Abstract
The retrosplenial cortex is anatomically positioned to integrate sensory, motor, and visual information and is thought to have an important role in processing spatial information and guiding behavior through complex environments. Anatomical and theoretical work has argued that the retrosplenial cortex participates in spatial behavior in concert with its primary input, the parietal cortex. Although the nature of these interactions is unknown, the central position is that the functional connectivity is hierarchical with egocentric spatial information processed at parietal cortex, and higher-level allocentric mappings generated in the retrosplenial cortex. Here, we review the evidence supporting this proposal. We begin by summarizing the key anatomical features of the retrosplenial-parietal network, and then review studies investigating the neural correlates of these regions during spatial behavior. Our summary of this literature suggests that the retrosplenial-parietal circuitry does not represent a strict hierarchical parcellation of function between the two regions, but instead a heterogeneous mixture of egocentric-allocentric coding and integration across frames of reference. We also suggest that this circuitry should be represented as a gradient of egocentric-to-allocentric information processing from parietal to retrosplenial cortices, with more specialized encoding of global allocentric frameworks within the retrosplenial cortex and more specialized egocentric and local allocentric representations in parietal cortex. We conclude by identifying the major gaps in this literature and suggest new avenues of research.
Keywords: spatial navigation, reference frame, parietal cortex, posterior parietal cortex, retrosplenial cortex
1. Introduction
Accurate navigation requires that animals make use of a variety of stimulus sources and frames of reference to guide behavior (Knierim & Hamilton, 2011). One of the more widely studied forms of navigation is the capacity of an animal to monitor position and direction with reference to global or allocentric cues (O’Keefe & Nadel, 1978). This may involve the use of landmarks associated with the background or distal portions of the environment. Animals can also self-localize on the basis of proximal landmarks or objects as well as the geometric contours that define the local environment (Cheng, 1988; Collett, Cartwright, & Smith, 1986). Secondly, animals also use internal stimuli to self-localize, including those derived from one’s movements through the environment (e.g., vestibular, motor, optic flow; Gallistel, 1990; McNaughton, Battaglia, Jensen, Moser, & Moser, 2006). The processing of self-motion stimuli can operate by tracking the animal’s current location in relation to a known location (i.e., path integration) or in the service of executing a sequence of movements or body turns to a goal (i.e., egocentric navigation). In general, these external and internal frames of reference can be processed in parallel or sequentially, which allows for rapid changes in the sequence of movements, or route, to the goal (Hamilton, Rosenfelt, & Whishaw, 2004; Maaswinkel & Whishaw, 1999; McDonald & White, 1994).
The neurobiological mechanisms by which information is coordinated between spatial reference frames is poorly understood, but experimental and computational studies have argued for a central role of the densely interconnected retrosplenial (RSC) and parietal (PC) cortices (Bicanski & Burgess, 2016; Byrne, Becker, & Burgess, 2007; Oess, Krichmar, & Röhrbein, 2017; Wilber et al., 2015). Specifically, this network has been hypothesized to operate as a coordinate transformation system by which viewer-dependent (egocentric) frameworks are transformed into viewer-independent (allocentric) representations, and vice versa. While the PC has long been linked to egocentric encoding based on the subjects body (i.e., viewer dependent or body centered), the RSC has often been hypothesized as a source of translational computations between reference frames (Andersen, Essick, & Siegel, 1985; Bicanski & Burgess, 2016; Bremner & Andersen, 2012; Byrne et al., 2007; Byrne & Crawford, 2010; Hay & Redon, 2006; McNaughton, Knierim, & Wilson, 1995; Oess et al., 2017; Vann, Aggleton, & Maguire, 2009; Wilber, Clark, Forster, Tatsuno, & McNaughton, 2014; Xing & Andersen, 2000).
A recently expanding literature has emerged investigating the relative role of RSC and PC in coordinating information between internal and external spatial frames of reference. The aim of this review is to synthesize this literature. We begin our summary by describing the reciprocal connectivity of the RSC and PC, and their position within cortical and subcortical networks (Olsen & Witter, 2016; Wilber et al., 2015; Zingg et al., 2014). We then describe studies that have targeted electrophysiological recordings from neurons in the RSC and PC, but with specific emphasis on work in freely behaving rodents (Table 1). Additionally, we have limited our review to studies that inform the relationship between internal and external stimuli and the functional organization of the RSC-PC network. From this work, we conclude that although the evidence strongly supports a linkage with spatial processing at multiple frames of reference, the traditional topographical arrangement of egocentric-to-allocentric processing along the PC-to-RSC circuit, while quite useful in modeling the network, does not appear to be supported by current animal literature. Rather, we suggest the evidence supports the following conclusions: (1) there is considerable overlap in allocentric and egocentric coding by RSC and PC neurons, (2) each region participates in the integration of information across reference frames, and (3) spatial information is organized along a gradient from PC to RSC, with greater encoding of global allocentric information within the RSC and more specialized encoding of self-motion and egocentric information in the PC. We conclude by identifying major gaps and unanswered questions in this body of work.
Table 1.
Parietal and Retrosplenial Electrophysiological Correlates of Spatial Reference Frames
| Frame of Reference | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Parietal Cortex | Self-motion | Egocentric | Route-centric | Allocentric head direction | Allocentric location | Test Apparatus |
| Chen et al 1994a |
|
|
RAM | |||
| Chen et al 1994b |
|
|
RAM | |||
| McNaughton 1994 |
|
RAM | ||||
| Nitz 2006 |
|
RT | ||||
| Nitz 2012 |
|
SM | ||||
| Whitlock et al 2012 |
|
HM | ||||
| Wilber et al 2014 |
|
|
|
ST | ||
| Wilber et al 2017 |
|
ST | ||||
| Retrosplenial Cortex | ||||||
|
| ||||||
| Chen et al 1994a |
|
RAM | ||||
| Chen et al 1994b |
|
RAM | ||||
| Cho & Sharp 2001 |
|
|
|
Cylinder | ||
| Alexander & Nitz, 2015 |
|
|
|
|
WM | |
| Jacob et al 2017 |
|
Connect-DC | ||||
| Alexander & Nitz, 2017 |
|
|
|
|
|
CT, PM |
| Lozano et al 2017 |
|
Cylinder | ||||
Notes. RAM = radial arm maze, RT= route task, SM = Spiral Maze, HM = Hairpin Maze, ST = Sequence Task, WM= ‘W’ Maze, Connect-DC = Connected-Dual Chamber, CT=Circular Track, PM=Plus Maze.
2. Subdivisions and Connectivity of the Retrosplenial-Parietal Network
Parietal Cortex
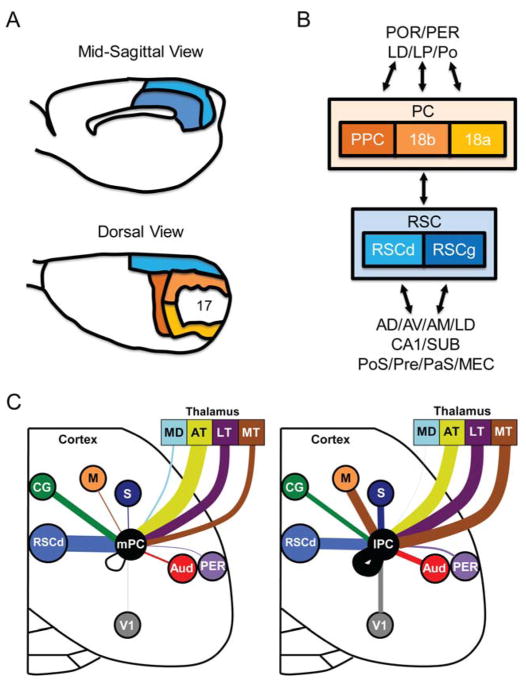
While in primates the PC includes a large cortical surface (Broadman’s areas 5 and 7), the extent and location of the rodent PC has been the subject of some debate (reviewed in Corwin & Reep, 1998). This lack of clarity has led to a number of methodological differences in functional assessment of PC and behavior (summarized by Calton & Taube, 2009; Whitlock, Sutherland, Witter, Moser, & Moser, 2008). Nevertheless, it is generally agreed that, based on cyto- and myeloarchitectural criteria, the rodent PC occupies a thin strip of cortex equivalent to Broadman’s area 7, and is located between primary somatosensory (Broadman’s area 2) and secondary visual cortices (Broadman’s areas 18), and lateral to the RSC (Corwin & Reep, 1998; Kolb & Walkey, 1987; Krieg, 1946; Vogt & Miller, 1983). The rodent PC can be divided into a dorsal and ventral limb, which can be further subdivided into two or three regions: medial, lateral, and posterior zones (Olsen & Witter, 2016; Paxinos & Watson, 2007; Whitlock, 2017). Secondary visual areas, which include Broadman’s areas 18a and 18b, form a ring around primary visual cortex (Broadman’s area 17) and are frequently included in functional descriptions of PC (Fig. 1A) (Glickfeld & Olsen, 2017; Wilber et al., 2014). While some have disagreed on grounds of cyto- and chemo-architectural differences (Kolb & Walkey, 1987; Olsen & Witter, 2016), recent work has supported the idea based on evidence of significant overlap in connectivity and function (Wilber et al., 2015; Wilber et al., 2014). In the present review, we include these secondary visual areas in our description of PC, but make note of regional differences in the sections below. To avoid causing confusion with existing literature we refer to the broader region as PC and the more narrowly defined region (when necessary to be more specific) as posterior parietal cortex (PPC; Fig. 1A).
Figure 1.
Cytoarchitectural organization of the retrosplenial (RSC) and parietal (PC) cortices. A, Panels adapted from Kolb and Walkey (1987) showing the cytoarchitectural organization of the retrosplenial and parietal cortex of the rat brain. B, Circuit diagram showing the primary differences in cortical and subcortical inputs to the retrosplenial and parietal cortex (PC). Note: anatomical and electrophysiological work have largely focused on PPC and the anterior regions of areas 18a and 18b (V2MM and V2ML in Paxinos & Watson, 2007). For clarity, we refer to the collection of these areas as PC. C, The density of cortical and thalamic inputs to PC have been precisely mapped in rats as illustrated here, more work is needed to map additional connections in the rat for this brain network (adapted from Wilber et al., 2015). For this study density of inputs to PC were shown to be similar in the anterior to posterior direction, but to vary considerably in the medial/lateral direction, therefore PC data was collapsed across the anterior/posterior direction and shown separately for medial (mPC) and lateral regions (lPC). Each brain region is indicated by a different color and the line thickness represents the strength of thalamic and cortical projections to the medial PC (left panel) and lateral PC (right panel). Only regions with significant medial versus lateral differences in projection strength are shown (i.e., non-overlapping error bars). Note that projections to lateral PC involve stronger projections from the somatosensory, motor, visual, auditory cortex, and motor thalamus. The medial PC receives stronger inputs from the dorsal retrosplenial cortex and cingulate region. Mediodorsal thalamus, anterior thalamus, lateral thalamus, perirhinal cortex. Key: AD, anterodorsal thalamus; AV, anteroventral thalamus; AM, anteromedial thalamus; Broadmann’s area 17, primary visual cortex; Broadmann’s areas 18a and 18b, secondary visual cortex; AT, anterior thalamus; AUD, auditory cortex; CG, cingulate region; lPC, lateral posterior parietal cortex and the rostral portion of area 18a; LD, laterodorsal thalamus; LP, lateroposterior thalamus; LT, lateral thalamus; mPC, medial posterior parietal cortex and the rostral portion of area 18b; MD, mediodorsal thalamus; M, motor; MT, motor thalamus; MEC, medial entorhinal cortex; PaS, parasubiculum; PER, perirhinal cortex; Po, posterior thalamic complex; POR, postrhinal cortex; PoS, postsubiculum; PPC, posterior parietal cortex; Pre, presubiculum; RSCg, retrosplenial cortex-granular; RSCd, retrosplenial cortex-dysgranular; S, somatosensory; SUB, subiculum; V1, visual.
With respect to PC connectivity, inputs from the hippocampal formation are relatively sparse, with the exception of axons stemming from the postrhinal and perirhinal cortices (Fig. 1B & C) (Agster & Burwell, 2009; Olsen, Ohara, Iijima, & Witter, 2017; Reep, Chandler, King, & Corwin, 1994; Wilber et al., 2015). Projections from these regions appear to be distributed to all PC subdivisions, with slightly greater input provided by the postrhinal cortex (Agster & Burwell, 2009; Furtak, Wei, Agster, & Burwell, 2007). As noted above, and described further below, the RSC has one of the densest limbic system connections with the PC (Olsen et al., 2017; Wilber et al., 2015). In addition, the limbic thalamus sends large inputs to the PC, including the anterior and lateral nuclei (Clark & Harvey, 2016; Wilber et al., 2015). Quantitative mappings of the projection density between thalamus and PC (see Fig. 1C) suggests a topographical organization with anterior nuclei targeting the medial zones of the PC, and lateral thalamic nuclei targeting the lateral PC (Wilber et al., 2015). Olsen and Witter (2016) presented a detailed investigation of the projections from PC to thalamus and concluded that axons largely targeted lateral and posterior thalamic nuclei. The authors suggested that this relationship was topographically organized with medial PC sending greater projections to the lateroposterior thalamic group, while the lateral PC sending greater input to the posterior thalamic complex. Given that direct projections from the primary visual cortex are relatively weak (Wilber et al., 2015), it is likely that laterodorsal and lateroposterior thalamic input, which are embedded within the tecto-pulvinar pathway, serves as a conduit of visual information to the PC.
Retrosplenial Cortex
In primates, the RSC is located posterior to the splenium of the corpus callosum and hidden deep below the midline cortical surface. As in the primate, the rodent RSC is situated along the midline and caudal half of the corpus callosum, but occupies a more superficial, and less hidden, cortical position relative to primates (Fig. 1A). The rodent RSC can be divided into two subdivisions based on cytoarchitectural differences, the granular (RSCg) and dysgranular cortices (RSCd; van Groen & Wyss, 1992, 2003; Wyss & Van Groen, 1992). The RSCg can be further subdivided into two (granular-a, granular-b) or three subdivisions (granular-a, granular-b, and granular-c; reviewed in Sugar, Witter, van Strien, & Cappaert, 2011).
The topography of inputs-outputs with each RSC subregions is an important feature for functional considerations with respect to its role in navigation (Vann et al., 2009). Notably, the RSCg has strong connectivity with limbic thalamus and limbic cortical structures thought to participate in spatial problem solving (Jones & Witter, 2007; van Groen & Wyss, 2003; van Groen & Wyss, 1990), including the anterior thalamic nuclei (anterodorsal, anteroventral, anteromedial, and laterodorsal) and hippocampal formation (CA1, subiculum, presubiculum, parasubiculum, postsubiculum, and medial entorhinal cortex; Fig 1B). The RSCd sends and receives axons from some of the same thalamic (anteromedial, laterodorsal, and lateroposterior) and hippocampal structures (subiculum, postsubiculum, and medial entorhinal cortex), but this division has a much stronger connectivity profile with dorsal-posterior cortical regions such as primary and secondary visual cortices (Broadman’s area 17 and 18b, respectively) and PC (van Groen & Wyss, 1992). The greater selectivity of input from higher visual cortices, and laterodorsal-lateroposterior thalamus, is speculated to endow the RSCd a privileged role in visual-spatial processing (Vann & Aggleton, 2005). Nevertheless, given the dense intrinsic connectivity across RSC subdivisions, it seems likely that information can be integrated between subregions (Shibata, Honda, Sasaki, & Naito, 2009; Sugar et al., 2011).
RSC-PC Connectivity
As noted above, several studies have emphasized a strong reciprocal relationship between the RSC and PC (Olsen et al., 2017; Reep et al., 1994; van Groen & Wyss, 1992; Wilber et al., 2015). However, only recently has the projection density of these cortical connections been evaluated in rodents (Glickfeld & Olsen, 2017; Mesina et al., 2016; Oh et al., 2014; Wang, Sporns, & Burkhalter, 2012; Wilber et al., 2015; Zingg et al., 2014). For instance, Wilber et al. (2015) utilized a custom software platform that calculated the proportion of tracer positive cells within the boundaries of the RSC, its granular and dysgranular subregions, as well as other cortical and subcortical structures. Using this analysis, the authors determined that the rat RSC formed the largest cortical input to the PC and established at least two patterns of anatomical topography between the regions. First, the RSCd largely dominated the input-output relationship with the PC, with lesser involvement by the RSCg. This observation is consistent with recent work showing that the mouse RSCg and RSCd form distinct subnetworks, with each having greater interaction with the dorsal hippocampus and PPC, respectively (Zingg et al., 2014). Secondly, although the RSCd sends and receives axons from all regions of the PC, the medial PC has a relatively stronger relationship (Wang et al., 2012; Wilber et al., 2015). Thus, connectivity with the RSCd appears to increase along a gradient from lateral-to-medial PC.
3. Reference Frame Coding by Parietal Cortical Neurons
The PC is situated in a pivotal position between sensory and motor cortical regions, suggesting that the structure may play a critical role in both integrating incoming sensory information and performing sensorimotor transformations. Such a function would allow the PC to utilize the current sensory information available to the body to execute a trajectory to a goal location. However, the exact function of the PC in navigating animals has been difficult to establish concretely. This has been due in large part to the fact that PC neurons are sensitive to differences in motion-states (e.g., forward or turning movements; McNaughton et al., 1994), testing procedures (e.g., spontaneous foraging vs traversing a learned route; Whitlock, Pfuhl, Dagslott, Moser, & Moser, 2012), and the high dimensionality of single cell and population coding within the PC (Nitz, 2009; Nitz, 2006; Whitlock, 2014; Whitlock, 2017; Whitlock et al., 2012; Wilber, Skelin, Wu, & McNaughton, 2017).
Early lesion studies began to unveil the intricate functions of the PC. For instance, Kolb, Sutherland, and Whishaw (1983) investigated the impact of large lesions of the PC on the spatial behavior of rats in three tasks: the Morris water maze, 8-arm radial arm maze, and a spatial alternation test. In short, each task required animals to navigate to a specific spatial location. The authors reported that PC lesioned animals were capable of learning the reward location; however, their ability to adjust initial heading toward the correct goal location was robustly impaired in both the water maze and spatial alternation task. This was particularly apparent during spatial reversal in the alternation task, in which animals had to change their trajectory toward the opposite lane which was now rewarded. Some studies have shown that animals can utilize several frames of reference sequentially toward a goal location (Hamilton et al., 2008; Hamilton, Akers, Weisend, & Sutherland, 2007; Hamilton et al., 2004). Thus, the observations by Kolb et al. (1983) may be indicative of the role the PC may play in encoding the proper sequence of movements (e.g., left turn then right turn) to form the entire route to the goal location.
In addition, Save and Poucet (2000) examined differences in the use of proximal and distal landmarks by comparing groups of rats with PPC or hippocampal lesions in the Morris water maze. The results showed that PPC lesioned rats were significantly impaired at locating a hidden platform in the presence of only proximal landmarks, but their ability to use distal landmarks to find the platform was relatively intact. In contrast, lesions of the hippocampus impaired the use of distal cues to find the platform, and to a lesser extent, impaired performance in relation to the proximal cues. The finding of impairments in proximal cue processing after lesions of the PPC is consistent with a follow-up study in which recordings of hippocampal place cells were performed in animals with extensive PPC lesions (Save, Paz-Villagran, Alexinsky, & Poucet, 2005). In control animals, when the proximal cues were rotated, an equivalent rotation of the hippocampal place field was observed. In animals with PPC lesions, hippocampal place fields failed to rotate consistently with the proximal cues, but were controlled by distal cues. Collectively, these findings point to the functional importance the PC has in processing the local spatial frame of reference.
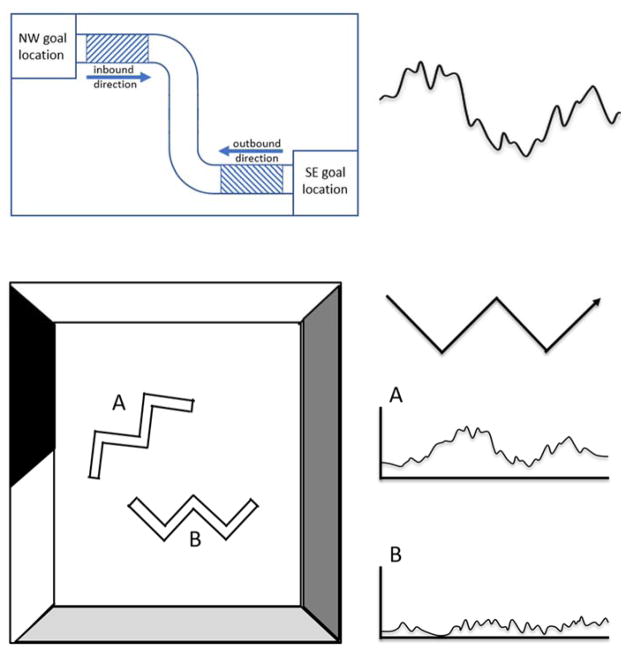
Nitz (2006) expanded on the ideas above by investigating how PPC neurons might encode information along spatial trajectories. Recordings from PPC neurons were acquired while rats locomoted between two different sites in a maze that involved a series of alternating straight runs and turns. For example, in one direction, an animal would follow a path composed of a sequence of three straight runs separated by a right and then left turn, with the same sequence encountered when locomoting in the opposite direction. Neural activity was assessed in both directions of travel, termed ‘inbound’ and ‘outbound’ directions, allowing for an assessment of the contributions of allocentric (specific location), egocentric (body-turn), and cues associated with the direction of travel. In this task, PC neuronal activity was dependent on both the position of the animal in context of the local frame of reference, but were independent of the global allocentric frame. For example, some cells were active in the first segment of the path in each direction of travel (inbound and outbound paths) rather than at a specific location along the route as is the case for hippocampal place cells (Fig. 2, top panel). These route-centered representations also tended to scale (i.e., an increase or decrease in their field width) in correspondence to a change in the length of path. An additional set of experiments conducted by Nitz (2012) recorded PPC neurons while rats traversed a spiral track composed of 5 loops, which could be defined in three distinct reference frames: a path segment, an individual loop, and the full spiral route. PPC neurons showed activity that distinguished between similar path segments, but also between individual loops. Thus, neural activity was best modeled by a combination of all three route-centered reference frames. Collectively, the findings highlight the importance of the local allocentric frame of reference and route-centered information to PPC cell activity.
Figure 2.
Reference frames in PC and RSC. Top, Schematic of route-centered neuronal firing during maze traversal (adapted from Nitz, 2006). Shaded regions depict the locations of increased firing rate in cells tuned to the starting segment of the whole route (left) which corresponds to the peaks in firing rate on the line plot (right). The particular cell fires independently of both world-centered location (SE versus NW quadrant) and allocentric direction (inbound versus outbound). Note, this is an example of one of the “simplest” coding frames reported by Nitz and colleagues, see text for more complex forms of route centered coding in parietal cortex. Bottom, Schematic of allocentric modulation by an RSC neuron during locomotion across the W-shaped track when positioned in two locations (position A and position B) in the environment (adapted from Alexander & Nitz, 2015). Note that the line plot representing neural activity for an individual RSC neuron (right) fires robustly cell after the animal performs left-turns along the track when in maze position A, but not when in maze position B.
McNaughton and colleagues (Chen, Lin, Green, Barne, & McNaughton, 1994a; 1994b; McNaughton et al., 1994) have previously shown that PC neurons could be modulated by the speed of angular and linear movements, or a combination of both. In a recent study, Whitlock et al. (2012) replicated these previous observations, but also demonstrated that the preferred motion-state of a PPC cell could be modulated by a particular behavioral context. For example, a cell that was once tuned to high-speed right turns in an open field, were now tuned to left turns in a “hairpin” maze that restricted movements to straight runs and alternating turns. Interestingly, the authors reported that these motion-sensitive cells did not retune when animals performed the same task, but in a different environment. These findings indicate that changes in motion-state occurred only after modifications to the spatial structure of the task, and not after a change in the global allocentric location of the task at hand. More recent work has shown that such preferred motion tuning in the PC is also present in the multi-unit activity recorded from the region around a single tetrode and is invariant across depth. The topography of motion-tuning across the surface of the PC is “patchy” with sharp boundaries representing neighboring regions, suggesting a modular organization. Adjacent “modules” had dramatically different self-motion tuning while other adjacent locations had similar tuning (Wilber et al., 2017). Further, most single cells within a module were generally more specific and fell within the broader range of the tuning for that module, but some single cells within a module had tuning that was quite different than the general tuning for that module. This suggests that motion state tuning exists at larger or multiple scales. Lastly, these observations suggest a potential neural mechanism for the observations of impaired spatial performance after lesions of the PPC (Kolb et al., 1983).
The work above points to the broad conclusion that the PC has a prominent role in processing information at the local behavioral context, and has a less prominent role in processing information at the global level. However, it is important to note that some studies have observed directional modulation of PC cells in relation to distal allocentric cues, including head direction cells (Chen et al., 1994a; 1994b; Wilber et al., 2014) and allocentric sound-direction cells (Nakamura, 1999). Wilber et al. (2014) investigated these allocentric signals in the PC while rats navigate toward a random set of locations marked by a light cue along the perimeter of a circular maze. Consistent with previous studies, the authors identified populations of PC cells that were active as a function of the animal’s head direction in global allocentric coordinates, similar to that observed in other limbic cortical regions. Coexisting with head direction cells, the authors identified populations of neurons that were more active at an egocentric relationship with the light, or a specific combination of head direction and viewer dependent (egocentric) light direction. Cells that were conjunctive for both head direction and egocentric direction also showed anticipatory firing such that they were most robustly modulated up to 1s before the onset of movement towards the goal location (e.g., cell fires before and during left turns only; also see Whitlock et al., 2012).
4. Reference Frame Coding by Retrosplenial Cortical Neurons
The observations summarized in the sections above are suggestive of a key role for the PC in at least the first stages of transforming egocentric, viewer-dependent coordinates, into allocentric, viewer-independent coordinates. The findings also indicate that the PC plays a more limited role in processing global allocentric information. This notion is in line with the hypothesis that the RSC has a greater role in this latter stage of processing, participating in the transformation of sensory rich information at the local level to a coherent representation of allocentric position in an environment. While there is a recently emerging literature supporting this hypothesis, it is important to note that several early studies have laid the groundwork. For instance, Sutherland and colleagues (1988) demonstrated that large lesions of the RSC impaired the ability of animals to learn to navigate to a hidden platform in a Morris water task; a behavior that requires animals learn the allocentric relationship between distal cues. Several subsequent studies, using a variety of behavioral tasks, have largely confirmed these observations after lesions of RSC (reviewed in Harker & Whishaw, 2004; Hunsaker & Kesner, 2018; Mitchell, Czajkowski, Zhang, Jeffery, & Nelson, 2018; Vann et al., 2009). In addition, studies have shown that the RSC plays a role in processing a wide range of stimulus sources for allocentric-based navigation, including environmental landmarks (Auger, Zeidman, & Maguire, 2015, 2017; Miller, Vedder, Law, & Smith, 2014; Vedder, Miller, Harrison, & Smith, 2017), directional heading (Aguirre & D’Esposito, 1999; Clark, Bassett, Wang, & Taube, 2010; Marchette, Vass, Ryan, & Epstein, 2014; Pothuizen, Aggleton, & Vann, 2008; Shine, Valdés-Herrera, Hegarty, & Wolbers, 2016), and computing spatial position on the basis of self-motion cues or path integration (Chrastil, Sherrill, Hasselmo, & Stern, 2015; Cooper & Mizumori, 2001; Elduayen & Save, 2014; Whishaw, Maaswinkel, Gonzalez, & Kolb, 2001; Wolbers & Buchel, 2005).
McNaughton and colleagues (Chen et al., 1994a; 1994b) provided early support for the strong linkage between the RSC and allocentric information processing. In these experiments, the authors described populations of head direction cells—neurons that fire preferentially in specific allocentric heading orientations—within the medial PC and in both subregions of the RSC (see also Cho & Sharp, 2001; Wilber et al., 2014). However, McNaughton and colleagues determined that some head direction cells in the medial PC were also sensitive to movement variables such as high speed straight movements or turn related movements (e.g., a right turn). In contrast, modulation by self-motion was more limited in the RSC, with some head direction cells in the RSCg, but not RSCd, demonstrating sensitivity to motor stimuli. These observations suggest a possible lateral-to-medial functional gradient for motion modulation in posterior cortices, and could be related to the fact that lateral regions receive stronger motor cortical input (see Fig. 1C for pattern of connectivity between PC and motor cortex).
Other work has pointed to an orthogonal pattern of greater allocentric landmark processing in the RSC relative to PC (Calton, Turner, Cyrenne, Lee, & Taube, 2008; Clark et al., 2010; Yoder, Clark, & Taube, 2011). For instance, Clark et al. (2010) showed that large lesions of the RSC can impair control over anterior thalamic head direction cell activity typically exerted by a prominent environmental landmark. Briefly, when a landmark is manipulated in an environment, limbic head direction cells maintain their orientation in relation to the cue (Clark & Taube, 2012; Lozano et al., 2017; Yoder et al., 2011). However, in animals with RSC lesions, anterior thalamic head direction cells were no longer controlled by the cue, and would adopt other orientations, perhaps in reference to other stimulus sources (Clark et al., 2010). Using the same procedures, Calton et al. (2008) found that large lesions of the PC generally failed to impair landmark control over head direction cells. However, because the cue occupied a position along the chamber wall, it is unclear whether the landmark in these studies is considered a proximal or distal landmark, or as a boundary given the large angular span of the cue card (Bicanski & Burgess, 2016; Yoder et al., 2011). As noted in the preceding section, the work by Poucet and colleagues have placed local and distal cues in conflict and have shown that lesions of the PC can impair navigation and place cell orientation in relation to local objects, but not in relation to distal cues. Thus, these findings are consistent with the interpretation that the PC may have a greater involvement in processing proximal cues, and point to a greater involvement of RSC in allocentric orientation (Save et al., 2005; Save & Poucet, 2000).
A recent study by Jacob et al. (2017) expanded on this idea. The study investigated the responses of RSC head direction cells while animals locomoted between two rectangular enclosures with a doorway between. Each enclosure could be distinguished based on a unique odor cue in each chamber, as well as identical landmarks occupying opposite short walls in each rectangular enclosure. The authors reported cell populations that adopted three types of firing patterns: first, the authors reported that populations of head direction cells fired in the same allocentric direction regardless of the animal’s position in the two-chambered apparatus. This firing pattern suggests that RSC cell activity was modulated by the global frame of reference, consistent with previous studies on limbic head direction cell activity (Taube & Burton, 1995; Yoder et al., 2011), and consistent with recent human neuroimaging work (Shine et al., 2016). The second form of RSC cell response was in relation to the local environment. Notably, head direction cells were found to change their preferred orientation, often adopting opposing 180 degree orientations, in each enclosure. This finding suggests that local environmental cues could also modulate RSC neural activity; again, an observation consistent with recent human neuroimaging work (Marchette et al., 2014), and studies showing that local cues can control head direction cell activity (Clark, Harris, & Taube, 2012; Knight, Hayman, Ginzberg, & Jeffery, 2011). However, what was particularly striking was the observation that cell responses to local cues co-existed with cells that preferentially responded to the global reference frame. Thus, both local and global frames of reference were simultaneously represented by RSC head direction cell populations. Consistent with these observations, the authors reported that many RSC head direction cells, specifically in the RSCd, expressed bi-directional tuning in the two-chambered environment. These cells therefore adopted two simultaneous preferred orientations (180 degrees apart) that were apparent in either enclosure.
The collective results above point to a prominent role for the RSC in integrating information across local and global allocentric frameworks. An animal may utilize this information to monitor their position within the local environment while at the same time guide their behavior toward a distant goal. Animals can also guide their behavior through an environment by referencing a sequence of movements to a goal, or a route. Changes to the structure of a route—for example in the service of by-passing a detour or executing a short-cut—may involve keeping track of the route structure at the local and global level. (Alexander & Nitz, 2015) addressed this possibility in a recent series of studies. First, Alexander and Nitz (2015) recorded from RSC neurons while animals navigated along a W-shaped maze (Fig. 2, bottom panel). Thus, the animal’s route through the maze was composed of alternating left-right-left turns in one direction, and right-left-right turns in the opposite direction. To determine whether cell activity was sensitive to global position in the environment, on separate trial blocks, the maze was moved to one of two locations in the environment. As in previous research, the authors observed that populations of RSC neurons were sensitive to specific body turns along the path, for example, neurons that were selective to left-turns but not right-turns and vice versa (Chen et al., 1994; Cho & Sharp, 2001). In addition, the authors identified populations of RSC neurons that fired preferentially in particular locations along a route. This took on two forms: first, neural firing in specific locations along the route, but not associated with a specific action; second, activity that reflected a “gain” in spiking at specific actions along a path. Thus, RSC neurons could at the same time be modulated by body-turns or route-centered behaviors, while at the same time increasing their firing at specific locations in the environment. Lastly, the authors reported that RSC neurons were sensitive to the location of the route in relation to the global environment (see Fig. 2, bottom panel). In other words, some neurons would show elevated spiking when the maze would occupy one of the two maze locations. In sum, RSC neurons can simultaneously process information regarding egocentric movements and their allocentric position within local, route-centered, and global frameworks.
Alexander and Nitz (2017) recently expanded on these findings by recording RSC neurons while animals navigated through a plus maze track. While replicating the observations made in their previous study, the authors also determined that RSC cells are active at different spatial scales. In other words, some RSC neurons tend to express a gain in spiking along broad path segments, while others along shorter repeating segments. In some cases, these RSC cell responses were conjunctive for more than one spatial scale. This was particularly apparent for cells that elevated their firing along a broad single path segment, while at the same time expressing neural activity in one or more additional path segments. Of particular interest was the finding that neural activity displayed symmetry between the two halves of the plus maze, suggesting a novel role for the RSC in encoding the relative distance between route segments.
A recent study by Vedder and colleagues (2017) investigated the relationship between RSC neural activity and the acquisition of spatial trajectories. Vedder et al recorded populations of RSC neurons while rats navigated to one of two reward locations in a T-maze task. A flashing light was used to indicate which of the two locations contained a reward. As described in the previous section, neurons in the PC can be modulated by the animal’s egocentric orientation in relation to a light cue (Wilber et al., 2014). While Vedder et al reported that populations of RSC neurons spiked in relation to the presentation of the light cue, cell firing was robust regardless of the location of the reward. In other words, RSC neurons appeared to exhibit view-independent modulation by the light cue; an observation consistent with the hypothesis that the RSC has a prominent role in processing landmarks (Miller et al., 2014). In addition, Vedder et al reported that RSC neurons spiked in anticipation of the cue presentation, and with some neurons preferentially spiking before the onset of a turn in a specific direction of trial (e.g., a neuron fires strongly only before the onset of a left turn). This differential activity on the set of the T-maze, also known as “splitter” firing, has been well documented in the firing characteristics of hippocampal neurons, which points to the possibility that the RSC and hippocampus participate in a shared network computing trajectories to future goal locations within an allocentric framework (Chrastil et al., 2015).
Lastly, it is important to make note of a recent study by Mao et al. (2017) showing that RSC neurons can fire in a single restricted region of the environment similar to hippocampal place cells. In this study, calcium imaging was conducted on RSC neurons from head-fixed mice while locomoting on a treadmill. In general, place-like firing by RSC cells could be maintained in darkness (in the absence of salient external cues), but appeared to show greater sensitivity to external tactile cues placed along the track. Although these place-like cells were observed throughout RSC subregions, the expression of place modulation was greatest in the superficial layers (II–III) compared to deep layers. The CA1 region of the hippocampus sends extensive projections to the superficial layers of cortex (Cenquizca & Swanson, 2007), and direct projections to the RSCg (Wyss & Van Groen, 1992; Zingg et al., 2014), many of which are GABAergic (Miyashita & Rockland, 2007). While inputs from CA1 may influence place-sensitivity of RSC cell populations, spatial coding could be a direct result of the fixed path, and perhaps route-like structure, of the treadmill apparatus. The fact that studies have generally failed to observe robust spatial firing by RSC neurons in freely moving animals seems to support this latter possibility (Alexander & Nitz, 2015, 2017; Lozano et al., 2017). Nevertheless, some studies have observed place-like firing in open environments, with some cells exhibiting conjunctive properties with head direction or turn direction (Cho & Sharp, 2001; Jacob et al., 2017 supplemental Figure 12), leaving the possibility of precise spatial coding by RSC neurons an open question for future investigation.
5. Models of Reference Frame Transformation in the Retrosplenial-Parietal Network
The mechanisms by which spatial information might be integrated across reference frames, and transformed from one frame to another, has been explored in several theoretical and computational studies (Bicanski & Burgess, 2016; Burgess, 2008; Byrne et al., 2007; McNaughton et al., 1995; Oess et al., 2017). McNaughton, Knierim and Wilson (1995) provided an early conceptualization of how this might be done. The basic structure of their model is a three-layered network of distinct neural populations. The first layer is composed of two neural populations: one with neurons that represent an animals allocentric heading in the environment (i.e., head direction cells), and a second that is modulated by an animals egocentric heading relative to a landmark. An example cell in this population might be to spike preferentially when a landmark is located right of one’s body. These two types of “input variables” converge post-synaptically on a second layer of cells that encode combinations, or conjunctions, of the two reference frames. Conjunctive cells are associated with a third layer that encodes the bearing, a view-invariant representation, of a landmark. This latter signal, in combination with information regarding the relative distance from the landmark, would produce a signal reflecting the specific distance and direction relative to a landmark (i.e., a landmark-vector representation; Deshmukh & Knierim, 2013; Wilber et al., 2014). For example, each combination of viewer-dependent landmark direction (e.g., empire state building is on my right) and allocentric head direction (e.g., I am facing NE) is associated with a single map-like or world-centered representation of the direction of the landmark (e.g., the landmark is due east from this spot on the map; Fig. 3). Thus, the model demonstrates how sensory information, for example the view of a landmark, which is body-centered in nature, can be transformed into map-like coordinates.
Figure 3.
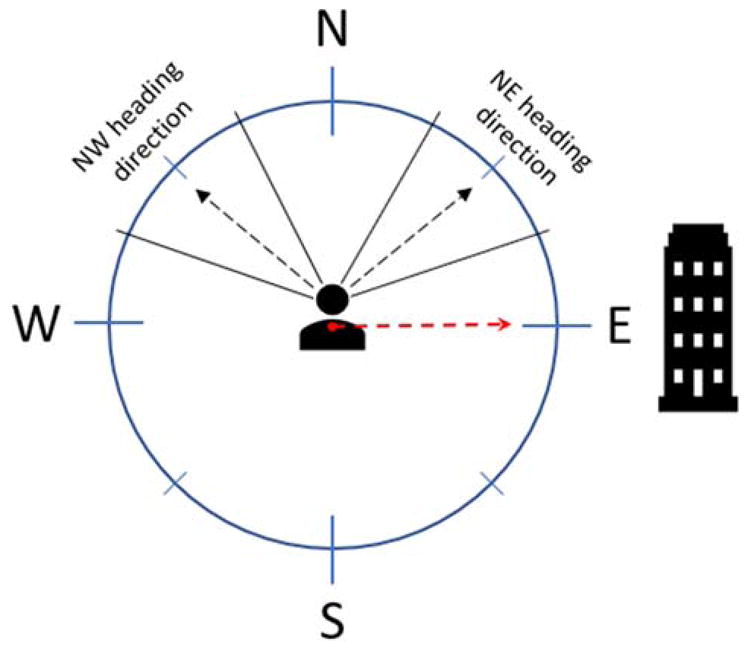
Diagram illustrating allocentric heading direction, allocentric landmark location, and egocentric translation of landmark location. The black dotted lines depict two examples of possible heading directions – NW and NE respectively. The red dotted line depicts the allocentric location of the building from where the person is standing. When facing NW, the person may think, “The building is slightly behind me and to the right.” When facing NE the person may think, “The building is slightly to my right.” However, for these two different egocentric representations of the building location, its allocentric representation is always the same from this point – “The building is east from where I am.”
Wilber et al. (2014) investigated whether the components of this coding scheme might be expressed by PC neurons. As briefly described earlier, the authors found evidence for neurons modulated by the animal’s egocentric orientation relative to a landmark, head direction cells in global allocentric coordinates, and cells that are conjunctive for both of these firing characteristics. In other words, the PC solves the first stages of the model above—taking in landmarks as we view them at the sensory level, and beginning the process of translating the position of that landmark into map-like coordinates. It should be noted that Wilber et al. (2014) did not find evidence for other model components in PC, particularly neurons associated with the third layer or model output. Characteristic firing by these neurons would be the bearing to a landmark (i.e., viewer invariant direction of the landmark). These ‘landmark bearing’ cells could be combined with other cells that encode the distance to a landmark, and the conjunction of these two features would result in a code for a vector to a landmark. Some preliminary evidence has been collected pointing to neurons with distance encoding features in the medial portions of the PC (Alexander, 2016). In addition, as noted in the previous section, neurons sensitive to the relative distance between maze segments have been identified within the RSC (Alexander & Nitz, 2017). Regardless of the location of this hypothesized distance signal, neural activity resembling landmark vectors have been observed in the hippocampus (Deshmukh & Knierim, 2013; Wilber et al., 2014) and more recently in the entorhinal cortex with features essentially identical to those reported in the hippocampus (Høydal, Skytøen, Moser, & Moser, 2017; Wilber et al., 2014). Alternatively, cells that in some ways have encoding that resembles viewer-dependent (egocentric) landmark directions have been observed in medial entorhinal cortex, suggesting this brain network may be quite broad (Chen et al., 2018; Wang, Xiaojing, Deshmukh, & Knierim, 2018). Lastly, to date there have been no reports of neurons with landmark bearing, or view invariant responses within the RSC-PC network. Further, we are unaware of studies investigating the possibility of this form of cellular activity in more medial regions of the PC, or in RSC, where they have been hypothesized to emerge (Wilber et al., 2014).
Subsequent modelling work continued to focus on a role for the RSC-PC network in coordinate transformations (Bicanski & Burgess, 2016; Byrne et al., 2007). In general, the components of the model are similar to McNaughton et al. (1995)—egocentric representations of environmental landmarks are combined with allocentrically modulated head direction cells to form conjunctions of the two features, which are then transformed into allocentric representations of specific environmental locations. However, a few differences are worth noting. First, the model provides some specific hypotheses regarding the neuroanatomical basis of the computations. In particular, it is hypothesized that the transformation involves the RSC with egocentric information conveyed by visual cortical regions or the PC. The product of this transformation is a global allocentric representation by hippocampal neurons (i.e., place cells). Second, instead of precise landmarks, the model focuses on transformations to and from allocentric representations of environmental boundaries computed by hippocampal neurons. Nonetheless, the same system could conceptually apply to distinct landmarks or other non-spatial features (Bicanski & Burgess, 2016; Dhindsa et al., 2014). Lastly, the translation is additionally featured to operate in reverse, i.e., a transformation from world-centered information into egocentric coordinates. For example, if you are driving in an unfamiliar city you might need to translate your location on a map into the appropriate body-centered action (e.g., turn right) for that world-centered location (e.g., at an intersection in a city).
While the emphasis of the modelling work above is placed on coordinate transformations between body-centered and map-like representations, it is important to note that the initial computations require input from an allocentrically modulated head direction circuit (summarized in Byrne et al., 2007; McNaughton et al., 1995). Thus, in one sense, this work additionally describes a framework for translating information from an allocentric head direction coordinate system to a precise allocentric location (see also Peyrache, Schieferstein, & Buzsáki, 2017). Head direction cells have been identified within a broad network of limbic thalamic and cortical regions and are generally thought to derive their allocentric orientation from a combination of angular path integration and distant landmark cues (see Taube, 2007 for review). Supporting the models summarized above, lesions of limbic head direction cell regions (the anterior thalamus or postsubiculum) can produce deficits in the spatial precision of hippocampal place cell firing (Calton et al., 2003; Harland et al., 2017) and grid cell coding (Winter, Clark, & Taube, 2015). Whether directional modulation within the RSC and PC is endowed by these same inputs, or is generated intrinsically within RSC-PC circuitry, is presently unclear. However, because head direction cells in RSC share some characteristics with postsubiculum cells (Lozano et al., 2017), it seems likely that external inputs from limbic head direction cell circuitry play a key role.
A recent model (Oess, Krichmar, & Rohrbein, 2017; Oess et al., 2017) has extended the work by Byrne and McNaughton by similarly proposing a world-centered to body-centered coordinate transformation in a hypothetical RSC-PC circuit. Although similar in concept to the models above, Oess et al. (2017) include a number of additional elements. First, the authors feature a specific PC based module devoted to processing egocentric and route-centered information; the selection of which is dependent on the navigation task at hand. This latter feature is consistent with the summary above indicating that PC neuronal activity can represent either route-centered, egocentric, or self-motion tuning depending on the behavioral task (Alexander, 2016; Whitlock et al., 2012). Secondly, Oess et al. (2017) include a RSC based decision-making module, which provides a mechanism for selecting the most reliable spatial reference frame given the spatial task. This feature is consistent with work demonstrating that neurons in RSC and PC can respond to the onset of reward or other salient task features (Licata et al., 2017; Vedder et al., 2017; Yang, Jacobson, & Burwell, 2017). It is important to note that the model does not include self-motion modulation as a feature which is a dominant characteristic of neural activity in PC (Whitlock et al., 2012; Wilber et al., 2014; Wilber et al., 2017). Indeed, Wilber et al. (2014) hypothesized that self-motion likely contributes to the unusual shape of egocentrically modulated tuning curves in the PC, which appeared to be absent in model simulations (see Fig. 7 of Oess et al., 2017). A complete replication of self-motion tuning would be useful to include in future modelling work.
6. Conclusions
The central aim of the present review was to summarize recent electrophysiological and computational work investigating a coordinated role for the RSC-PC circuit in spatial information processing. We offer three general conclusions. First, studies have identified considerable overlap in the types of internal and external spatial information processed by RSC and PC neurons. For instance, neurons expressing, sensory, self-motion, and egocentric modulation have been characterized within each (Alexander & Nitz, 2015; Chen et al., 1994; Whitlock et al., 2012; Wilber et al., 2014). Further, route-centered coding by RSC and PC neurons, which is organized in relation to the actions and local stimulus sources comprising the path, suggests that at least some features of local allocentric processing is shared between RSC and PC (Alexander & Nitz, 2015; Nitz, 2006).
A second general conclusion is that the RSC has a relatively greater role in processing information at the global allocentric level. Neurons sensitive to the distal environmental context have been identified throughout both granular and dysgranular subregions of the RSC (Alexander & Nitz, 2015; Jacob et al., 2017), along with neurons that appear to encode the allocentric distance between routes in a complex environment (Alexander & Nitz, 2017). It is important to make note that this specialization in global allocentric coding is not likely a consequence of strict parcellation along anatomical lines. For instance, PC neurons are often conceptualized as predominantly egocentrically modulated—however, studies have identified PC cells with directional sensitivity in global coordinates (Chen et al., 1994a; 1994b; Nakamura, 1999; Wilber et al., 2014). We suggest that spatial information processing is instead organized along a gradient across the two regions, with perhaps greater self-motion and egocentric processing at the PC end of the circuit, and greater global allocentric processing in the RSC. Although this notion is consistent with the lateral-to-medial gradient-like anatomical relationship between the two regions (Wang et al., 2012; Wilber et al., 2015), comparative studies of neural coding between the regions is lacking.
A final conclusion, based on the observed conjunctive coding of neurons throughout the RSC-PC network, is that each region likely plays a role in coordinating information across reference frames. Notable examples of this type of coding are studies demonstrating conjunctive signals in the RSC, which can represent combinations of egocentric, route-centric, and allocentric frameworks (Alexander & Nitz, 2015, 2017; Clark, 2017). Similar conjunctive signals have been observed in the PC representing both egocentric and allocentric head direction information (Wilber et al., 2014). These findings raise questions of how such coding schemes might be generated within this network. One possibility is that conjunctive signals are a consequence of network connectivity between RSC and PC. Certainly, the strong reciprocal connectivity between the two regions supports this possibility (Wilber et al., 2014). Nevertheless, neurons with similar conjunctive characteristics have been identified in afferents of the RSC-PC circuit and closely associated regions of the limbic system, and may also participate in this processing (Chen et al., 2018; Hinman, 2016; Hoydal, Skytoen, Moser, & Moser, 2018; Peyrache et al., 2017; Wang et al., 2018).
The research summarized above is likely to have an impact on future theoretical and computational studies. In general, models to date have argued for a more dichotomous view of RSC and PPC. Some studies have been supportive of this functional organization—for instance, human neuroimaging studies have shown strong RSC engagement when switching between, or associating, different spatial viewpoints (Dhindsa et al., 2014; Robertson, Hermann, Mynick, Kravitz, & Kanwisher, 2016; Zhang, Copara, & Ekstrom, 2012). On the one hand, the electrophysiological studies summarized in the sections above are inconsistent with this view in showing that the PPC-RSC network is better conceptualized as a coordinate transformation system with a high degree of functional overlap and integration at each anatomical level. On the other hand, the summary also shows that some functional specialization exists in each region. Indeed, model components such as neurons with conjunctive modulation for egocentric and allocentric reference frames have been identified in each region. One issue that remains unanswered is whether other hypothesized model features might be represented within the RSC-PC circuitry. For instance, studies have discovered neurons in hippocampal and parahippocampal regions with landmark vector correlates (Deshmukh & Knierim, 2013; Høydal et al., 2017; Wilber et al., 2014). Although similar neural correlates have not been observed in the PC (Wilber et al., 2014), it seems possible that the RSC may participate given the relatively greater allocentric modulation within its neuronal population.
One of the aims of this review was to highlight avenues for future research. We suggest that studies could be directed toward three general gaps in the current literature: (1) work could be aimed at better understanding the functional organization of spatial information processing along the RSC-PC network, (2) studies might be directed at determining the topographical arrangement of spatial frames within this circuitry, and (3) studies should address the precise mechanisms underlying how information is coordinated across spatial frameworks. These questions could be investigated utilizing methods for simultaneous recording of neural activity across the RSC-PC circuit along with reference frame manipulation (e.g., Clark et al., 2012; Knierim & Neunuebel, 2016; Wilber et al., 2014). Utilizing targeted circuit manipulations, other studies could address the necessity of external and intrinsic connectivity to the expression of reference frame representations in the RSC-PC network. It is likely that the large cortical surface of RSC and PC has served as a barrier for studies along these lines. However, recent innovations in viral-mediated methods allows greater selectivity and targeting of large cortical regions such as the RSC (Robinson et al., 2014; Smith, Bucci, Luikart, & Mahler, 2016). Additionally, methods that can investigate the functional connectivity within precise circuits may be utilized, such as those that couple immediate early gene expression with neuroanatomical tracing (Mesina et al., 2016). Such methods could help better understand the arrangement of reference frame processing intrinsically, but also between, PC and RSC circuitry. In sum, it is our hope that the present review will provide a framework in which future investigation can be motivated, and facilitate a better understanding of the role of the RSC-PC in spatial behavior.
Acknowledgments
The manuscript was written while the authors were supported by grants from NIAAA P50AA022534, R21AA024983, and Alzheimer’s Association AARG-17-531572 to BJC and NIA R00AG049090 to AAW.
References
- Agster KL, Burwell RD. Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus. 2009;19(12):1159–1186. doi: 10.1002/hipo.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre GK, D’Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999;122(9):1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- Alexander AS, Conner AM, Tung JC, Nitz DA. Hippocampal and Posterior Parietal Cortex Spatial Encoding During Pursuit. Paper presented at the Society for Neuroscience; San Diego, CA. 2016. [Google Scholar]
- Alexander AS, Nitz DA. Retrosplenial cortex maps the conjunction of internal and external spaces. Nat Neurosci. 2015;18(8):1143–1151. doi: 10.1038/nn.4058. http://www.nature.com/neuro/journal/v18/n8/abs/nn.4058.html#supplementary-information. [DOI] [PubMed] [Google Scholar]
- Alexander AS, Nitz DA. Spatially Periodic Activation Patterns of Retrosplenial Cortex Encode Route Sub-spaces and Distance Traveled. Current Biology. 2017;27(11):1551–1560. e1554. doi: 10.1016/j.cub.2017.04.036. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Essick GK, Siegel RM. Encoding of Spatial Location by Posterior Parietal Neurons. Science. 1985;230(4724):456–458. doi: 10.2307/1696086. [DOI] [PubMed] [Google Scholar]
- Auger SD, Zeidman P, Maguire EA. A central role for the retrosplenial cortex in de novo environmental learning. eLife. 2015;4:e09031. doi: 10.7554/eLife.09031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Zeidman P, Maguire EA. Efficacy of navigation may be influenced by retrosplenial cortex-mediated learning of landmark stability. Neuropsychologia. 2017;104:102–112. doi: 10.1016/j.neuropsychologia.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicanski A, Burgess N. Environmental Anchoring of Head Direction in a Computational Model of Retrosplenial Cortex. The Journal of Neuroscience. 2016;36(46):11601–11618. doi: 10.1523/JNEUROSCI.0516-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner Lindsay R, Andersen Richard A. Coding of the Reach Vector in Parietal Area 5d. Neuron. 2012;75(2):342–351. doi: 10.1016/j.neuron.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N. Spatial Cognition and the Brain. Annals of the New York Academy of Sciences. 2008;1124(1):77–97. doi: 10.1196/annals.1440.002. [DOI] [PubMed] [Google Scholar]
- Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol Rev. 2007;114(2):340–375. doi: 10.1037/0033-295x.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne PA, Crawford JD. Cue Reliability and a Landmark Stability Heuristic Determine Relative Weighting Between Egocentric and Allocentric Visual Information in Memory-Guided Reach. Journal of Neurophysiology. 2010;103(6):3054–3069. doi: 10.1152/jn.01008.2009. [DOI] [PubMed] [Google Scholar]
- Calton JL, Stackman RW, Goodridge JP, Archey WB, Dudchenko PA, Taube JS. Hippocampal place cell instability after lesions of the head direction cell network. Journal of Neuroscience. 2003;23(30):9719–9731. doi: 10.1523/JNEUROSCI.23-30-09719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton JL, Taube JS. Where am I and how will I get there from here? A role for posterior parietal cortex in the integration of spatial information and route planning. Neurobiology of Learning and Memory. 2009;91(2):186–196. doi: 10.1016/j.nlm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton JL, Turner CS, Cyrenne DLM, Lee BR, Taube JS. Landmark control and updating of self-movement cues are largely maintained in head direction cells after lesions of the posterior parietal cortex. Behavioral Neuroscience. 2008;122(4):827–840. doi: 10.1037/0735-7044.122.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Research Reviews. 2007;56(1):1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Green EJ, Barne CA, McNaughton BL. Head-direction cells in the rat posterior cortex I. anatomical distribution and behavioral modulation. Experimental Brain Research. 1994;101:8–23. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Green EJ, Barne CA, McNaughton BL. Head-direction cells in the rat posterior cortex II. anatomical distribution and behavioral modulation. Experimental Brain Research. 1994;101:24–34. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang C, Lee H, Rao G, Yoganarasimha D, Savelli F, Knierim JJ. Egocentric bearing selectivity in lateral entorhinal cortex. April 22, 2018; Paper presented at the International Conference on Learning and Memory; Huntington Beach, Californnia, USA. 2018. [Google Scholar]
- Cheng K. Some psychophysics of the pigeon’s use of landmarks. J Comp Physiol A. 1988;162(6):815–826. doi: 10.1007/BF00610970. [DOI] [PubMed] [Google Scholar]
- Cho J, Sharp PE. Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behav Neurosci. 2001;115(1):3–25. doi: 10.1037/0735-7044.115.1.3. [DOI] [PubMed] [Google Scholar]
- Chrastil ER, Sherrill KR, Hasselmo ME, Stern CE. There and Back Again: Hippocampus and Retrosplenial Cortex Track Homing Distance during Human Path Integration. The Journal of Neuroscience. 2015;35(46):15442. doi: 10.1523/JNEUROSCI.1209-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B, Taube J. Vestibular and attractor network basis of the head direction cell signal in subcortical circuits. Frontiers in Neural Circuits. 2012;6(7) doi: 10.3389/fncir.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ. Spatial Navigation: Retrosplenial Cortex Encodes the Spatial Structure of Complex Routes. Current Biology. 2017;27(13):R649–R651. doi: 10.1016/j.cub.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Bassett JP, Wang SS, Taube JS. Impaired Head Direction Cell Representation in the Anterodorsal Thalamus after Lesions of the Retrosplenial Cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(15):5289–5302. doi: 10.1523/JNEUROSCI.3380-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Harris MJ, Taube JS. Control of anterodorsal thalamic head direction cells by environmental boundaries: Comparison with conflicting distal landmarks. Hippocampus. 2012;22(2):172–187. doi: 10.1002/hipo.20880. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Harvey RE. Do the anterior and lateral thalamic nuclei make distinct contributions to spatial representation and memory? Neurobiology of Learning & Memory. 2016;133:69–78. doi: 10.1016/j.nlm.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Collett TS, Cartwright BA, Smith BA. Landmark learning and visuo-spatial memories in gerbils. Journal of Comparative Physiology A. 1986;158(6):835–851. doi: 10.1007/BF01324825. [DOI] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJY. Temporary Inactivation of the Retrosplenial Cortex Causes a Transient Reorganization of Spatial Coding in the Hippocampus. The Journal of Neuroscience. 2001;21(11):3986. doi: 10.1523/JNEUROSCI.21-11-03986.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JV, Reep RL. Rodent posterior parietal cortex as a component of a cortical netowrk mediating directed spatial attention. Psychobiology. 1998;26(2):87–102. [Google Scholar]
- Deshmukh SS, Knierim JJ. Influence of local objects on hippocampal representations: Landmark vectors and memory. Hippocampus. 2013;23(4):253–267. doi: 10.1002/hipo.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhindsa K, Drobinin V, King J, Hall GB, Burgess N, Becker S. Examining the role of the temporo-parietal network in memory, imagery, and viewpoint transformations. Frontiers in Human Neuroscience. 2014;8:709. doi: 10.3389/fnhum.2014.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elduayen C, Save E. The retrosplenial cortex is necessary for path integration in the dark. Behavioural Brain Research. 2014;272:303–307. doi: 10.1016/j.bbr.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: The perirhinal and postrhinal cortices. Hippocampus. 2007;17(9):709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The Organization of Learning. Cambridge: Bradform Books/MIT Press; 1990. [Google Scholar]
- Glickfeld LL, Olsen SR. Higher-Order Areas of the Mouse Visual Cortex. Annual Review of Vision Science. 2017;3(1):251–273. doi: 10.1146/annurev-vision-102016-061331. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Johnson TE, Rice JP, Candelaria FT, Sutherland RJ, … Redhead ES. The relative influence of place and direction in the Morris water task. J Exp Psychol Anim Behav Process. 2008;34(1):31–53. doi: 10.1037/0097-7403.34.1.31. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Weisend MP, Sutherland RJ. How do room and apparatus cues control navigation in the Morris water task? Evidence for distinct contributions to a movement vector. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33(2):100–114. doi: 10.1037/0097-7403.33.2.100. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Rosenfelt CS, Whishaw IQ. Sequential control of navigation by locale and taxon cues in the Morris water task. Behavioural Brain Research. 2004;154(2):385–397. doi: 10.1016/j.bbr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Harker TK, Whishaw IQ. A reaffirmation of the retrosplenial contribution to rodent navigation: reviewing the influences of lesion, strain, and task. Neuroscience & Biobehavioral Reviews. 2004;28(5):485–496. doi: 10.1016/j.neubiorev.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Harland B, Grieves RM, Bett D, Stentiford R, Wood ER, Dudchenko PA. Lesions of the Head Direction Cell System Increase Hippocampal Place Field Repetition. Curr Biol. 2017;27(17):2706–2712. e2702. doi: 10.1016/j.cub.2017.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay L, Redon C. Response delay and spatial representation in pointing movements. Neuroscience Letters. 2006;408(3):194–198. doi: 10.1016/j.neulet.2006.08.080. [DOI] [PubMed] [Google Scholar]
- Hinman J, Chapman G, Hasselmo Michael. Representation of environmental boundaries within an egocentric reference frame. Paper presented at the Society for Neuroscience; San Diego, CA. 2016. [Google Scholar]
- Hoydal OA, Skytoen ER, Moser M-B, Moser EI. Object-vector coding in the medial entorhinal cortex. bioRxiv. 2018 doi: 10.1038/s41586-019-1077-7. [DOI] [PubMed] [Google Scholar]
- Høydal ØA, Skytøen ER, Moser M-B, Moser EI. Object-vector cells in the medial entorhinal cortex. Paper presented at the Society for Neuroscience; Washington D.C. 2017. [Google Scholar]
- Hunsaker MR, Kesner RP. Unfolding the cognitive map: The role of hippocampal and extra-hippocampal substrates based on a systems analysis of spatial processing. Neurobiology of Learning & Memory. 2018;147:90–119. doi: 10.1016/j.nlm.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Jacob YP, Casali G, Spieser L, Page H, Overington D, Jeffery K. An independent, landmark-dominated head-direction signal in dysgranular retrosplenial cortex. Nat Neurosci. 2017;20(2):173–175. doi: 10.1038/nn.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob PY, Casali G, Spieser L, Page H, Overington D, Jeffery K. An independent, landmark-dominated head direction signal in dysgranular retrosplenial cortex. Nature Neuroscience. 2017;20(2):173–175. doi: 10.1038/nn.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BF, Witter MP. Cingulate cortex projections to the parahippocampal region and hippocampal formation in the rat. Hippocampus. 2007;17(10):957–976. doi: 10.1002/hipo.20330. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Hamilton DA. Framing Spatial Cognition: Neural Representations of Proximal and Distal Frames of Reference and Their Roles in Navigation. Physiological Reviews. 2011;91(4):1245–1279. doi: 10.1152/physrev.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP. Tracking the Flow of Hippocampal Computation: Pattern Separation, Pattern Completion, and Attractor Dynamics. Neurobiology of Learning and Memory. 2016;129:38–49. doi: 10.1016/j.nlm.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R, Hayman R, Ginzberg LL, Jeffery K. Geometric cues influence head direction cells only weakly in non-disoriented rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(44):15681–15692. doi: 10.1523/JNEUROSCI.2257-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Sutherland RJ, Whishaw IQ. A comparison of the contributions of the frontal and parietal association cortex to spatial localization in rats. Behav Neurosci. 1983;97(1):13–27. doi: 10.1037//0735-7044.97.1.13. [DOI] [PubMed] [Google Scholar]
- Kolb B, Walkey J. Behavioural and anatomical studies of the posterior parietal cortex in the rat. Behavioural Brain Research. 1987;23(2):127–145. doi: 10.1016/0166-4328(87)90050-7. [DOI] [PubMed] [Google Scholar]
- Krieg WJS. Connections of the cerebral cortex. I. The albino rat. B. Structure of the cortical areas. The Journal of Comparative Neurology. 1946;84(3):277–323. doi: 10.1002/cne.900840302. [DOI] [PubMed] [Google Scholar]
- Licata AM, Kaufman MT, Raposo D, Ryan MB, Sheppard JP, Churchland AK. Posterior parietal cortex guides visual decisions in rats. The Journal of Neuroscience. 2017 doi: 10.1523/JNEUROSCI.0105-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano YR, Page H, Jacob PY, Lomi E, Street J, Jeffery K. Retrosplenial and postsubicular head direction cells compared during visual landmark discrimination. Brain and Neuroscience Advances. 2017;1:2398212817721859. doi: 10.1177/2398212817721859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaswinkel H, Whishaw IQ. Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behavioural Brain Research. 1999;99(2):143–152. doi: 10.1016/S0166-4328(98)00100-4. [DOI] [PubMed] [Google Scholar]
- Mao D, Kandler S, McNaughton BL, Bonin V. Sparse orthogonal population representation of spatial context in the retrosplenial cortex. Nature Communications. 2017;8(1):243. doi: 10.1038/s41467-017-00180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchette SA, Vass LK, Ryan J, Epstein RA. Anchoring the neural compass: coding of local spatial reference frames in human medial parietal lobe. Nature Neuroscience. 2014;17:1598. doi: 10.1038/nn.3834. https://www.nature.com/articles/nn.3834#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: Evidence for independent memory systems involving dorsal striatum and hippocampus. Behavioral and Neural Biology. 1994;61(3):260–270. doi: 10.1016/S0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7(8):663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Knierim JJ, Wilson MA. Vector encoding and the vestibular foundations of spatial cognition: Neurophysiological and computational mechanisms. In: Gazzaniga MS, editor. The Cognitive Neurosciences. Cambridge: The MIT Press; 1995. pp. 585–595. [Google Scholar]
- McNaughton BL, Mizumori SJY, Barnes CA, Leonard BJ, Marquis M, Green EJ. Cortical Representation of Motion during Unrestrained Spatial Navigation in the Rat. Cerebral Cortex. 1994;4(1):27–39. doi: 10.1093/cercor/4.1.27. [DOI] [PubMed] [Google Scholar]
- Mesina L, Wilber AA, Clark BJ, Dube S, Demecha AJ, Stark CEL, McNaughton BL. A Methodological Pipeline for Serial-Section Imaging and Tissue Realignment for Whole-brain Functional and Connectivity Assessment. Journal of Neuroscience Methods. 2016;266:151–160. doi: 10.1016/j.jneumeth.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AMP, Vedder LC, Law LM, Smith DM. Cues, context, and long-term memory: the role of the retrosplenial cortex in spatial cognition. Frontiers in Human Neuroscience. 2014;8:586. doi: 10.3389/fnhum.2014.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, Czajkowski R, Zhang N, Jeffery K, Nelson AJD. Retrosplenial cortex and its role in spatial cognition. Brain and Neuroscience Advances. 2018;2:2398212818757098. doi: 10.1177/2398212818757098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Rockland KS. GABAergic projections from the hippocampus to the retrosplenial cortex in the rat. Eur J Neurosci. 2007;26(5):1193–1204. doi: 10.1111/j.1460-9568.2007.05745.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K. Auditory Spatial Discriminatory and Mnemonic Neurons in Rat Posterior Parietal Cortex. Journal of Neurophysiology. 1999;82(5):2503–2517. doi: 10.1152/jn.1999.82.5.2503. [DOI] [PubMed] [Google Scholar]
- Nitz D. Parietal cortex, navigation, and the construction of arbitrary reference frames for spatial information. Neurobiology of Learning and Memory. 2009;91(2):179–185. doi: 10.1016/j.nlm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Nitz DA. Tracking Route Progression in the Posterior Parietal Cortex. Neuron. 2006;49(5):747–756. doi: 10.1016/j.neuron.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Nitz DA. Spaces within spaces: rat parietal cortex neurons register position across three reference frames. Nature Neuroscience. 2012;15(10):1365–1367. doi: 10.1038/nn.3213. doi: http://www.nature.com/neuro/journal/vaop/ncurrent/abs/nn.3213.html#supplementary-information. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon; 1978. [Google Scholar]
- Oess T, Krichmar JL, Rohrbein F. A Computational Model for Spatial Navigation Based on Reference Frames in the Hippocampus, Retrosplenial Cortex, and Posterior Parietal Cortex. Front Neurorobot. 2017;11:4. doi: 10.3389/fnbot.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oess T, Krichmar JL, Röhrbein F. A Computational Model for Spatial Navigation Based on Reference Frames in the Hippocampus, Retrosplenial Cortex, and Posterior Parietal Cortex. Frontiers in Neurorobotics. 2017;11(4) doi: 10.3389/fnbot.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, … Zeng H. A mesoscale connectome of the mouse brain. Nature. 2014;508(7495):207–214. doi: 10.1038/nature13186. http://www.nature.com/nature/journal/v508/n7495/abs/nature13186.html#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GM, Ohara S, Iijima T, Witter MP. Parahippocampal and retrosplenial connections of rat posterior parietal cortex. Hippocampus. 2017;27(4):335–358. doi: 10.1002/hipo.22701. [DOI] [PubMed] [Google Scholar]
- Olsen GM, Witter MP. Posterior parietal cortex of the rat: Architectural delineation and thalamic differentiation. Journal of Comparative Neurology. 2016;524(18):3774–3809. doi: 10.1002/cne.24032. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. New York: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Schieferstein N, Buzsáki G. Transformation of the head-direction signal into a spatial code. Nature Communications. 2017;8(1):1752–1752. doi: 10.1038/s41467-017-01908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothuizen HHJ, Aggleton JP, Vann SD. Do rats with retrosplenial cortex lesions lack direction? European Journal of Neuroscience. 2008;28(12):2486–2498. doi: 10.1111/j.1460-9568.2008.06550.x. [DOI] [PubMed] [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Experimental Brain Research. 1994;100(1):67–84. doi: 10.1007/bf00227280. [DOI] [PubMed] [Google Scholar]
- Robertson Caroline E, Hermann Katherine L, Mynick A, Kravitz Dwight J, Kanwisher N. Neural Representations Integrate the Current Field of View with the Remembered 360° Panorama in Scene-Selective Cortex. Current Biology. 2016;26(18):2463–2468. doi: 10.1016/j.cub.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, Bucci DJ. Chemogenetic Silencing of Neurons in Retrosplenial Cortex Disrupts Sensory Preconditioning. The Journal of Neuroscience. 2014;34(33):10982. doi: 10.1523/JNEUROSCI.1349-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save E, Paz-Villagran V, Alexinsky T, Poucet B. Functional interaction between the associative parietal cortex and hippocampal place cell firing in the rat. European Journal of Neuroscience. 2005;21(2):522–530. doi: 10.1111/j.1460-9568.2005.03882.x. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B. Involvement of the hippocampus and associative parietal cortex in the use of proximal and distal landmarks for navigation. Behavioural Brain Research. 2000;109(2):195–206. doi: 10.1016/S0166-4328(99)00173-4. [DOI] [PubMed] [Google Scholar]
- Shibata H, Honda Y, Sasaki H, Naito J. Organization of intrinsic connections of the retrosplenial cortex in the rat. Anatomical Science International. 2009;84(4):280. doi: 10.1007/s12565-009-0035-0. [DOI] [PubMed] [Google Scholar]
- Shine JP, Valdés-Herrera JP, Hegarty M, Wolbers T. The Human Retrosplenial Cortex and Thalamus Code Head Direction in a Global Reference Frame. The Journal of Neuroscience. 2016;36(24):6371. doi: 10.1523/JNEUROSCI.1268-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV. DREADDs: Use and Application in Behavioral Neuroscience. Behavioral Neuroscience. 2016;130(2):137–155. doi: 10.1037/bne0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar J, Witter MP, van Strien NM, Cappaert NL. The retrosplenial cortex: intrinsic connectivity and connections with the (para)hippocampal region in the rat. An interactive connectome. Front Neuroinform. 2011;5:7. doi: 10.3389/fninf.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Kolb B. Contributions of cingulate cortex to two forms of spatial learning and memory. The Journal of Neuroscience. 1988;8(6):1863. doi: 10.1523/JNEUROSCI.08-06-01863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. The Head Direction Signal: Origins and Sensory-Motor Integration. Annual Review of Neuroscience. 2007;30(1):181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Taube JS, Burton HL. Head direction cell activity monitored in a novel environment and during a cue conflict situation. Journal of Neurophysiology. 1995;74(5):1953–1971. doi: 10.1152/jn.1995.74.5.1953. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. The Journal of Comparative Neurology. 1992;315(2):200–216. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. The Journal of Comparative Neurology. 2003;463(3):249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss MJ. Connections of the retrosplenial granular a cortex in the rat. The Journal of Comparative Neurology. 1990;300(4):593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Selective dysgranular retrosplenial cortex lesions in rats disrupt allocentric performance of the radial-arm maze task. Behav Neurosci. 2005;119(6):1682–1686. doi: 10.1037/0735-7044.119.6.1682. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10(11):792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vedder LC, Miller AMP, Harrison MB, Smith DM. Retrosplenial Cortical Neurons Encode Navigational Cues, Trajectories and Reward Locations During Goal Directed Navigation. Cereb Cortex. 2017;27(7):3713–3723. doi: 10.1093/cercor/bhw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Miller MW. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. The Journal of Comparative Neurology. 1983;216(2):192–210. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- Wang C, Xiaojing C, Deshmukh S, Knierim JJ. Influence of objects on egocentric bearing tuning in lateral entorhinal cortex. April 22, 2018; Paper presented at the International Conference on Learning and Memory; Huntington Beach, California, USA. 2018. [Google Scholar]
- Wang Q, Sporns O, Burkhalter A. Network Analysis of Corticocortical Connections Reveals Ventral and Dorsal Processing Streams in Mouse Visual Cortex. The Journal of Neuroscience. 2012;32(13):4386–4399. doi: 10.1523/jneurosci.6063-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Maaswinkel H, Gonzalez CLR, Kolb B. Deficits in allothetic and idiothetic spatial behavior in rats with posterior cingulate cortex lesions. Behavioural Brain Research. 2001;118(1):67–76. doi: 10.1016/S0166-4328(00)00312-0. [DOI] [PubMed] [Google Scholar]
- Whitlock JR. Navigating actions through the rodent parietal cortex. Frontiers in Human Neuroscience. 2014;8 doi: 10.3389/fnhum.2014.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR. Posterior parietal cortex. Curr Biol. 2017;27(14):R691–r695. doi: 10.1016/j.cub.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Whitlock Jonathan R, Pfuhl G, Dagslott N, Moser MB, Moser Edvard I. Functional Split between Parietal and Entorhinal Cortices in the Rat. Neuron. 2012;73(4):789–802. doi: 10.1016/j.neuron.2011.12.028. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Sutherland RJ, Witter MP, Moser MB, Moser EI. Navigating from hippocampus to parietal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(39):14755–14762. doi: 10.1073/pnas.0804216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Clark BJ, Demecha AJ, Mesina L, Vos JM, McNaughton BL. Cortical connectivity maps reveal anatomically distinct areas in the parietal cortex of the rat. Frontiers in Neural Circuits. 2015;8(146) doi: 10.3389/fncir.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Clark BJ, Forster TC, Tatsuno M, McNaughton BL. Interaction of Egocentric and world centered reference frames in the rat posterior parietal cortex. The Journal of Neuroscience. 2014;34(16):5431–5446. doi: 10.1523/JNEUROSCI.0511-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Skelin I, Wu W, McNaughton BL. Laminar Organization of Encoding and Memory Reactivation in the Parietal Cortex. Neuron. 2017;95(6):1406–1419. e1405. doi: 10.1016/j.neuron.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SS, Clark BJ, Taube JS. Spatial navigation. Disruption of the head direction cell network impairs the parahippocampal grid cell signal. Science. 2015;347(6224):870–874. doi: 10.1126/science.1259591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbers T, Buchel C. Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. Journal of Neuroscience. 2005;25(13):3333–3340. doi: 10.1523/jneurosci.4705-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM, Van Groen T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus. 1992;2(1):1–11. doi: 10.1002/hipo.450020102. [DOI] [PubMed] [Google Scholar]
- Xing J, Andersen RA. Models of the Posterior Parietal Cortex Which Perform Multimodal Integration and Represent Space in Several Coordinate Frames. Journal of Cognitive Neuroscience. 2000;12(4):601. doi: 10.1162/089892900562363. [DOI] [PubMed] [Google Scholar]
- Yang FC, Jacobson TK, Burwell RD. Single neuron activity and theta modulation in the posterior parietal cortex in a visuospatial attention task. Hippocampus. 2017;27(3):263–273. doi: 10.1002/hipo.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Clark BJ, Brown JE, Lamia MV, Valerio S, Shinder ME, Taube JS. Both visual and idiothetic cues contribute to head direction cell stability during navigation along complex routes. J Neurophysiol. 2011;105(6):2989–3001. doi: 10.1152/jn.01041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Clark BJ, Taube JS. Origins of landmark encoding in the brain. Trends Neurosci. 2011;34(11):561–571. doi: 10.1016/j.tins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Copara M, Ekstrom AD. Differential Recruitment of Brain Networks following Route and Cartographic Map Learning of Spatial Environments. PLoS ONE. 2012;7(9):e44886. doi: 10.1371/journal.pone.0044886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Hintiryan H, Gou L, Song Monica Y, Bay M, Bienkowski Michael S, … Dong H-W. Neural Networks of the Mouse Neocortex. Cell. 2014;156(5):1096–1111. doi: 10.1016/j.cell.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]