Abstract
This work summarizes evidence for the role of RSC in processing fear-inducing context memories. Specifically, we discuss molecular, cellular, and network mechanisms by which RSC might contribute the processing of contextual fear memories. We focus on glutamatergic and cholinergic mechanisms underlying encoding, retrieval, and extinction of context-dependent fear. RSC mechanisms underlying retrieval of recently and remotely acquired memories are compared to memory mechanisms of anterior cortices. Due to the strong connectivity between hippocampus and RSC, we also compare the extent to which their mechanisms of encoding, retrieval, and extinction show overlap. At a theoretical level, we discuss the role of RSC in the framework of systems consolidation as well as retrieval-induced memory modulation. Lastly, we emphasize the implication of these findings for psychopathologies associated with neurological and psychiatric disorders.
Keywords: retrosplenial cortex, conttext memory, local field potential, NMDA, muscarinic
Hippocampus, Cortex, and Episodic memories
The most prevalent view of memory storage is framed by the systems consolidation theory, which suggests that recently acquired memories are dependent on the hippocampus, but in time become fully dependent on the cortex and independent of the hippocampus (Squire & Alvarez, 1995). This theory is largely based on studies demonstrating temporally graded retrograde amnesia following hippocampal lesions (Kim & Fanselow, 1992; Scoville & Milner, 1957; Squire, 1992) and disengagement of the hippocampus long after a memory formation in favor of medial prefrontal cortical (mPFC) regions including the anterior cingulate (Restivo, Vetere, Bontempi, & Ammassari-Teule, 2009), infralimbic (Vetere et al., 2011), and orbitofrontal cortices (Lesburgueres et al., 2011). Several recent lines of evidence show that hippocampal-cortical interactions underlying memory may be more complex than originally postulated, and several key questions remain. For example, hippocampal damage can impair detailed and vivid episodic memories regardless of memory age, causing flat retrograde amnesia. This phenomenon provides the basis for the multiple trace theory of memory (Nadel & Moscovitch, 1997), which proposes a time-unlimited role of the hippocampus in processing such memories. Similar findings were recently obtained with optogenetic aproaches (Goshen et al., 2011), but rather than emphasizing a role of memory detail/richness as the main determinant of hippocampal involvement, the authors focused on the duration of hippocampal inactivation, allowing or disallowing compensatory cortical mechanisms to take place. Although the causes of time-dependent and independent effects of hippocampal inactivation are not fully understood, it is possible that these differences rely, at least in part, on hippocampal-cortical mechanisms underlying memory processing.
The dorsal hippocampus, which is the main hippocampal subdivision involved in the formation of contextual memories (Fanselow & Dong, 2010; Strange, Witter, Lein, & Moser, 2014), does not seem to be strongly connected to anterior cortices, and thus it is likely that hippocampal information is predominantly transferred to those areas indirectly, via posterior cortices, which are the main dorsohippocampal targets (Cenquizca & Swanson, 2007). Unlike anterior cortices, which show preferential involvement in processing remote memories, posterior cortices are required for both recently and remotely acquired memories and, thus, seem to play a role in processing recent memories that is non-redundant with the hippocampus (Burwell, Bucci, Sanborn, & Jutras, 2004; Burwell, Saddoris, Bucci, & Wiig, 2004). Among posterior cortices, the entorhinal cortex has been the focus of the majority of experimental, theoretical and computational research on episodic memories (Gluck, Meeter, & Myers, 2003; Kesner & Rolls, 2015; Norman, Polyn, Detre, & Haxby, 2006), however, it is increasingly recognized that posterior cortices, in addition to providing sensory input, also subserve mnemonic processes (Todd & Bucci, 2015). Nevertheless, there is mounting evidence for significant contributions of posterior cortices, including RSC, to processing memories, in particular stress related, fear-inducing context memories acquired through classical fear conditioning (Burwell, Bucci, et al., 2004; Burwell, Saddoris, et al., 2004; Corcoran et al., 2011; Keene & Bucci, 2008c, 2009; Vann & Aggleton, 2002).
RSC and memory: Brief overview of animal experiments
Some of the first evidence of RSC’s role in associative learning comes from eyeblink conditioning and discrimination reversal learning in rabbits following RSC lesions (Berger, Weikart, Bassett, & Orr, 1986; Gabriel et al., 1983). Berger and Bassett (1986) were the first to identify, using electrophysiological approaches in vivo, a disynaptic DH-RSC pathway through the subiculum. Because of the exclusive distribution of subicular efferents to RSC, they suggested that only brain areas receiving efferents from RSC could be influenced by learning-dependent hippocampal activity. RSC was later shown to play a role in spatial processing and navigation (Cooper & Mizumori, 1999, 2001; Vann & Aggleton, 2002; Whishaw, Maaswinkel, Gonzalez, & Kolb, 2001), as well as instrumental and associative learning of negatively valenced stimuli (Keene & Bucci, 2008b; Lukoyanov & Lukoyanova, 2006). Recent focus has been on contextual and trace fear conditioning, paradigms commonly used to study processing of stress-related episodic memories within hippocampal-cortical networks. These studies, which revealed important roles of RSC in encoding, retrieval, and extinction of context-dependent fear (Corcoran et al., 2011; Corcoran, Leaderbrand, & Radulovic, 2013; Cowansage et al., 2014; Jovasevic et al., 2015; Keene & Bucci, 2008a; Kwapis, Jarome, Lee, & Helmstetter, 2015; Leaderbrand et al., 2016), are discussed below in the framework of the overall cytoarchitecture of RSC and its major connections.
Cytoarchitecture and connectivity
RSC is composed of two major cytoarchitectural areas: granular and agranular (RSCg and RSCa, also known as A30, Fig. 3a), which represent the ventral and dorsal subdivisions, respectively. RSCg is further composed of areas A29 a–c along the rostro-caudal axis (Vogt & Miller, 1983). These areas differ in their connectivity to other brain regions (Shibata, 1998; Van Groen & Wyss, 2003; Vogt & Miller, 1983) and their functional roles, as illustrated by the finding that lesions of RSCg but not RSCa impair spatial learning and memory (van Groen, Kadish, & Wyss, 2004). The RSCg layers have highly organized laminar and modular geometry, consisting of dendritic bundles (layer 1), small pyramidal neurons (layer 2/3), sparse layer 4, large pyramidal neurons (layer 5) and polymorphic layer 6 (Vogt & Miller, 1983). A distinctive feature of the rodent RSCg is an accentuated layer 2 consisting of closely packed small pyramidal neurons (Ichinohe et al., 2008; Sripanidkulchai & Wyss, 1987), which predominantly receive thalamic input. In addition to excitatory neurons, RSC contains GABAergic parvalbumin (PV)-positive neurons. Some are localized in layer 2/3 (Ichinohe & Rockland, 2002), and we also identified a large population of these interneurons in layer 5 (unpublished data).
Different RSCg layers receive distinct long-range projections that support its role in memory processing. The key projections originate from two regions in DH, the subiculum and the CA1. Subicular projections originate from pyramidal neurons that densely innervate RSCg layers 1 and 3 (Van Groen & Wyss, 2003; Wyss & Van Groen, 1992), whereas a long-range GABAergic projection into RSC layer 1 (Jinno, 2009; Miyashita & Rockland, 2007) originate from stratum lacunosum-moleculare interneurons. Another major projection implicated in memory function of RSCg originates from anterior thalamic nuclei, and particularly from the anteroventral (AV) and anterodorsal (AD) nuclei. Their projection terminates in layers 1 and 3, with AV more preferentially innervating layer 1 (Odagiri, Meguro, Asano, Tani, & Ichinohe, 2011; Wyss & Van Groen, 1992). In addition to these non-sensory afferents, sensory afferents from visual cortex have also been described, however they predominantly project to the agranular RSC (Vogt and Miller 1983).
In turn, RSC communicates back to thalamus and parahippocampal regions via projection neurons located in specific layers. For example, corticothalamic neurons in layer 6 project back to AV and AD (Mathiasen, Dillingham, Kinnavane, Powell, & Aggleton, 2017; Shibata, 2000; Sripanidkulchai & Wyss, 1987), closing the inter-areal recurrent loop. Projection neurons in layer 5 send their axons to pre- and post-subiculum (Van Groen & Wyss, 2003). RSCg also projects to layer 5 pyramidal neurons in entorhinal cortex (Czajkowski et al., 2013), although a laminar source of this projection is unclear. Other areas to which RSCg distributes information to other regions, via projections originating from neurons in layers 2, 3, 5, and 6, include contralateral/ipsilateral RSCg (Sripanidkulchai & Wyss, 1987) and secondary motor cortex (Yamawaki, Radulovic, & Shepherd, 2016). Furthermore, a subset of pyramidal neurons in layer 5 send their long-range axons to the ventrolateral potion of the pontine nuclei (Yamawaki et al., 2016).
Molecular RSC mechanisms of memory
To investigate the role of RSC in memory processing, we have focused on memories learned through contextual fear conditioning using footshocks as unconditioned stimuli. Although in this task learning itself is seemingly simple – a neutral box is paired with a footshock so that subsequent exposure to that box will yield freezing responses that serve as indices of fear– the neural processes underlying this type of learning are quite complex. Memory for contextual fear conditioning requires the integration of information from multiple sensory modalities to form a representation of the context, along with emotional and mnemonic information. Because of its connectivity with the regions listed above, which mediate these types of information, RSC is poised to play a critical role in contextual memory processing.
Glutamate and downstream signaling pathways
In our initial experiments testing the role of RSC in memory, we observed that glutamatergic antagonism yielded profound retrieval deficits for both recent and remote memories (Corcoran et al., 2011), and the extent of these deficits was comparable to findings with hippocampal inactivation. Unexpectedly, the roles of NMDAR were completely reversed relative to their known roles in hippocampus with respect to memory formation versus retrieval. Namely hippocampal NR2A NMDAR are required for memory formation but not retrieval (Gao et al., 2010), whereas RSC NR2A NMDAR are required for retrieval without affecting memory formation. Furthermore, antagonism of RSC NMDAR impaired retrieval of both recent and remote memory, thus demonstrating that the role of RSC in memory retrieval is not time limited (Corcoran et al., 2011). Collectively, these data show that NMDARs in RSC are necessary for the retrieval of both remote and recent memories of fear-evoking contexts.
In addition to retrieval, glutamatergic RSC mechanisms also contribute to fear extinction, induced by repeated exposures to the context without shock. Unlike the hippocampus, however, wherein extinction requires NR2A (Leaderbrand, Corcoran, & Radulovic, 2014), RSC mechanisms involved NR2B (Corcoran et al., 2013) and required NR2B-mediated downregulation of the cAMP-dependent protein kinase (PKA)/cAMP response element-binding protein pathway. Consistent with this finding, remote extinction was enhanced by uncoupling of NR2B from receptor for activated C kinase 1 or PKA from A-kinase anchor proteins (Corcoran et al., 2015). Thus, targeting NR2B interactions with its scaffolds might be effective in modulation of remote memories.
RSC cholinergic signaling
Similar to glutamate, signaling through acetylcholine receptors (AChR) has long been understood to play a role in learning and memory processes. In the hippocampus, inactivation of AChR disrupts memory formation (Kremin et al., 2006; Newman, Gillet, Climer, & Hasselmo, 2013), whereas their role in memory retrieval is somewhat more contentious (Huang et al., 2011; Souza, Bruning, Acker, Neto, & Nogueira, 2013). We used pharmacological inactivation and subunit-specific genetic knockdown of muscarinic AChR (mAChR) in both hippocampus and RSC to directly compare their roles in encoding and retrieval of memory for contextual fear conditioning (Leaderbrand et al., 2016). We found that M3 subunit-containing mAChR are similarly necessary for formation of context memory in both regions, although in RSC, this role requires the additional co-activation of M1 subunit-containing receptors. In contrast, retrieval mechanisms showed notable regional differences. Retrieval of recent memories was dependent on M2/M4 signaling in RSC, but on M1/M3 signaling in hippocampus. Interestingly, all M1–M4 subunits in RSC were involved in remote memory retrieval. The shift of RSC cholinergic mechanisms from M1/M3 to M1/M4 suggest that despite the time-independent role for RSC in retrieval of contextual memories, the mechanisms that underlie this role change as memories age.
RSC network mechanisms of memory
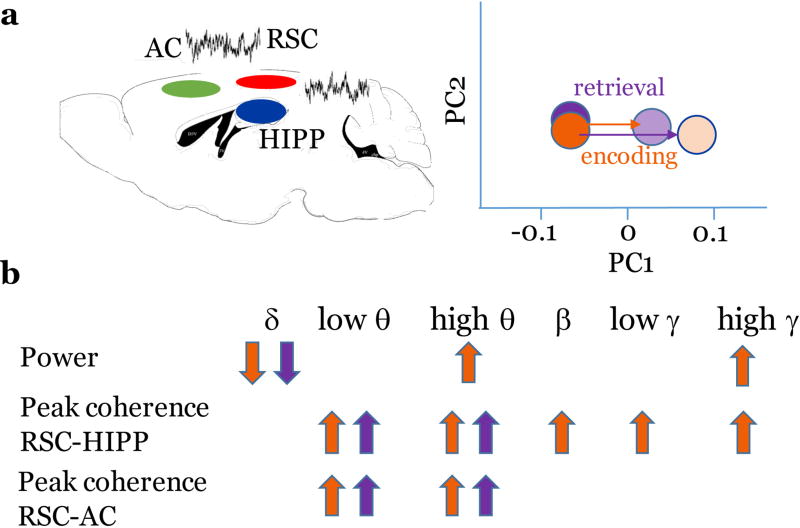
We recorded local field potentials (LFPs) from RSC, hippocampus, anterior dorsal thalamus, and anterior cingulate cortex to better understand how activity within this interconnected network of structures correlates with contextual learning and memory (Corcoran, Frick, Radulovic, & Kay, 2016). During both exposure to a novel context and retrieval of recently-acquired memory for contextual fear conditioning, coherence of LFPs in the theta frequency range was increased between all structures, reflecting enhanced connectivity among these areas. We recently performed a more extensive analysis among multiple frequencies and replicated this phenomenon, suggesting that its underlying mechanisms are rather robust. Interestingly, although we found multiple changes of power (in delta, theta, beta, and gamma frequencies), coherence related to encoding and retrieval of context memories was consistently increased only in the theta range (Miller, Frick, Smith, Radulovic, & Corcoran, 2017) (Figure 1).
Figure 1.
Changes of RSC oscillatory activity related to memory processing. (a) Shift of LFP states in RSC, dorsal hippocampus and anterior cingulate cortex (left) during encoding and retrieval of context memory (right). Overall, encoding results in more pronounced changes of LFP power and coherent activity between RSC and other brain areas. (b) Decreased delta power and increased peak coherence in the theta frequency are the most consistent indicators of recent memory retrieval (purple arrow), whereas multiple changes are seen during encoding (orange arrow). Based on data presented in (Miller et al., 2017). AC, anterior cingulate cortwex, HIPP, hippocampus.
In contrast to recent memories, RSC-hippocampal and RSC-thalamic theta coherence was decreased in mice that successfully retrieved, relative to mice that failed to retrieve, remote memory (Corcoran et al., 2016). This suggests not only that processing remote memories requires a different degree of interdependent activity between RSC and other memory processing areas as does processing of recent memories, but that a failure to decrease RSC-hippocampal theta coherence in the long term might be linked to remote retrieval deficits. Thus, just as specific neurotransmitters are involved in different phases of memory processing, distinct patterns of activity among the connections between RSC and other cortical and subcortical regions seem to be associated with contextual fear memory encoding, retrieval, and extinction.
What is unique about the role of RSC in memory processing?
Given that RSC and entorhinal cortex are the main cortical targets of dorsohippocampal projections, it is not surprising that both cortices play important roles in the consolidation and retrieval of fear-inducing context memories. It is not yet clear, however, whether DH-RSC and DH-entorhinal circuits interact or operate in parallel, and whether their roles in memory are redundant (completely or partially) or unique. Although many questions remain open, existing evidence points to some interesting and unique features of RSC-mediated memory processes relevant for contextual fear inducing memories.
Although initial inactivation studies suggested a time-limited role of RSC in memory processing (Maviel, Durkin, Menzaghi, & Bontempi, 2004), accumulating evidence supports a time-unlimited role of RSC in the retrieval of fear-inducing context memory from earliest stages of memory consolidation to remote retrieval (Corcoran et al., 2011; Tayler, Tanaka, Reijmers, & Wiltgen, 2013; Todd, Mehlman, Keene, DeAngeli, & Bucci, 2016). In that respect, RSC’s role resembles those of the perirhinal and postrhinal cortices (Burwell, Bucci, et al., 2004). On the contrary, the DH-entorhinal circuit seems to be predominantly engaged in earlier stages of memory processing (Frankland & Bontempi, 2005; Kitamura et al., 2017), whereas anterior cortices (orbitofrontal, anterior cingulate, and medial prefrontal cortices) become involved at later stages (Frankland, Bontempi, Talton, Kaczmarek, & Silva, 2004; Kitamura et al., 2017). Thus, the role of RSC appears to be less dynamic, possibly providing a more stable system for memory storage and retrieval. Given the still controversial issue on the temporal involvement of the hippocampus in memory, with evidence for both temporary (Kim & Fanselow, 1992; Winocur, Sekeres, Binns, & Moscovitch, 2013) and permanent ((Lehmann, Lacanilao, & Sutherland, 2007; Sparks, Spanswick, Lehmann, & Sutherland, 2013) involvement, it is worth exploring whether these discrepancies are due to differential utilization of DH-RSC and DH-entorhinal circuits across discrete paradigms, allowing permanent involvement through the DH-RSC and temporary involvement through the DH-entorhinal cortical pathway.
In addition to its cortical connections, RSC is densely connected to multiple anterior thalamic nuclei (Jankowski et al., 2013), which play a major role in maintaining episodic memories in humans (Nishio et al., 2014), as well as memories of fear-inducing contexts in rodents (Lopez, Gamache, Milo, & Nader, 2017; Marchand, Faugere, Coutureau, & Wolff, 2014). The role of RSC interactions with individual thalamic nuclei may thus provide further detail on the mechanisms underlying sustained RSC involvement in memory processing.
At the molecular level, a similar stability is found with glutamatergic RSC mechanisms of memory retrieval, whereas hippocampal mechanisms seem to vary with time. For example, hippocampally-mediated retrieval of fear-inducing context memories relies on the time-limited involvement of AMPA (Goshen et al., 2011; Kitamura et al., 2009) and beta-adrenergic receptors (Ouyang & Thomas, 2005) whereas RSC-mediated retrieval relies on the time-independent involvement of NMDAR (Corcoran et al., 2011). Thus, the roles of RSC NMDAR and AMPAR in contextual fear conditioning appear to be dissociable, as it has been previously found with reward learning (Di Ciano, Cardinal, Cowell, Little, & Everitt, 2001; Di Ciano & Everitt, 2001), and reversed when compared to the hippocampus (Bast, da Silva, & Morris, 2005; Kim, DeCola, Landeira-Fernandez, & Fanselow, 1991). NMDAR are known for their important role in synaptic plasticity, reflected both at the level of physiological activity and gene expression (Platenik, Kuramoto, & Yoneda, 2000) of memory-processing neurons. This suggests that RSC could be an important site for retrieval-induced plasticity, such as memory stabilization, update, or extinction (Alberini, Milekic, & Tronel, 2006). This is consistent with the demonstrated role of RSC in extinction of both recently and remotely acquired contextual freezing (Corcoran et al., 2013), and needs to be further examined in other retrieval-mediated phenomena.
Implications of RSC in human brain disorders
The key role of RSC NMDAR in retrieval and extinction of fear-inducing context memories may have important implications for psychiatric disorders rooted in stress-related memories, such as post-traumatic stress disorder (PTSD). Abnormal regulation and function of NMDAR, which has been implicated in PTSD, could contribute to retrieval-mediated phenomena, such as flashbacks and re-experiencing of traumatic memories, as well as resistance to extinction, resulting in persistent fear and avoidance. Hyper- or hypoactivation of RSC is found in patients suffering from post-traumatic stress disorder (Liberzon et al., 1999; Sartory et al., 2013), schizophrenia (Mitelman, Shihabuddin, Brickman, Hazlett, & Buchsbaum, 2005), and bipolar disorder (Nugent et al., 2006), suggesting that RSC dysfunction contributes to cognitive and affective pathologies associated with psychiatric illnesses. This could be due to deficits of RSC function in evaluating the emotional salience of information from the environment (Cato et al., 2004), in the retrieval of autobiographical memories (Piefke, Weiss, Zilles, Markowitsch, & Fink, 2003), or in reflection upon emotionally toned experiences (Oddo et al., 2010). RSC dysfunction is likely to impact neuroanatomically associated structures, such as the anterior cingulate cortex, which also shows abnormal activity in patients with PTSD and depression along with abnormal processing of emotionally valenced memories (Rauch, Shin, & Phelps, 2006) (Davey, Harrison, Yucel, & Allen, 2012).
RSC also shows abnormalities in the most common neurological disorders with memory deficits (Vann & Albasser, 2009). For example, early memory loss in patients with Alzheimer’s disease is associated with metabolic decline (Minoshima et al., 1997) and hypoactivity (Desgranges et al., 2002) in RSC. Retrosplenial pathology is sufficient to induce human amnesia (Valenstein et al., 1987) and retrosplenial hypoactivity is also seen in the amnesic Korsakoff’s syndrome (Joyce & Robbins, 1993; Reed et al., 2003). It has been proposed that this is due to the dense connections of RSC with thalamic nuclei, the hippocampus (Vann & Albasser, 2009), and the prefrontal cortex (Shibata, 1998; Shibata, Kondo, & Naito, 2004; Wyss & Van Groen, 1992). Consequently, RSC is seen as a key link for temporal-diencephalic, temporal-frontal, and thalamic-hippocampal interactions (Kobayashi & Amaral, 2007).
Understanding the molecular, cellular, and circuit mechanisms by which RSC and its circuits contribute to the processing of memories in general as well as stress-related episodic memories will facilitate our understanding of the role of memory in these neuropsychopathologies and reveal the potential of RSC manipulations to alleviate them.
Summary
Converging molecular, cellular, and network evidence suggests that RSC acts as a hub that integrates and coordinates the activity of distinct brain regions to mediate acquisition and time-independent retrieval of contextual memories. In humans, this role has been hinted at by imaging studies that have identified RSC as a node of connectivity between regions of the default mode network. Activity within this network is associated with emotional regulation and autobiographical memory retrieval (Bressler & Menon, 2010), whereas dysfunction is seen in a number of mental disorders, including major depression (Hamilton et al., 2011) and anxiety (Carlson, Rubin, & Mujica-Parodi, 2017). Disruptions in RSC-dependent inter-regional connectivity may yield susceptibility to extinction failure, which has been posited as a mechanism underlying the persistence of symptoms in post-traumatic stress disorder (Rothbaum & Davis, 2003), or to aging-related impairments in memory retrieval, as RSC is among the first areas of the brain to show metabolic decline (Minoshima et al., 1997), and functional connectivity with RSC is disrupted (Andrews-Hanna et al., 2007; Jones et al., 2011), in both mild cognitive impairment and Alzheimer’s disease. Thus, RSC and RSC-related networks may provide critical avenues for understanding and treating pathological memory states.
Acknowledgments
This work was supported by NIMH grant to J.R. (MH078064).
References
- Alberini CM, Milekic MH, Tronel S. Mechanisms of memory stabilization and de-stabilization. Cell Mol Life Sci. 2006;63(9):999–1008. doi: 10.1007/s00018-006-6025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, da Silva BM, Morris RG. Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J Neurosci. 2005;25(25):5845–5856. doi: 10.1523/JNEUROSCI.0698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger TW, Weikart CL, Bassett JL, Orr WB. Lesions of the retrosplenial cortex produce deficits in reversal learning of the rabbit nictitating membrane response: implications for potential interactions between hippocampal and cerebellar brain systems. Behav Neurosci. 1986;100(6):802–809. doi: 10.1037//0735-7044.100.6.802. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Bucci DJ, Sanborn MR, Jutras MJ. Perirhinal and postrhinal contributions to remote memory for context. J Neurosci. 2004;24(49):11023–11028. doi: 10.1523/JNEUROSCI.3781-04.2004. doi:24/49/11023 [pii] 10.1523/JNEUROSCI.3781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Saddoris MP, Bucci DJ, Wiig KA. Corticohippocampal contributions to spatial and contextual learning. J Neurosci. 2004;24(15):3826–3836. doi: 10.1523/JNEUROSCI.0410-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Rubin D, Mujica-Parodi LR. Lost emotion: Disrupted brain-based tracking of dynamic affective episodes in anxiety and depression. Psychiatry Res. 2017;260:37–48. doi: 10.1016/j.pscychresns.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Cato MA, Crosson B, Gokcay D, Soltysik D, Wierenga C, Gopinath K, Briggs RW. Processing words with emotional connotation: an FMRI study of time course and laterality in rostral frontal and retrosplenial cortices. J Cogn Neurosci. 2004;16(2):167–177. doi: 10.1162/089892904322984481. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56(1):1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJ. Retrosplenial cortex inactivation selectively impairs navigation in darkness. Neuroreport. 1999;10(3):625–630. doi: 10.1097/00001756-199902250-00033. [DOI] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJ. Temporary inactivation of the retrosplenial cortex causes a transient reorganization of spatial coding in the hippocampus. J Neurosci. 2001;21(11):3986–4001. doi: 10.1523/JNEUROSCI.21-11-03986.2001. doi:21/11/3986 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzman YF, Gao C, Jovasevic V, Radulovic J. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J Neurosci. 2011;31(32):11655–11659. doi: 10.1523/JNEUROSCI.2107-11.2011. 31/32/11655 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Frick BJ, Radulovic J, Kay LM. Analysis of coherent activity between retrosplenial cortex, hippocampus, thalamus, and anterior cingulate cortex during retrieval of recent and remote context fear memory. Neurobiol Learn Mem. 2016;127:93–101. doi: 10.1016/j.nlm.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Leaderbrand K, Jovasevic V, Guedea AL, Kassam F, Radulovic J. Regulation of fear extinction versus other affective behaviors by discrete cortical scaffolding complexes associated with NR2B and PKA signaling. Transl Psychiatry. 2015;5:e657. doi: 10.1038/tp.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Leaderbrand K, Radulovic J. Extinction of remotely acquired fear depends on an inhibitory NR2B/PKA pathway in the retrosplenial cortex. J Neurosci. 2013;33(50):19492–19498. doi: 10.1523/JNEUROSCI.3338-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84(2):432–441. doi: 10.1016/j.neuron.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski R, Sugar J, Zhang SJ, Couey JJ, Ye J, Witter MP. Superficially projecting principal neurons in layer V of medial entorhinal cortex in the rat receive excitatory retrosplenial input. J Neurosci. 2013;33(40):15779–15792. doi: 10.1523/JNEUROSCI.2646-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Harrison BJ, Yucel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med. 2012;42(10):2071–2081. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- Desgranges B, Baron JC, Lalevee C, Giffard B, Viader F, de La Sayette V, Eustache F. The neural substrates of episodic memory impairment in Alzheimer's disease as revealed by FDG-PET: relationship to degree of deterioration. Brain. 2002;125(Pt 5):1116–1124. doi: 10.1093/brain/awf097. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21(23):9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25(3):341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6(2):119–130. doi: 10.1038/nrn1607. doi:nrn1607 [pii] 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304(5672):881–883. doi: 10.1126/science.1094804. 304/5672/881 [pii] [DOI] [PubMed] [Google Scholar]
- Gabriel M, Lambert RW, Foster K, Orona E, Sparenborg S, Maiorca RR. Anterior thalamic lesions and neuronal activity in the cingulate and retrosplenial cortices during discriminative avoidance behavior in rabbits. Behav Neurosci. 1983;97(5):675–696. doi: 10.1037//0735-7044.97.5.675. [DOI] [PubMed] [Google Scholar]
- Gluck MA, Meeter M, Myers CE. Computational models of the hippocampal region: linking incremental learning and episodic memory. Trends Cogn Sci. 2003;7(6):269–276. doi: 10.1016/s1364-6613(03)00105-0. [DOI] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. Dynamics of retrieval strategies for remote memories. Cell. 2011;147(3):678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZB, Wang H, Rao XR, Zhong GF, Hu WH, Sheng GQ. Different effects of scopolamine on the retrieval of spatial memory and fear memory. Behav Brain Res. 2011;221(2):604–609. doi: 10.1016/j.bbr.2010.05.032. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Knight A, Ogawa M, Ohshima T, Mikoshiba K, Yoshihara Y, Rockland KS. Unusual patch-matrix organization in the retrosplenial cortex of the reeler mouse and Shaking rat Kawasaki. Cereb Cortex. 2008;18(5):1125–1138. doi: 10.1093/cercor/bhm148. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Rockland KS. Parvalbumin positive dendrites co-localize with apical dendritic bundles in rat retrosplenial cortex. Neuroreport. 2002;13(6):757–761. doi: 10.1097/00001756-200205070-00005. [DOI] [PubMed] [Google Scholar]
- Jankowski MM, Ronnqvist KC, Tsanov M, Vann SD, Wright NF, Erichsen JT, O'Mara SM. The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front Syst Neurosci. 2013;7:45. doi: 10.3389/fnsys.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S. Structural organization of long-range GABAergic projection system of the hippocampus. Front Neuroanat. 2009;3:13. doi: 10.3389/neuro.05.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Machulda MM, Vemuri P, McDade EM, Zeng G, Senjem ML, Jack CR., Jr Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011;77(16):1524–1531. doi: 10.1212/WNL.0b013e318233b33d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovasevic V, Corcoran KA, Leaderbrand K, Yamawaki N, Guedea AL, Chen HJ, Radulovic J. GABAergic mechanisms regulated by miR-33 encode state-dependent fear. Nat Neurosci. 2015;18(9):1265–1271. doi: 10.1038/nn.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce EM, Robbins TW. Memory deficits in Korsakoff and non-Korsakoff alcoholics following alcohol withdrawal and the relationship to length of abstinence. Alcohol Alcohol Suppl. 1993;2:501–505. [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behav Neurosci. 2008a;122(1):89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Involvement of the retrosplenial cortex in processing multiple conditioned stimuli. Behav Neurosci. 2008b;122(3):651–658. doi: 10.1037/0735-7044.122.3.651. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behav Neurosci. 2008c;122(5):1070–1077. doi: 10.1037/a0012895. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Damage to the retrosplenial cortex produces specific impairments in spatial working memory. Neurobiol Learn Mem. 2009;91(4):408–414. doi: 10.1016/j.nlm.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Rolls ET. A computational theory of hippocampal function, and tests of the theory: new developments. Neurosci Biobehav Rev. 2015;48:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Kim JJ, DeCola JP, Landeira-Fernandez J, Fanselow MS. N-methyl-D-aspartate receptor antagonist APV blocks acquisition but not expression of fear conditioning. Behav Neurosci. 1991;105(1):126–133. doi: 10.1037//0735-7044.105.1.126. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, Tonegawa S. Engrams and circuits crucial for systems consolidation of a memory. Science. 2017;356(6333):73–78. doi: 10.1126/science.aam6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139(4):814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. J Comp Neurol. 2007;502(5):810–833. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Kremin T, Gerber D, Giocomo LM, Huang SY, Tonegawa S, Hasselmo ME. Muscarinic suppression in stratum radiatum of CA1 shows dependence on presynaptic M1 receptors and is not dependent on effects at GABA(B) receptors. Neurobiol Learn Mem. 2006;85(2):153–163. doi: 10.1016/j.nlm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Helmstetter FJ. The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiology of Learning and Memory. 2015;123:110–116. doi: 10.1016/j.nlm.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaderbrand K, Chen HJ, Corcoran KA, Guedea AL, Jovasevic V, Wess J, Radulovic J. Muscarinic acetylcholine receptors act in synergy to facilitate learning and memory. Learn Mem. 2016;23(11):631–638. doi: 10.1101/lm.043133.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaderbrand K, Corcoran KA, Radulovic J. Co-activation of NR2A and NR2B subunits induces resistance to fear extinction. Neurobiol Learn Mem. 2014;113:35–40. doi: 10.1016/j.nlm.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H, Lacanilao S, Sutherland RJ. Complete or partial hippocampal damage produces equivalent retrograde amnesia for remote contextual fear memories. Eur J Neurosci. 2007;25(5):1278–1286. doi: 10.1111/j.1460-9568.2007.05374.x. [DOI] [PubMed] [Google Scholar]
- Lesburgueres E, Gobbo OL, Alaux-Cantin S, Hambucken A, Trifilieff P, Bontempi B. Early tagging of cortical networks is required for the formation of enduring associative memory. Science. 2011;331(6019):924–928. doi: 10.1126/science.1196164. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Lopez J, Gamache K, Milo C, Nader K. Differential role of the anterior and intralaminar/lateral thalamic nuclei in systems consolidation and reconsolidation. Brain Struct Funct. 2017 doi: 10.1007/s00429-017-1475-2. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Lukoyanova EA. Retrosplenial cortex lesions impair acquisition of active avoidance while sparing fear-based emotional memory. Behav Brain Res. 2006;173(2):229–236. doi: 10.1016/j.bbr.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Marchand A, Faugere A, Coutureau E, Wolff M. A role for anterior thalamic nuclei in contextual fear memory. Brain Struct Funct. 2014;219(5):1575–1586. doi: 10.1007/s00429-013-0586-7. [DOI] [PubMed] [Google Scholar]
- Mathiasen ML, Dillingham CM, Kinnavane L, Powell AL, Aggleton JP. Asymmetric cross-hemispheric connections link the rat anterior thalamic nuclei with the cortex and hippocampal formation. Neuroscience. 2017;349:128–143. doi: 10.1016/j.neuroscience.2017.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305(5680):96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- Miller AMP, Frick BJ, Smith DM, Radulovic J, Corcoran KA. Network oscillatory activity driven by context memory processing is differently regulated by glutamatergic and cholinergic neurotransmission. Neurobiol Learn Mem. 2017;145:59–66. doi: 10.1016/j.nlm.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophr Res. 2005;72(2–3):91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Rockland KS. GABAergic projections from the hippocampus to the retrosplenial cortex in the rat. Eur J Neurosci. 2007;26(5):1193–1204. doi: 10.1111/j.1460-9568.2007.05745.x. doi:EJN5745 [pii] 10.1111/j.1460-9568.2007.05745.x. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7(2):217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Newman EL, Gillet SN, Climer JR, Hasselmo ME. Cholinergic blockade reduces theta-gamma phase amplitude coupling and speed modulation of theta frequency consistent with behavioral effects on encoding. J Neurosci. 2013;33(50):19635–19646. doi: 10.1523/JNEUROSCI.2586-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio Y, Hashimoto M, Ishii K, Ito D, Mugikura S, Takahashi S, Mori E. Multiple thalamo-cortical disconnections in anterior thalamic infarction: implications for thalamic mechanisms of memory and language. Neuropsychologia. 2014;53:264–273. doi: 10.1016/j.neuropsychologia.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10(9):424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, Drevets WC. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30(2):485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Odagiri S, Meguro R, Asano Y, Tani T, Ichinohe N. Single axon branching analysis in rat thalamocortical projection from the anteroventral thalamus to the granular retrosplenial cortex. Front Neuroanat. 2011;5:63. doi: 10.3389/fnana.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Lux S, Weiss PH, Schwab A, Welzer H, Markowitsch HJ, Fink GR. Specific role of medial prefrontal cortex in retrieving recent autobiographical memories: an fMRI study of young female subjects. Cortex. 2010;46(1):29–39. doi: 10.1016/j.cortex.2008.07.003. S0010-9452(08)00250-5 [pii] [DOI] [PubMed] [Google Scholar]
- Ouyang M, Thomas SA. A requirement for memory retrieval during and after long-term extinction learning. Proc Natl Acad Sci USA. 2005;102(26):9347–9352. doi: 10.1073/pnas.0502315102. doi:0502315102 [pii] 10.1073/pnas.0502315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126(Pt 3):650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Platenik J, Kuramoto N, Yoneda Y. Molecular mechanisms associated with long-term consolidation of the NMDA signals. Life Sci. 2000;67(4):335–364. doi: 10.1016/s0024-3205(00)00632-9. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. doi:S0006-3223(06)00796-7 [pii] 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Lasserson D, Marsden P, Stanhope N, Stevens T, Bello F, Kopelman MD. FDG-PET findings in the Wernicke-Korsakoff syndrome. Cortex. 2003;39(4–5):1027–1045. doi: 10.1016/s0010-9452(08)70876-1. [DOI] [PubMed] [Google Scholar]
- Restivo L, Vetere G, Bontempi B, Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J Neurosci. 2009;29(25):8206–8214. doi: 10.1523/JNEUROSCI.0966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Sartory G, Cwik J, Knuppertz H, Schurholt B, Lebens M, Seitz RJ, Schulze R. In search of the trauma memory: a meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD) PLoS One. 2013;8(3):e58150. doi: 10.1371/journal.pone.0058150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H. Organization of projections of rat retrosplenial cortex to the anterior thalamic nuclei. Eur J Neurosci. 1998;10(10):3210–3219. doi: 10.1046/j.1460-9568.1998.00328.x. [DOI] [PubMed] [Google Scholar]
- Shibata H. Organization of retrosplenial cortical projections to the laterodorsal thalamic nucleus in the rat. Neurosci Res. 2000;38(3):303–311. doi: 10.1016/s0168-0102(00)00174-7. [DOI] [PubMed] [Google Scholar]
- Shibata H, Kondo S, Naito J. Organization of retrosplenial cortical projections to the anterior cingulate, motor, and prefrontal cortices in the rat. Neurosci Res. 2004;49(1):1–11. doi: 10.1016/j.neures.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Souza AC, Bruning CA, Acker CI, Neto JS, Nogueira CW. 2-Phenylethynyl-butyltellurium enhances learning and memory impaired by scopolamine in mice. Behav Pharmacol. 2013;24(4):249–254. doi: 10.1097/FBP.0b013e32836353a5. [DOI] [PubMed] [Google Scholar]
- Sparks FT, Spanswick SC, Lehmann H, Sutherland RJ. Neither time nor number of context-shock pairings affect long-term dependence of memory on hippocampus. Neurobiol Learn Mem. 2013;106:309–315. doi: 10.1016/j.nlm.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5(2):169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Sripanidkulchai K, Wyss JM. The laminar organization of efferent neuronal cell bodies in the retrosplenial granular cortex. Brain Res. 1987;406(1–2):255–269. doi: 10.1016/0006-8993(87)90790-6. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15(10):655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol. 2013;23(2):99–106. doi: 10.1016/j.cub.2012.11.019. S0960-9822(12)01328-0 [pii] [DOI] [PubMed] [Google Scholar]
- Todd TP, Bucci DJ. Retrosplenial Cortex and Long-Term Memory: Molecules to Behavior. Neural Plast. 2015;2015:414173. doi: 10.1155/2015/414173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Mehlman ML, Keene CS, DeAngeli NE, Bucci DJ. Retrosplenial cortex is required for the retrieval of remote memory for auditory cues. Learn Mem. 2016;23(6):278–288. doi: 10.1101/lm.041822.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. Retrosplenial cortex lesions of area Rgb (but not of area Rga) impair spatial learning and memory in the rat. Behav Brain Res. 2004;154(2):483–491. doi: 10.1016/j.bbr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol. 2003;463(3):249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behav Neurosci. 2002;116(1):85–94. [PubMed] [Google Scholar]
- Vann SD, Albasser MM. Hippocampal, retrosplenial, and prefrontal hypoactivity in a model of diencephalic amnesia: Evidence towards an interdependent subcortical-cortical memory network. Hippocampus. 2009;19(11):1090–1102. doi: 10.1002/hipo.20574. [DOI] [PubMed] [Google Scholar]
- Vetere G, Restivo L, Novembre G, Aceti M, Lumaca M, Ammassari-Teule M. Extinction partially reverts structural changes associated with remote fear memory. Learn Mem. 2011;18(9):554–557. doi: 10.1101/lm.2246711. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Miller MW. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol. 1983;216(2):192–210. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Maaswinkel H, Gonzalez CL, Kolb B. Deficits in allothetic and idiothetic spatial behavior in rats with posterior cingulate cortex lesions. Behav Brain Res. 2001;118(1):67–76. doi: 10.1016/s0166-4328(00)00312-0. [DOI] [PubMed] [Google Scholar]
- Winocur G, Sekeres MJ, Binns MA, Moscovitch M. Hippocampal lesions produce both nongraded and temporally graded retrograde amnesia in the same rat. Hippocampus. 2013;23(5):330–341. doi: 10.1002/hipo.22093. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Van Groen T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus. 1992;2(1):1–11. doi: 10.1002/hipo.450020102. [DOI] [PubMed] [Google Scholar]
- Yamawaki N, Radulovic J, Shepherd GM. A Corticocortical Circuit Directly Links Retrosplenial Cortex to M2 in the Mouse. J Neurosci. 2016;36(36):9365–9374. doi: 10.1523/JNEUROSCI.1099-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]