Abstract
Background
People experiencing strong feelings of fatigue during exercise sometimes subconsciously yell to refocus their efforts and, thus, maintain exercise performance. The present study examined the influence of yelling during high-intensity exercise by analysing cardiorespiratory reactions and integrated electromyography (iEMG) changes in the vastus lateralis during a cycle ergometer test.
Methods
A total of 23 moderately trained people were recruited. The cycling test began with a resistance of 25 W/min, which was gradually increased. During the experimental trial, the participants were required to yell at least 3 times when they felt exhausted; during the controlled trial, they were not allowed to produce any yelling sounds. The testing order was randomly assigned and the 2 trials were completed within an interval between 3–10 days. Two-way repeated measures ANOVA was applied to analyse the differences within and between the trials, and interaction of trial and time.
Results
The peak power and time to exhaustion (p < 0.01) in the yelling trial were higher than those in the control trial. However, the vastus lateralis iEMG values of both trials at peak power were not significantly different. During the yelling period at 90%–100% of the maximal effort, a significant time-by-trial interaction (p < 0.05) was observed in oxygen consumption (VO2), CO2 production, O2 pulse, ventilation, and respiratory rate. All the above measures showed a significant between-trial difference (p < 0.02). However, heart rate, respiratory exchange ratio, end-tidal oxygen pressure, and ventilatory equivalent for oxygen showed only significant between-trial difference (p < 0.05), but without interaction of trial and time.
Conclusion
Yelling enhances the peak O2 pulse and VO2 and maintains CO2-exclusion efficiency during high-intensity exercise. It may enable maintaining muscle activation without stronger EMG signals being required during high-intensity exercise.
Keywords: Aerobic exercise, Cardiopulmonary exercise test, Intense exercise, Performance-enhancing effect, Respiratory–cardiac activities, Shouting
1. Introduction
When experiencing substantial exercise fatigue, some athletes attempt to maintain a strong athletic performance by yelling. For example, a track-and-field runner might yell during a final sprint. However, the physiologic mechanism of the effect of yelling on sports performance remains unclear. The early study of Ikai and Steinhaus1 demonstrated that simple shouts during exertion can increase the parameter that was previously believed to be maximal strength. However, that study focused primarily on investigating the effect of yelling on the performance of anaerobic exercises (e.g., weight lifting); hence, whether similar effects occur during extreme aerobic exercise remains unclear. Bunn and Mead2 suggested that phonation can be regarded as a subsidiary of respiration. During vocalisation, the tidal volume (VT) and expiratory time are increased, whereas inspiratory time is reduced. During high-effort whispers, the end-expiratory thoracic volume is substantially reduced because the volume of all compartments decreases, impinging on the maximal expiratory flow–volume curve.3 Aliverti et al.4 showed that, during exercise, the expired volume is entirely attributable to the abdomen, whereas during phonation, all 3 chest-wall compartments contribute to the expired volume. Therefore, we speculated that, during high-intensity exercise involving vigorous ventilation, forceful yelling may exert a considerable effect on thoracic and abdominal pressure as well as cause capacity changes that affect ventilation effectiveness during extreme exercise. Dempsey et al.5 proposed that the control of the respiratory system contributes to exercise limitation, and that its primary effect originates from complex respiratory–cardiac interactive effects. Their study showed that respiratory muscle work and fatigue, cyclical fluctuations in intrathoracic pressure, and cardiac output are crucial determinants of performance. Effective respiratory control facilitates the promotion of the cardiac output required to meet the demands of limb activities during exercise, affects the progress of peripheral muscular fatigue, and affects the sense of central fatigue through the perception of effort. However, previous studies have investigated only the interaction between respiration and vocalisation at rest and respiratory muscle actions during exercise. The immediate effect of yelling (a loud vocalisation) during exercise has not been reported.
The purpose of the present study was to quantify participants' exercise performance by using an incremental maximal cycling test. We investigated the effects of yelling on lower-extremity muscle power and electromyography (EMG) signals when the participants experienced extreme feelings of exhaustion. In addition, we extensively investigated the effects of abdominal yelling on cardiorespiratory system changes and attempted to clarify how the physiologic mechanisms of yelling affect the performance of extreme aerobic exercises.
2. Methods
2.1. Participants
A total of 23 moderately trained people (19 men and 4 women) participated in this study. Their mean age, height, weight, and body mass index (BMI) were 20.3 ± 1.5 years, 170.3 ± 7.1 cm, 64.4 ± 7.4 kg, and 22.2 ± 2.0 kg/m2, respectively. All participants were routinely involved (5.8 ± 2.2 h/week) in various intermittent activities (e.g., volleyball, tennis, basketball, and soccer), were familiar with maximal training, and had no history or clinical signs of cardiopulmonary diseases or orthopaedic from each participant injury in the lower extremities. Written consent was obtained and the Ethics Committee of I-Shou University granted ethical approval.
2.2. Design
The participants were not allowed to eat or drink coffee for 4 h before the exercise tests, and vigorous exercise and alcohol were forbidden for 24 h before the day of testing. Each participant visited our laboratory twice to participate in the incremental cycling test. The control and experimental trials (with yelling) were performed in random order. Each exercise test was conducted in an air-conditioned laboratory with an atmospheric temperature of 20°C–24°C and a relative humidity of 50%–60%. Each participant completed the experimental protocol within a period of 10 days, with at least 3 days between each exercise test, to ensure that the participants' level of physical fitness had not changed.
The participants produced a yell by forcefully contracting the abdominal muscles and emitting a short and loud tone (participants were asked to yell “Er”) while exerting maximal effort in the final stage of the exercise test. The following criteria were used to determine the time point during each test at which the yelling occurred, with at least one of the criteria required to initiate the yelling: (1) the participant started to feel fatigued; (2) the participant reached a respiratory exchange ratio (RER) of 1.1 and achieved 85% of the age-predicted maximal heart rate (HR) (220 − age) (Fig. 1). Three to 5 yells at intervals of 1–3 s were required, and the participant was asked to sustain each yell 1–2 s. EMG was applied to the rectus abdominals to ensure that the yells were not only vocalised from the throat. By contrast, the participants in the control trial were encouraged by simulated yelling sounds (about 100 decibels emitted by the surveying person) in the final stage, but not allowed to produce any yelling sounds by themselves (Fig. 1). All participants were asked to practice the yelling maneuver before the first exercise test to ensure they can properly use abdominal muscles to issue the yelling volume up to 100 decibels. The yelling volume was measured by using a decibel meter (DSL-333; TECPEL, Taipei, Taiwan, China). However, we could not measure the actual decibel value of each yell emitted by the participants during the exercise test, because the detector of the decibel meter could not be inserted into the inside of the face mask.
Fig. 1.
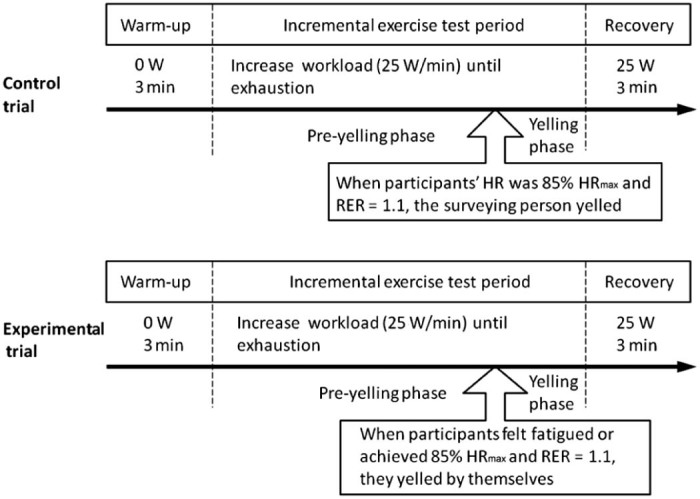
Diagram of the experimental design and the point during the exercise test at which the yelling occurred. HR = heart rate; RER = respiratory exchange ratio; W = watt.
2.3. Methodology
The participants performed incremental maximal exercise tests on a bicycle ergometer (ANGIO with a reclining chair, Lode, Groningen, The Netherlands). The exercise began after a 3 min warm-up period at 0 W, after which the workload was increased by 25 W/min (ramp protocol) until the participant felt exhausted.6 The workload of the ergometer was subsequently returned to 25 W, and the participant continued to cycle for a 3 min recovery period. The pedalling rate was maintained at approximately 60 rpm for each participant to prevent the participants from varying the rate and, thus, potentially influencing the results. A pedal-frequency meter that provided visual feedback was used by the participants to maintain the aforementioned rate. Tests were terminated when the participants could not continue because of exhaustion or when the target pedal rate could not be maintained for 10 s despite verbal encouragement. All participants achieved at least 2 of the following criteria for determining maximal effort: (1) a plateau in oxygen consumption (VO2) with an increased work rate; (2) an HR >85% of the age-predicted maximum; or (3) an RER greater than 1.1.7
To assess the physiologic responses during the exercise, we measured the HR, systolic blood pressure (SBP), and rate-pressure product (RPP) by using an electrocardiographic device (Tango+, SunTech, Raleigh, NC, USA). The participants breathed through a face mask, which allowed breath-by-breath analysis of the expired air using an automated gas-analysis system (Vmax 29c; Sensor Medics, Yorba Linda, CA, USA). The cardiorespiratory parameters measured during exercise were VO2, carbon dioxide production (VCO2), the RER, minute ventilation (VE), VT, the respiratory rate (RR), ventilatory equivalents for O2 (VE/VO2) and CO2 (VE/VCO2), end-tidal partial pressures of O2 (PetO2) and CO2 (PetCO2), and oxygen pulse (O2 pulse). The O2 pulse was defined as the ratio of VO2 to the HR. which, according to the Fick equation, is numerically equal to the product of stroke volume and the arteriovenous oxygen difference.8 Because the changes in the arteriovenous oxygen difference during progressive maximal exercise testing are uniform for almost all healthy people, the O2 pulse was used as a surrogate marker for the stroke volume.9
Myoelectric activity was determined using surface EMG and recorded using bipolar silver–silver chloride electrodes that were 7 mm in diameter and fixed at a 20 mm interelectrode distance (Norotrode 20; Myotronics-Noromed, Inc., Tukwila, WA, USA). The EMG signals were amplified at a gain of 1000 and a frequency passband of 1–5 kHz (EMG 100C; Biopac, Goleta, CA, USA), sampled using a data acquisition system (MP 150; Biopac) at a rate of 5 kHz, and subsequently stored on a computer disc for subsequent analysis. The electrodes were placed on the distal half of the musculus vastus lateralis of the right leg and rectus abdominis. Each electrode site was prepared by abrading and swabbing the site by using an ether pad. The EMG signals were examined for movement artefacts, and the electrodes were secured using surgical tape to minimise displacement during movement. To ensure that the electrodes were placed at precisely the same location for each testing session, the electrode sites were marked using a pen. However, large amounts of sweat interfered with the acquired EMG signals; thus, the signals exhibited noise that caused difficulties in differentiating among the 8 sets of EMG signals used in this study; therefore, we analysed only the 15 EMG recordings of which the signals were the clearest. EMG signals were recorded continually during the incremental cycling test; the raw EMG signals were full-wave rectified and integrated using commercially available software (MATLAB, Release 2013a; The MathWorks Inc., Natick, MA, USA). The integrated electromyography (iEMG) signal of the maximal contraction was averaged for the final 10 s and used as the value of peak power in the exercise test.
2.4. Statistical analysis
Data are presented as the mean and standard error of the mean (mean ± SEM). Prior to the statistical analysis, tests for normality (Kolmogorov–Smirnov) were carried out on all variables. A nonparametric test was not necessary, since all distributions were normal. Statistical differences in the peak-power performance, time to exhaustion, and EMG activation between the yelling and control trials were calculated using paired-sample t tests. A two-way (trial × time) repeated-measures analysis of variance (RM-ANOVA) was used to analyse the dependant variables over time between the yelling and control trials. In the exercise test durations, the VO2, HR, and O2 pulse values were calculated at every 10% of the maximal cycling time for each evaluation. First, the analyses were performed using the data from 10% to 80% of the maximal effort time to determine the difference in the baseline responses between the 2 trials (preyelling period). Second, the analyses were performed using the data from 90% to 100% of the maximal effort time during the final stages (yelling period) of the 2 trials. Statistical analyses were conducted using SPSS Version 18.0 (IBM, Armonk, NY, USA) software, and the significance level was set at p < 0.05.
3. Results
When the participants exerted themselves to the point of exhaustion during the incremental cycling test, yelling was observed to improve their peak-power performance and time to exhaustion by 6.0% (p = 0.006) and 5.8% (p = 0.003), respectively, compared with the values recorded in the control trial (Table 1). We analysed the iEMG signals of the vastus lateralis muscle at peak power. The results indicated that iEMG signals (p = 0.061) and iEMG/power ratio (p = 0.297) did not statistically differ between the 2 trials (Table 2), although the peak power was significantly higher (8.8%) in the yelling trial than in the control trial (p = 0.011, n = 15; 8 sets of EMG signals with noise were excluded).
Table 1.
Maximal values of variables measured during a maximal exercise test in the 2 trials (n = 23) (mean ± SEM).
| Control trial | Yelling trial | p value | |
|---|---|---|---|
| Peak power (W) | 174.5 ± 7.0 | 185.0 ± 5.7 | 0.006 |
| Time to exhaustion (s) | 451.9 ± 16.6 | 478.0 ± 14.0 | 0.003 |
Table 2.
iEMG activation of the vastus lateralis muscle at exhaustion (n = 15) (mean ± SEM).
| Control trial | Yelling trial | p value | |
|---|---|---|---|
| Power (W) | 161.53 ± 7.98 | 175.67 ± 5.98 | 0.011 |
| iEMG (mV) | 50.67 ± 4.07 | 63.26 ± 8.84 | 0.061 |
| iEMG/power | 0.313 ± 0.020 | 0.357 ± 0.051 | 0.297 |
Abbreviation: iEMG= integrated electromyography.
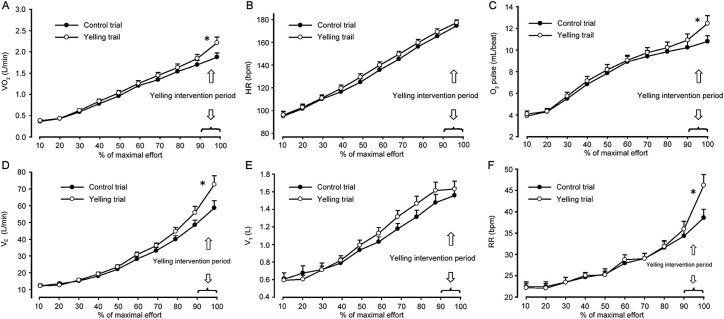
The RM-ANOVA indicated that the overall main effect of the values of VO2, HR, and O2 pulse progressively increased from 10% to 100% of the maximal effort in both trials (p < 0.001) (Fig. 2A–C). The responses of VO2, HR, and O2 pulse during 10%–80% of the maximal effort (preyelling phase) were similar between trials (p > 0.05), and no significant time-by-trial interaction (p > 0.05) was observed. During the yelling period (90%–100% of the maximal effort), a significant time-by-trial interaction effect on the VO2 (p = 0.010) and O2 pulse (p = 0.012) responses was observed. The responses in VO2 (p = 0.004) and the O2 pulse (p = 0.015) differed significantly between the 2 trials.
Fig. 2.
Cardiac and ventilatory responses during the incremental cycling test for the VO2 (A), HR (B), O2 pulse (C), VE (D), VT (E), and RR (F) (n = 23) (mean ± SEM). HR = heart rate; O2 pulse = oxygen pulse; RR = respiratory rate; VE = minute ventilation; VO2 = oxygen consumption; VT = tidal volume. * p < 0.05, a significant time-by-trial interaction effect.
Time exerted a significant main effect on most cardiorespiratory variables during the yelling period, except the changes in the VT and VE/VCO2. This showed that the variables at the maximal effort were significantly (p < 0.007) higher than 90% of the maximal effort (Table 3). Moreover, the significant time-by-trial interaction effect was observed only for VO2, VCO2, the O2 pulse, VE, and the RR. No significant interaction effect was observed for the RER, HR, SBP, and RPP between the yelling and the control trials at 90%–100% of the maximal effort time. The increase in maximal VE (p = 0.032) was primarily caused by an increase in the RR (p = 0.009) and not by the VT (p = 0.312) (Table 3, Fig. 2D–F).
Table 3.
Comparison of cardiac and metabolic variables at 90% and 100% maximal exercise before and after yelling, and the time-by-trial interaction effect according to RM-ANOVA (n = 23) (mean ± SEM).
| Variable | 90% of maximal effort | 100% of maximal effort | p (F) value | ||||
|---|---|---|---|---|---|---|---|
| Control trial | Yelling trial | Control trial | Yelling trial | Time | Trial | Time × trial | |
| Cardiac and metabolic | |||||||
| VO2 (L/min) | 1.70 ± 0.52 | 1.84 ± 0.46 | 1.87 ± 0.49 | 2.21 ± 0.64 | <0.001(40.620) | 0.004(10.203) | 0.010(7.996) |
| VCO2(L/min) | 2.01 ± 0.59 | 2.25 ± 0.61 | 2.36 ± 0.63 | 2.81 ± 0.76 | <0.001(96.327) | 0.001(13.174) | 0.025(5.763) |
| RER | 1.19 ± 0.09 | 1.22 ± 0.13 | 1.26 ± 0.12 | 1.29 ± 0.14 | <0.001(41.461) | 0.038(4.847) | 0.887(0.021) |
| HR(bpm) | 165.43 ± 13.06 | 169.35 ± 13.25 | 174.52 ± 10.41 | 178.65 ± 12.79 | <0.001(66.921) | 0.035(5.073) | 0.901(0.016) |
| SBP(mmHg) | 169.39 ± 20.95 | 165.83 ± 20.78 | 179.13 ± 20.75 | 173.35 ± 22.96 | <0.001(26.198) | 0.136(2.396) | 0.437(0.626) |
| RPP(1/1000) | 28.10 ± 4.70 | 28.16 ± 4.70 | 30.79 ± 4.55 | 30.44 ± 4.89 | <0.001(71.284) | 0.826(0.050) | 0.380(0.802) |
| O2 pulse(mL/beat) | 10.32 ± 2.85 | 11.07 ± 2.73 | 10.78 ± 2.52 | 12.44 ± 3.45 | <0.001(18.529) | 0.015(6.913) | 0.012(7.502) |
| Ventilator efficiency | |||||||
| VE(L/min) | 48.04 ± 14.02 | 55.88 ± 18.17 | 58.70 ± 20.91 | 72.71 ± 25.56 | <0.001(46.662) | 0.001(14.453) | 0.032(5.225) |
| VT(L) | 1.48 ± 0.45 | 1.61 ± 0.46 | 1.56 ± 0.42 | 1.63 ± 0.43 | 0.168(2.036) | 0.096(3.024) | 0.312(1.071) |
| RR(bpm) | 34.30 ± 8.35 | 35.91 ± 8.94 | 38.57 ± 9.69 | 46.17 ± 12.18 | <0.001(37.950) | 0.005(9.753) | 0.009(8.082) |
| VE/VO2 | 29.04 ± 4.28 | 30.70 ± 6.02 | 31.35 ± 6.34 | 34.09 ± 8.38 | 0.001(15.329) | 0.002(12.486) | 0.207(1.690) |
| VE/VCO2 | 24.52 ± 2.33 | 24.65 ± 2.67 | 24.74 ± 3.12 | 25.83 ± 4.05 | 0.104(2.868) | 0.061(3.908) | 0.056(3.989) |
| PetO2(mmHg) | 103.21 ± 5.36 | 105.27 ± 4.88 | 105.60 ± 6.74 | 108.38 ± 6.32 | <0.001(34.827) | 0.010(7.938) | 0.347(0.925) |
| PetCO2(mmHg) | 46.74 ± 4.41 | 46.32 ± 5.96 | 45.93 ± 4.89 | 44.38 ± 6.50 | 0.007(9.009) | 0.241(1.450) | 0.066(3.738) |
Abbreviations: HR = heart rate; O2 pulse = oxygen pulse; PetCO2 = end-tidal partial pressures of CO2; PetO2 = end-tidal partial pressures of O2; RER = respiratory exchange ratio; RM-ANOVA = repeated measures analysis of variance; RPP = rate-pressure product; RR = respiratory rate; SBP = systolic blood pressure; VCO2 = carbon dioxide production; VE = minute ventilation; VO2 = oxygen consumption; VT = tidal volume.
Although there were no significant interaction effects, significant elevations were observed in the HR (p = 0.035), RER (p = 0.038), PetO2 (p = 0.010), and VE/VO2 (p = 0.002) in the yelling trial relative to those in the control trial (Table 3). This demonstrated that the linear trends of the 2 trials were parallel. The maximal exercise performance (including the peak power and time to exhaustion) in the yelling trial was significantly higher than that in the control trial; we suggest that this significant increase in the HR and RER was attributable to the workload difference between the 2 trials. Moreover, the PetO2 was higher in the yelling trial than in the control trial (p = 0.010) when the participants were exhausted. However, no statistical difference in the PetCO2 (p = 0.241) was observed between the trials. Similarly, the VE/VO2 was significantly higher in the yelling trial than in the control trial (p = 0.002), whereas no statistically significant difference in the VE/VCO2 (p = 0.061) was observed (Table 3).
Recovery of the participants after maximal exercise was observed during a 3 min cool-down. The RM-ANOVA results indicated no time-by-trial interaction effects or between-trial differences in the cardiac responses (including the O2 pulse and HR) and respiratory responses (VE, VT, RR, VO2, and VCO2) during the recovery period (data not shown). These results indicated that although yelling improved the exercise performance, it did not significantly affect the recovery status of cardiorespiratory functions.
4. Discussion
In this study, we determined that yelling during high-intensity exercise increases the power output and prolongs the time to exhaustion during incremental cycling tests. Yelling increases the peak VO2, stroke volume (estimated according to the O2 pulse), and ventilatory efficiency. However, the fatigue status (estimated according to iEMG/power) of the vastus lateralis muscle in the yelling trial did not significantly differ from that in the control trial. We speculate that yelling plays a role in preventing muscle fatigue from causing rapid degradation, thereby promoting exercise performance. Hug et al.10 reported that the EMG signal intensity of the vastus lateralis significantly increased as the exercise intensity increased after the anaerobic threshold for an incremental cycling test was passed. Hautier et al.11 observed that iEMG/force can be used as a representative parameter for determining the extent of muscle fatigue and that a higher value indicates greater muscle fatigue. In this study, we requested the participants to maintain a fixed pedalling rate (distance/time fixed) throughout the test, even as the resistance gradually increased; therefore, we were able to use iEMG/power as an indicator of muscle fatigue.
Yelling promotes maximal muscular power and exerts a significant effect on the intensity of cardiorespiratory responses. We demonstrated that abdominal yelling did not affect the HR or the RPP but increased the VO2 and O2 pulse response to maximal exercise. Therefore, we infer that the stroke volume plays a greater role than the HR and the RPP do in the contribution of yelling to elevating the peak VO2. During an incremental exercise test, when the exercise intensity increases by approximately 40%–50% of the VO2max, the stroke volume should plateau or increase only slightly in both moderately trained and untrained people. By contrast, the stroke volume in elite athletes does not plateau but increases continuously as the intensity of the exercise increases over the full range of the incremental exercise test.12 The results from the moderately trained students in our study are consistent with those of previous findings (Fig. 2, control trial). The unique finding of the present study is that the stroke volume can be further elevated by abdominal yelling during maximal exercise (Fig. 2, yelling trial); this is similar with a trend that has been observed in elite athletes in previous studies.12
Elite athletes appear to rely on enhancements in both ventricular filling and emptying to augment the stroke volume. Turkevich et al.13 hypothesised that ventricular filling is limited in nonelite athletes and attributed to progressively higher HR levels, potentially resulting in an increase in blunted stroke volume during exercise as a result of the Frank–Starling mechanism. In the present study, no significant time-by-trial interaction effect on the HR during 90%–100% of the time ranges analysed was evident. It appears that the yelling effect is not involved in ventricular filling. Another factor that could explain the increased stroke volume in elite athletes is the increased force of contraction at high intensities of exercise.14 We speculate that the stroke volume increased in our study because of the action of the abdominal muscles during yelling, which causes cyclical fluctuations in the intrathoracic pressure that rhythmically compress the heart. The aforementioned effect of abdominal yelling could reduce the difference between elite and nonelite athletes. The promotion of the O2 pulse by yelling was observed only in moderately trained people in the present study; further evidence is required to determine the effect of yelling on elite athletes.
Because nearly all inspiratory muscles performed at full capacity at the maximal exercise intensity, it is conceivable that the peak VT did not differ between the 2 trials and that there was no time-by-trial interaction. Yelling primarily promotes the efficiency of expiratory muscles, shortening the expiration time (increasing the RR) and thereby increasing the peak VE (Fig. 2D). At the maximal exercise intensity, because of extreme shortness of breath, dead-space ventilation increases,15 thus reducing the O2 exchange rate and CO2 removal efficiency and causing an increase in the PetO2 and a decrease in the PetCO2.16 In our study, the peak PetO2 was significantly higher in the yelling trial than in the control trial, but no significant difference in the peak PetCO2 was observed. This result indicates that yelling promotes the efficiency of expiratory muscles in stabilising the removal efficiency of CO2 (e.g., VE/VCO2) at a high exercise intensity and preventing the rapid deterioration of dead-space ventilation, thus enabling maximal exercise performance. This phenomenon corresponds to the increase in the peak VCO2 that is significantly higher than that in the VO2 observed in the yelling trial (e.g., the difference in the RER between the 2 trials, p = 0.038) (Table 3).
We summarised our findings to explain the physiologic mechanisms through which abdominal yelling promotes exercise performance. Persistent high-intensity exercise can cause metabolic accumulation that leads to muscle acidification. Relevant information is sent to the motor cortex by sensory nerves, stimulating the brain to generate the sensation of fatigue and increasing hyperventilation to promote the removal of CO2 and, thus, counter the effect of metabolic acidification.17, 18 To maintain exercise performance, α-motor neuron discharges increase as EMG signals increase to fight muscle fatigue caused by metabolic accumulation. Yelling can effectively increase the peak VE and efficiency of CO2 removal through the rhythmic contraction of expiratory muscles. However, cyclical fluctuations in intrathoracic pressure can induce respiratory–cardiac interactive effects that increase the stroke volume, thus causing the peak VO2 to increase. The aforementioned benefits facilitate alleviating the accumulation of anaerobic metabolites and slowing muscle fatigue. Therefore, muscular performance during exercise can be maintained or even increased without requiring the cerebral motor cortex to increase EMG signals substantially to activate fatigued muscle groups.
This study had several limitations: (1) The multiple effects of yelling on the human body occur on both physiologic and psychological levels. This study investigated the effects on cardiorespiratory physiologic parameters and EMG during high-intensity exercise; however, the effects of yelling on psychological and electroencephalographic parameters have yet to be studied. (2) This study evaluated the effects of yelling only on the performance of the lower limbs. Cerny and Ucer19 suggested that work performed by the upper extremities interferes with the recruitment of respiratory muscles, leading to maximal VE limitation. Consequently, the effects of yelling on the exercise performance of the lower extremities cannot be directly applied to the upper extremities, and further evidence is required. (3) This study investigated only the responses to yelling in moderately trained people during a maximal exercise test. Because elite athletes have a distinct O2 pulse response during maximal exercise tests,12 it is uncertain whether yelling effectively enhances the performance of their stroke volume. (4) We did not directly measure cardiac output, arteriovenous O2 differences, or stroke volume. Therefore, the behavior of the stroke volume was inferred using only the O2 pulse data.
5. Conclusion
This study revealed that abdominal yelling contributes substantially to the promotion of respiratory–cardiac interactive effects by increasing the peak stroke volume (represented by the O2 pulse) and VO2, thereby increasing exercise performance. Moreover, yelling during high-intensity exercise contributes considerably in increasing VCO2 by facilitating the expulsion of CO2. We suggest that yelling enables maintaining steady-state PetCO2 and VE/VCO2 levels during high-intensity exercise, preventing the increase of dead-space ventilation.
These findings can be used as a reference in developing a simple coping strategy for relieving exercise fatigue. Coaches should be aware that efficient breathing is a crucial contributor to performance in high-intensity exercise. In addition, amateur athletes can benefit from yelling as a means of breathing control.
Authors' contributions
CLC and JST conceived of and designed the experiment; CLC, NYY, and JST completed the acquisition, analysis, or interpretation of data for the work. CLC, NYY, JST, SHC, YRY, and LW drafted the work or revised it critically for important intellectual content. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
None of the authors declare competing financial interests.
Acknowledgment
This study was supported by a Grant-in-Aid for Scientific Research (ISU99-04-03) from I-Shou University, Kaohsiung, Taiwan, China. The authors wish to thank Dr. Te-Sheng Wen for his encouragement and guidance throughout this project.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Ikai M., Steinhaus A.H. Some factors modifying the expression of human strength. J Appl Physiol. 1961;16:157–163. doi: 10.1152/jappl.1961.16.1.157. [DOI] [PubMed] [Google Scholar]
- 2.Bunn J.C., Mead J. Control of ventilation during speech. J Appl Physiol. 1971;3:870–872. doi: 10.1152/jappl.1971.31.6.870. [DOI] [PubMed] [Google Scholar]
- 3.Binazzi B., Lanini B., Bianchi R., Romagnoli I., Nerini M., Gigliotti F. Breathing pattern and kinematics in normal subjects during speech, singing and loud whispering. Acta Physiol. 2006;186:233–246. doi: 10.1111/j.1748-1716.2006.01529.x. [DOI] [PubMed] [Google Scholar]
- 4.Aliverti A., Cala S.J., Duranti R., Ferrigno G., Kenyon C.M., Pedotti A. Human respiratory muscle actions and control during exercise. J Appl Physiol. 1997;83:1256–1269. doi: 10.1152/jappl.1997.83.4.1256. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey J.A., Amann M., Romer L.M., Miller J.D. Respiratory system determinants of peripheral fatigue and endurance performance. Med Sci Sports Exerc. 2008;40:457–461. doi: 10.1249/MSS.0b013e31815f8957. [DOI] [PubMed] [Google Scholar]
- 6.Pate R.R., Blair S.N., Durstine J.L., Eddy D.O., Hanson P., Painter P. In: Guidelines for exercise testing and prescription. 4th ed. American College of Sports Medicine, editor. Lea & Febiger; Philadelphia, PA: 1991. [Google Scholar]
- 7.Forman D.E., Myers J., Lavie C.J., Guazzi M., Celli B., Arena R. Cardiopulmonary exercise testing: relevant but underused. Postgrad Med. 2010;122:68–86. doi: 10.3810/pgm.2010.11.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasserman K., Hansen J.E., Sue D.Y., Casaburi R., Whipp B.J. 3rd ed. Lippincott William & Wilkins; Philadelphia, PA: 1999. Principles of exercise testing and interpretation; pp. 2–192. [Google Scholar]
- 9.Oliveira R.B., Myers J., Araujo C.G. Long-term stability of the oxygen pulse curve during maximal exercise. Clinics. 2011;66:203–209. doi: 10.1590/S1807-59322011000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hug F., Faucher M., Kipson N., Jammes Y. EMG signs of neuromuscular fatigue related to the ventilatory threshold during cycling exercise. Clin Physiol Funct Imaging. 2003;23:208–214. doi: 10.1046/j.1475-097x.2003.00497.x. [DOI] [PubMed] [Google Scholar]
- 11.Hautier C.A., Arsac L.M., Deghdegh K., Souquet J., Belli A., Lacour J.R. Influence of fatigue on EMG/force ratio and cocontraction in cycling. Med Sci Sports Exerc. 2000;32:839–843. doi: 10.1097/00005768-200004000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Zhou B., Conlee R.K., Jensen R., Fellingham G.W., George J.D., Fisher A.G. Stroke volume does not plateau during graded exercise in elite male distance runners. Med Sci Sports Exerc. 2001;33:1849–1854. doi: 10.1097/00005768-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Turkevich D., Micco A., Reeves J.T. Noninvasive measurement of the decrease in left ventricular filling time during maximal exercise in normal subjects. Am J Cardiol. 1988;62:650–652. doi: 10.1016/0002-9149(88)90676-5. [DOI] [PubMed] [Google Scholar]
- 14.Urhausen A., Holpes R., Kindermann W. One- and two-dimensional echocardiography in body builders and endurance-trained subjects. Int J Sports Med. 1989;10:139–144. doi: 10.1055/s-2007-1024891. [DOI] [PubMed] [Google Scholar]
- 15.Sun X.G., Hansen J.E., Garatachea N., Storer T.W., Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med. 2002;166:1443–1448. doi: 10.1164/rccm.2202033. [DOI] [PubMed] [Google Scholar]
- 16.Wasserman K., Whipp B.J., Koyl S.N., Beaver W.L. Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol. 1973;35:236–243. doi: 10.1152/jappl.1973.35.2.236. [DOI] [PubMed] [Google Scholar]
- 17.Woods J.J., Furbush F., Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol. 1987;58:125–137. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]
- 18.McCloskey D.I., Mitchell J.H. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerny F.J., Ucer C. Arm work interferes with normal ventilation. Appl Ergon. 2004;35:411–415. doi: 10.1016/j.apergo.2004.05.001. [DOI] [PubMed] [Google Scholar]