Abstract
Purpose
The aim of this study was to investigate the effects of fluid replacement by water or sports drinks on serum heat shock protein 70 (HSP70) levels and DNA damage during exercise at a high ambient temperature.
Methods
Ten male college athletes with an athletic career ranging from 6 to 11 years were recruited from Yonsei University. The subjects ran on a treadmill at 75% of heart rate reserve during 4 different trials: thermoneutral temperature at 18°C (T), high ambient temperature at 32°C without fluid replacement (H), high ambient temperature at 32°C with water replacement (HW), and high ambient temperature at 32°C with sports drink replacement (HS). During each condition, blood samples were collected at the pre-exercise baseline (PEB), immediately after exercise (IAE), and 60 min post-exercise.
Results
Skin temperature significantly increased during exercise and was significantly higher in H compared to T and HS at IAE. Meanwhile, serum HSP70 was significantly increased in all conditions at IAE compared to PEB and was higher in H compared to T at the former time point. Significantly increased lymphocyte DNA damage (DNA in the tail, tail length, tail moment) was observed in all trials at IAE compared to PEB, and attenuated DNA damage (tail moment) was observed in HS compared to H at IAE.
Conclusion
Acute exercise elevates serum HSP70 and induces lymphocyte DNA damage. Fluid replacement by sports drink during exercise at high ambient temperature can attenuate HSP response and DNA damage by preventing dehydration and reducing thermal stress.
Keywords: Acute exercise, DNA damage, Fluid replacement, Heat stress, HSP70, Sports drink
1. Introduction
Increased external temperature can exert an influence on cardiovascular, hormonal, and immune responses, and can also negatively affect performance during endurance exercise.1 Hyperthermia and dehydration are also considered major limiting factors of exercise performance in the heat.2 Previous studies have suggested that hyperthermia and dehydration, together with oxidative stress, are associated with heat-related exercise response,1, 3 and that the production of free radicals resulting from exercise and/or heat stress is the cause of leukocyte DNA damage after exercise.4, 5 DNA damage due to severe exercise stress in turn can lead to apoptosis and could partly account for exercise-related lymphopenia.6
The induction of heat shock proteins (HSPs) may function in leukocytes as a protective mechanism against exercise, heat, and oxidative stress.1 Fehrenbach et al.1 suggested that a cause-and-effect relation was present between exercise-induced oxidative stress and HSP response. The protective functions of HSPs include the protection of cellular homeostasis and immune function through chaperoning protein assembly, degradation, translocation, and stabilization.7 HSPs can be classified according to molecular weight (kDa) into the categories of 10 kDa, 20–30 kDa, 40 kDa, 60 kDa, 70 kDa, 90 kDa, and 100 kDa proteins.8 Of these, HSP70 showed the greatest variation in expression in response to exercise stimuli.8 In addition, HSP70 has been found to play a crucial role in thermotolerance9 and to be induced rapidly by physiological stress factors such as pH changes in the body or glycogen depletion.10 Furthermore, HSP70 expression in response to stress has been shown to play an important protective role against apoptosis11 and in the regulation of apoptotic cell signaling.12 Nuclear translocation of HSP72, a member of the HSP70 kDa family, was also reported to suppress the occurrence of apoptosis in DNA-damaged cells.13
On the other hand, fluid replacement during exercise in the heat could attenuate the rise in body temperature.14 Additionally, a previous study by Hillman et al.3 suggested that exercise-induced dehydration could increase oxidative stress but that fluid replacement could decrease thermal and oxidative stress during prolonged exercise in the heat. Sports drinks have also been reported to be more effective than water in fluid replacement for attenuating the increase in oxidative stress caused by dehydration.15 However, to date, no in vivo study has assessed the heat shock response and DNA damage in leukocytes in a comparison between different fluid replacements.
Therefore, the present study aimed to investigate the effects of fluid replacement by water or sports drinks on serum HSP70 and lymphocyte DNA damage during exercise at a high ambient temperature among college athletes.
2. Methods
2.1. Subjects
Ten male college athletes (soccer, n = 5; rugby, n = 5) with athletic careers ranging from 6 to 11 years were recruited from Yonsei University. Subjects were non-smoking, non-drinking, and injury- and disease-free as determined by a health history questionnaire and physical examination. The baseline physical characteristics of the subjects are shown in Table 1. All subjects provided informed consent, and the study protocol was approved by the Institutional Ethics Review Board of the Department of Physical Education at Yonsei University.
Table 1.
Physical characteristics of the subjects at baseline (n = 10, mean ± SD).
| Variable | Value |
|---|---|
| Age (year) | 18.8 ± 0.8 |
| Height (cm) | 175.6 ± 7.0 |
| Weight (kg) | 70.4 ± 6.7 |
| Body fat (%) | 12.1 ± 2.4 |
| LBM (kg) | 62.0 ± 5.9 |
| VO2max (mL/kg/min) | 56.6 ± 5.3 |
| RBC (×106 µL) | 5.2 ± 0.3 |
| WBC (×103 µL) | 5.9 ± 2.1 |
| Platelets (×103 µL) | 209.7 ± 20.4 |
| Hgb (g/dL) | 15.7 ± 0.8 |
| Hct (%) | 50.1 ± 3.0 |
| Neutrophil (%) | 56.1 ± 9.8 |
| Lymphocyte (%) | 33.2 ± 9.1 |
| Monocyte (%) | 8.0 ± 1.7 |
| Glucose (mg/dL) | 88.8 ± 6.2 |
Abbreviations: Hct = hematocrit; Hgb = hemoglobin; LBM = lean body mass; RBC = red blood cell; VO2max = maximal oxygen consumption; WBC = white blood cell.
2.2. Preliminary tests
Preliminary tests included measurements of height, body composition (body weight, fat mass, and fat free mass), maximal exercising capacities (maximal oxygen consumption (VO2max), maximal heart rate (HRmax)) and hematologic parameters. Height was measured with a stadiometer (HD; STDK, Tokyo, Japan), and body composition was measured through bio-impedance body composition analysis with Inbody220 (Biospace, Seoul, Korea). The VO2max measurement was performed on a treadmill (Q65; Quinton, Seattle, WA, USA) according to the Bruce protocol, in which an increase of 2% in the incline and 0.8 mph in the speed occurs every 3 min from an initial incline level of 10% and a speed of 1.7 mph.16 Gas analyses were performed with MetaMax 3B (Cortex, Leipzig, Germany) every 10 s to determine the volume of single ventilation, oxygen consumption, carbon dioxide emission, respiratory exchange ratio, and breathing rate per minute. HRmax was measured during the VO2max measurement with Polar a5 (Polar, Kempele, Finland). The following hematologic parameters were accessed using an automatic hematology analyzer (Sysmex XE-2100; Sysmex, Kobe, Japan): red blood cell count, white blood cell count, platelet count, hemoglobin concentration, hematocrit value, and neutrophil, lymphocyte, and monocyte percentages. Serum glucose concentration was determined by a hexokinase method using a commercially available glucose/hexokinase assay kit (Pointe Scientific, Canton, MI, USA). The absorbance was measured at 340 nm with an automatic analyzer (Hitachi, Tokyo, Japan).
2.3. General experimental design
The exercise intensity for the trials was set at 75% of heart rate reserve for each subject based on data obtained from screening tests. The subjects ran under 4 different conditions: a thermoneutral temperature at 18°C (T), high ambient temperature at 32°C without fluid replacement (H), high ambient temperature at 32°C with water replacement (HW), and high ambient temperature at 32°C with sports drink replacement (HS). The exercise intensity and humidity (50%) were identical in all trials. The trials were performed in the order of trial T, H, HW, and HS, and were each separated by 7 days to avoid any transient effects on the physiological and psychological conditions of the subjects.
The experimental condition of 18°C and 50% relative humidity is considered to be thermoneutral for physical activity and to be a comfort zone, since a low heat stress index has been reported for this condition.17 In contrast, the condition at 32°C and 50% relative humidity is considered to be adverse because it frequently results in heat cramps. Based on the wet-bulb globe temperature index, sports events conducted under this condition are subject to cancellation.17, 18, 19 Both experimental conditions (a thermoneutral environment and a high ambient temperature) were set up using an E series walk-in temperature and humidity chamber (ESPEC, Osaka, Japan).
Skin temperature was calculated using the equation introduced by Watson et al.20 based on the data collected from the chest, forearm, thigh, and calf with MSR12 (MSR Electronics GmbH, Henggart, Switzerland). The measurement timings were 8 times during rest, every 10 min during the exercise trial, immediately after the exercise trial, and 60 min post-exercise. Dehydration levels were calculated by the difference in the body weight between pre-exercise and immediately after the trial in the H group. An amount of plain water (Evian, Danone, France) or sports drink (Gatorade; Pepsi Co., Inc., Barrington, IL, USA) equal to the body weight loss after trial H was given to the subjects for fluid replacement, with multiple separations during exercise. Plain water and sports drink were prepared at 15°C, the temperature suggested providing the best absorption rate and sensation of refreshment.21 In terms of nutritional information, the sports drink used in the current study contained 6% carbohydrate, 20.9 mEq/L Na+, 6.1 mEq/L K+, and 9.5 mEq/L Cl−.
2.4. Blood sampling and analyses
Blood samples were collected at the pre-exercise baseline (PEB), immediately after the exercise (IAE), and 60 min post-exercise (60MPE) from the antecubital vein. For each of the blood sampling time points, serum pH levels, plasma lactate, HSP70, and lymphocyte DNA damage were determined.
2.4.1. Serum pH and plasma lactate analyses
Serum pH was detected with a Corning pH/Ion analyzer model 355 (Corning Inc., Corning, NY, USA). For analyzing the plasma concentration of lactate, potassium oxalate/sodium fluoride was added to the blood samples. The blood samples were subsequently centrifuged at 3000 rpm for 20 min, and the plasma was extracted for analysis. Ten microliters of the collected plasma samples was pipetted on an LAC DT slide using a DT pipette (Eastman Kodak, Rochester, NY, USA) with a microtip (Ortho-Clinical Diagnostics, Rochester, NY, USA). The absorbance was measured at 555 nm for detecting lactate using an Ektachem DT 60 clinical chemistry analyzer (Eastman Kodak).
2.4.2. Serum HSP70 analysis
The serum HSP70 concentrations were determined by an enzyme-linked immunosorbent assay (EIA) using a commercially available HSP70 high-sensitivity EIA kit (Assay Designs, Ann Arbor, MI, USA). The absorbance was measured at 450 nm with a microplate reader (Molecular Device, Palo Alto, CA, USA).
2.4.3. Lymphocyte DNA damage analyses
DNA damage was analyzed as described by Singh et al.22 and Green et al.23 For the Comet assay, whole blood was mixed with phosphate-buffered saline and poured gently over a peripheral blood lymphocyte separation solution (Histopaque-1077; Sigma Chemical Co., St. Louis, MO, USA). After centrifugation at 1450 rpm for 25 min, peripheral blood lymphocytes were transferred into another tube, mixed with 0.7% low-melting agarose, and added to slides precoated with 0.5% agarose. The cells were then lysed for 1 h at 4°C in a lysis buffer containing 2.5 mol/L NaCl, 100 mmol/L EDTA, 1% Triton X-100, 10% DMSO, and 10 mmol/L Tris, pH 10. After the lysis, the DNA was allowed to unwind for 40 min in an electrophoretic solution containing 300 mmol/L NaOH and 1 mmol/L EDTA (pH > 13). Electrophoresis was carried out at 30 V for 30 min. The slides were then neutralized with 0.4 mol/L Tris, pH 7.5, and fixed with ethanol. Finally, the DNA was stained by adding 60 µL of ethidium bromide (20 µg/mL) to each slide. Measurements were made using image analysis software (Komet 5.0; Kinetic Imaging, Liverpool, UK) and a fluorescence microscope (Leica, Wetzlar, Germany) equipped with an excitation filter of 515–560 nm and a barrier filter of 590 nm. Images from 50 cells were analyzed for each slide, and the parameters recorded were DNA in the tail (%), tail length (µm), and tail moment (% of DNA in tail × tail length). All steps were performed under dimmed light, and the electrophoresis tank was covered with black paper to avoid additional light-induced DNA damage.
2.5. Statistical analyses
Statistical analyses were performed with SPSS Version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± SD unless otherwise stated. For identifying differences in normally distributed results, two-way repeated analysis of variance (ANOVA) was employed. When a significant interaction was apparent, the simple main effects on measured variables were determined using one-way ANOVA. Tukey's post hoc test was subsequently used to conservatively locate significant differences. Statistical significance was set at p < 0.05.
3. Results
3.1. Skin temperature
In trial T, the skin temperature was significantly increased from Ex.20 min (20 min of exercise) to IAE relative to that at Ex.10 min (p < 0.05; Fig. 1). Additionally, trials H, HW, and HS showed significantly increased temperatures from Ex.10 min to IAE in comparison to PEB (p < 0.05). Further, the skin temperature was significantly higher from Ex.10 min to IAE in trials H, HW, and HS as compared to that in trial T (p < 0.05). At IAE, the skin temperature was significantly higher in trial H compared to that of trials T and HS (p < 0.05). However, there was no significant between trials H and HW (p > 0.05).
Fig. 1.
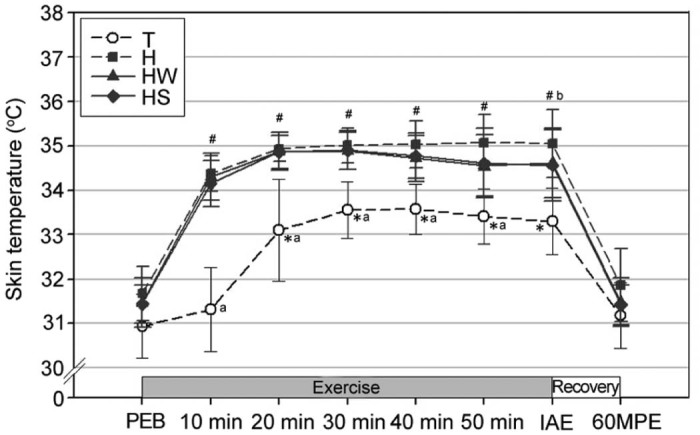
Changes in skin temperature (mean ± SD). *Significantly different from the skin temperature at 10 min of exercise in T (p < 0.05). #Significantly different from the skin temperature at PEB in H, HW, and HS (p < 0.05). aSignificantly lower in T than H, HW, and HS (p < 0.05). bSignificantly higher in H than T and HS (p < 0.05). H = exercise at high ambient temperature; HS = exercise at high ambient temperature with fluid replacement by sports drink; HW = exercise at high ambient temperature with fluid replacement by water; IAE = immediately after exercise; PEB = pre-exercise baseline; T = exercise in a thermoneutral environment; 60MPE = 60 min post-exercise.
3.2. Serum pH level and plasma lactate
The pH and plasma lactate levels were significantly decreased and increased, respectively, at IAE relative to those at PEB in all trials (p < 0.05; Fig. 2). Further, the pH levels were significantly lower at IAE in trial H as compared to trials T and HS (p < 0.05); however, there was no significant difference between trials H and HW (p > 0.05), while no significant differences in plasma lactate were observed between the trials at any of the blood sampling points.
Fig. 2.
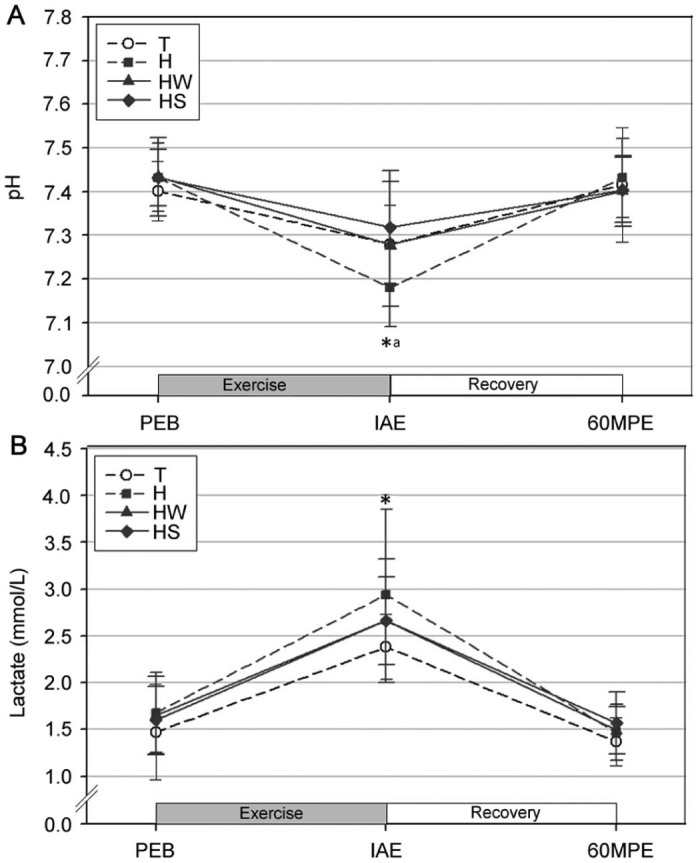
Changes in the serum pH level (A) and plasma lactate (B) (mean ± SD). *Significantly different from PEB in all trials (p < 0.05). aSignificantly lower in H than T and HS (p < 0.05). H = exercise at high ambient temperature; HS = exercise at high ambient temperature with fluid replacement by sports drink; HW = exercise at high ambient temperature with fluid replacement by water; IAE = immediately after exercise; PEB = pre-exercise baseline; T = exercise in a thermoneutral environment; 60MPE = 60 min post-exercise.
3.3. Serum HSP70
The serum HSP70 levels were significantly increased at IAE as compared to PEB in all trials (p < 0.05; Fig. 3). In addition, the serum HSP70 at IAE was significantly higher in trial H as compared to trial HS (p < 0.05).
Fig. 3.
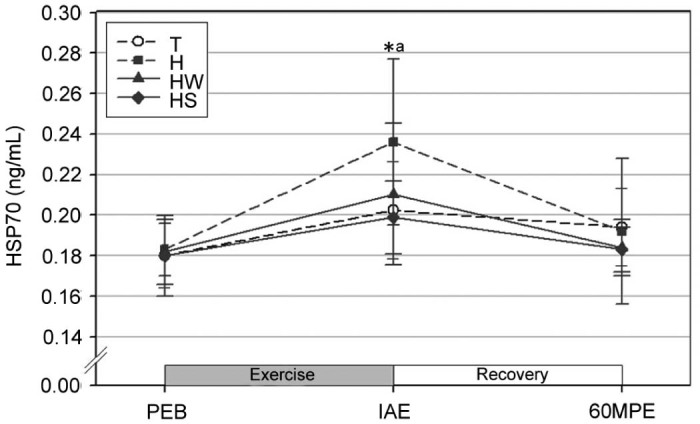
Changes in serum HSP70 (mean ± SD). *Significantly different from PEB in all trials (p < 0.05). aSignificantly higher in H than HS (p < 0.05). H = exercise at high ambient temperature; HS = exercise at high ambient temperature with fluid replacement by sports drink; HW = exercise at high ambient temperature with fluid replacement by water; IAE = immediately after exercise; PEB = pre-exercise baseline; T = exercise in a thermoneutral environment; 60MPE = 60 min post-exercise.
3.4. Lymphocyte DNA damage
Significantly increased DNA in the tail (Fig. 4A), tail length (Fig. 4B), and tail moment (Fig. 4C) relative to the levels at rest were observed at IAE for all trials (p < 0.05). Further, the tail moment was significantly higher in trial H at IAE as compared to trials T and HS (p < 0.05); there was no significant difference between trials H and HW (p > 0.05). However, no significant differences in DNA in the tail and tail length were observed between the trials.
Fig. 4.
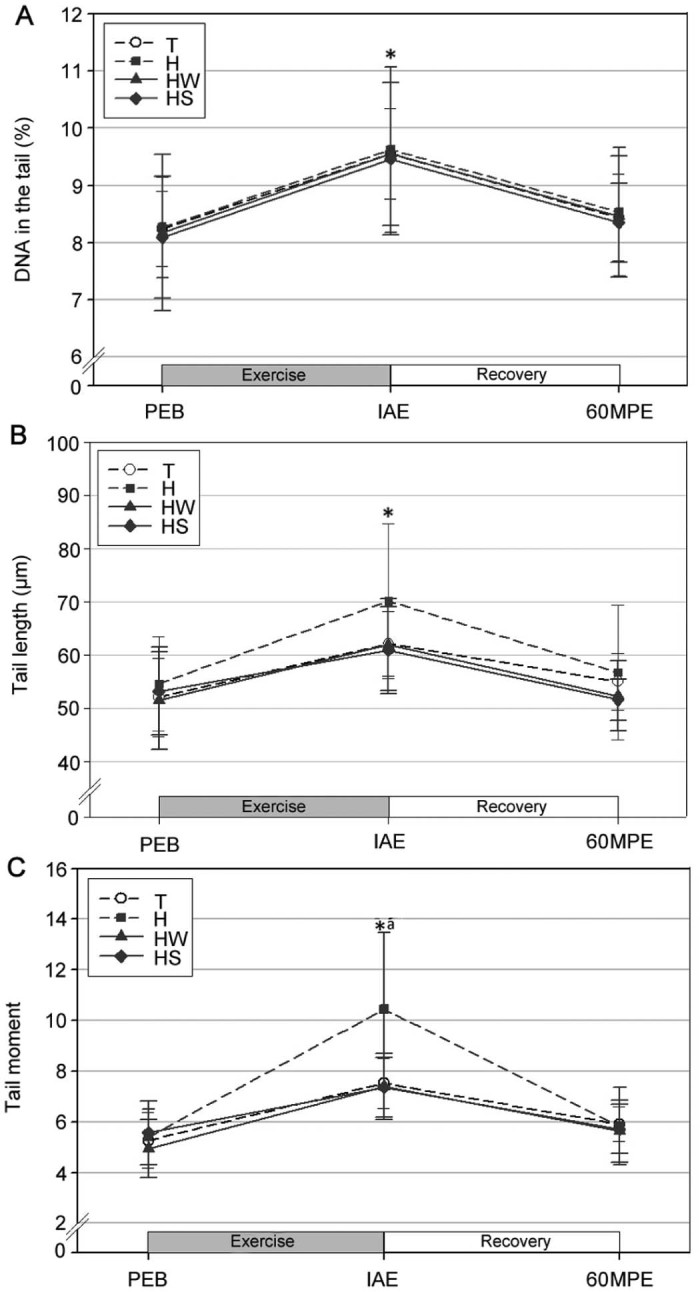
Changes in DNA in the tail (A), tail length (B), and tail moment (C) (mean ± SD). *Significantly different from PEB in all trials (p < 0.05). aSignificantly higher in H than T and HS (p < 0.05). H = exercise at high ambient temperature; HS = exercise at high ambient temperature with fluid replacement by sports drink; HW = exercise at high ambient temperature with fluid replacement by water; IAE = immediately after exercise; PEB = pre-exercise baseline; T = exercise in a thermoneutral environment; 60MPE = 60 min post-exercise.
4. Discussion
4.1. Skin temperature
In the present study, the skin temperature was significantly increased after exercise, with trials H, HW, and HS showing significantly higher values than trial T. These results are consistent with previous studies reporting that exercise at high ambient temperature and humidity as compared to a thermoneutral condition results in impaired heat-control capacity, thereby leading to higher body temperature and increased body fluid loss.24, 25 We assume that the high ambient temperature condition (32°C, 50%) set for the current study impaired heat control capacity and thus lead to impaired homeostasis of body temperature. In addition, the skin temperature appeared to be significantly lower immediately after exercise in trial HS compared to trial H. These findings support the results of a previous study which showed that a rise in body temperature caused by prolonged exercise could be attenuated more effectively by sports drinks containing carbohydrates and electrolytes than by water, at the time of fluid replacement during exercise.26
Glucose administered with fluid during exercise was previously reported to spare liver glycogen storage and to reduce the rate of gluconeogenesis, resulting in ~6% reduction in heat production within the liver.27, 28 Moreover, the study of Shi and Gisolfi29 showed that the combination of increased osmotic pressure and unbalanced sodium levels results in a rise in body temperature, and that a drink containing glucose and electrolytes (e.g., sodium) can attenuate an increase in osmotic pressure and maintain body fluid volume by balancing electrolyte levels. Based on these previous findings, the relatively attenuated rise in body temperatures in trial HS as compared to the other trials in the current study can be attributed to the consumption of glucose and electrolytes contained in the sports drink.
4.2. Serum pH and plasma lactate
Generally accepted physiological variables that impair exercising capacity include pH and lactate levels.30, 31 Lactate does not directly impair physiological functions; however, the increased hydrogen ion (H+) levels that occur with lactate accumulation contribute to acidification, which can disrupt enzymatic activities associated with ATP production. Hence, the increased acidification can eventually induce fatigue and impair a number of physiological functions.30, 32
In the current study, the pH was significantly decreased at IAE relative to PEB in all trials but was restored at 60MPE. The decreased pH induced by exercise eventually results from an accumulation of H+ due to accelerated glycolysis. This decreased pH impairs enzymatic activities and cross-bridge formation during muscle contraction, resulting in the early onset of fatigue. At IAE, the pH in trial H was significantly lower than that in trials T and HS. Abbiss et al.33 showed a linear inverse coefficient (r = −0.69) between core temperature and pH levels based on experiments involving 3 different conditions: hot (34°C), neutral (22°C), and cold (10°C). Consistent with this previous report, the current study showed that the lowest pH level after exercise occurred under the condition that induced the highest rise in body temperature.
An inefficient oxygen supply to the working muscles is well known to limit oxidative energy production and to increase the dependency on glycolysis, a shift that can result in high lactate levels.34, 35 In the present study, the plasma lactate levels were significantly increased at IAE in all trials relative to PEB and had decreased to baseline levels at 60MPE. Although no significant difference between the trials was observed at IAE, plasma lactate levels were highest to lowest in the order of trials H, HW, HS, and T. These results are consistent with the previous study of Hargreaves et al.36 which reported that during submaximal exercise, a non-hydration group had higher glycogen usage for energy production compared to a hydration group. Furthermore, increased blood viscosity due to decreased blood plasma results in higher cardiac stress, leading to the impaired clearance of metabolic by-products, including lactate. In the current study, trials HW and HS had relatively lower plasma lactate than trial H, and thus support the findings of Ali et al.37 In this previous study, fluid replacement during high-intensity exercise prevented excessive plasma loss, attenuated cardiac stress, and prevented a substantial decrease in oxygen supply to working muscles, which resulted in a lower production of plasma lactate. Given that lactate production is known to increase with every rise in body temperature,37, 38 fluid replacement during exercise is assumed to contribute to lower plasma lactate and delay fatigue through attenuating an elevation in body temperature.
Previous studies have reported that the administration of fast-absorptive drink containing electrolytes restores oxygen supply to the working muscles by increasing cardiac output and subsequently the peripheral blood supply, as well as delaying acidification.39, 40 Therefore, these previous studies and the current one support that administering sports drink during dehydrating conditions is more effective in attenuating acidification and delaying the onset of fatigue than administering water.
4.3. Serum HSP70
In the present study, serum HSP70 appeared to be significantly higher at IAE in all trials compared to PEB, consistent with the findings of previous studies that acute exercise increases serum HSP70.41, 42 According to Puntschart et al.,42 30 min of treadmill running significantly increased serum HSP70 within skeletal muscle cells. Meanwhile, Suzuki et al.41 reported significantly increased serum HSP70 after a triathlon race. Of interest in the current study, the serum HSP70 in trial HS appeared to be significantly lower than that in trial H. According to Sandström et al.,43 monocyte serum HSP70 is positively associated with body temperature with a moderate coefficient (r = 0.44). Moreover, Lovell et al.44 reported higher serum HSP70 within peripheral blood mononuclear cells when heat shock was administered and suggested that serum HSP70 is temperature dependent. Given these reports, the lower serum HSP70 at IAE in trial HS as compared to trial H of the current study is likely attributable to the relatively lower rise in body temperature. Other studies support this discussion that HSP70 expression is induced during increasing oxidative stress.9 An increase in oxidative stress by exercise-induced dehydration was determined to be independent of environmental conditions (e.g., atmospheric temperature) and was able to be attenuated through the maintenance of euhydration by fluid replacement during exercise.4 A previous study has reported that after excessive dehydration (3% body weight), the intake of sports drinks rather than water was more effective for fluid replacement in the attenuation of oxidative stress.15 In addition, serum HSP70 was reported to be influenced not only by thermal stress but also by pH45 and glucose levels.46 Taking these results together, the lower serum HSP70 in trial HS as compared to trial H can be attributed to rehydration attenuating a rise in body temperature and oxidative stress and to electrolytes and glucose in the sports drink possibly delaying the acidification of working muscles and increasing the efficiency of energy utilization.
4.4. Lymphocyte DNA damage
All 3 parameters for lymphocyte DNA damage (DNA in the tail, tail length, and tail moment) were significantly increased at IAE compared to PEB in all trials. Based on previous reports, the lymphocyte DNA damage likely resulted from the increased production of reactive oxygen species (ROS), particularly by the superoxide radicals generated by the high oxygen supply during high-intensity exercise.47, 48 Previous studies have reported that acute exercise increases the parameters for oxidative stress.49, 50, 51 Moreover, some studies have reported that increased DNA damage, as assessed by the Comet assay, follows acute exercise,15, 52, 53 supporting the results of the current study. In particular, Paik et al.15 reported increased DNA in the tail, DNA tail length, and DNA tail moment in lymphocytes at the completion of high-intensity treadmill running at 80% VO2max until exhaustion. Meanwhile, Mastaloudis et al.53 reported a significantly increased DNA in the tail of leukocytes during a 50 km ultra-marathon for 7 h. In addition, Hartmann et al.52 reported a significantly increased tail moment at the completion of a triathlon.
In the current study, the tail moment in trial H was significantly higher than that in trials T and HS at IAE. Pereira Ede et al.54 suggested that dehydration is the most deleterious factor for oxidative stress based on their study results, which showed a 10-fold increase in oxidative stress after dehydration in yeast cells. Zuo et al.55 showed higher ROS production in the diaphragm of rats under heat stress conditions (42°C) as compared to the control condition (37°C). In this previous study, a greater elevation in the core temperature was observed under the heat stress condition, suggesting a potential association between core temperature and ROS production. Given the findings of these previous reports, the elevated body temperatures and the subsequent dehydration possibly contributed to the increased lymphocyte DNA damage in the present study. By contrast, the fluid replacement by water or sports drinks may have attenuated lymphocyte DNA damage by reducing the 2 stressors of dehydration and exercise. Sports drinks were reported to be 4-fold more efficient in preserving plasma volume as compared to water, since sports drinks control osmotic pressure by replenishing electrolyte loss and enhancing the re-absorption of electrolytes by supplying glucose. Glucose functions by acting as a co-carrier for water and sodium in the proximal tubule of the glomerulus.56, 57 Therefore, in the current study, attenuated DNA damage with fluid replacement by sports drinks during exercise in high ambient temperature is thought to be the result of a reduction in ROS production through the prevention of hypoxia and elevated body temperatures during exercise.
5. Conclusion
These results suggest that (a) acute exercise elevates serum HSP70 and induces lymphocyte DNA damage; and (b) fluid replacement by sports drinks during exercise at high ambient temperature can attenuate HSP response and DNA damage by preventing dehydration and reducing thermal stress.
Authors' contributions
HTR and SYC participated in study design, subject recruitment, data collection, data processing, data analysis, and drafted the manuscript; WYS participated in data analysis, and drafted the manuscript; IYP and SHS conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
None of the authors declare competing financial interests.
Acknowledgment
This work was supported by the Dong-A University research fund. This article is a partly condensed form of the first author's doctoral thesis (Dr. Hee-Tae Roh) from Yonsei University.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Contributor Information
Il-Young Paik, Email: ppaik@yonsei.ac.kr.
Sang-Hoon Suh, Email: ssh@yonsei.ac.kr.
References
- 1.Fehrenbach E., Veith R., Schmid M., Dickhuth H.H., Northoff H., Niess A.M. Inverse response of leukocyte heat shock proteins and DNA damage to exercise and heat. Free Radic Res. 2003;37:975–982. doi: 10.1080/10715760310001595748. [DOI] [PubMed] [Google Scholar]
- 2.Cheuvront S.N., Kenefick R.W., Montain S.J., Sawka M.N. Mechanisms of aerobic performance impairment with heat stress and dehydration. J Appl Physiol. 2010;109:1989–1995. doi: 10.1152/japplphysiol.00367.2010. [DOI] [PubMed] [Google Scholar]
- 3.Hillman A.R., Vince R.V., Taylor L., McNaughton L., Mitchell N., Siegler J. Exercise-induced dehydration with and without environmental heat stress results in increased oxidative stress. Appl Physiol Nutr Metab. 2011;36:698–706. doi: 10.1139/h11-080. [DOI] [PubMed] [Google Scholar]
- 4.Niess A.M., Baumann M., Roecker K., Horstmann T., Mayer F., Dickhuth H.H. Effects of intensive endurance exercise on DNA damage in leukocytes. J Sports Med Phys Fitness. 1997;38:111–115. [PubMed] [Google Scholar]
- 5.Niess A.M., Dickhuth H.H., Northoff H., Fehrenbach E. Free radicals and oxidative stress in exercise-immunological aspects. Exerc Immunol Rev. 1999;5:22–56. [PubMed] [Google Scholar]
- 6.Mars M., Govender S., Weston A., Naicker V., Chuturgoon A. High intensity exercise: a cause of lymphocyte apoptosis? Biochem Biophys Res Commun. 1998;249:366–370. doi: 10.1006/bbrc.1998.9156. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto R.I., Tissieres A., Georgopoulos C. Progress and perspectives on the biology of heat shock proteins and molecular chaperones. In: Morimoto R.I., Tissieres A., Georgopoulos C., editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press; New York, NY: 1994. pp. 1–30. [Google Scholar]
- 8.Noble E.G., Milne K.J., Melling C.W. Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab. 2008;33:1050–1065. doi: 10.1139/H08-069. [DOI] [PubMed] [Google Scholar]
- 9.Piper P. Induction of heat shock proteins and thermotolerance. Methods Mol Biol. 1996;53:313–317. doi: 10.1385/0-89603-319-8:313. [DOI] [PubMed] [Google Scholar]
- 10.Morton J.P., Holloway K., Woods P., Cable N.T., Burniston J., Evans L. Exercise training-induced gender-specific heat shock protein adaptations in human skeletal muscle. Muscle Nerve. 2009;39:230–233. doi: 10.1002/mus.21182. [DOI] [PubMed] [Google Scholar]
- 11.Siu P.M., Bryner R.W., Martyn J.K., Alway S.E. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J. 2004;18:1150–1152. doi: 10.1096/fj.03-1291fje. [DOI] [PubMed] [Google Scholar]
- 12.Creagh E.M., Sheehan D., Cotter T.G. Heat shock proteins—modulators of apoptosis in tumour cells. Leukemia. 2000;14:1161–1173. doi: 10.1038/sj.leu.2401841. [DOI] [PubMed] [Google Scholar]
- 13.Kato K., Yamanaka K., Hasegawa A., Okada S. Dimethylarsinic acid exposure causes accumulation of Hsp72 in cell nuclei and suppresses apoptosis in human alveolar cultured (L-132) cells. Biol Pharm Bull. 1999;22:1185–1188. doi: 10.1248/bpb.22.1185. [DOI] [PubMed] [Google Scholar]
- 14.Horswill C.A. Effective fluid replacement. Int J Sport Nutr. 1998;8:175–195. doi: 10.1123/ijsn.8.2.175. [DOI] [PubMed] [Google Scholar]
- 15.Paik I.Y., Jeong M.H., Jin H.E., Kim Y.I., Suh A.R., Cho S.Y. Fluid replacement following dehydration reduces oxidative stress during recovery. Biochem Biophys Res Commun. 2009;383:103–107. doi: 10.1016/j.bbrc.2009.03.135. [DOI] [PubMed] [Google Scholar]
- 16.Bruce R.A., Blackmon J.R., Jones J.W., Strait G. Exercising testing in adult normal subjects and cardiac patients. Pediatrics. 1963;32:742–756. [PubMed] [Google Scholar]
- 17.McArdle W.D., Katch F.I., Katch V.L. 3rd ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. Essentials of exercise physiology. [Google Scholar]
- 18.American College of Sports Medicine Prevention of thermal injuries during distance running. Med J Aust. 1984;141:876–879. [PubMed] [Google Scholar]
- 19.American College of Sports Medicine. Armstrong L.E., Casa D.J., Millard-Stafford M., Moran D.S., Pyne S.W. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39:556–572. doi: 10.1249/MSS.0b013e31802fa199. [DOI] [PubMed] [Google Scholar]
- 20.Watson P., Shirreffs S.M., Maughan R.J. Blood-brain barrier integrity may be threatened by exercise in a warm environment. Am J Physiol Regul Integr Comp Physiol. 2005;288:1689–1694. doi: 10.1152/ajpregu.00676.2004. [DOI] [PubMed] [Google Scholar]
- 21.American College of Sports Medicine. Sawka M.N., Burke L.M., Eichner E.R., Maughan R.J., Montain S.J. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007;39:377–390. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 22.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 23.Green M.H., Lowe J.E., Harcourt S.A., Akinluyi P., Rowe T., Cole J. UV-C sensitivity of unstimulated and stimulated human lymphocytes from normal and xeroderma pigmentosum donors in the comet assay: a potential diagnostic technique. Mutat Res. 1992;273:137–144. doi: 10.1016/0921-8777(92)90075-e. [DOI] [PubMed] [Google Scholar]
- 24.Powers S.K., Howley E.T., Cox R. Blood lactate concentrations during submaximal work under differing environmental conditions. J Sports Med Phys Fitness. 1985;25:84–89. [PubMed] [Google Scholar]
- 25.Rasch W., Samson P., Cote J., Cabanac M. Heat loss from the human head during exercise. J Appl Physiol. 1991;71:590–595. doi: 10.1152/jappl.1991.71.2.590. [DOI] [PubMed] [Google Scholar]
- 26.Bergeron M.F., Waller J.L., Marinik E.L. Voluntary fluid intake and core temperature responses in adolescent tennis players: sports beverage versus water. Br J Sports Med. 2006;40:406–410. doi: 10.1136/bjsm.2005.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton M.T., Gonzalez-Alonso J., Montain S.J., Coyle E.F. Fluid replacement and glucose infusion during exercise prevent cardiovascular drift. J Appl Physiol. 1991;71:871–877. doi: 10.1152/jappl.1991.71.3.871. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen B., Savard G., Richter E.A., Hargreaves M., Saltin B. Muscle blood flow and muscle metabolism during exercise and heat stress. J Appl Physiol. 1990;69:1040–1046. doi: 10.1152/jappl.1990.69.3.1040. [DOI] [PubMed] [Google Scholar]
- 29.Shi X., Gisolfi C.V. Fluid and carbohydrate replacement during intermittent exercise. Sports Med. 1998;25:157–172. doi: 10.2165/00007256-199825030-00003. [DOI] [PubMed] [Google Scholar]
- 30.Finsterer J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet Disord. 2012;13:218. doi: 10.1186/1471-2474-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juel C. Changes in interstitial K+ and pH during exercise: implications for blood flow regulation. Appl Physiol Nutr Metab. 2007;32:846–851. doi: 10.1139/H07-065. [DOI] [PubMed] [Google Scholar]
- 32.Parkhouse W.S. The effects of ATP, inorganic phosphate, protons, and lactate on isolated myofibrillar ATPase activity. Can J Physiol Pharmacol. 1992;70:1175–1181. doi: 10.1139/y92-163. [DOI] [PubMed] [Google Scholar]
- 33.Abbiss C.R., Nosaka K., Laursen P.B. Hyperthermic-induced hyperventilation and associated respiratory alkalosis in humans. Eur J Appl Physiol. 2007;100:63–69. doi: 10.1007/s00421-007-0405-z. [DOI] [PubMed] [Google Scholar]
- 34.Chwalbinski-Moneta J., Krysztofiak H., Ziemba A., Nazar K., Kaciuba-Uscilko H. Threshold increases in plasma hormone in relation to plasma catecholamine and blood lactate concentrations during progressive exercise in endurance-trained athletes. Eur J Appl Physiol Occup Physiol. 1996;73:117–120. doi: 10.1007/BF00262819. [DOI] [PubMed] [Google Scholar]
- 35.Moquin A., Mazzeo R.S. Effect of mild dehydration on the lactate threshold in women. Med Sci Sports Exerc. 2000;32:396–402. doi: 10.1097/00005768-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Hargreaves M., Dillo P., Angus D., Febbraio M. Effect of fluid ingestion on muscle metabolism during prolonged exercise. J Appl Physiol. 1996;80:363–366. doi: 10.1152/jappl.1996.80.1.363. [DOI] [PubMed] [Google Scholar]
- 37.Ali A., Gardiner R., Foskett A., Gant N. Fluid balance, thermoregulation and sprint and passing skill performance in female soccer players. Scand J Med Sci Sports. 2011;21:437–445. doi: 10.1111/j.1600-0838.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 38.Cooper K.E. Some responses of the cardiovascular system to heat and fever. Can J Cardiol. 1994;10:444–448. [PubMed] [Google Scholar]
- 39.Bell A.W., Hales J.R., King R.B., Fawcett A.A. Influence of heat stress on exercise-induced changes in regional blood flow in sheep. J Appl Physiol. 1983;55:1916–1923. doi: 10.1152/jappl.1983.55.6.1916. [DOI] [PubMed] [Google Scholar]
- 40.Hultman E., Sahlin K. Acid–base balance during exercise. Exerc Sport Sci Rev. 1980;8:41–128. [PubMed] [Google Scholar]
- 41.Suzuki K., Peake J., Nosaka K., Okutsu M., Abbiss C.R., Surriano R. Changes in markers of muscle damage, inflammation and HSP70 after an Ironman Triathlon race. Eur J Appl Physiol. 2006;98:525–534. doi: 10.1007/s00421-006-0296-4. [DOI] [PubMed] [Google Scholar]
- 42.Puntschart A., Vogt M., Widmer H.R., Hoppeler H., Billeter R. Hsp70 expression in human skeletal muscle after exercise. Acta Physiol Scand. 1996;157:411–417. doi: 10.1046/j.1365-201X.1996.512270000.x. [DOI] [PubMed] [Google Scholar]
- 43.Sandström M.E., Madden L.A., Taylor L., Siegler J.C., Lovell R.J., Midgley A. Variation in basal heat shock protein 70 is correlated to core temperature in human subjects. Amino Acids. 2009;37:279–284. doi: 10.1007/s00726-008-0144-4. [DOI] [PubMed] [Google Scholar]
- 44.Lovell R., Madden L., Carroll S., McNaughton L. The time-profile of the PBMC HSP70 response to in vitro heat shock appears temperature-dependent. Amino Acids. 2007;33:137–144. doi: 10.1007/s00726-006-0400-4. [DOI] [PubMed] [Google Scholar]
- 45.Milne K.J., Noble E.G. Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol. 2002;93:561–568. doi: 10.1152/japplphysiol.00528.2001. [DOI] [PubMed] [Google Scholar]
- 46.Yamada P., Amorim F., Moseley P., Schneider S. Heat shock protein 72 response to exercise in humans. Sports Med. 2008;38:715–733. doi: 10.2165/00007256-200838090-00002. [DOI] [PubMed] [Google Scholar]
- 47.Niess A.M., Simon P. Response and adaptation of skeletal muscle to exercise-the role of reactive oxygen species. Front Biosci. 2007;12:4826–4838. doi: 10.2741/2431. [DOI] [PubMed] [Google Scholar]
- 48.Radak Z. Human Kinetics; Champaign, IL: 2000. Free radicals in exercise and aging. [Google Scholar]
- 49.Bloomer R.J., Davis P.G., Consitt L.A., Wideman L. Plasma protein carbonyl response to increasing exercise duration in aerobically trained men and women. Int J Sports Med. 2007;28:21–25. doi: 10.1055/s-2006-924140. [DOI] [PubMed] [Google Scholar]
- 50.Miyata M., Kasai H., Kawai K., Yamada N., Tokudome M., Ichikawa H. Changes of urinary 8-hydroxydeoxyguanosine levels during a two-day ultramarathon race period in Japanese non-professional runners. Int J Sports Med. 2008;29:27–33. doi: 10.1055/s-2007-965072. [DOI] [PubMed] [Google Scholar]
- 51.Sureda A., Ferrer M.D., Tauler P., Romaguera D., Drobnic F., Pujol P. Effects of exercise intensity on lymphocyte H2O2 production and antioxidant defences in soccer players. Br J Sports Med. 2009;43:186–190. doi: 10.1136/bjsm.2007.043943. [DOI] [PubMed] [Google Scholar]
- 52.Hartmann A., Pfuhler S., Dennog C., Germadnik D., Pilger A., Speit G. Exercise-induced DNA effects in human leukocytes are not accompanied by increased formation of 8-hydroxy-2′-deoxyguanosine or induction of micronuclei. Free Radic Biol Med. 1998;24:245–251. doi: 10.1016/s0891-5849(97)00249-9. [DOI] [PubMed] [Google Scholar]
- 53.Mastaloudis A., Tian W.Y., Obert P., O'donnell F.B., Roderick H., Dashwood R.H. Endurance exercise results in DNA damage as detected by the COMET ASSAY. Free Radic Biol Med. 2004;36:966–975. doi: 10.1016/j.freeradbiomed.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 54.Pereira Ede J., Panek A.D., Eleutherio E.C. Protection against oxidation during dehydration of yeast. Cell Stress Chaperones. 2003;8:120–124. doi: 10.1379/1466-1268(2003)008<0120:paoddo>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuo L., Christofi F.L., Wright V.P., Liu C.Y., Merola A.J., Berliner L.J. Intra- and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. Am J Physiol Cell Physiol. 2000;279:1058–1066. doi: 10.1152/ajpcell.2000.279.4.C1058. [DOI] [PubMed] [Google Scholar]
- 56.Madara J.L., Pappenheimer J.R. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- 57.Nose H., Mack G.W., Shi X.R., Nadel E.R. Involvement of sodium retention hormones during rehydration in humans. J Appl Physiol. 1988;65:332–336. doi: 10.1152/jappl.1988.65.1.332. [DOI] [PubMed] [Google Scholar]


