Abstract
Phosphatidylethanol is a direct alcohol biomarker for identifying alcohol misuse. It carries several advantages over other alcohol biomarkers including a detection half-life of several weeks and little confounding by patient characteristics or organ dysfunction. The aim of this study is to derive an optimal phosphatidylethanol cutpoint to identify organ donors with alcohol misuse, and to assess the impact of alcohol misuse on organ allocation. Discrimination of phosphatidylethanol was evaluated using the area under the ROC curve from a mixed effects logistic regression model. Phosphatidylethanol had an area under the ROC curve of 0.89 (95% CI 0.80–0.98). A phosphatidylethanol cutpoint of ≥84 ng/mL provided optimal discrimination for the identification of alcohol misuse with a sensitivity of 75% (95% CI 52.9%–89.4%) and a specificity of 97% (95% CI 91%–99%), a positive predictive value of 82% (95% CI 59%–94%), and a negative predictive value of 95% (95% CI 89%–98%). In critically ill deceased organ donors phosphatidylethanol had good test characteristics to discriminate alcohol misuse. Other alcohol biomarkers performed poorly in deceased organ donors. Liver allocation was decreased in donors with alcohol misuse by proxy history, but not in those with phosphatidylethanol >84 ng/ml, revealing possible information bias in liver allocation.
INTRODUCTION
Alcohol misuse accounts for 88,000 deaths in the United States (US) every year, making it the fourth leading preventable cause of death in the US and the 12th leading cause of death overall.1,2 Alcohol misuse encompasses two patterns, binge drinking and chronic heavy drinking, which contribute to a dysregulated immune response in acutely ill patients and is associated with more severe organ dysfunction than patients without alcohol misuse.1,3 Twenty seven percent of the US population admits to alcohol misuse.4 Of all driving fatalities, 31% are attributable to excessive alcohol use, along with an overall increased morbidity and mortality from injuries such as falls, violence and burns.5–8 Therefore, it is not surprising that almost 1 out of every 6 organ donor has a history of alcohol misuse.9 Nearly one third of organ donors are 18–34 years old, an age demographic with known high risk drinking behaviors, where 50% admit to recent binge drinking.4
Solid organ transplantation from deceased donors is a complicated and aggressive treatment for advanced organ failure. A donor’s alcohol consumption history is obtained by-proxy after the donor is deceased but prior to organ allocation and recovery.10 Alcohol misuse in organ donors may have implications during the transplant process and has been associated with an increased risk of primary graft dysfunction in lung and heart transplant recipients.11–13 While there are no investigations into the accuracy and reliability of proxy history data in deceased organ donors, the addition of objective alcohol biomarkers to proxy history in organ donors could improve the accuracy and understanding of a donor’s recent alcohol use. Alcohol biomarkers routinely utilized in the clinical setting to screen for alcohol misuse, including blood alcohol content (BAC), gamma-glutamyltransferase (GGT), aspartate aminotransferase (AST), alanine amino transferase (ALT), and mean corpuscular volume (MCV), are metabolized quickly or are confounded by a donor’s demographics, comorbidities and organ dysfunctions.14–16 Phosphatidylethanol (PEth), a direct alcohol biomarker, is a byproduct formed on the phospholipid membrane of red blood cells in the presence of alcohol.17,18 Measurable in the blood for up to three weeks in heavy daily consumers, PEth is not confounded by demographics, comorbidities or organ dysfunction. With a sensitivity frequently reported above 90%, PEth has also repeatedly exhibited better sensitivity for estimating heavy alcohol consumption than other alcohol biomarkers, and the specificity of PEth, which reaches nearly 100%, is an extremely reliable test for recent alcohol abstention.17,19–23 PEth has been previously investigated for assessing alcohol misuse in various settings including the outpatient clinic, alcohol treatment programs, and in the intensive care unit.17,24–26
The aim of this investigation is to assess alcohol misuse in the organ donor population. Our hypothesis is that PEth will be an effective biomarker for identifying alcohol misuse in organ donors. Our primary aim is to identify an optimal PEth cutpoint for identifying alcohol misuse in this cohort. A secondary aim includes assessing whether identification of alcohol misuse in organ donors leads to a decrease in the number of organs recovered for transplantation. The results from this study may better inform the role of alcohol misuse on transplant recipient outcomes and improve the organ allocation process.
METHODS
Between August 2016 and March 2017, whole blood samples were collected from deceased organ donors at the time of transplantation by the local organ procurement organization (OPO), Gift of Hope (GOH), in Itasca, IL. Additionally, whole blood samples were collected from a second cohort of brain dead organ donors who were deemed suitable for lung donation at Loyola University Medical Center (LUMC) between February 2015 and February 2017. Demographic, medical history, proxy alcohol history, and organ allocation data were collected from donor charts provided by the OPO or obtained through DonorNet. The Institutional Review Board of Loyola University Chicago approved this study (LU204490). All donors were consented for research by-proxy before samples were collected.
Alcohol Biomarkers
Values for clinically utilized alcohol biomarkers such as AST, ALT, GGT, and BAC were obtained from donor charts. The standard range derived from the clinical laboratory (Quest Diagnostics, Chantilly, VA) that serves health systems was used to identify the upper and lower limits of normal for AST, ALT, and GGT. The first measured values after hospital admission were chosen for inclusion into the study. Whole blood and serum were collected on donors at procurement and brought back to the OPO or directly to LUMC, and maintained at 4°C for less than 24 hours.27,28 40µL of vortexed whole blood was then used to create dried blood spots which are stable at room temperature for up to one year.27,29 These samples were then sent to the United States Drug Testing Laboratories (USDTL, Des Plaines, IL) for analysis of PEth by liquid chromatography–mass spectrometry (LC-MS).29 This method measures the most prevalent PEth isomer (palmitoyl/oleoyl) in human blood, a phospholipid containing 16:0 and 18:1 fatty acids. The limit of detection was 2 ng/mL, the limit of quantitation was 8ng/ml and the assay is linear up to 800 ng/mL. Frozen aliquoted serum which was received within six hours of procurement was sent to Quest laboratories (Quest Diagnostics, Chantilly, VA) to determine serum levels of percent carbohydrate deficient transferrin (%CDT). Serum analysis was conducted in a manner similar to that proposed by Schellenberg et al. using rate-nephelpometric determination after anion-exchange separation.30 Values are reported if greater than 1.5%.
Alcohol Use Classification and Reference Standard
The alcohol use reference standard utilized in this investigation is by proxy history. Since this cohort is composed of brain dead organ donors who are unable to participate with more accepted reference standards, such as alcohol consumption recall and validated questionnaires such as the Alcohol Use and Disorder Identification Test, the reference standard is limited by bias’s inherent to the proxy and their relationship to the donor. Alcohol misuse in the donor cohort was defined according to the Centers for Disease Control (CDC) definition for excessive alcohol use: >1 drink per day on average or ≥4 drinks consumed on one occasion in one month for women; >2 drinks per day on average or ≥5 drinks consumed on one occasion in one month for men.3 The UNOS definition of heavy alcohol use is ≥ 2 drinks per day for either sex. Alcohol intake from proxy histories was quantified by the proxy responses on the Uniform Donor Risk Assessment Interview.10
Statistical Analysis
Donor demographics and clinical characteristics were presented as counts and percentages or medians and interquartile ranges for the overall sample and for each cohort (GOH, LUMC), and cohorts were compared with the Wilcoxon rank sum tests for continuous characteristics and chi-square or Fisher’s exact tests for nominal variables. Discrimination of PEth for identifying alcohol misuse was evaluated using the area under the receiver operating characteristic (ROC) curve from a mixed effects logistic regression model with random intercepts for cohort. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were compared for select cut-points in PEth predicting alcohol misuse based on proxy history, including the PEth level with the highest Youden index (J).31 The area under the ROC (c-statistic) was computed for the optimal cut-point based on Youden’s J, and 5,000 bootstrap samples were used to estimate the 95% confidence intervals (CI) for the overall sample and demographic subgroups. The nonparameteric approach of DeLong et al. was used to compare the ROC curves between biomarkers against the reference proxy-history.32 Analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC).
RESULTS
A total of 140 brain dead organ donors had consent by proxy and were included in this study, with 62% (n=87) from the Gift of Hope (GOH) donor cohort and 38% (n=53) from the Loyola University Medical Center (LUMC) cohort. As expected of a cohort comprised of entirely lung donors, the LUMC cohort was younger than the GOH cohort (median [IQR] years: 27 [21–46] vs. 42 [26–55] years, p<0.001), and had lower body mass index (median [IQR]: 25 [23–29] vs. 28 [25–34], p=0.006). Cause of death also differed by cohort (p=0.001), as the LUMC cohort had higher rates of head trauma as cause of death (28% vs. 23%) and lower rates of anoxia (23% vs. 43%). The median time from hospital admission to organ recovery was longer in the LUMC cohort (median [IQR] 3 [3–4] days vs 2 [2–5] days, p=0.038), and blood samples retrieved for biomarker testing were performed at this time.
Based on proxy histories for the mixed-cohort, 15% (n=21) were found to have heavy alcohol use based on the UNOS definition (≥2 drinks/day) and 17% (n=24) were found to have alcohol misuse based on the CDC definition. Any alcohol use was identified in 66% (n=93). Using the UNOS definition, the proportions of heavy alcohol use were 20% (n=17) in the GOH cohort and 7.5% (n=4) in the LUMC cohort (p=0.054). Using the CDC definitions, the rates of alcohol misuse were 21% (n=18) in the GOH cohort and 11% (n=6) in the LUMC cohort (p=0.154). Demographics for the mixed cohort comparing alcohol misuse versus no misuse in organ donors are contained in Table 1. The donors in the ‘alcohol misuse group’ were older and more likely smokers than the ‘no misuse group’.
Table 1.
Donor demographics and characteristics
| Overall 140 |
No Alcohol misuse 116 (83%) |
Alcohol misuse 24 (17%) |
p-value | |
|---|---|---|---|---|
| Age, median (IQR) | 36 (23–53) | 32 (22–53) | 47 (40–52) | 0.012 |
| Female, n (%) | 52 (37) | 45 (39) | 7 (29) | 0.374 |
| Race, n (%) | ||||
| African American | 37 (26) | 33 (28) | 4 (17) | 0.375 |
| White | 83 (59) | 68 (59) | 15 (63) | |
| Other | 20 (14) | 15 (13) | 5 (21) | |
| BMI, median (IQR) | 27 (24–31) | 27 (24–31) | 26 (24–31) | 0.468 |
| Smoker, n (%) | 78 (56) | 59 (51) | 19 (79) | 0.011 |
| Drug use, n (%) | 57 (41) | 44 (38) | 13 (54) | 0.141 |
| ≥ 2 drinks per day, n (%) | 21 (15) | 1 (1) | 20 (83) | |
| Cause of death, n (%) | ||||
| CVA/Stroke | 45 (32) | 36 (31) | 9 (38) | 0.862 |
| Anoxia | 49 (35) | 40 (35) | 9 (38) | |
| Head trauma | 35 (25) | 29 (25) | 6 (25) | |
| Gunshot wound | 5 (4) | 5 (4) | 0 (0) | |
| Other | 6 (4) | 6 (5) | 0 (0) | |
| Days from admission to organ recovery, median (IQR) | 3 (2–4.5) | 2 (2–4) | 3 (2–5) | 0.203 |
IQR: interquartile range; BMI: body mass index; CVA: cerebrovascular accident;
Alcohol Biomarkers Analysis
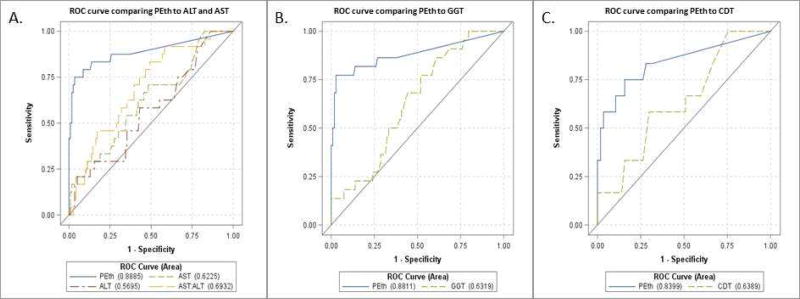
Median values of the measured direct and indirect alcohol biomarkers are contained in Table 2. BAC is not routinely obtained at hospital admission, and included are those values that were available. Only %CDT where serum was processed within six hours of procurement was included. BAC, PEth, and AST:ALT were significantly different between the ‘no alcohol misuse’ and ‘alcohol misuse’ groups. As a continuous measure, PEth had an area under the ROC curve of 0.89 (95% CI 0.80–0.98). A PEth cutpoint of ≥84 ng/mL provided optimal discrimination by Youden’s index (J) for the identification of alcohol misuse with a sensitivity of 75% (95% CI 53%–89%), a specificity of 97% (95% CI 91%–99%), a positive predictive value (PPV) of 82% (95% CI 59%–94%), and a negative predictive value (NPV) of 95% (95% CI 89%–98%). Sensitivity, specificity, PPV, and NPV were calculated for alternative cut points for PEth and are included in Table 3. Based on dichotomized PEth (≥ 84 vs. < 84 ng/ml), the area under the ROC was 0.86 (95% CI: 0.76–0.94) overall and > 0.80 when examined by race, gender, and age subgroups (Table 4). Comparison ROC curves were performed for additional alcohol biomarkers including ALT (AUC=0.5695, n=137, p<0.001), AST (AUC=0.6225, n=137, p<0.001), AST:ALT (AUC=0.6932, n=137, p=0.007), GGT (AUC=0.6319, n=128, p<0.001), and CDT (AUC=0.6389, n=69, p=0.091), all with inferior AUC values to PEth (Figure 1). Table 5 contains the alcohol use, categorization of alcohol misuse according to CDC definition and heavy alcohol use according to UNOS based on PEth less than or greater than 84.
Table 2.
Alcohol biomarkers based on proxy history of drinking
| No Alcohol Misuse | Alcohol Misuse | ||||
|---|---|---|---|---|---|
| Median (IQR) | No. | Median (IQR) | No. | Median (IQR | p-value |
| Direct Alcohol Biomarkers | |||||
| BAC (mg/dL) | 46 | 0 (0–0) | 11 | 25.0 (0–235) | 0.010 |
| PEth (ng/mL) | 116 | 0 (0–17) | 24 | 176 (70–481) | <0.001 |
| Indirect Alcohol Biomarkers | |||||
| %CDT | 57 | 1.9 (1.5–2.2) | 12 | 1.75 (1.7–2.0) | 0.987 |
| AST | 113 | 42 (26–101) | 24 | 77 (30–341) | 0.05 |
| ALT | 113 | 36 (22–100) | 24 | 45 (24–250) | 0.248 |
| AST:ALT | 113 | 1.17 (0.91–1.5) | 24 | 1.52 (1.30–1.89) | 0.003 |
| GGT | 106 | 31 (17–68) | 22 | 49 (26–66) | 0.078 |
BAC: blood alcohol content; PEth: phosphatidyethanol; CDT: carbohydrate-deficient transferrin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma-glutamyl transferase; IQR: interquartile range.
Table 3.
Range of sensitivities and specificities for PEth
| PEth (ng/ml) | n positive | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| ≥ 50 | 29 | 79% | 91% | 66% | 96% |
| ≥ 75 | 24 | 75% | 95% | 75% | 95% |
| ≥ 84* | 22 | 75% | 97% | 82% | 95% |
| ≥ 100 | 19 | 67% | 97% | 84% | 93% |
| ≥ 125 | 16 | 58% | 98% | 88% | 92% |
Optimal Youden’s J
PEth: phosphatidyethanol; PPV: positive predictive value; NPV: negative predictive value
Table 4.
Subgroup analysis of PEth ≥84ng/ml
| No. | Area under the ROC (95% Confidence Interval) |
|
|---|---|---|
| Overall | 140 | 0.86 (0.76–0.94) |
| Age | ||
| < 40 | 65 | 0.88 (0.77–0.97) |
| ≥ 40 | 75 | 0.82 (0.60–0.99) |
| Gender | ||
| Male | 88 | 0.86 (0.75–0.96) |
| Female | 52 | 0.85 (0.65–0.99) |
| Race/ethnicity | ||
| White | 83 | 0.85 (0.73–0.96) |
| Non-white | 57 | 0.88 (0.71–0.99) |
PEth: phosphatidyethanol
Figure 1. ROC Curves comparing PEth versus other commonly utilized alcohol biomarkers for predicting alcohol misuse.
Predictive accuracy of PEth as a continuous measure with area under the ROC at 0.8885 for alcohol misuse per CDC definitions compared to other commonly utilized alcohol biomarkers. A) ALT with area under the ROC at 0.5695 for EAU, AST with area under the ROC at 0.6225 for EAU, and AST:ALT with area under the ROC at 0.6932 for EAU. B) GGT with area under the ROC at 0.6319 for EAU with n=130/140 cohort with measured values available. C) %CDT with area under the ROC at 0.6389 for EAU with n=69/140 cohort with measured values available.
Table 5.
| Alcohol use, n (%) | Overall* 122 |
PEth ≥ 84 18 (15%) |
PEth < 84 104 (85%) |
p- value |
|---|---|---|---|---|
| Any use | 83 (68) | 18 (100.0) | 65 (63) | <0.001 |
| Alcohol misuse | 19 (16) | 14 (78) | 5 (5) | <0.001 |
| ≥ 2 drinks per day, n (%) | 17 (14) | 12 (67) | 5 (5) | <0.001 |
p-values from Wilcoxon rank sum tests (medians) and chi-square or Fisher’s exact test (all others)
Donors with allocation ≥1 organ
Impact of alcohol use on organ allocation
Overall, the median number of organs recovered per donor was 5 (IQR 2.5–6). The median number of solid organs recovered for the GOH cohort was 3 organs (IQR 1–6) compared to 6 (IQR 6–7) in the LUMC cohort. Comparing the GOH and LUMC cohorts, there was a difference in organ-specific recovery for all solid organs, with the LUMC cohort allocating more organs than the GOH donors (data not shown). Table 6 contains organ allocation based on a history of alcohol misuse, as well as on PEth grouping ≥84 or <84. Only liver allocation was significantly different based on a history of alcohol misuse compared to no alcohol misuse. Analysis was also conducted for the whole cohort (n=140), including those who were not suitable for any solid organ donations, and there was no significant difference (data not shown).
Table 6.
Organ allocation based on EAU proxy history and PEth
| Overall 140 |
No Misuse 116 (83%) |
Alcohol Misuse 24 (17%) |
p-value | PEth < 84 104 (85%) |
PEth ≥ 84 18 (15%) |
p- value |
|
|---|---|---|---|---|---|---|---|
| Organs transplanted, median (IQR) | 5 (2.5–6) | 5 (3–6) | 4 (2–6) | 0.269 | 6 (4–7) | 6 (4–7) | 0.553 |
| Organ, n (%) | |||||||
| Lung | 85 (61) | 71 (61) | 14 (58) | 0.793 | 71 (69) | 14 (78) | 0.418 |
| Liver | 105 (75) | 92 (79) | 13 (54) | 0.010 | 92 (89) | 13 (73) | 0.131 |
| Renal | 110 (79) | 91 (78) | 19 (79) | 0.938 | 92 (89) | 18 (100) | 0.210 |
| Heart | 81 (58) | 70 (60) | 11 (46) | 0.190 | 68 (65) | 13 (72) | 0.788 |
IQR: interquartile range; EAU: excessive alcohol use; PEth: phosphatidylethanol
DISCUSSION
This investigation is the first to examine use of alcohol biomarkers to screen for alcohol misuse in deceased organ donors. Using proxy histories as a reference standard, this study identifies the direct alcohol biomarker, PEth, with a cut point of 84 ng/ml to discriminate alcohol misuse in the deceased organ donor. PEth carried high sensitivity and specificity in the overall cohort as well as in subgroups by age, sex and race. Conversely, more commonly utilized alcohol biomarkers, such as ALT, AST and GGT, as well as %CDT had worse discrimination. Liver allocation was decreased in those with an alcohol misuse history by proxy, but it was not different between groups using a PEth cut point of 84. This signifies that PEth could provide additional information that may affect organ allocation.
PEth was first discovered in the blood of ethanol-treated rats in 1983 and since then has been studied in a variety of populations as a promising marker for assessing alcohol consumption.21 The half-life of PEth, reported as 4 days, is not affected by age, sex, or body mass index.19,33 With its longer half-life, PEth provides a larger window of recent alcohol use unlike BAC which becomes undetectable within hours of consumption.18 In our cohort of deceased organ donors, indirect biomarkers for alcohol use, such as ALT, AST, GGT and %CDT were unable to reliably identify alcohol misuse. Since indirect alcohol biomarkers are confounded by non-alcohol related comorbidities this finding was not surprising.14–16
Previous investigations have identified various PEth cut points for a range of drinking patterns in a wide array of settings.23,26,34–37 In the outpatient setting, cut point levels for PEth have resulted in proposed PEth levels ranging from 45 ng/ml to 700 ng/ml for chronic heavy daily consumption.23,35,36 While there is a growing body of knowledge with validated PEth cutpoint levels in the outpatient population, these cutpoints are not as well developed for inpatient populations with only two investigations proposing PEth cut points of 155 and 250 ng/ml, respectively, for identifying inpatients with alcohol misuse.34,38 In the intensive care unit (ICU), volume resuscitation, blood transfusions and medication interactions would be expected to interfere with the reliability of any blood-based alcohol biomarker, as these therapies could affect the availability and degradation of PEth. Despite the presence of these confounders, PEth was still able to reliably identify those with alcohol misuse in the ICU, both in our investigation and in a recent cohort of medical and burn ICU patients reported by Afshar et al.26 In our investigation, median time between donor hospital admission and sample acquisition was 3 days during which time the donor is unable to freely consume alcohol. The half-life of PEth is approximately four days, yet individual variation can exist.18,19 We anticipated and observed that our PEth cutpoint is lower than PEth cut points reported in other patient settings, as the organ donors abstained from alcohol for nearly one half-life prior to PEth sample acquisition. Given the limited reports of PEth use in the ICU, this investigation is adding to the growing body of evidence confirming PEth can be reliably utilized in critically ill patients to help identify alcohol misuse instead of solely relying on proxy histories.26
The prevalence of alcohol misuse in deceased organ donors in this investigation was 17%. This is lower than reported in the general population (27%), which could be due to underreporting by proxy.4 Substance use, such as alcohol misuse, has been associated with an increased risk of developing primary graft dysfunction (PGD), and therefore it is important to accurately understand a donor’s alcohol use history.11,12 The addition of an accurate objective direct alcohol biomarker such as PEth to a proxy history is desirable for transplant physicians and surgeons in order to make educated decisions about organ utilization in deceased organ donors. PEth is easy to obtain as a venipuncture or dried blood spot sample, but is limited in use given a slow turnaround time of approximately seven days. Transplant decisions need to be made within hours to days. Utilization of PEth as part of an organ allocation/utilization decision would require that the assay improve to permit point-of-care analysis, allowing for results in real-time.
Alcohol misuse in deceased organ donors did not lead to an overall decrease in rates of organ recovery for transplant by proxy history or by PEth cutoff ≥84 ng/ml. The median number of organs recovered for transplant in the cohort was 6.0 which is almost double the national average of 3.03 organs per donor9, suggesting that the donors within our cohort were healthier than the general donor pool. Based on the findings of this investigation, history of significant alcohol use does not impact organ utilization decisions. Future investigations utilizing PEth in a broader pool of eligible organ donors may be able to further delineate whether alcohol affects organ recovery, as only 72/100 eligible donors are actually able to donate organs.9 Finally, we did find cases of alcohol misuse by proxy history, but not by PEth level, which was associated with lower liver allocation suggesting that the history alone of alcohol misuse may lead to bias in liver allocation. There exists large variations and subjectivity in the decision of whether to utilize an organ for transplantation. Objective data to corroborate alcohol histories may be most valuable in liver allocation with a negative or low PEth level potentially leading to additional livers allocated.
In conclusion, we found that in a critically ill brain dead organ donor cohort that a PEth cut point of ≥84 ng/ml carries good test characteristics to discriminate alcohol misuse. Indirect alcohol biomarkers such as AST, ALT, GGT, and %CDT had poor discrimination for alcohol misuse in deceased organ donors. Proxy histories may limit the reliability of obtaining alcohol misuse histories as one third of donors with PEth ≥84 ng/ml failed to be defined as heavy drinkers under the UNOS definition. There was a decrease in liver allocation amongst donors with a proxy history of alcohol misuse, however there was no differences in organ allocation utilizing PEth >84 ng/ml. Therefore, alcohol misuse was not associated with a decrease in the allocation of organs in deceased donors and the addition of PEth to proxy donor histories may reduce the information bias in organ donors with alcohol misuse proxy histories.
Highlights.
PEth ≥84 ng/mL had optimal discrimination for identification of alcohol misuse in organ donors.
Other commonly used alcohol biomarkers performed poorly in deceased organ donors.
Liver allocation was decreased in donors with alcohol misuse by history but not by PEth.
Acknowledgments
This research was supported by the National Institute of Alcoholism and Alcohol Abuse, K23AA022126 (EML), K23AA024503 (MA), T32AA013527 (MW), and the National Institute of General Medical Sciences R01GM115257 (EJK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation. No conflicts of interest.
References
- 1.(CDC) CfDCaP. Alcohol and Public Health: Alcohol-Related Disease Impact (ARDI) [Accessed 8/10/2017, 2017];Average for United States 2006–2010 Alcohol-Attributable Deaths Due to Excessive Alcohol Use. https://nccd.cdc.gov/DPH_ARDI/Default/Report.aspx?T=AAM&P=f6d7eda7-036e-4553-9968-9b17ffad620e&R=d7a9b303-48e9-4440-bf47-070a4827e1fd&M=8E1C5233-5640-4EE8-9247-1ECA7DA325B9&F=&D=
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.Prevention CfDCa. Fact-Sheets-Alcohol Use and Your Health. https://www.cdc.gov/alcohol/fact_sheets/alcohol_use.htm.
- 4.Quality CfBHSa. Key Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. 2016 [Google Scholar]
- 5.Analysis NCfSa. [Accessed 8/10/17, 2017];2014 Crash Data Key Findings. 2015; (Traffic Safety Facts Crash Stats. Report No. DOT HS 812 219) Available at: https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/812219.
- 6.Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis. 2014;11:E109. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(CDC) CfDC. [Accessed 8/10/2017, 2017];At a glance 2016: Excessive Alcohol Use, preventing a leading risk for Death, Disease, and Injury. 2016 https://www.cdc.gov/chronicdisease/resources/publications/aag/pdf/2015/alcohol-aag.pdf.
- 8.Afshar M, Netzer G, Murthi S, Smith GS. Alcohol exposure, injury, and death in trauma patients. J Trauma Acute Care Surg. 2015;79(4):643–648. doi: 10.1097/TA.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Israni AK, Zaun D, Bolch C, et al. OPTN/SRTR 2015 Annual Data Report: Deceased Organ Donation. Am J Transplant. 2017;17(Suppl 1):503–542. doi: 10.1111/ajt.14131. [DOI] [PubMed] [Google Scholar]
- 10.Implementation Guidance Document: Uniform Donor Risk Assessment Interview Forms. 2015 https: http://www.aatb.org/sites/default/files/AATB-EBAA Implementation Guidance Document, v2 (5.20.15).pdf.
- 11.Lowery EM, Kuhlmann EA, Mahoney EL, Dilling DF, Kliethermes SA, Kovacs EJ. Heavy alcohol use in lung donors increases the risk for primary graft dysfunction. Alcohol Clin Exp Res. 2014;38(11):2853–2861. doi: 10.1111/acer.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houyel L, Petit J, Nottin R, Duffet JP, Mace L, Neveux JY. Adult heart transplantation: adverse role of chronic alcoholism in donors on early graft function. J Heart Lung Transplant. 1992;11(6):1184–1187. [PubMed] [Google Scholar]
- 13.Pelaez A, Mitchell PO, Shah NS, et al. The role of donor chronic alcohol abuse in the development of primary graft dysfunction in lung transplant recipients. Am J Med Sci. 2015;349(2):117–123. doi: 10.1097/MAJ.0000000000000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conigrave KM, Degenhardt LJ, Whitfield JB, et al. CDT, GGT, and AST as markers of alcohol use: the WHO/ISBRA collaborative project. Alcohol Clin Exp Res. 2002;26(3):332–339. [PubMed] [Google Scholar]
- 15.Mundle G, Ackermann K, Munkes J, Steinle D, Mann K. Influence of age, alcohol consumption and abstinence on the sensitivity of carbohydrate-deficient transferrin, gamma-glutamyltransferase and mean corpuscular volume. Alcohol Alcohol. 1999;34(5):760–766. doi: 10.1093/alcalc/34.5.760. [DOI] [PubMed] [Google Scholar]
- 16.Mundle G, Munkes J, Ackermann K, Mann K. Sex differences of carbohydrate-deficient transferrin, gamma-glutamyltransferase, and mean corpuscular volume in alcohol-dependent patients. Alcohol Clin Exp Res. 2000;24(9):1400–1405. [PubMed] [Google Scholar]
- 17.Wurst FM, Thon N, Yegles M, Schruck A, Preuss UW, Weinmann W. Ethanol metabolites: their role in the assessment of alcohol intake. Alcohol Clin Exp Res. 2015;39(11):2060–2072. doi: 10.1111/acer.12851. [DOI] [PubMed] [Google Scholar]
- 18.Gnann H, Weinmann W, Thierauf A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36(9):1507–1511. doi: 10.1111/j.1530-0277.2012.01768.x. [DOI] [PubMed] [Google Scholar]
- 19.Wurst FM, Thon N, Aradottir S, et al. Phosphatidylethanol: normalization during detoxification, gender aspects and correlation with other biomarkers and self-reports. Addict Biol. 2010;15(1):88–95. doi: 10.1111/j.1369-1600.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- 20.Walther L, de Bejczy A, Lof E, et al. Phosphatidylethanol is superior to carbohydrate-deficient transferrin and gamma-glutamyltransferase as an alcohol marker and is a reliable estimate of alcohol consumption level. Alcohol Clin Exp Res. 2015;39(11):2200–2208. doi: 10.1111/acer.12883. [DOI] [PubMed] [Google Scholar]
- 21.Alling C, Gustavsson L, Anggard E. An abnormal phospholipid in rat organs after ethanol treatment. FEBS Lett. 1983;152(1):24–28. doi: 10.1016/0014-5793(83)80474-8. [DOI] [PubMed] [Google Scholar]
- 22.Viel G, Boscolo-Berto R, Cecchetto G, Fais P, Nalesso A, Ferrara SD. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788–14812. doi: 10.3390/ijms131114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrock A, Hernandez Redondo A, Martin Fabritius M, Konig S, Weinmann W. Phosphatidylethanol (PEth) in blood samples from "driving under the influence" cases as indicator for prolonged excessive alcohol consumption. Int J Legal Med. 2016;130(2):393–400. doi: 10.1007/s00414-015-1300-5. [DOI] [PubMed] [Google Scholar]
- 24.Winkler M, Skopp G, Alt A, et al. Comparison of direct and indirect alcohol markers with PEth in blood and urine in alcohol dependent inpatients during detoxication. Int J Legal Med. 2013;127(4):761–768. doi: 10.1007/s00414-012-0812-5. [DOI] [PubMed] [Google Scholar]
- 25.Allen JP, Wurst FM, Thon N, Litten RZ. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl. 2013;19(4):369–376. doi: 10.1002/lt.23596. [DOI] [PubMed] [Google Scholar]
- 26.Afshar M, Burnham EL, Joyce C, et al. Cut-point Levels of Phosphatidylethanol to Identify Alcohol Misuse in a Mixed Cohort including Critically Ill Patients. Alcohol Clin Exp Res. 2017 doi: 10.1111/acer.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakhireva LN, Shrestha S, Gutierrez HL, Berry M, Schmitt C, Sarangarm D. Stability of Phosphatidylethanol in Dry Blood Spot Cards. Alcohol Alcohol. 2016;51(3):275–280. doi: 10.1093/alcalc/agv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faller A, Richter B, Kluge M, Koenig P, Seitz HK, Skopp G. Stability of phosphatidylethanol species in spiked and authentic whole blood and matching dried blood spots. Int J Legal Med. 2013;127(3):603–610. doi: 10.1007/s00414-012-0799-y. [DOI] [PubMed] [Google Scholar]
- 29.Jones JJM, Plate C, Lewis D. The Detection of 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol and Ethyl Glucuronide in Human Umbilical Cord. American Journal of Analytical Chemistry. 2012;3:800–810. [Google Scholar]
- 30.Schellenberg F, Schwan R, Mennetrey L, Loiseaux MN, Pages JC, Reynaud M. Dose-effect relation between daily ethanol intake in the range 0–70 grams and %CDT value: validation of a cut-off value. Alcohol Alcohol. 2005;40(6):531–534. doi: 10.1093/alcalc/agh194. [DOI] [PubMed] [Google Scholar]
- 31.Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163(7):670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 33.Varga A, Hansson P, Johnson G, Alling C. Normalization rate and cellular localization of phosphatidylethanol in whole blood from chronic alcoholics. Clin Chim Acta. 2000;299(1–2):141–150. doi: 10.1016/s0009-8981(00)00291-6. [DOI] [PubMed] [Google Scholar]
- 34.Aradottir S, Asanovska G, Gjerss S, Hansson P, Alling C. PHosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol. 2006;41(4):431–437. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- 35.Kip MJ, Spies CD, Neumann T, et al. The usefulness of direct ethanol metabolites in assessing alcohol intake in nonintoxicated male patients in an emergency room setting. Alcohol Clin Exp Res. 2008;32(7):1284–1291. doi: 10.1111/j.1530-0277.2008.00696.x. [DOI] [PubMed] [Google Scholar]
- 36.Stewart SH, Law TL, Randall PK, Newman R. Phosphatidylethanol and alcohol consumption in reproductive age women. Alcohol Clin Exp Res. 2010;34(3):488–492. doi: 10.1111/j.1530-0277.2009.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart SH, Koch DG, Willner IR, Anton RF, Reuben A. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38(6):1706–1711. doi: 10.1111/acer.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kummer N, Ingels AS, Wille SM, et al. Quantification of phosphatidylethanol 16:0/18:1, 18:1/18:1, and 16:0/16:0 in venous blood and venous and capillary dried blood spots from patients in alcohol withdrawal and control volunteers. Anal Bioanal Chem. 2016;408(3):825–838. doi: 10.1007/s00216-015-9169-1. [DOI] [PubMed] [Google Scholar]