Abstract
Many uncommon Candida spp. (species other than C. albicans, C. parapsilosis, C. glabrata, C. tropicalis, and C. krusei) have been shown to emerge in tertiary care facilities. We aimed to investigate these uncommon candidemia in children. Forty-six cases of candidemia caused by uncommon Candida spp. were identified during 2003–2015 from a medical center in Taiwan. The most common specie was C. guilliermondii (31.2%), followed by C. lusitaniae (18.8%) and C. metapsilosis (18.8%). These cases were analyzed and compared with 148 episodes of C. albicans candidemia. The incidence density of uncommon Candida spp. candidemia and the proportion to all candidemia episodes increased substantively during the study period. Prior exposure to azoles was uncommon in the 30 days prior to infection, but fluconazole resistant strains were significantly more common (n = 19, 41.3%). The increased incidence density of uncommon Candida spp. candidemia was associated with increasing use of antifungal agents. No differences in demographics, underlying comorbidities, risk factors, clinical features, dissemination, and 30-day mortality were found between uncommon Candida spp. and C. albicans candidemia. Patients with uncommon Candida spp. candidemia were more likely to require modifications in antifungal treatment and receive echinocandin drugs (43.5% vs 21.6%, p = 0.007). Candidemia caused by uncommon Candida spp. had poorer response to antifungal treatment, led to longer duration of candidemia (median 4.0 versus 2.5 days, p = 0.008), and had a higher treatment failure rate (56.5% vs 38.5%, p = 0.040).
Introduction
Candidemia is a major cause of morbidity and mortality in the health care setting, especially among critically ill or immunocompromised patients or those with complicated medical conditions1–3. Among all Candida-associated invasive fungal diseases, C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, and C. krusei account for nearly 90% of isolates from blood or sterile site cultures1–6. Candidemia caused by other uncommon species, including C. haemulonii, C. guilliermondii, and C. lusitaniae, is less well known and data have been reported only in small case series7–12. However, these uncommon fungal species have emerged as a new health threat to hospitalized patients and are endemic in some areas10–12. The widespread use of immunosuppressive therapies, broad-spectrum antibiotics, and antifungal prophylaxis may further increase the role of Candidal species as the causative pathogens among high-risk patients13–15.
Recently, clinical isolates of C. haemulonii and C. guilliermondii have been reported to exhibit decreased in vitro susceptibility to antifungal agents9,16, which highlights the importance of early identification and more updated treatment strategies. C. lusitaniae and C. famata have also presented as breakthrough candidemia in immunocompromised patients and lead to unfavorable outcomes11,17,18. Clinical data of invasive candidiasis caused by these uncommon yeasts have mostly come from adult patients10–12,17,18, whereas relevant studies reported in children are rare9. Because most institutions have had limited experience with candidemia caused by these uncommon Candida spp. in children, we conducted an observational study of all candidemia cases caused by these pathogens that occurred at our institution during a 13-year period.
Materials and Methods
Study design, collection of isolates and antifungal susceptibility
This study was part of a collaborative, combined retrospective and prospective collected database, laboratory-based, single-center study of invasive yeast infection as previously described19. We identified patients younger than 18 years of age with Candida bloodstream infection (BSI) caused by uncommon Candida spp. between January 2003 and December 2015. All Candida isolates were phenotypically identified by using the API 32C AUX yeast identification kit (bioMérieux SA, Marcy l’Étoile, France) and chromogenic culture media (CHROMagar, Becton Dickinson and Company, Franklin Lakes, NJ, USA). Beginning in December 2013, we applied Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF, Bruker Biotype, software version 3.0, USA), ITS1-5.8S-ITS2 rDNA gene sequencing and large-subunit (18S) ribosomal RNA gene D1/D2 domain sequencing to re-confirm all these species. The study was approved by the Institutional Review Board and Human Research Ethics Committee of Chang Gung Memorial Hospital (CGMH), and a waiver of informed consent for anonymous data collection was also approved. All methods in this current study were performed in accordance with the relevant guidelines mentioned in this manuscript.
We also enrolled all cases of candidemia in children caused by C. albicans during the study period for comparisons. We excluded cases of unidentified Candida spp. and selected only the first isolate recovered from the blood if a patient had several cultures positive for the same Candida spp. Antifungal susceptibility of all these Candida isolates to nine antifungal agents was determined by broth microdilution method using a Sensititre YeastOne system (Trek Diagnostic Systems Ltd., East Grinstead, UK) according to the manufacturer’s instructions20,21. Minimum inhibitory concentration (MIC) was recorded as the highest concentration of antifungal agent resulting in the development of a blue color. The criteria for susceptibility of these Candida isolates to nine antifungal agents were based on MIC breakpoints of Candida spp. recommended by the Clinical & Laboratory Standards Institute (CLSI) guidelines22. For uncommon Candida spp., other than C. guilliermondii, clinical breakpoints are undefined; therefore, isolates that showed MICs higher than the epidemiologic cutoff value were considered potentially resistant23.
Data collection and definitions
An incident episode of candidemia was defined as the first positive blood culture drawn from a peripheral vein yielding a Candida species, with clinical symptoms and signs compatible with Candida BSI24,25. Episodes were considered to be separate if they occurred at least 1 month apart and when at least one negative blood culture was noted between them. The clinical information was accumulated from a review of medical charts and included demographic characteristics, predisposing risk factors within the preceding 30 days from the onset of Candida BSI (defined as the day of first positive blood culture for Candida spp), underlying diseases, and the presence of an intravenous catheter or any other artificial device at the time candidemia appeared. The clinical manifestations at the time of blood culture collection, ICU admission, and the antimicrobial regimens used were also collected.
An episode of candidemia was considered catheter-related only if the same Candida species was cultured from the catheter tip during the episode; the definition was suggested by the guidelines of the Infectious Diseases Society of America26,27. Persistent candidemia was defined as repeated positive blood cultures for Candida spp. for more than 3 days after antifungal agents were administered. Candidemia-attributable mortality was defined as patients died within 7 days after onset of candidemia or in the presence of persistent clinical sepsis or persistent candidemia, or those died of candidemia associated complications28,29. Breakthrough candidemia was defined as new occurrence of candidemia while the patient was on antifungal agents for more than three days30,31. The primary study outcome was clinical treatment failure, which was defined according to the Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria32 as the following: (1) all-cause mortality between days 3 and 30 after the initial positive blood culture, or (2) persistent fungal BSI for ≥72 hours after the initiation of antifungal therapy. The secondary outcome was all cause in-hospital mortality. Patients’ response to antifungal therapy following candidemia was defined according to the consensus criteria of the Mycoses Study Group and European Organization for Research and Treatment of Cancer33.
Statistical analysis
The demographic, clinical, outcome variables and the in vitro susceptibility data were summarized using the descriptive statistics. All statistical analyses were performed using IBM SPSS software (version 22.0; IBM SPSS Inc., New York, USA). Categorical variables were compared using the χ2 or Fisher’s exact test, and continuous variables by the Mann-Whitney U test. A P value of 0.05 was considered significant. Poisson regression and the Cochran-Armitage test were used for trend analysis of the annual BSI incidence densities and the proportions of candidemia caused by uncommon Candida spp., respectively. We also compared BSI incidence densities for three time periods; 2003–2006, 2007–2011, and 2012–2015, using Poisson distribution and test-based methods. The correlation between the annual use of antifungals and time was evaluated by using the Spearman correlation. The associations between the incidence densities of uncommon Candida spp. BSI and the annual use of antifungals (defined as daily doses per 1,000 patient-days) were evaluated by using Poisson regression.
We used Cox regression analysis to identify factors that were significantly associated with death. Clinically relevant parameters in the univariate analysis (P < 0.1) were included at multivariate regression analysis. The full model was reduced to a final model by using a stepwise elimination procedure. The proportional hazards assumption was tested graphically and by building time-dependent variables.
Results
We identified 323 episodes of candidemia that occurred in hospitalized children over the 13-year study period. A total of 25 cultures that previously grew unspecified Candida spp. were rechecked by our ITS1-5.8S-ITS2 rDNA gene sequencing and MALDI-TOF and large-subunit (18S) ribosomal RNA gene D1/D2 domain sequencing. Twenty-one of the cultures were documented, and four unidentified Candida spp. were excluded. A total of 46 episodes of candidemia in 45 patients were caused by 10 uncommon Candida spp (Table 1). These data were compared with those reported for 148 episodes of candidemia caused by C. albicans in 136 patients.
Table 1.
The uncommon Candida species causing 46 episodes of candidemia in children.
| Pathogens | Total episode number, n (%) | Age category& | Years of occurrence | Treatment outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Newborn | Children | 2003–2006 | 2007–2011 | 2012–2015 | Persistent candidemia* | Attributable mortality | ||
| C. guilliermondii | 15 (31.2) | 7 | 8 | 3 | 4 | 8 | 9 (60.0) | 3 (20.0) |
| C. lusitaniae | 7 (18.8) | 2 | 5 | 2 | 3 | 2 | 4 (57.1) | 1 (14.3) |
| C. metapsilosis | 9 (18.8) | 3 | 6 | 0 | 2 | 7 | 6 (66.7) | 3 (33.3) |
| C. orthopsilosis | 3 (6.3) | 1 | 2 | 0 | 0 | 3 | 3 (100) | 1 (33.3) |
| C. haemulonii | 4 (8.3) | 1 | 3 | 1 | 1 | 2 | 2 (50.0) | 1 (25.0) |
| C. lipolytica | 2 (4.2) | 1 | 1 | 0 | 2 | 0 | 1 (50.0) | 0 (0) |
| C. dubliniensis | 2 (4.2) | 0 | 2 | 1 | 0 | 1 | 1 (50.0) | 0 (0) |
| C. pelliculosa | 2 (4.2) | 1 | 1 | 0 | 0 | 2 | 1 (50.0) | 1 (50.0) |
| C. duobushaemulonii | 1 (2.1) | 0 | 1 | 0 | 1 | 0 | 1 (100) | 1 (100) |
| C. famata | 1 (2.1) | 0 | 1 | 0 | 0 | 1 | 0 (0) | 0 (0) |
| Total | 46 (100) | 16 (34.8) | 30 (65.2) | 7 (15.2) | 13 (28.3) | 26 (56.5) | 28 (60.9) | 11 (23.9) |
| Controls: C. albicans | 148 (100) | 50 (33.8) | 98 (66.2) | 58 (39.2) | 53 (35.8) | 37 (25.0) | 58 (39.2) | 35 (23.6) |
&Newborn: from neonatal intensive care unit, age <3 months old; children: ward or pediatric intensive care unit, age 3 months-18 years old.
*Defined as repeated positive blood cultures for Candida spp. for more than 3 days after antifungal agents.
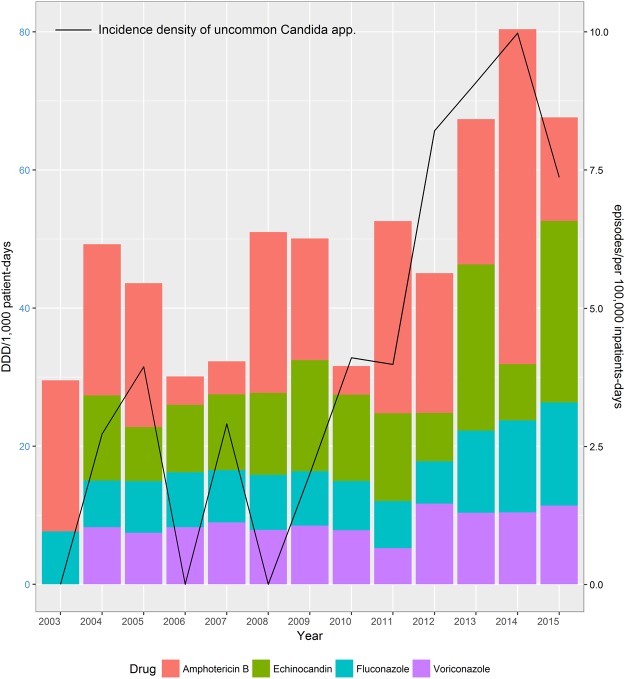
The overall incidence of uncommon Candida spp. candidemia and their proportion relative to all episodes of candidemia increased significantly during 2003–2015 (incidence density p < 0.001; proportion p < 0.001) (Fig. 1). The overall incidence density of uncommon Candida spp. BSI was 3.61 episodes per 100,000 inpatient days, which increased from 1.48 (2003–2006) to 2.47 (2007–2011) and then to 7.29 (2012–2015; p < 0.001). Twenty-nine (63.4%) of the 46 episodes of uncommon Candida spp. candidemia occurred after January 2012. The overall proportion of uncommon Candida spp. candidemia relative to all episodes of candidemia in children was 14.4% and increased from 6.2% (2003–2006) to 9.3% (2007–2011) and then to 29.1% (2012–2015; p < 0.001). During 2012–2015, C. guilliermondii and C. metapsilosis had the highest incidence density (both 1.76 episodes/100,000 inpatient days) and had increased significantly compared with cases during 2003–2006 and 2007–2011. The incidence density rate of other uncommon Candida spp. did not increase.
Figure 1.
Increasing incidence density and proportion relative to all episodes of invasive candidiasis caused by uncommon Candida species and increasing annual use of overall antifungal drugs in Chang Gung Memorial Hospital, Taiwan, R.O.C. January 2003–December 2015. Spearman correlation coefficient r = 0.59, p = 0.033. DDD, defined dai.
Echinocandins have been available in our institute since 2004. Although the annual use of echinocandins, azole antifungals, and amphotericin B increased gradually during 2003–2015, there was no statistically significant increase in their use. The overall use of antifungal agents did increase significantly during this study period (Spearman r = 0.68; p = 0.040) (Fig. 1). The increase in incidence density of uncommon Candida spp. candidemia was correlated with the trend of increased voriconazole (VFEND®, Pfizer, New York, NY, USA) use (p = 0.098) and was significantly associated with the continuous increase in overall antifungal agent use (p = 0.033).
Most chronic comorbid conditions and associated risk factors were comparable between cases of candidemia caused by uncommon Candida spp. and C. albicans groups (Table 2). Although uncommon Candida spp. had a significantly higher MIC to fluconazole and relatively more commonly presented as breakthrough candidemia (17.4% vs 8.1%, P = 0.094), previous exposure to azoles was comparable between these two groups. The clinical characteristics, therapeutic regimens and treatment responses of uncommon Candida spp. candidemia and C. albicans candidemia are compared in Table 3. The severity of illness, judged by rates of severe sepsis and septic shock, were comparable between cases of candidemia caused by uncommon Candida spp. and those due to C. albicans. However, candidemia caused by uncommon Candida spp. led to significantly higher rates of persistent candidemia compared to C. albicans candidemia (76.1% vs 56.8%, p = 0.024).
Table 2.
Demographic and clinical characteristics of 46 episodes of candidemia caused by uncommon Candida spp. versus 148 episodes of C. albicans candidemia.
| Characteristic | Uncommon Candida spp. candidemia (total n = 46) | C. albicans candidemia (total n = 148) | P value |
|---|---|---|---|
| Neonatal episodes, n (%) | 16 (34.8) | 50 (33.8) | 0.809 |
| Patient age (days) of neonatal episodes, median (IQR) | 28.5 (18.5–65.8) | 25.5 (11.8–58.5) | 0.213 |
| Non-neonatal episodes, n (%) | 30 (65.2) | 98 (66.2) | 0.809 |
| Patient age (years) of non-neonatal episodes, years (IQR) | 3.9 (1.1–11.1) | 4.2 (1.0–8.2) | 0.711 |
| Sex, male subjects/female subjects | 21 (45.7)/25 (54.3) | 71 (48.0)/77 (52.0) | 0.866 |
| Underlying conditions* | |||
| Congenital or genetic anomalies | 5 (10.9) | 15 (10.1) | 0.886 |
| Neurological sequelae | 18 (39.1) | 48 (32.4) | 0.476 |
| Cardiovascular disease | 5 (10.9) | 17 (11.5) | 0.908 |
| Chronic lung disease and/or pulmonary hypertension | 16 (34.8) | 48 (32.4) | 0.858 |
| Gastrointestinal sequelae | 11 (23.9) | 44 (29.7) | 0.575 |
| Renal sufficiency with/without dialysis | 8 (17.4) | 19 (12.8) | 0.467 |
| Hematological/Oncology cancer | 9 (19.6) | 23 (15.5) | 0.503 |
| Immunodeficiency | 2 (4.3) | 2 (1.4) | 0.212 |
| Autoimmune disease | 1 (2.2) | 5 (3.4) | 0.680 |
| Hepatic failure or cholestasis | 0 (0) | 6 (4.1) | 0.165 |
| Days of hospitalization before candidemia onset, median (IQR) | 29.0 (14.8–50.0) | 29.0 (14.3–55.5) | 0.787 |
| Sequences of episodes | 0.608 | ||
| First episode | 39 (84.8) | 131 (88.5) | |
| Recurrent episode | 7 (15.2) | 17 (11.5) | |
| Associated risk factors | |||
| Receipt of systemic antibiotics& | 44 (95.7) | 138 (93.2) | 0.735 |
| Prior bacteremia& | 27 (58.7) | 67 (45.3) | 0.130 |
| Prior azoles exposure& | 6 (13.0) | 10 (6.8) | 0.176 |
| Presence of central venous catheter | 45 (97.8) | 141 (95.3) | 0.683 |
| Stay in an intensive care unit | 31 (67.4) | 110 (74.3) | 0.549 |
| Receipt of parenteral nutrition | 30 (65.2) | 94 (63.5) | 0.863 |
| Receipt of immunosuppressive drugs | 14 (30.4) | 29 (19.6) | 0.154 |
| Presence of artificial device other than central venous catheter | 27 (58.7) | 68 (46.0) | 0.176 |
| Prior surgery& | 16 (34.8) | 46 (31.1) | 0.719 |
| Neutropenia¶ | 14 (30.4) | 31 (20.9) | 0.230 |
All data were expressed as number (percentage %), unless indicated otherwise; IQR: interquartile range.
*Indicated the presence of underlying condition or risk factor at onset of candidemia, and most patients with candidemia had >1 underlying condition and/or risk factor.
&Within one month prior onset of candidemia, prior azoles exposure indicated patients received azoles drug in addition to the antifungal agents at time of candidemia.
¶Absolute neutrophil count ≤ 500 cells/μL.
Table 3.
Clinical features, treatment and outcomes of candidemia caused by uncommon Candida spp. versus C. albicans candidemia.
| Uncommon Candida spp. candidemia (total n = 46) | C. albicans candidemia (total n = 148) | P value | |
|---|---|---|---|
| Clinical features | |||
| Severe sepsis | 18 (39.1) | 55 (37.2) | 0.862 |
| Septic shock | 15 (32.6) | 44 (29.7) | 0.717 |
| Progressive and deteriorated candidiasis¶ | 6 (13.0) | 33 (22.3) | 0.209 |
| Disseminated candidiasis# | 0 (0) | 7 (4.7) | 0.133 |
| Breakthrough candidemia | 8 (17.4) | 12 (8.1) | 0.094 |
| Duration of candidemia (days), median (interquartile range) | 4.0 (1.8–8.3) | 2.5 (1.0–5.0) | 0.008 |
| ≤2 days | 16 (34.8) | 74 (50.0) | |
| 3–7 days | 17 (37.0) | 52 (35.1) | |
| ≥8 days | 13 (28.3) | 22 (14.9) | |
| Ultimate antifungal regimens for treatment | 0.054 | ||
| Fluconazole/Voriconazole | 16 (34.8) | 63 (42.6) | |
| Amphotericin B | 9 (19.6) | 44 (29.7) | |
| Echinocandins | 20 (43.5) | 32 (21.6) | 0.007 |
| Combination antifungal treatment | 0 (0) | 2 (1.4) | |
| None | 1 (2.2) | 7 (4.7) | |
| Effective antifungal agents given within 48 hours after onset of candidemia (based on antifungal susceptibility testing) | 16/46 (34.8) | 58/144 (40.3) | 0.601 |
| Total treatment duration (days), mean (interquartile range) | 20.0 (14.0–27.5) | 16.0 (14.0–22.0) | 0.116 |
| Catheter removal | 23/45 (51.1) | 91/141 (64.5) | 0.107 |
| Removal of central venous catheter within 3 days of onset | 16/45 (35.6) | 60/141 (42.6) | 0.406 |
| Treatment outcomes | |||
| Responsiveness after initiation of antifungal treatment* | 0.007 | ||
| Within 72 hours | 8 (17.4) | 72 (48.6) | |
| 4–7 days | 15 (32.6) | 22 (14.9) | |
| More than 7 days | 10 (21.7) | 20 (13.5) | |
| Treatment failure | 26 (56.5) | 54 (36.5) | 0.040 |
| Modification of antifungal treatment | 29/45 (64.4) | 54 (36.5) | 0.003 |
| Duration of candidemia after effective antifungal agents (days), median (IQR) | 3.0 (1.0–7.5) | 1.0 (1.0–4.0) | <0.001 |
| Candidemia attributable mortality | 11 (23.9) | 35 (19.0) | 0.971 |
| Early mortality (≤7 days) | 6 (13.0) | 17 (11.5) | 0.941 |
| Late mortality (8–30 days) | 5 (10.9) | 18 (12.2) | 0.929 |
| Final in-hospital mortality | 19/45 (42.2) | 52/136 (38.2) | 0.486 |
All data were expressed as number (percentage %), unless indicated otherwise.
¶Defined as candidemia episodes with more disseminated candidiasis and/or progressive multi-organ failure even after effective antifungal agents.
#Indicated positive Candida isolates recovered from more than two sterile sites, in addition to primary bloodstream infection.
*Responsiveness to antifungal agents was defined according to the consensus criteria of the Mycoses Study Group and European Organization for Research and Treatment of Cancer33.
Overall, 186 episodes (95.9%) of candidemia were treated with specific antifungal agents. The mean duration between onset of candidemia (time of the first positive blood culture for Candida spp.) and initiation of antifungal agents was 2.3 days (range 0–8 days). In 83 episodes (44.6%) of the 186 episodes, antifungal regimens were modified during the treatment course. Patients with uncommon Candida spp. candidemia had a significantly higher rate of antifungal regimens modification than did those with C. albicans candidemia, and were more often treated with echinocandins (43.5% vs 21.6%, p = 0.007). After modification of antifungal regimens, uncommon Candida spp. led to a significantly longer duration of candidemia (median 3.0 versus 1.0 days, p < 0.001), were slower to respond to antifungal agents, and had significantly higher rates of clinical treatment failure (56.5% versus 38.5%, p = 0.040), although the deaths due to fungemia and final in-hospital mortality were comparable between these two groups.
MICs for uncommon Candida spp. of eight antifungal agents are shown in Table 4. Overall, 19 (47.5%) of 40 available uncommon Candida spp. isolates were intermediate or resistant (minimum inhibitory concentration [MIC] ≥ 4 mg/L). With the exception of three C. haemulonii isolates that had high MICs of amphotericin B (2.0 mg/L), all other strains were susceptible to amphotericin B (MIC ≤ 1.0 mg/L). Seven C. guilliermondii isolates, three C. haemulonii isolates, and one isolate each of C. lusitaniae, C. metapsilosis, C. orthopsilosis, C. pelliculosa and C. duobushaemulonii were resistant (MIC ≥ 1 mg/L) or susceptible-dose-dependent (MIC 0.25–0.5 mg/L) to itraconzole. Except for 2 C. haemulonii strains, all isolates were susceptible to voriconazole (MIC ≤ 1 mg/L) and demonstrated posaconazole MICs of ≤0.5 mg/L. Caspofungin and micafungin demonstrated good activity against all species and isolates; an exception to this was one episode of C. guilliermondii with MICs of 8 mg/L and 2.0 m/L, respectively.
Table 4.
Available susceptibility data for uncommon Candida isolates associated with candidemia in children.
| Agents | MIC (mg/L) | Candida guilliermondii | Candida lusitaniae | Candida metapsilosis | Candida orthopsilosis | Candida haemulonii | Candida lipolytica | Candida dubliniensis | Candida pelliculosa | C.duobushaemulonii |
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 15) | (n = 7) | (n = 8) | (n = 3) | (n = 3) | (n = 1) | (n = 2) | (n = 2) | (n = 1) | ||
| AMB | MIC50 | 0.25 | 0.25 | 0.5 | 0.5 | 2.0 | 0.25 | 0.5 | 0.25 | 1 |
| MIC90 | 0.25 | 0.5 | 1 | 1 | 2.0 | 0.25 | 0.5 | 0.25 | 1 | |
| Range | ≤0.12–0.5 | ≤0.12–0.5 | 0.5–1 | 0.5–1.0 | 2.0 | 0.25 | 0.5 | 0.25 | 1 | |
| FLU | MIC50 | 4 | 1 | 2 | 2 | >128 | 2 | 1 | 4 | 32 |
| MIC90 | 8 | 1 | 8 | 2 | >128 | 2 | 1 | 4 | 32 | |
| Range | 2–8 | 0.5–16 | 1–8 | 1–2 | 16–>128 | 2 | 1 | 4 | 32 | |
| ITC | MIC50 | 0.25 | 0.06 | 0.12 | 0.12 | >16 | 0.12 | 0.06 | 0.25 | 0.5 |
| MIC90 | 0.5 | 0.25 | 0.25 | 0.25 | >16 | 0.12 | 0.06 | 0.25 | 0.5 | |
| Range | 0.12–0.5 | 0.03–0.25 | 0.06–0.25 | 0.12–0.25 | 1–>16 | 0.12 | 0.06 | 0.25 | 0.5 | |
| VOR | MIC50 | 0.06 | 0.015 | 0.06 | 0.06 | >8 | 0.06 | 0.015 | 0.12 | 0.25 |
| MIC90 | 0.12 | 0.12 | 0.25 | 0.12 | >8 | 0.06 | 0.015 | 0.25 | 0.25 | |
| Range | 0.03–0.25 | ≤0.008–0.12 | 0.03–0.25 | 0.06–0.25 | 0.5–>8 | 0.06 | 0.015 | 0.12–0.25 | 0.25 | |
| POS | MIC50 | 0.25 | 0.03 | 0.06 | 0.12 | >8 | 0.25 | 0.03 | 0.5 | 0.5 |
| MIC90 | 0.25 | 0.06 | 0.12 | 0.12 | >8 | 0.25 | 0.03 | 0.5 | 0.5 | |
| Range | 0.06–0.5 | 0.015–0.06 | 0.015–0.12 | 0.06–0.12 | 0.5–>8 | 0.25 | 0.03 | 0.5 | 0.5 | |
| 5-FC | MIC50 | ≤0.06 | ≤0.06 | ≤0.06 | 0.12 | <=0.06 | 0.5 | <=0.06 | <=0.06 | >64 |
| MIC90 | ≤0.06 | ≤0.06 | 0.12 | 0.25 | <=0.06 | 0.5 | <=0.06 | <=0.06 | >64 | |
| Range | ≤0.06 | ≤0.06 | ≤0.06–0.12 | ≤0.06–0.25 | <=0.06 | 0.5 | <=0.06 | <=0.06 | >64 | |
| CAS | MIC50 | 0.25 | 0.25 | 0.25 | 0.5 | 0.12 | 0.06 | 0.12 | 0.03 | 0.06 |
| MIC90 | 0.5 | 0.5 | 0.25 | 0.5 | 0.25 | 0.06 | 0.12 | 0.06 | 0.06 | |
| Range | 0.06–>8 | 0.12–0.5 | 0.12–0.25 | 0.5 | 0.06–0.25 | 0.06 | 0.12 | 0.03–0.06 | 0.06 | |
| MIC | MIC50 | 0.5 | 0.06 | 0.5 | 0.5 | 0.25 | 0.25 | 0.015 | 0.015 | 0.12 |
| MIC90 | 1 | 0.06 | 0.5 | 1 | 0.25 | 0.25 | 0.015 | 0.03 | 0.12 | |
| Range | 0.25–2 | 0.03–0.06 | 0.12–0.5 | 0.25–1 | 0.06–0.25 | 0.25 | 0.015 | 0.015–0.03 | 0.12 |
AMB: amphotericin B; CAS: caspofungin; FLU: fluconazole; 5-FC: 5-flucytosine; ITC: itraconazole; MIC: micafungin; POS: posaconazole; VOR: voriconazole.
Discussion
Population-based surveillance studies have documented the shift of candidemia from C. albicans to non-albicans species over the past two decades2,4,5. Uncommon Candida species, which generally account for less than 10% of all cases of candidemia17,18, are emerging among critically ill patients. The reported prevalence of uncommon Candida spp. in children varies widely between 3.2–22%, depending on definitions, geographic region, and patient characteristics34–37. We found that uncommon Candida spp. accounted for 14.4% of all cases of candidemia in children, and our result is in agreement with a recent study that documented the incidence and proportion of uncommon Candida spp. BSIs has risen during the past decade. We found uncommon Candida spp. candidemia were more frequently associated with treatment failure than candidemia caused by C. albicans, as these isolates were more commonly resistant to azoles, which led to poorer response and longer duration of candidemia.
In contrast to non-albicans candidemia in adults, in which prior fluconazole exposure was often concluded as an independent risk factor18,38,39, studies in the pediatric populations found no difference between C. albicans and non-albicans candidemia in terms of demographics, underlying disease, risk factors, clinical features and outcomes32,40. However, previous studies attempting to investigate the risk factors of non-albicans candidemia in children have focused on the more common Candida spp., such as C. parapsilosis and C. glabrata32,41,42. To our knowledge, this is the first study to investigate uncommon Candida spp. candidemia in children. We found similar patients characteristics, risk factors and comparable outcomes between uncommon Candida spp. and C. albicans. These results are in agreement with other reports concluding host characteristics and underlying medical illness are the most powerful predictors of final outcomes14,42–44.
The overall incidence of candidemia during the study period remained stable and was not affected by the changes in antifungal treatment policies45,46. It is tempting to speculate that an increase in strains resistant to fluconazole and higher MICs values were associated with the changes of in antifungal treatment policies. Published guidelines encourage empiric use of echinocandins in patients with severe illness, history of azole exposure, or neutropenia47. This may have influenced the anti-fungal prescribing practices. It is possible that a significant increase in consumption of antifungal agents during the first half of the study period accounted for the emergence of uncommon Candida species, which required longer periods of antifungal treatment and led to a vicious cycle of more uncommon Candida species. Therefore, the incidence density of uncommon Candida spp. BSIs was noted to be significantly higher after 2012.
During the 13-year study period, the approach and antifungal treatment policies at our institute changed in two aspects. In the neonatal intensive care unit, antifungal prophylaxis with fluconazole for extremely low birth weight infants was launched in 2011, and echinocandins became available since 2004. Caspofungin (Cancidas®, Merck, Sharp & Dohme, Kenilworth, NJ, USA) has been widely used since 2005 and micafungin (Micamine®, Astellas Pharma, Inc., Tokyo, Japan) became more common beginning in 2009. These changes in antifungal regimens may account for the changing epidemiologic characteristics of candidemia in children since 2011. Several studies have concluded that the increase of certain non-albicans or uncommon Candida species, such as C. glabrata and C. kefyr, are associated with the increasing use of echinocandin drugs17,42,45. Another study found significant positive correlation between use of itraconzaole and the increased incidence of C. parapsilosis and C. guilliermondii candidemia45. However, no antifungal agent can account for the emergence of uncommon Candida spp. candidemia found in this study. In addition, our cases of uncommon Candida spp. candidemia were less commonly breakthrough candidemia17 or due to previous treatment with specific antifungal agents.
In several studies, C. guilliermondii has been the most commonly isolated uncommon Candida spp. among pediatric patients16,24,48, and C. lusitaniae was common in another international study in children33. In other recent reports, more than half of all patients with candidemia caused by uncommon Candida spp. had breakthrough infections or underlying hematological malignancies15,17. In our cohort, almost all cases of pediatric candidemia had specific chronic comorbidities and the majority of breakthrough candidemia cases were due to C. parapsilosis and C. glabrata. These differences are a further reflection of the changing epidemiologic characteristics of pediatric candidemia and unique features of uncommon Candida spp. in children. Therefore, we concluded that uncommon Candida spp. distributions and clinical characteristics vary by patient population, geographic region, and antifungal practices15,17,49.
Our study had some limitations. First, it was a retrospective study from a single institution with a small number of episodes caused by individual uncommon Candida spp.; therefore, further multicenter, prospective studies, or systemic review with meta-analysis are required to update information applicable to different geographic areas or specific groups at risk for candidemia caused by uncommon Candida species. Second, although we used the MALDI-TOF and DNA sequencing to re-identify all Candida isolates in the past 13 years, some Candida strains of pediatric candidemia more than five years ago were not available and were identified phenotypically at that time. Therefore, it is possible that during 2003–2011, some C. dublinensis and other Candida isolates were mis-identified as C. albicans, and the frequency of uncommon Candida spp was thus underestimated. Although MALDI-TOF has strengths of rapid, sensitive, and economical in terms of both costs and labor involved, it is also limited that the spectral database of MALDI-TOF must contain peptide mass fingerprints of the specific species before it can correctly identify new species50. Finally, the uncommon Candida species are a heterogeneous “mixture” of many different organisms. Therefore, they may not share common clinical characteristics and treatment strategies should depend on individual cases.
In conclusion, uncommon Candida spp. causing candidemia are emerging among hospitalized children. We did not find clinical variables that enable us early recognition or prediction of candidemia caused by these pathogens. Although clinical outcomes at day 30 are similar to those caused by C. albicans, uncommon Candida spp. more frequently result in prolonged fungemia and treatment failure. Because uncommon Candida species frequently show fluconazole MICs above the epidemiologic cutoff values, identification of all Candida organisms at the species level by advanced molecular methods is of value in guiding treatment directions.
Conclusion
Uncommon Candida species have now emerged among hospitalized children. These pathogens frequently are not susceptible to fluconazole and had higher rate of treatment failure; echinocandins are the treatment choice.
Acknowledgements
This work was supported by a grant from Chang Gung Memorial Hospital (CMRPG3B1302, CMRPG3D1241, and CMRPG3E1491). The authors acknowledge the statistical assistance provided by the Clinical Trial Center, Chang Gung Memorial Hospital, Linkou, Taiwan, which was founded by the Ministry of Health and Welfare of Taiwan; MOHW106-TDU-B-212-113005. Chang Gung Medical Research Program Foundation (grants CMRPG3E1491).
Author Contributions
Ming-Horng Tsai: Dr. Tsai conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. Jen-Fu Hsu: Dr. Hsu designed the data collection instruments, and coordinated and supervised data collection and the whole study. Lan-Yan Yang: Dr. Yang performed the statistical analysis of this study. Yu-Bin Pan: Dr. Pan helped to perform the statistical analysis of this study and complete the figure 1. Mei-Ying Lai: Dr. Lai helped to collect and verify the data. Shih-Ming Chu: Dr. Chu performed the microbiological characteristics of this study. Hsuan-Rong Huang: Dr. Huang took care of these patients, and carried out the initial analyses. Ming-Chou Chiang: Dr. Chiang took care of these patients, and helped data verification. Ren-Huei Fu: Dr. Fu took care of these patients, and helped data verification. Jang-Jih Lu: Dr. Lu critically reviewed the manuscript, revised the manuscript, and approved the final manuscript as submitted.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Russo A, et al. Risk factors and clinical outcomes of candidaemia in patients treated for Clostridium difficile infection. Clin Microbiol Infect. 2015;21:493.e1–493.e4. doi: 10.1016/j.cmi.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Puig-Asensio M, et al. Epidemiology and outcome of candidaemia in patients with oncological and haematological malignancies: results from a population-based surveillance in Spain. Clin Microbiol Infect. 2015;21:491.e1–491.e10. doi: 10.1016/j.cmi.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Oeser C, et al. Neonatal invasive fungal infection in England 2004-2010. Clin Microbiol Infect. 2014;20:936–941. doi: 10.1111/1469-0691.12578. [DOI] [PubMed] [Google Scholar]
- 4.Hesstvedt L, et al. Twenty-two years of candidaemia surveillance: results from a Norwegian national study. Clin Microbiol Infect. 2015;21:938–945. doi: 10.1016/j.cmi.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Tan BH, et al. Incidence and species distribution of candidaemia in Asia: a laboratory-based surveillance study. Clin Microbiol Infect. 2015;21:946–953. doi: 10.1016/j.cmi.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Klingspor L, et al. Invasive Candida infections in surgical patients in intensive care units: a prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006–2008) Clin Microbiol Infect. 2015;21:87.e1–87.e10. doi: 10.1016/j.cmi.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Lockhart SR, Messer SA, Pfaller MA, Diekema DJ. Lodderomyces elongisporus Masquerading as Candida parapsilosis as a cause of bloodstream infections. J Clin Microbiol. 2008;46:374–376. doi: 10.1128/JCM.01790-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alcoba-Flórez J, et al. Phenotypic and molecular characterization of Candida nivariensis sp. nov., a possible new opportunistic fungus. J Clin Microbiol. 2005;43:4107–4111. doi: 10.1128/JCM.43.8.4107-4111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan ZU, et al. Outbreak of fungemia among neonates caused by Candida haemulonii resistant to amphotericin B, itraconazole, and fluconazole. J Clin Microbiol. 2007;45:2025–2027. doi: 10.1128/JCM.00222-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MN, et al. Candida haemulonii and closely related species at 5 university hospitals in Korea: identification, antifungal susceptibility, and clinical features. Clin Infect Dis. 2009;48:e57–e61. doi: 10.1086/597108. [DOI] [PubMed] [Google Scholar]
- 11.Minari A, Hachem R, Raad I. Candida lusitaniae: a cause of breakthrough fungemia in cancer patients. Clin Infect Dis. 2001;32:186–190. doi: 10.1086/318473. [DOI] [PubMed] [Google Scholar]
- 12.Ramos LS, et al. Candida haemulonii complex: species identification and antifungal susceptibility profiles of clinical isolates from Brazil. J Antimicrob Chemother. 2015;70:111–115. doi: 10.1093/jac/dku321. [DOI] [PubMed] [Google Scholar]
- 13.Cuervo G, et al. Breakthrough candidemia in the era of broad-spectrum antifungal therapies. Clin Microbiol Infect. 2015;22:181–188. doi: 10.1016/j.cmi.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Puig-Asensio M, et al. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect. 2014;20:O245–O254. doi: 10.1111/1469-0691.12380. [DOI] [PubMed] [Google Scholar]
- 15.Gamaletsou MN, et al. A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin Microbiol Infect. 2014;20:O50–O57. doi: 10.1111/1469-0691.12312. [DOI] [PubMed] [Google Scholar]
- 16.González GM, et al. Species distribution and antifungal susceptibility of bloodstream fungal isolates in paediatric patients in Mexico: a nationwide surveillance study. J Antimicrob Chemother. 2013;68:2847–2851. doi: 10.1093/jac/dkt283. [DOI] [PubMed] [Google Scholar]
- 17.Jung DS, Farmakiotis D, Jiang Y, Tarrand JJ, Kontoyiannis DP. Uncommon Candida species fungemia among cancer patients, Houston, Texas, USA. Emerg Infect Dis. 2015;21:1942–1950. doi: 10.3201/eid2111.150404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SC, et al. Candidaemia with uncommon Candida species: predisposing factors, outcome, antifungal susceptibility, and implications for management. Clin Microbiol Infect. 2009;15:662–669. doi: 10.1111/j.1469-0691.2009.02821.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsai MH, et al. Clinical and molecular characteristics of bloodstream infections caused by Candida albicans in children from 2003 to 2011. Clin Microbiol Infect. 2015;21:1018.e1–1018.e8. doi: 10.1016/j.cmi.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Alexander BD, et al. Comparative evaluation of Etest and Sensititre YeastOne panels against the Clinical and Laboratory Standards Institute M27-A2 reference broth microdilution method for testing Candida susceptibility to seven antifungal agents. J Clin Microbiol. 2007;45:698–706. doi: 10.1128/JCM.01840-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orasch C, et al. Candida species distribution and antifungal susceptibility testing according to European Committee on Antimicrobial Susceptibility Testing and new vs. old Clinical and Laboratory Standards Institute clinical breakpoints: a 6-year prospective candidaemia survey from the fungal infection network of Switzerland. Clin Microbiol Infect. 2014;20:698–705. doi: 10.1111/1469-0691.12440. [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts: 4th informational supplement. Document M27-S4. Wayne, PA: CLSI (2012).
- 23.Pfaller MA, Diekema DJ. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol. 2012;50:2846–2856. doi: 10.1128/JCM.00937-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santolaya ME, et al. Active surveillance of candidemia in children from Latin America: a key requirement for improving disease outcome. Pediatr Infect Dis J. 2014;33:e40–e44. doi: 10.1097/INF.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen MH, et al. Performance of Candida real-time polymerase chain reaction, β-D-Glucan Assay, and blood cultures in the diagnosis of invasive candidiasis. Clin Infect Dis. 2012;54:1240–1248. doi: 10.1093/cid/cis200. [DOI] [PubMed] [Google Scholar]
- 26.Mermel LA, et al. Clinical practice gudelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muñoz P, et al. Risk factors for late recurrent candidaemia. A retrospective matched case-control study. Clin Microbiol Infect. 2016;22:277.e11–277.e20. doi: 10.1016/j.cmi.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Tsai MH, et al. Risk factors and outcomes for multidrug-resistant gram-negative bacteremia in the NICU. Pediatrics. 2014;133:e322–e329. doi: 10.1542/peds.2013-1248. [DOI] [PubMed] [Google Scholar]
- 29.Tsai MH, et al. Clinical and microbiological characteristics, and impact of therapeutic strategies on the outcomes of children with candidemia. Sci Rep. 2017;7:1083. doi: 10.1038/s41598-017-01123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerolle N, et al. Breakthrough invasive fungal disease in patients receiving posaconazole primary prophylaxis: a 4-year study. Clin Microbiol Infect. 2014;20:O952–O959. doi: 10.1111/1469-0691.12688. [DOI] [PubMed] [Google Scholar]
- 31.Brosh-Nissimov T, Ben-Ami R. Differential association of fluconazole does and does/MIC ratio with mortality in patients with Candida albicans and non-albicans bloodstream infection. Clin Microbiol Infect. 2015;21:1011–1017. doi: 10.1016/j.cmi.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Dotis J, Prasad PA, Zaoutis T, Roilides E. Epidemiology, risk factors and outcome of Candida parapsilosis bloodstream infection in children. Pediatr Infect Dis J. 2012;31:557–560. doi: 10.1097/INF.0b013e31824da7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segal BH, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008;47:674–683. doi: 10.1086/590566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinbach WJ, et al. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr Infect Dis J. 2012;31:1252–1257. doi: 10.1097/INF.0b013e3182737427. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Nunez A. Incidence and mortality of proven invasive Candida infections in pediatric intensive care patients. Infect Control Hosp Epidemiol. 2001;22:477–478. doi: 10.1086/503410. [DOI] [PubMed] [Google Scholar]
- 36.Vogiatzi L, et al. Invasive candidiasis in pediatric intensive care in Greece: a nationwide study. Intensive Care Med. 2013;39:2188–2195. doi: 10.1007/s00134-013-3057-y. [DOI] [PubMed] [Google Scholar]
- 37.Jordan I, et al. Per-species risk factors and predictors of invasive Candida infections in patients admitted to pediatric intensive care units: development of ERICAP scoring systems. Pediatr Infect Dis J. 2014;2014:e187–e193. doi: 10.1097/INF.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 38.Chow JK, et al. Factors associated with candidemia caused by non-albicans Candida species versus Candida albicans in the intensive care unit. Clin Infect Dis. 2008;46:1206–1213. doi: 10.1086/529435. [DOI] [PubMed] [Google Scholar]
- 39.Playford EG, et al. Candidemia in nonneutropenic critically ill patients: risk factors for non-albicans Candida spp. Crit Care Med. 2008;36:2034–2039. doi: 10.1097/CCM.0b013e3181760f42. [DOI] [PubMed] [Google Scholar]
- 40.Dutta A, Palazzi DL. Candida non-albicans versus Candida albicans fungemia in the non-neonatal pediatric population. Pediatr Infect Dis J. 2011;30:664–668. doi: 10.1097/INF.0b013e318213da0f. [DOI] [PubMed] [Google Scholar]
- 41.Pemán J, et al. Epidemiology and antifungal susceptibility of bloodstream fungal isolates in pediatric patients: a Spanish multicenter prospective survey. J Clin Microbiol. 2011;49:4158–4163. doi: 10.1128/JCM.05474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puig-Asensio M, et al. Impact of therapeutic strategies on the prognosis of candidemia in the ICU. Crit Care Med. 2014;42:1423–1432. doi: 10.1097/CCM.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 43.Fernández-Ruiz M, et al. Initial use of echinocandins does not negatively influence outcome in Candida parapsilosis bloodstream infection: a propensity score analysis. Clin Infect Dis. 2014;58:1413–1421. doi: 10.1093/cid/ciu158. [DOI] [PubMed] [Google Scholar]
- 44.Bassetti M, et al. A multicenter multinational study of abdominal candidiasis: epidemiology, outcomes and predictors of mortality. Intensive Care Med. 2015;41:1601–1610. doi: 10.1007/s00134-015-3866-2. [DOI] [PubMed] [Google Scholar]
- 45.Lai CC, et al. Association between incidence of candidemia and consumption of antifungal agents at a medical center in Taiwan. Int J Antimicrob Agents. 2012;40:349–353. doi: 10.1016/j.ijantimicag.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 46.Bassetti M, et al. Incidence of candidemia and relationship with fluconazole use in an intensive care unit. J Antimicrob Chemother. 2009;64:625–629. doi: 10.1093/jac/dkp251. [DOI] [PubMed] [Google Scholar]
- 47.Pappas PG, et al. Clinical practice guideline for the management of candidiasis: 2016 updated by the Infectious Disease Society of America. Clin Infect Dis. 2016;62:e1–50. doi: 10.1093/cid/civ1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dufresne SF, et al. Epidemiology of Candida kefyr in patients with hematologic malignancies. J Clin Microbiol. 2014;52:1830–1837. doi: 10.1128/JCM.00131-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charsizadeh A, et al. Candidemia in children caused by uncommon species of Candida. Arch Pediatr Infect Dis. 2018;6(2):e11895. doi: 10.5812/pedinfect.11895. [DOI] [Google Scholar]
- 50.Singhal N, Kumar M, Kanaujai PK, Virdi JS. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol. 2015;6:791. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]