Abstract
Purpose
The aim of this study was to investigate the impact of total soy saponins (TS) on the free radical metabolism from the quadriceps femoris muscle, serum testosterone, lactate dehydrogenase (LDH), and blood urea nitrogen (BUN) in rats exercised to exhaustion.
Methods
A one-time exhausted treadmill exercise session was used. Sprague-Dawley rats were divided into 4 groups: a control group—animals receiving no TS and no exercise (NTSNE), animals receiving TS but no exercise group (TSNE), animals receiving no TS but exercised to exhaustion group (NTSE), and animals receiving TS and exercised to exhaustion group (TSE). The TSNE and TSE groups were fed TS at a dosage of 20 mg/kg body weight once per day for 2 weeks. The NTSE group was given a placebo, and the NTSNE group was not given any treatment. The NTSE and TSE groups were exercised at speed of 30 m/min on treadmill until exhausted. The exercise time and exercise distance were recorded when the rats became exhausted and the rats were then decapitated and anatomized immediately. A 10% homogenate of the quadriceps femoris tissue was prepared. The levels of superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), glutathione reductase (GR), reduced glutathione (GSH), total antioxidant capacity (T-AOC), LDH, BUN, and serum testosterone were tested.
Results
TS significantly increased the exercise time to exhaustion by 20.62% (p < 0.05). The MDA levels were decreased significantly in the TSNE group than in NTSNE group (p < 0.05); the T-AOC levels increased significantly in the TSNE group than in the other 3 groups (p < 0.01, p < 0.05, p < 0.05). The LDH activity significantly increased in the NTSE group than in TSNE group (p < 0.05). The BUN levels significantly increased in the NTSE group than in the other 3 groups (p < 0.01, p < 0.01, p < 0.05), and significantly increased in the TSE group than in NTSNE and TSNE groups (both p < 0.01). The serum testosterone levels increased significantly in the TSNE group than in the other 3 groups (all p < 0.01). SOD, CAT, GSH-Px, GR, and GSH were not statistically different among the groups.
Conclusion
TS can significantly improve the exercised rats' serum testosterone level and antioxidant activity in their quadriceps femoris to varying degrees, decrease MDA and serum LDH and BUN levels, increase the exercise time, and delay the occurrence of the fatigue.
Keywords: Exercised rat, Free radical, Quadriceps femoris, Serum enzymes, Testosterone, Total soy saponins
1. Introduction
Total soy saponins (TS) are a subset of pentacyclic triterpenoid glycosides with a variety of biological activities. According to the different sapogenins, TS can be divided into 4 groups: the A group, B group, E group, and 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP) group. The A group can be divided into Aa–Ah; the B group can be divided into Ba, Bb, Bc, Bb′, and Bc′; the E group can be divided into Bd and Be; the DDMP group can be divided into αg, βg, βa, γg, and γa subgroups (Fig. 1, Fig. 2).
Fig. 1.
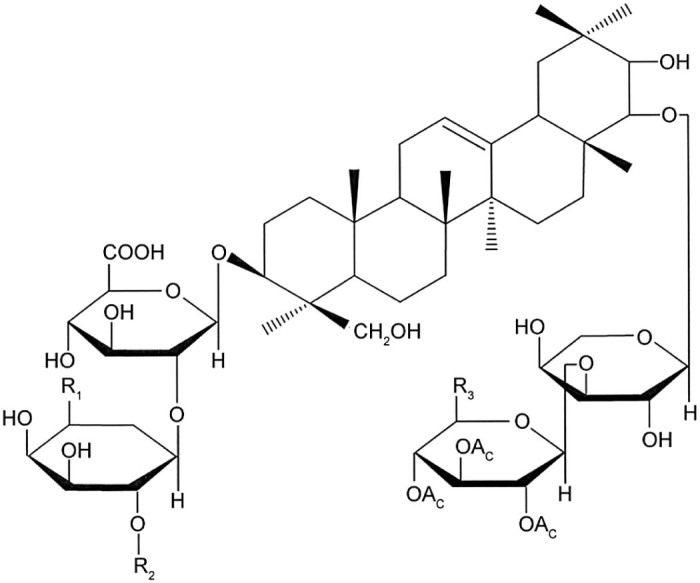
Total soy saponins structure of A group.
Fig. 2.
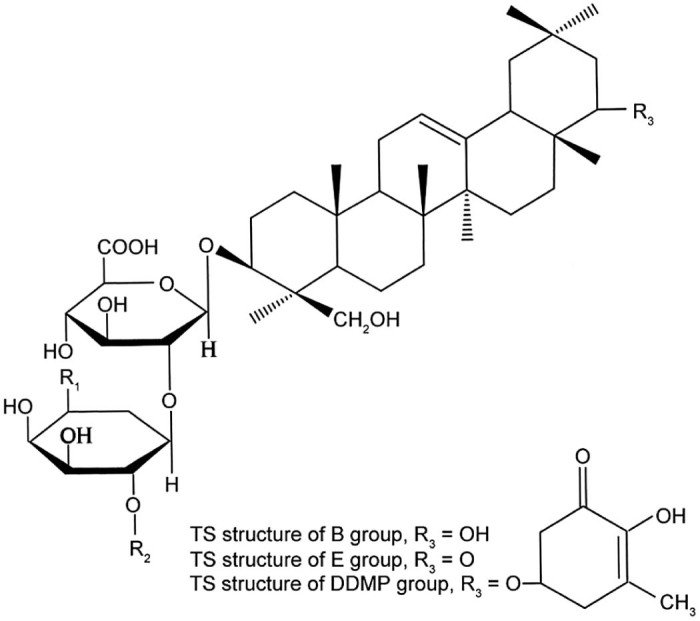
Total soy saponins structure of B, E, and DDMP groups. DDMP = 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one; TS = total soy saponins.
There are 2 free radical (FR) defense systems in the human body. One type is an enzymatic defense system such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and glutathione reductase (GR). The other is a non-enzymatic defense system such as vitamin C, vitamin E, and glutathione (GSH). Typically, the body keeps a dynamic balance between the generation and the removal of FR. However, under the condition of exhausted exercise, FR in the body increases significantly. When the level of lipid peroxidation exceeds the body's antioxidant capacity, this will result in the occurrence of oxidative stress and directly cause biofilm injury, the degeneration of intracellular proteins, and lead to cell death, apoptosis, tissue damage, and disease.1
TS have a variety of biological activities, such as antioxidant2 and immune-enhancing activity.3, 4 They can also improve the rats' macrophage phagocytic capacity5 and humoral and cellular immunity.3 By inhibiting the activity of α-glucosidase6 and α-amylase,7 TS significantly reduced the level of blood sugar in diabetic rats, and significantly improved the glucose tolerance in both the diabetic and healthy rats.8 TS also have significant effects on anti-aging9 and the inhibition of tumor cell DNA,10 herpes simplex virus (HSV-1), human cytomegalovirus (HCMV), polio virus, influenza virus, measles virus, mumps virus, and coxsackie virus.11, 12, 13, 14 Further anti-aging studies on human embryonic lung diploid fibroblasts in vitro confirmed that the cells treated with TS can grow extended to 80 generations, whereas the longest survival time of the control group was only until 51 generations.15 TS also have a significant capacity of anti-lipid peroxidation activity on plasma lipoprotein16 and can prevent low-density lipoprotein cholesterol (LDL-C) from oxidizing and decrease their susceptibility to oxidation, thus hindering the conversion of LDL-C to oxidized low-density lipoprotein, which is an important factor in atherosclerosis risk. TS protected not only the heart, but also the vascular smooth muscle. TS can significantly reduce the generation of lipid peroxides, protect endothelial integrity, and maintain the normal cardiovascular function. The aim of this study was to investigate the impact of TS on the free radical metabolism from the quadriceps femoris muscle, serum testosterone, lactate dehydrogenase (LDH), and blood urea nitrogen (BUN) in rats exercised to exhaustion.
2. Methods
2.1. Experimental design and subjects
Thirty-two Sprague-Dawley (SD) healthy 2-month-old male rats were used (weight 190–210 g) and were provided care as directed by the Experimental Animal Center of the Medical School, Xi'an Jiao Tong University (animal certificate No.: Shaanxi Medical Animal No. 08-005). This study was performed according to the international, national, and institutional rules considering animal experiments, clinical studies and biodiversity rights, and had been approved by Xijing Hospital Ethic Committee in Fourth Military Medical University.
All rats were randomly divided into 4 groups: a control group—animals receiving no TS and no exercise (NTSNE), animals receiving TS but no exercise group (TSNE), animals receiving no TS but exercised to exhaustion group (NTSE), and animals receiving TS and exercised to exhaustion group (TSE). Eight rats from each group were fed in divided cages. The temperature varied from 22°C to 28°C, the relative humidity was 45%–65%, the cages were illuminated by natural light, the ambient noise was no higher than 45 dB and all rats had free access to water and basic rodent chow.
North China Pharmaceutical Co., Ltd (Shijiazhuang, China) provided TS with a purity of 90% and 10% ash. The rats were started on TS gavage after 3 days of adaptation to the environment. Each rat in the supplement groups (TSNE and TSE groups) was fed a 2 mL aliquot of TS dissolved in normal saline at a fixed time between 9:00 a.m. and 9:30 a.m., once per day for 2 weeks, with TS dosage of 20 mg/kg body weight. During the supplement gavage, the rats were weighed every 3 days and the dosage was adjusted in time according to the body weight. The NTSE group was fed the same volume of normal saline vehicle. The NTSNE (control) group received no treatments.
2.2. Exhaustive exercise protocol
An acute exhaustive exercise session was completed. The rats were not given any prior training; the NTSE and TSE groups underwent an acute exhaustive exercise session on the treadmill only before dissection. The treadmill was horizontal and gradually increased to the predetermined exercise intensity (30 m/min) within 3 min. The treadmill speed was set at 10 m/min for the first minute, 20 m/min for the second minute, and 30 m/min for the third minute. The exercise time to exhaustion and exercise distance for each rat were recorded. We judged whether the rats exercised to exhaustion according to the following criteria: the rats could not maintain a predetermined treadmill speed, squatted against the back wall of the treadmill lane on their buttocks, and both the current stimulus and the brush driving could not force the rats to continue exercising. The exhaustive behavior was characterized by shortness of breath, mental fatigue, and a prone nutation.
2.3. Dissection and index test
The rats were anesthetized with ether immediately after reaching a state of exhaustion and killed by decapitation. Blood was collected and the serum was separated after coagulation. The quadriceps femoris was removed immediately and the remaining blood was washed away with 4°C normal saline and placed in a clean culture dish, marked according to each group. The weight of the quadriceps femoris was measured and the quadriceps femoris was ground in 4°C normal saline. Quadriceps femoris tissue homogenates of 10% mass concentration were prepared and the supernatant was separated after centrifugation (7.99 × g, 5 min). Finally, antioxidant indicators, testosterone, LDH, and BUN were assayed accordingly.
2.4. Methods of testing
The antioxidant indicators were tested with reagent kits provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). SOD was tested by the xanthine oxidase method; malondialdehyde (MDA) was tested by the thiobarbituric (TBA) method; CAT was tested by the ultraviolet spectroscopy method; GSH-Px, GR, and GSH were tested by the dithiobis nitrobenzoic acid method; total antioxidant capacity (T-AOC) was tested by a spectrophotometry method; the testosterone was tested by the radioimmunoassay method; and the serum BUN and LDH were tested by an automatic biochemistry analyzer (Hitachi 7060; Hitachi Corporation of Japan, Tokyo, Japan; LabStar 2.5; Beijing Zhifang Technology Development Co. Ltd., Beijing, China).
A 721B spectrophotometer (Shanghai Jingke Instrument Co., Ltd., Shanghai, China), a 752B spectrophotometer (Shanghai Jingke), an FJ-2008Pγ radioimmunoassay counter (Xi'an Nuclear Instrument Factory, Xi'an, China), a Hitachi 7060 automatic biochemical analyzer, a TGL-16G refrigerated centrifuge (Flying Pigeon, Shanghai Anting Scientific Instrument Factory, Shanghai, China), a DK-98-1A water bath (Taisite, Tianjin City Taisite Instrument Co., Ltd., Tianjin, China) and a DSPT-202 treadmill (Duanshi, Shanghai Xinruan Information Technology Co. Ltd., Hangzhou, China) were used.
2.5. Data processing
The experimental data were processed with statistical software SigmaStat Version 3.5 (SYSTAT Software Inc., San Jose, CA, USA) and the results were shown as mean ± SD. A p < 0.05 or p < 0.01 was considered statistically significant after a one-way analysis of variance (ANOVA) Student–Newman–Keuls test (S–N–K test). The exhaustive time was processed by a t test and the results of the t test were measured by Cohen's d value.
3. Results
With velocity of 30 m/min, TS can significantly improve time to exhaustion of the rats by 20.62% (86 ± 12 min vs. 103 ± 19 min, p < 0.05, Cohen's d = 1.07), which indicated that the present t test was trustworthy (Cohen standards, the t test has a small effect size, medium effect size, and large effect size when Cohen's d value is 0.2, 0.5, and >0.8, respectively. The Cohen's d value of present t test was 1.07 > 0.8).
Data presented in Table 1 show that TS can improve the rats' antioxidant capacity of their quadriceps femoris to varying degrees. By S–N–K test of a one-way ANOVA, as compared with the NTSNE group, the MDA level significantly decreased in the TSNE group (p < 0.05). As compared with the NTSNE group (p < 0.01), the NTSE group (p < 0.05), and the TSE group (p < 0.05), T-AOC activities significantly increased in the TSNE group.
Table 1.
Impact of TS on the antioxidant capacity of the quadriceps femoris in exercised rats (n = 8 in each group, mean ± SD).
| Indices | NTSNE | TSNE | NTSE | TSE |
|---|---|---|---|---|
| SOD (U/mg prot) | 50.21 ± 8.72 | 59.43 ± 10.84 | 54.93 ± 8.99 | 56.61 ± 6.61 |
| MDA (nmol/mg prot) | 3.98 ± 0.55* | 2.83 ± 0.54 | 3.83 ± 0.83 | 3.52 ± 1.19 |
| CAT (U/g prot) | 0.08 ± 0.03 | 0.08 ± 0.02 | 0.07 ± 0.02 | 0.08 ± 0.02 |
| GSH-Px (U/0.1 mL) | 8.09 ± 4.48 | 8.89 ± 3.34 | 9.27 ± 1.81 | 9.56 ± 4.09 |
| GR (U/g prot) | 7.65 ± 3.57 | 10.57 ± 3.85 | 5.94 ± 2.68 | 8.58 ± 3.47 |
| GSH (mg/mg prot) | 2.76 ± 0.29 | 2.93 ± 0.29 | 2.96 ± 0.63 | 3.13 ± 0.25 |
| T-AOC (U/mg prot) | 0.13 ± 0.18** | 1.14 ± 0.82 | 0.50 ± 0.15* | 0.53 ± 0.35* |
Note: *p < 0.05, **p < 0.01, compared with TSNE group; by S–N–K test of a one-way ANOVA.
Abbreviations: CAT = catalase; GR = glutathione reductase; GSH = reduced glutathione; GSH-Px = glutathione peroxidase; MDA = malondialdehyde; NTSE = animals receiving no TS but exercised to exhaustion group; NTSNE = animals receiving no TS and no exercise; SOD = superoxide dismutase; T-AOC = total antioxidant capacity; TS = total soy saponins; TSE = animals receiving TS and exercised to exhaustion group; TSNE = animals receiving TS but no exercise group.
Under the intervention of TS, whether quiet or exercised, the activities of SOD, CAT, GSH-Px, GR, and GSH showed a tendency to increase, but this difference was not statistically significant.
As shown in Table 2, by S–N–K test of a one-way ANOVA, LDH in NTSE group significantly increased in response to exhausted exercise compared with the TSNE group (p < 0.05). As compared with the NTSNE group (p < 0.01), the TSNE group (p < 0.01), and the TSE group (p < 0.05), the BUN level significantly increased in the NTSE group. As compared with both NTSNE and TSNE groups, the BUN level in TSE group significantly increased (both p < 0.01). The serum testosterone level significantly increased in the TSNE group compared with the other 3 groups (p < 0.01).
Table 2.
Impact of TS on the serum LDH activity, BUN, and testosterone level in exercised rats (n = 8 in each group, mean ± SD).
| Indices | NTSNE | TSNE | NTSE | TSE |
|---|---|---|---|---|
| LDH (U/L) | 1345.83 ± 86.61 | 1212.83 ± 97.01 | 1512.00 ± 273.42† | 1409.17 ± 237.10 |
| BUN (mmol/L) | 7.17 ± 0.83##,** | 6.58 ± 0.57##,** | 11.13 ± 1.42 | 10.02 ± 1.22* |
| Testosterone (ng/mL) | 19.75 ± 14.51†† | 73.95 ± 55.01 | 8.13 ± 4.31†† | 12.46 ± 10.69†† |
Abbreviations: BUN = blood urea nitrogen; LDH = lactate dehydrogenase; NTSE = animals receiving no TS but exercised to exhaustion group; NTSNE = animals receiving no TS and no exercise; TS = total soy saponins; TSE = animals receiving TS and exercised to exhaustion group; TSNE = animals receiving TS but no exercise group.
p < 0.05, **p < 0.01, compared with NTSE group.
p < 0.01, compared with TSE group.
p < 0.05, ††p < 0.01, compared with TSNE group.
4. Discussion
The occurrence of fatigue is highly related to the working ability of the skeletal muscle. The FR was one of the key factors which resulted in the decline of skeletal muscle contractibility and the occurrence of fatigue.14 According to the catastrophe theory of muscular fatigue, the fatigue can occur in any links from the cerebral cortex excitement to the contractile proteins of the skeletal muscle.15 As the body exercises to exhaustion, approximately 2% O2 of the body intake is converted to the superoxide anion FR in the way of one-electron reduction,16 and the oxidative stress is enhanced. By the way of Haber–Weiss reaction, the superoxide anion FR and H2O2 can generate hydroxyl radical, while the latter is more toxic to cells. The FR is mainly produced in the mitochondrion and endoplasmic reticulum of the skeletal muscle. The FR and its metabolite MDA can injure the quadriceps femoris cells, include lipid peroxidation, and attack nucleic acid and protein, and finally the biological functions of the cell are also damaged.17 A mass of FR produced in exhaustive exercise not only consumed the antioxidant enzymes, but also attacked enzyme molecules, thus the synthesis and regeneration of antioxidant enzymes are affected. The results of the present study showed TS could significantly improve the time to exhaustion in rats. This improved performance may be associated with the antioxidant effect of TS. As the experimental data showed in Table 1, TS can improve the rats' antioxidant capacity of their quadriceps femoris to varying degrees, and especially improve T-AOC activities and decrease MDA level and show a statistical difference. Compared with NTSNE group, the activity of T-AOC increased (p < 0.05) while MDA level decreased (p < 0.01), and the activity of SOD, GSH-Px, GR, and GSH showed a tendency of increase (but no statistical difference) in TSNE group. These results may be the outcome from a negative feedback regulation of the body: exhaustive exercise → oxidative stress↑ → the compensation reaction of the body↑ → antioxidative genetic expression↑→ the activity of antioxidative enzyme↑ → FR scavenging↑ → FR level↓→ MDA↓. We purpose that exercise provided a positive effect on the antioxidant capacity of the body, and this benefit may be one of the factors by which exercise can provide an anti-aging effect.18
Under the intervention of TS, the activity of SOD, CAT, GHS-Px, GR, GSH, and T-AOC in the TSE group rose slightly more than that found in the NTSE group while the MDA level was lower than in the NTSE group. The experimental results indicated that TS can improve the antioxidative enzymes activity of the quadriceps femoris, alleviate the oxidative stress, and delay the occurrence of fatigue. As the present data showed, exhaustive exercise stimulated FR increase, and prompted the occurrence of fatigue. The mechanism is likely found in the following points: (1) The activity of creatine kinase decreased and resulted in the increase of adenosine monophosphate (AMP). Under the catalysis of adenine trans-aminase, AMP converted to hypoxanthine nucleotide (IMP), IMP converted to hypoxanthine catalysis by 5-nucleotidase and nucleosidase and by catalysis of hypoxanthine oxidase, hypoxanthine produced a mass of superoxide anion.19 This set of reactions can cause a significant increase of lipid peroxidation levels and damage the skeletal muscle cell. (2) The FR metabolism was enhanced when the rats exercised. FR also likely caused lipid peroxidation, which damaged the cell membrane and possibly also caused gene mutation, which further led to synthesis error of protein and enzyme. Finally, the activities of many enzymes (such as phosphofructokinase, citroyl synthetase, isocitrate dehydrogenase, and oxoglutarate dehydrogenase complex) associated with energy metabolism are decreased causing reduced metabolism. Lipid peroxidation can cause a decrease of membrane fluidity and a change of membrane hydromechanics, making the membrane more fragile and may cause calcium overload.20 As a result, ATP synthesis decreased. (3) The change of structure of membrane lipid led to the pore of myolemma and mitochondrial membrane enlargement and a change of membrane permeability. This change will likely result in membrane injury, and thus the structures of subcellular organelle are also damaged and led to a series of disorders.21 (4) In the process of biological membrane lipid peroxidation, the metabolic pathways of arachidonic acid are activated and many highly active substances such as leukotrienes and prostaglandin are produced. Meanwhile the oxygen FR generated can cause lipid peroxidation and again can create a vicious cycle and further increase the skeletal muscle cell injury.22
As compared with the NTSNE group, the activity of T-AOC increased (p < 0.05) while MDA level decreased (p < 0.01), and the activity of SOD, GSH-Px, GR, and GSH showed a tendency of increase (but no statistical difference) in TSNE group. The results of the present experiment indicate that TS has the capacity of scavenging FR even in the rest state. The antioxidative effect of TS may relate to its chemical structure. Considering TS structure, the parent nuclear is rich in phenolic hydroxyl and can combine with FR and form a stable semiquinone. Thus, TS can break the chain reaction of FR. The enol and keto structure of TS can also capture and directly clear FR. Yoshiki et al.23 studied the TS of DDMP group by molecular orbital method and found the antioxidative activity of DDMP saponins related to C-6 and reported that reactive oxygen species were eliminated mainly in this area. Lee et al.24 reported that TS also has a strong total antioxidant capacity and anti-active oxygen capacity in vitro. TS can inhibit lipid peroxidation in liver tissue and alleviate the swelling of liver mitochondria, inhibit erythrocyte membrane lipid peroxidation, and reduce hemolysis of the red blood cells.
Typically, the body keeps a dynamic balance between the generation and the removal of FR. However, under the condition of exhausted exercise, FR in the body can increase significantly.25, 26 SOD, CAT, and GSH-Px are common antioxidative enzymes which can eliminate FR and reduce this imbalance. Under normal physiological conditions and appropriate exercise work rates, the antioxidant enzymes system of the body, by the way of their respective roles, can maintain a dynamic balance between the FR generation and removal (Fig. 3).
Fig. 3.
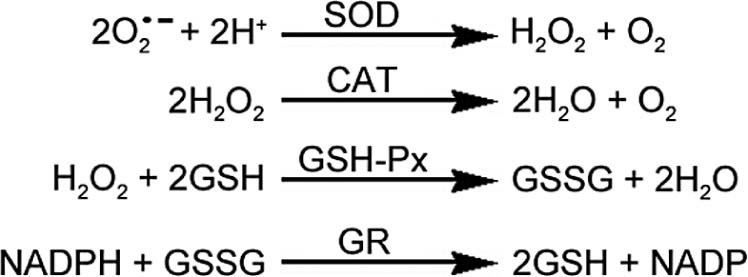
The scavenging effect of antioxidant enzymes on free radicals in vivo. CAT = catalase; GR = glutathione reductase; GSH = reduced glutathione; GSH–Px = glutathione peroxidase; GSSG = oxidized glutathione; NADP = oxidized nicotinamide adenine dinucleotide phosphate; NADPH = reduced nicotinamide adenine dinucleotide phosphate; SOD = superoxide dismutase.
Data found in Table 1 show that the T-AOC in the TSNE group was significantly higher than that in the other groups. This difference maybe in part due to TS eliminating FR and reducing the consumption of non-enzyme substance such as vitamin C, vitamin E, GSH, and cysteine. Thereby, the T-AOC of quadriceps femoris was increased significantly. According to the present experimental data, it can be inferred that the increase of T-AOC in quadriceps femoris of rats is mainly due to the increase of the non-enzyme system.
Data presented in Table 2 showed that TS decreased the activity of serum LDH and BUN while increasing blood testosterone level whether the rats were in a rested or an exhausted exercise state. There are 5 kinds of blood LDH isozyme. LDH1 mainly exists in cardiac muscle tissue; LDH5 mainly exists in skeletal muscle. The present study evaluated total LDH, which reflects the permeability and the damage extent of the sarcolemma. The results show that NTSE group led to an increase of LDH activities, which suggest increased sarcolemma permeability. This increased permeability may be due to myotasis or FR injury. TS intervention decreased LDH activity, which supports that TS provides skeletal muscle cell protection that is likely achieved through an antioxidative effect.
The serum BUN level can reflect kidney function and protein metabolism. Although protein can supply energy in exercise, protein's main biological functions are protection and reparation of the body, not supplying energy. When the body is short of glycogen, exercised at high intensities and for long time periods, protein metabolism can rise and serum BUN will increase. The experiment completed here and the data presented show that exhaustive exercise led to an increase of serum BUN level which indicates increased protein metabolism. TS intervention significantly decreased BUN level and a statistical difference among groups was found. This difference supports the contention that TS can provide protection from protein used by the body and TS may be an essential factor in the explanation for the increased exercise time to exhaustion reported in this study.
As seen from the data presented in Table 2, TS significantly increased rat serum testosterone level. As compared with the NTSNE group, the serum testosterone level sharply increased in the TSNE group (p < 0.01). This increase shows that TS can stimulate testosterone secretion and increase testosterone level in the rat. The SD of testosterone data in the TSNE group was larger than in other groups, indicating a higher dispersion degree for the testosterone data and a higher individual variation for this group. The reason for this greater degree of dispersion is not clear, but it may be caused in part by some random errors in the testing process, which could affect objectivity of the results. By the intervention of TS, compared with NTSE group, the serum testosterone increased in the TSE group, but this difference was not statistically significant. As the main androgen and anabolic steroid, testosterone can sustain muscle volume and muscle weight, sustain bone density and strength, and improve stamina. Testosterone can also affect hematopoiesis, calcium balance, bone mineralization, lipid metabolism, and glycometabolism. That TS can increase the exercise time to exhaustion in the rat is likely related to TS ability to stimulate the secretion of testosterone. This question is still in need of further study to better understand the proper mechanism responsible.
5. Conclusion
According to the data analysis and discussions of the experimental results presented in this study, the following conclusions were drawn: (1) TS can significantly increase the rats' exercise time to exhaustion, and TS has a positive effect on preventing fatigue; (2) TS can significantly increase the serum testosterone level and decrease serum LDH and BUN activity; these changes indicate that TS can provide protection of protein use, sustain moderate permeability of the sarcolemma, and stimulate synthesis and secretion of testosterone; (3) TS positively affected antioxidative capability of the rats' quadriceps femoris, especially by improved T-AOC and decreased MDA. The antioxidative effect of TS in the quadriceps femoris of exercised rats is likely dependent on the non-enzyme system.
Authors' contributions
ZGL designed the study and drafted the paper; RXN, YL, ZHL, and ZYX performed the experiments; CXY participated in the data analysis and drafted the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (No. 11101354).
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Song B., Zhao Y., Sun Y. Effects of soysaponins on blood lipid and antioxidation in hyperlipidemia population. Chin Gen Pract. 2010;13:3880–3881. in Chinese. [Google Scholar]
- 2.Dong W., Zhang D., Gao X., Jin L., Wang J. Enhancement effect of total soyasaponin on immune function. J Chin Cereal Oil Assoc. 2001;16:9–11. in Chinese. [Google Scholar]
- 3.Ye B., Wen C., Li X., Zhu Q., Song X. Effect of soyasaponins on levels of IL-2 and γ-IFN of spleen cell in diabetic rats. Chin J Lab Diagnosis. 2013;17:261–263. in Chinese. [Google Scholar]
- 4.Zhu X., Zhou C., Song B., Sun Y. Effect of soyasaponins on immunological function of tumor bearing mice. Prog Mod Biomed. 2013;13:4847–4850. in Chinese. [Google Scholar]
- 5.Quan J., Yin X., Jin M., Shen M. Study on the Inhibition of alpha-glucosidase by soyasaponins. J Chin Med Mater. 2003;26:654–656. in Chinese. [PubMed] [Google Scholar]
- 6.Quan J., Yin X., Tanaka M., Kanazawa T. The hypoglycemic effects of soybean hypocotyl extract in diabetic rats. Acta Nutr Sin. 2004;26:207–209. in Chinese. [Google Scholar]
- 7.Wang W., Li D., Li R. Experimental study of soyasaponins protective effect on diabetic kidney disease. Chin J Immunol. 2013;29:1272–1275. in Chinese. [Google Scholar]
- 8.Jin L., Gao X., Wang J., Dong W. A study on the anti-aging effect of soyasaponin II experiment of soyasaponin on the anti-aging function. Sci Technol Food Ind. 1999;20(Suppl. 1):S39–41. in Chinese. [Google Scholar]
- 9.Huang G., Xiao J., Du D., Qiu H. Study on the anti-tumor effect of soyasaponins on H22-bearing mice. Food Res Dev. 2009;30:52–54. in Chinese. [Google Scholar]
- 10.He Z., Zhang F., Deng W., Wu X., Li B. The inhibitory effects of soybean saponins and soybean trypsin inhibitor on simian immunodeficiency virus. Chin J Appl Environ Biol. 1998;4:383–385. in Chinese. [Google Scholar]
- 11.Nakashima H., Okubo K., Honda Y., Tamura T., Matsuda S., Yamamoto N. Inhibitory effect of glycosides like saponin from soybean on infectively of HIV in vitro. AIDS. 1989;3:655–658. doi: 10.1097/00002030-198910000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi K., Hayashi H., Hiraoka N. Inhibitory activity of soyasaponin II on virus replication in vitro. Planta Med. 1997;63:102–105. doi: 10.1055/s-2006-957622. [DOI] [PubMed] [Google Scholar]
- 13.Li J., Hu J., Chen B., Wang X., An Z., Wei Y. Inhibitory effect of total soyasaponin of virus replication and its clinical application. Chin J Exp Clin Virol. 1995;9:111–114. in Chinese. [Google Scholar]
- 14.Tian Y. Higher Education Press; Beijing: 2003. Advanced physiology of sport and exercise; pp. 234–244. [Google Scholar]
- 15.Edwards R.H.T. Biochemical bases of fatigue in exercise performance: catastrophe theory of muscular fatigue. In: Knuttgen H.G., Vogel R.D., Poortmans J.R., editors. Biochemistry of exercise. Human Kinetics; Champaign, IL: 1983. pp. 3–28. [Google Scholar]
- 16.Simmons K.J. Defence against free radicals has therapeutic implications. Am Med Assoc. 1984;251:2187–2192. doi: 10.1001/jama.251.17.2187. [DOI] [PubMed] [Google Scholar]
- 17.Hou C., Long J., Liu J. Research progress in antioxidant effect of hydrogen and its mitochondria mechanism. Med Recapitul. 2013;19:2889–2891. in Chinese. [Google Scholar]
- 18.Wang A., Chi J., Xiang Z., Wang N., Song C. Research on effects of aerobic training on anti-senility effects of swimming on free radical metabolism in mice of different months old. J Beijing Univ Phys Educ. 2001;24:179–182. in Chinese. [Google Scholar]
- 19.Lu Y., Cheng F., Wang X. Antioxidant activities of different extracts of cynomorium songaricum and their protective effects against hypoxanthine/xanthine oxidase-induced cell injury: a comparative study. J Anhui Coll Tradit Chin Med. 2012;31:57–58. in Chinese. [Google Scholar]
- 20.Xu S., Liu T., Su Q. Study on heavy load training-induced cardiac contractility changes of rats, and the relationship between these changes and the levels of cardiac free radical, calcium ions. Chin Sport Sci. 2011;31:73–77. in Chinese. [Google Scholar]
- 21.Piao Z., Zhao D., Zhang H., Guo S., Lu J., Wang J. Study on liver histopathological features in the acute-on-chronic liver failure model rat. Chin J Gastroenterol Hepatol. 2010;19:432–436. in Chinese. [Google Scholar]
- 22.Peng M., Hu J. Lipoxygenase-mediated oxidative metabolism and oxidative stress. Chin J Pharmacol Toxicity. 2014;28:449–452. in Chinese. [Google Scholar]
- 23.Yoshiki Y., Okubo K., Igarashi K. Chemiluminescence of oxygen radical scavengers such as DDMP saponins in the presence of radicals and aldehyde. Adv Exp Med Biol. 1996;405:231–239. doi: 10.1007/978-1-4613-0413-5_20. [DOI] [PubMed] [Google Scholar]
- 24.Lee S.J., Bae J., Kim S., Jeong S., Choi C.Y., Choi S.P. Saponins from soybean and mung bean inhibit the antigen specific activation of helper T cells by blocking cell cycle progression. Biotechnol Lett. 2013;35:165–173. doi: 10.1007/s10529-012-1060-y. [DOI] [PubMed] [Google Scholar]
- 25.Huang C.C., Lin T.J., Chen C.C., Lin W.T. Endurance training accelerates exhaustive exercise-induced mitochondrial DNA deletion and apoptosis of left ventricle myocardium in rats. Eur J Appl Physiol. 2009;107:697–706. doi: 10.1007/s00421-009-1177-4. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z., Liu Y., Xiong Z., Feng Y., Tang W. Total soy saponins improve the antioxidant capacity of the myocardium and exercise ability in exhausted rats. J Sport Health Sci. 2016;5:424–429. doi: 10.1016/j.jshs.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


