the tone was significantly reduced for Group Contra relative to Group Ipsi. If the lesions merely affected general levels of anxiety or movement, the behavior of the rats in the two lesions groups should have been comparable throughout the different phases of the experiment, since the lesion damage was matched across the two groups. Instead, the overall pattern favors the conclusion that RSC-AuV circuitry is necessary for the retrieval of remotely acquired auditory fear memories.
An additional issue is that areas beyond RSC and AuV were slightly damaged by the lesions. In particular, damage was observed in Au1 in rats in each group. Although worthy of consideration, it is unlikely that damage to Au1 contributed to the behavioral findings since it has been shown that in contrast to secondary sensory cortical regions, primary sensory cortex is unnecessary for recalling remotely-acquired conditioned fear (Sacco et al., 2010). Moreover, damage to areas such as Au1 or perirhinal cortex were present in both lesion groups in our study, yet the Contra lesions had greater effects on fear memory to the tone compared to the Ipsi lesions.
Finally, we note that the present study does not dissociate the circuitry necessary for retrieval of recently versus remotely-acquired fear memories. The current experiment consisted only of groups that were conditioned at a remote timepoint, i.e., 28-days prior to the lesions; therefore, no direct comparison was made between recent and remote memory. It seems unlikely, however, that RSC-AuV communication would be necessary for the retrieval of recently-acquired fear memories since prior studies have demonstrated that neither bilateral lesions of RSC (Keene & Bucci, 2008a) nor bilateral lesions of AuV (Sacco & Sacchetti, 2010) affect the expression of recently-acquired tone-fear memories. Nevertheless, the role of RSC-AuV communication in the retrieval of recently-acquired fear memoires was not explicitly tested here and awaits future consideration.
While previous studies have identified roles for both RSC and AuV in the retrieval of remotely acquired fear memories (e.g., Sacco & Sachetti, 2010; Todd et al., 2016), the current experiment demonstrates that communication between these regions is necessary for the retrieval of remotely-acquired cue-specific fear memories. Although further research is needed to fully understand the circuits responsible for the acquisition, storage, and retrieval of remotely-acquired fear memories, it is worth noting that a large portion of experimental work has focused on contextual fear memories rather than cue-specific fear memories. It is interesting to note that while organisms can often control or avoid particular contexts/environments, they often have little control over the presence of cues that might elicit fear. Thus, further investigation of the mechanisms of remote cue-specific memories may be especially valuable for our understanding of anxiety disorders, such as PTSD.
Acknowledgments
This work was supported by National Science Foundation Grant IOS1353137 (D.J.B.) and National Institutes of Health Grant F32MH105125 (T.P.T.).
References
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of Comparative and Physiological Psychology. 1969;67:370. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Cambiaghi M, Grosso A, Likhtik E, Mazziotti R, Concina G, Renna A, … Sacchetti B. Higher-order sensory cortex drives basolateral amygdala activity during the recall of remote, but not recently learned fearful memories. Journal of Neuroscience. 2016;36:1647–1659. doi: 10.1523/JNEUROSCI.2351-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran Ka, Donnan MD, Tronson NC, Guzmán YF, Gao C, Jovasevic V, … Radulovic J. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear, emory. The Journal of Neuroscience. 2011;31:11655–11659. doi: 10.1523/JNEUROSCI.2107-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84:432–441. doi: 10.1016/j.neuron.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Conditional and unconditional components of post-shock freezing. The Pavlovian Journal of Biological Science. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Grosso A, Cambiaghi M, Concina G, Sacco T, Sacchetti B. Auditory cortex involvement in emotional learning and memory. Neuroscience. 2015;299:45–55. doi: 10.1016/j.neuroscience.2015.04.068. [DOI] [PubMed] [Google Scholar]
- Jiang MY, DeAngeli NE, Bucci DJ, Todd TP. Retrosplenial cortex has a time-dependent role in memory for visual stimuli. 2018 doi: 10.1037/bne0000229. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behavioral Neuroscience. 2008a;122:89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behavioral Neuroscience. 2008b;122:1070–1077. doi: 10.1037/a0012895. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience and Biobehavioral Reviews. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Gilmartin MR, Helmstetter FJ. Extinguishing trace fear engages the retrosplenial cortex rather than the amygdala. Neurobiology of Learning and Memory. 2014;113:41–54. doi: 10.1016/j.nlm.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Helmstetter FJ. The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiology of Learning and Memory. 2015;123:110–116. doi: 10.1016/j.nlm.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioural Brain Research. 1997:88. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behavioral Neuroscience. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Robinson S, Poorman CE, Marder TJ, Bucci DJ. Identification of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. Journal of Neuroscience. 2012;32:12076–12078. doi: 10.1523/JNEUROSCI.2814-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco T, Sacchetti B. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science. 2010;329:649–656. doi: 10.1126/science.1183165. [DOI] [PubMed] [Google Scholar]
- Todd TP, Mehlman ML, Keene CS, Deangeli NE, Bucci DJ. Retrosplenial cortex is required for the retrieval of remote memory for auditory cues. Learning & Memory. 2016;23:278–288. doi: 10.1101/lm.041822.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. Journal of Comparative Neurology. 1990;300:593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nature Reviews Neuroscience. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Miller MW. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. Journal of Comparative Neurology. 1983;216:192–210. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Van Groen T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus. 1992;2:1–11. doi: 10.1002/hipo.450020102. [DOI] [PubMed] [Google Scholar]