Abstract
Background
Cardiovascular diseases and insufficient levels of vitamin D are risk factors for adverse surgical outcomes, and they are both commonly present among older adults undergoing orthopaedic surgery. Giving the cardiovascular effects of vitamin D, pre-operative diagnosis of hypovitaminosis D would be a valuable step for the implementation of supplementation protocols. We investigated if the normalization of serum 25 [OH] D could ameliorate cardiac performance of older adults suffering from cardiovascular diseases.
Methods
We enrolled 47 older adults scheduled for major orthopaedic surgery and suffering from hypovitaminosis D. Patients underwent 6-months calcifediol supplementation with a starting dose at first post-operative day of 50 µg/die in liquid preparation. Down-titration to 20 µg/die at 3-months assessment was planned. Cardiac performance was evaluated by measuring left ventricular ejection fraction (LVEF) and global longitudinal strain (GLS) during pre-operative assessments and at 1-month, 3-months, 6-months follow-ups.
Results
Six months of calcifediol supplementation were associated with a significant improvement of both LVEF (+ 3.94%; 95% CI: −4.0789 to −0.8232; P < 0.01) and GLS (+ 18.56%; Z = −5.895; P < 0.0001).
Conclusions
Calcifediol supplementation normalized serum 25 [OH] D concentration after 1-month treatment. GLS offered better insights into myocardial contractile amelioration than LVEF, thus being useful for detecting earlier subclinical changes that may anticipate hemodynamic modifications.
Keywords: Global longitudinal strain, Left ventricular ejection fraction, Orthopedic surgery, Transthoracic echocardiography, Vitamin D
1. Introduction
In orthopedic setting, surgeons consider pre-operative low serum level of 25 [OH] D a risk for adverse outcomes,[1] and supplementation protocols were implemented since years.[2] Likewise, cardiologists consider the presence of cardiovascular diseases, such as arrhythmia, heart failure, and acute myocardial infarction, a risk factor for post-operative complications.[3],[4] Recently, vitamin D supplementation received attention from cardiologists for its efficacy in improving previously compromised ventricular output.[5] With the highest prevalence of hypovitaminosis D found in older adults undergoing orthopedic surgery[6] and its correlation with co-morbid cardiovascular diseases,[7] pre-operative diagnosis of hypovitaminosis D would be a step of convergent interests between cardiologists and orthopedics, thus giving vitamin D supplementation a common role. Therefore, we investigated if the correction of hypovitaminosis D could ameliorate post-surgical cardiac performance of older adults in our orthopedic setting.
2. Methods
2.1. Study participants and evaluations
Screening and recruitment were conducted among consecutive older adults referring to inpatient Cardiology Unit prior major orthopedic surgery at IRCCS Orthopaedic Galeazzi in Milan. Inclusion criteria were Caucasian race, age between 50–90 years old, presence of cardiovascular disease, and hypovitaminosis D. Exclusion criteria were chronic kidney disease with glomerular filtration rate < 35 mL/min, tumours in the last five years, and endocrine diseases with the exception of obesity, diabetes mellitus type 1 or 2, or dyslipidaemia. The chemiluminescent immunoassay ARCHITECT 25-OH Vitamin D (Abbott Diagnostics, USA) was used to determine calcifediol plasma concentrations: values < 32 ng/mL were considered indicative of low levels.[8] All patients underwent transthoracic echocardiography (TTE), which provided pre-operative left ventricular ejection fraction (LVEF) and global longitudinal strain (GLS) by using EPIC 7 ultrasound system (Philips, USA). The X5–1 transducer was used at a frequency of 5–1 MHz, in tandem with electrocardiography monitoring. Images were elaborated through Q-lab 10.5 software. After surgery, blood tests and TTE were performed at 1-month, 3-months, and 6-months follow-ups.
2.2. Intervention and analysis
A liquid preparation of calcifediol (i.e., oil-in-water emulsion with propylene glycol) to be administered by oral drops was used, instead of the more commonly used tablets, capsules, or powders. The dose was set at 50 µg/die, which was lower than the one used in other studies because of the absence of the first-pass effect,[9] and the higher matrix bioavailability and bioaccessibility ensured by the liquid preparation compared to solid dosages.[5] Therefore, the starting dose at first post-operative day was set to 10 drops (i.e., one drop = 5 µg calcifediol). At 1-month supplementation, dose was planned to be reduced to four drops in order to keep range between 32–60 ng/mL over remaining trial extent. The intention-to-treat analysis compared LVEF and GLS between baseline and follow-ups by using paired samples t-test for normally distributed values or Wilcoxon signed-rank test for skewed values. All tests were performed by using SPSS 22 and 2-tailed tests. Intra- and inter-observer variability was calculated using Bland-Altmann methods.[10]
3. Results
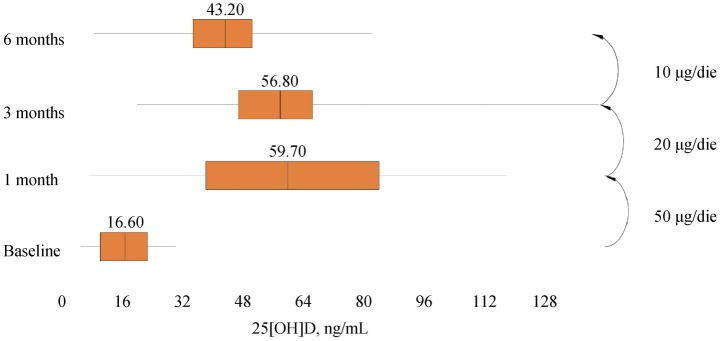
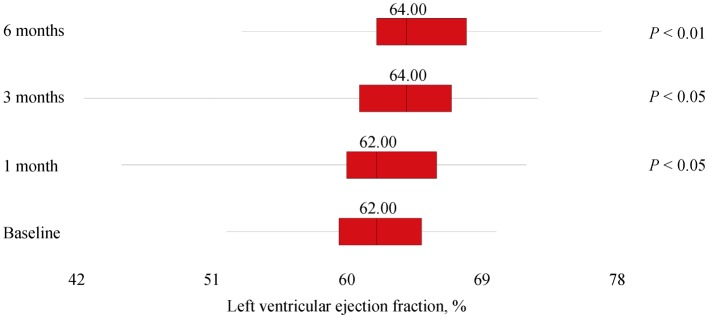
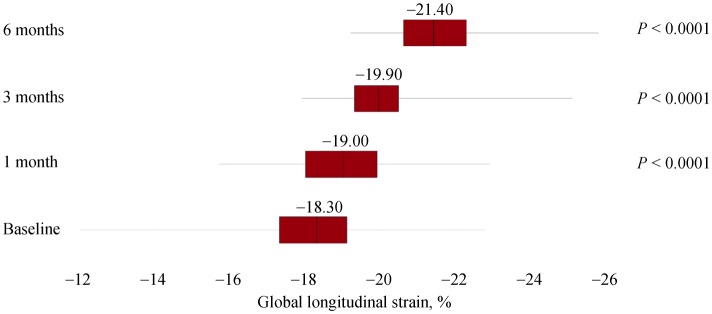
A total of 47 subjects completed the study, and baseline demographic and clinical data were expressed in Table 1. At 1-month assessment, blood withdrawals for 25 [OH] D measured 60.95 ± 28.40 ng/mL (7.20; 117.70) and, according to the protocol, drops were reduced to four per day. At 3-months assessment, serum 25 [OH] D concentrations showed a mean of 58.20 ± 20.72 ng/mL (19.00; 145.70) and, not according to the protocol, drops were reduced to two per day. After six months, measured values of serum 25[OH]D were 43.06 ± 16.46 ng/mL (8.30; 82.00) and statistical analyses revealed a significant difference for plasma vitamin D (P < 0.001; 95% CI: −31.3223 to −22.1458), as well as for both LVEF (95% CI: −4.0789 to −0.8232; P < 0.01) and GLS (Z = −5.895; P < 0.0001). However, LVEF at 1-month and 3-months follow-ups did not differ significantly from baseline: P = 0.644 (95% CI: −1.2230 to 1.9591) and P = 0.180 (95% CI: −3.1754 to 0.6137), respectively. See Figures 1, 2 and 3 for box plots.
Table 1. Baseline demographic and clinical data of cardiovascular older adults undergoing 6-months supplementation of calcifediol in daily drops.
| (n = 47) | |
| Age, yrs | 70.23 ± 8.41 (53.00; 90.00) |
| Gender (male/female) | 20 (43%) / 27 (57%) |
| Body mass index, kg/m2 | 30.25 ± 5.13 (20.54; 42.06) |
| Number of cardiovascular diseases | 2.00 (1.00/2.00) |
| High blood pressure | 37 (79%) |
| Ischaemic heart disease | 4 (8%) |
| Arrhythmia | 5 (11%) |
| Heart valve problem | 1 (2%) |
| Vascular disease | 6 (13%) |
| Number of comorbidities | 1.00 (0.00/2.00) |
| Obesity | 21 (45%) |
| Dyslipidaemia | 15 (32%) |
| Diabetes mellitus type 2 | 8 (17%) |
| Number of medications | 3.00 (2.00/5.00) |
| Beta-blockers | 18 (38%) |
| Ca blockers | 12 (26%) |
| ACEi/ARB/DRI | 32 (68%) |
| Diuretics | 22 (47%) |
| Anti-platelet, anti-integrin, anti-thrombotic | 12 (26%) |
| Anti-arrhythmics | 5 (11%) |
| 25 [OH] D, ng/mL | 16.33 ± 7.26 (4.90; 30.10) |
| Left ventricular ejection fraction, % | 62.26 ± 4.29 (52.00; 70.00) |
| Global longitudinal strain, % | −18.17 ± 1.75 (−11.90; −22.80) |
Continuous variables were reported as mean ± SD (min; max) or as median (Q1/Q3). Categorical variables were reported as frequencies and percentages. ACEi: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blockers; DRI: direct renin inhibitor.
Figure 1. Median and interquartile ranges of serum 25 [OH] D during 6-months supplementation of calcifediol in drops.
Figure 2. Median and interquartile ranges of left ventricular ejection fraction during 6-months supplementation of calcifediol in drops.
Figure 3. Median and interquartile ranges of global longitudinal strain during 6-months supplementation of calcifediol in drops.
4. Discussion
To investigate the influence of serum 25 [OH] D correction on cardiac performance, a total of 47 older adults suffering from hypovitaminosis D and cardiovascular diseases underwent 6-months supplementation of calcifediol. Unpredictable great bioavailability of the liquid preparation forced us to down-titrate the dosage at 3-months assessment in order to maintain serum 25 [OH] D concentration under 60 ng/mL. Both LVEF and GLS significantly changed, but the GLS resulted to be an earlier predictor for left ventricular function amelioration at 1-month assessment. After six months, subjects had an average increase of LVEF of 3.94%, and an increase of GLS of 18.56% from baseline values.
In this study, we may conclude that 50 µg/die of calcifediol from liquid preparation was able to increase serum 25 [OH] D concentration earlier and larger than other metabolic precursors.[9] Besides, 10 µg/die of liquid preparation may show better results than equivalent solid dosage forms.[11] At the same times, GLS offered better insights into myocardial contractile amelioration than LVEF after serum 25[OH]D correction, thus being useful for detecting earlier subclinical changes that may anticipate hemodynamic modifications.[12]
Since major orthopedic surgery was implemented as a whole-scale intervention,[13] pre-operative assessments became significantly important in order to identify risk factors for complications, such as the presence of hypovitaminosis D or cardiovascular disease. Aging may predispose to hypovitaminosis D and consequent hypocalcaemia could possibly interfere with cardiac function,[14],[15] thus negatively altering ventricular output.[16] Besides, the resulting activation of renin-angiotensin-aldosterone system from vitamin D deficiency was suggested to be involved in high blood pressure and atrial fibrillation.[17] Our data showed that pre-surgery vitamin D status evaluation could be a crucial step to an effective treatment plan in order to ameliorate cardiac performance. Possibly, its measurement should be done even 1-month prior surgery in order to allow time for treatment and obtain greater results. Moreover, we showed that a liquid preparation proved to be effective in normalizing plasma levels in a short time, thus highlighting the importance of considering the correct metabolite and dosage for supplementation protocols. More extensive studies should evaluate cardiac effects of vitamin D correction including larger sample size and a control group, and also augmenting technical information about dosage forms. Rigorous protocols for detection and discrimination between cardiac and musculoskeletal effects should be applied, but GLS definitely proved to be useful for detecting subclinical cardiac changes.
Acknowledgments
Bruno Farmaceutici S.p.A. supplied calcifediol in drops (Didrogyl). This study was part of the project “Ricerca Corrente del Ministero Della Salute”. There were no conflicts of interest for all authors to be declared.
References
- 1.Maier GS, Maus U, Lazovic D, et al. Is there an association between low serum 25-OH-D levels and the length of hospital stay in orthopaedic patients after arthroplasty? J Orthop Traumatol. 2016;17:297–302. doi: 10.1007/s10195-016-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sprague S, Petrisor B, Scott T, et al. What is the role of vitamin D supplementation in acute fracture patients? A systematic review and meta-analysis of the prevalence of hypovitaminosis D and Supplementation efficacy. J Orthop Trauma. 2016;30:53–63. doi: 10.1097/BOT.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 3.Sathiyakumar V, Avilucea FR, Whiting PS, et al. Risk factors for adverse cardiac events in hip fracture patients: an analysis of NSQIP data. Int Orthop. 2016;40:439–445. doi: 10.1007/s00264-015-2832-5. [DOI] [PubMed] [Google Scholar]
- 4.Matsen FA, 3rd, Li N, Gao H, et al. Factors affecting length of stay, readmission, and revision after shoulder arthroplasty: a population-based study. J Bone Joint Surg Am. 2015;97:1255–1263. doi: 10.2106/JBJS.N.01107. [DOI] [PubMed] [Google Scholar]
- 5.Witte KK, Byrom R, Gierula J, et al. Effects of vitamin D on cardiac function in patients with chronic HF: the VINDICATE study. J Am Coll Cardiol. 2016;67:2593–2603. doi: 10.1016/j.jacc.2016.03.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maier GS, Horas K, Seeger JB, et al. Vitamin D insufficiency in the elderly orthopaedic patient: an epidemic phenomenon. Int Orthop. 2015;39:787–792. doi: 10.1007/s00264-014-2519-3. [DOI] [PubMed] [Google Scholar]
- 7.Reddy Vanga S, Good M, Howard PA, et al. Role of vitamin D in cardiovascular health. Am J Cardiol. 2010;106:798–805. doi: 10.1016/j.amjcard.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 8.Alshahrani F, Aljohani N. Vitamin D: deficiency, sufficiency and toxicity. Nutrients. 2013;5:3605–3616. doi: 10.3390/nu5093605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shieh A, Ma C, Chun RF, et al. Effects of cholecalciferol vs calcifediol on total and free 25-hydroxyvitamin D and parathyroid hormone. J Clin Endocrinol Metab. 2017;102:1133–1140. doi: 10.1210/jc.2016-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 11.Vaes AMM, Tieland M, de Regt MF, et al. Dose-response effects of supplementation with calcifediol on serum 25-hydroxyvitamin D status and its metabolites: a randomized controlled trial in older adults. Clin Nutr. 2018;37:808–814. doi: 10.1016/j.clnu.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Kalam K, Otahal P, Marwick TH, et al. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 13.Leme LE, Sitta Mdo C, Toledo M, et al. Orthopedic surgery among the elderly: clinical characteristics. Rev Bras Ortop. 2011;46:238–246. doi: 10.1016/S2255-4971(15)30189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avsar A, Dogan A, Tavli T, et al. A rare cause of reversible dilated cardiomyopathy: hypocalcemia. Echocardiography. 2004;21:609–612. doi: 10.1111/j.0742-2822.2004.03149.x. [DOI] [PubMed] [Google Scholar]
- 15.Catalano A, Basile G, Lasco A, et al. Hypocalcemia: a sometimes overlooked cause of heart failure in the elderly. Aging Clin Exp Res. 2012;24:400–403. doi: 10.1007/BF03325272. [DOI] [PubMed] [Google Scholar]
- 16.Williams FM, Bergin JD. Cardiac screening before noncardiac surgery. Surg Clin North Am. 2009;89:747–762. doi: 10.1016/j.suc.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Majeed F. Low levels of Vitamin D an emerging risk for cardiovascular diseases: a review. Int J Health Sci. 2017;11:71–76. [PMC free article] [PubMed] [Google Scholar]