Abstract
This prospective British Ophthalmological Surveillance Unit (BOSU) study on dysthyroid optic neuropathy (DON) determines the incidence, presenting features and management throughout the UK. New cases were identified through the BOSU yellow card and an initial questionnaire and a subsequent 9-month follow-up questionnaire were posted out. From August 2015 to August 2016 DON was reported in 49 patients with 71 eyes affected, 22 patients had bilateral DON. The most common presenting symptom was blurred vision (83%) and the most common examination finding was upgaze restriction (85%). 85% of patients were initially treated with 3 days of either 1 g or 500 mg intravenous methyl prednisolone. We received 25 follow-up questionnaires (51% of the initial cohort) with 38 eyes treated for DON and 13 bilateral cases. The average steroid dose over 9 months was 4.5 g and 47% of patients had a surgical orbital decompression. The mean visual acuity gain after 9 months of follow-up for all patients was 0.25 LogMAR. The mean visual acuity gain after just medical therapy was 0.25 LogMAR and after both medical therapy and orbital decompression it was 0.24 LogMAR. In conclusion, the incidence of DON in the UK from this study is 0.75 per million population per annum. The majority of patients are treated with initial medical therapy and almost half of all patients subsequently went on to have an orbital decompression. With either medical therapy or medical and surgical therapy, vision can improve in patients with DON.
Introduction
Dysthyroid optic neuropathy (DON) is a sight-threatening condition, which occurs due to impaired optic nerve function secondary to Graves orbitopathy (GO). Sight-threatening GO is thought to account for about 2% of all cases of GO [1, 2]. The annual incidence of all cases of GO is estimated as 48 per million per year [3]. Sight-threatening GO includes DON, corneal exposure and globe subluxation, though DON makes up the majority of all cases of sight-threatening GO. If DON makes up approximately three-quarters of all cases of sight-threatening orbitopathy, the annual incidence of DON in the UK with a population of 65 million is ~62 cases per year.
The diagnosis of DON is often challenging and patients should be treated as soon as possible in order to reduce the risk of permanent visual compromise. The majority of the current literature supports initial treatment of DON with high dose intravenous steroids and surgical decompression if the response is poor or when high dose steroid therapy is not tolerated [4]. However, deviations from this management strategy were documented more than a decade ago and often seemed to be dictated by local access to ophthalmology and orbital input [5]. Due to the uncertainty around current UK trends in the management of DON, the British Ophthalmological Surveillance Unit (BOSU) funded and supported this study to identify the incidence of DON within the UK whilst determining typical presenting features and management.
The objectives of this BOSU study were to discover the incidence and distribution of DON in the United Kingdom and well as typical presenting features. The study would determine variations in management strategy of DON throughout the UK and their relation to clinical features and visual outcomes in treated DON.
Methods
Patients who presented to any hospital in the United Kingdom (UK) with DON from August 2015 to August 2016 were included within the study. New cases of DON were identified utilising the following criteria:
Any patient with Graves’ orbitopathy who has either disc swelling OR at least two of the following features not attributable to any other cause:
Impaired visual acuity subjectively or objectively.
Impaired colour vision: at least two errors on testing with Ishihara or HRR plates.
Relative afferent pupillary defect (RAPD) (patient with asymmetrical or unilateral DON).
Reproducible defect on automated perimetry in patient with VA of 6/18 or better, OR abnormal visual evoked potential (VEP).
All consultant ophthalmologists in the UK receive the BOSU yellow card, which advertises the current studies open to recruitment. Recruitment to studies is voluntary and data collection can only occur once the consultant has returned the yellow card. Ethics approval was obtained from the National Research Ethics Service (NRES) and no patient identifiable information was collected.
The DON study was placed on the yellow card in August 2015 for 1 year and consultants who reported a case of DON received an initial questionnaire to complete. A follow-up questionnaire was subsequently sent out at 9 months to collect data post treatment. These questionnaires were made available and presented at the British Oculoplastic Surgical Society annual scientific meeting in 2014.This helped ensure that consultants managing thyroid eye disease were aware that the study was about to commence and that they were satisfied with the questionnaires.
Results
This BOSU study recruited 49 patients (11 male, 38 female) with a mean age of 61 (SD = 13) (range 27–84). Figure 1 shows the distribution of patients throughout the UK. The largest number of reported cases was in Greater London (Table 1) and the number of cases reported in each region directly correlated to the regional population. Table 2 presents the incidence in each country within the United kingdom, with the highest rates of DON in Scotland. The initial data also revealed that 87% of patients were white Caucasian and Table 3 presents the mixture of ethnicities who presented with DON.
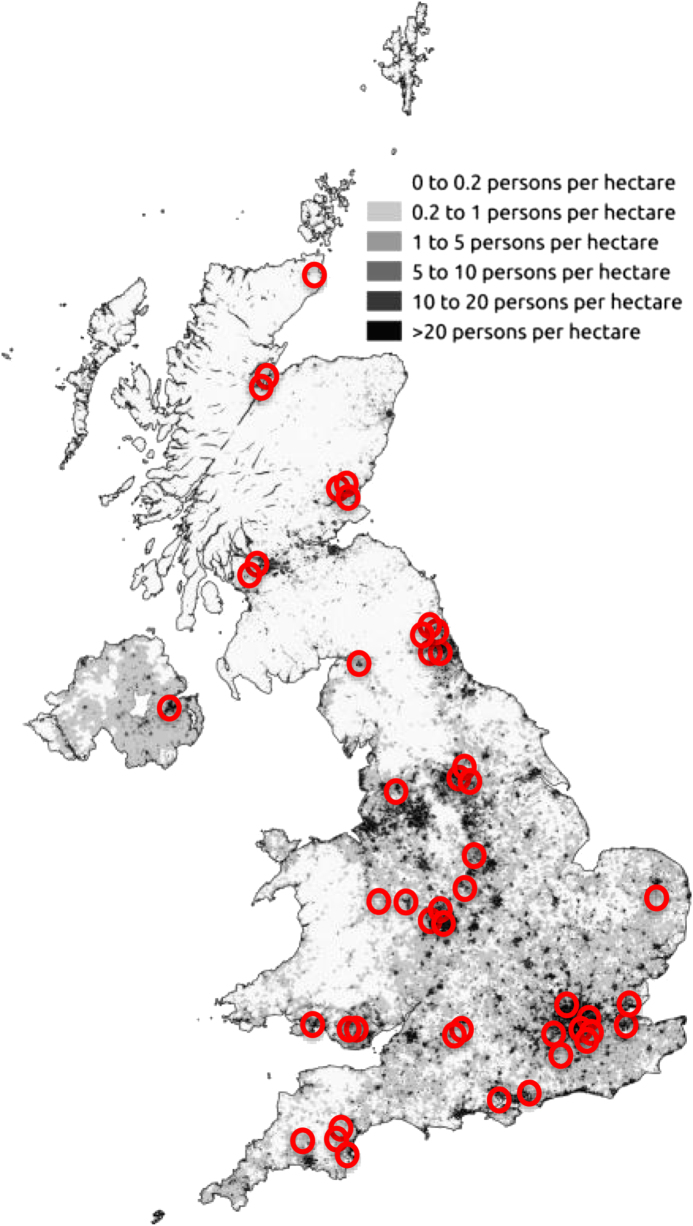
Fig. 1.
The number of cases of DON superimposed on a map from the 2011 census
Table 1.
The distribution of DON patients throughout the UK
| Location | Number of patients |
|---|---|
| North east | 6 |
| North west | 4 |
| Midlands | 5 |
| East | 2 |
| South West | 5 |
| South East | 2 |
| South | 3 |
| London | 9 |
| Wales | 5 |
| Scotland | 7 |
| Northern Ireland | 1 |
Table 2.
The incidence of DON in countries in the UK
| Country | Per million per annum |
|---|---|
| England | 0.7 |
| Northern Ireland | 0.55 |
| Scotland | 2.7 |
| Wales | 1 |
Table 3.
The ethnicities who presented with DON in the UK
| Ethnicity | Number of patients |
|---|---|
| White British | 43 |
| Chinese | 1 |
| Romanian | 1 |
| African | 2 |
| Caribbean | 1 |
| Maltese | 1 |
Following recruitment, twelve patients had been referred on to another hospital for further management of DON. Half of the patient’s blood tests revealed they were hyperthyroid at diagnosis and 42% were taking carbimazole. The initial questionnaire revealed 69% were smokers or ex smokers and only 6% had received radioactive iodine in the past 1 year. Three patients had glaucoma and three patients had known diabetic retinopathy. From the 49 reported patients, 71 eyes were recorded as having DON, 22 patients had bilateral DON, 17 in just the right eye and 10 in just the left eye.
The most common presenting symptom was blurred vision (83%) and 72% had reduced colour vision on assessment (Table 4). The average presenting vision was LogMAR 0.76 (range 0–2) in the right eye and LogMAR 0.46 in the left eye (range 0–2) (Supplementary Graph 1). Mean proptosis was 23.5 mm in the right eye and 23.5 mm in the left eye (range 16–32 mm, SD = 2.9). In our DON patient cohort 58% had proptosis >23 mm and 50% of the bilateral cases had no difference in proptosis between each eye.
Table 4.
Presenting symptoms in patients with DON in the UK
| Presenting symptom | Percentage of patients reporting symptom at presentation with DON (%) |
|---|---|
| Blurred vision | 83 |
| Change in colour vision | 72 |
| Awareness of visual field defect | 13 |
| Lid swelling | 1 |
| Diplopia | 1 |
| Asymtomatic | 13 |
The most common examination finding at presentation was upgaze restriction, present in 85% of patients; 71% also had chemosis. A relative afferent pupillary defect (RAPD) was present in 42% of patients and 63% of unilateral cases had an RAPD. When examining the optic nerve, 21% of patients were found to have optic disc swelling at presentation and only 7% of patients had choroidal folds. Imaging was completed in 86% of patients. Out of these patients who had imaging, 52% had a CT and 48% had an MRI. Visual fields were abnormal in 42% of DON patients and 14% of patients underwent a visual evoked potential as part of their investigations.
Of the 49 patients only 1 patient did not undergo treatment for DON due to mental health related issues. In 1 patient the initial treatment was not recorded, therefore 47 patients’ initial treatments in the first 72 h after diagnosis were recorded. Of these 51% received IV methyl prednisolone (IVMP) 1 g each day for 3 days, 34% received IVMP 500 mg each day for 3 days and 2% received IVMP 250 mg each day for 3 days. Only one patient received a primary orbital decompression and 6% of patients were commenced on an oral steroid regime. Orbital radiotherapy was organised for 12% of patients as part of their ongoing management and 6% had their initial steroid treatment reduced specifically because they were diabetic.
After 9 months the responsible clinicians were sent a follow-up questionnaire. Twenty-five questionnaires were returned which included 13 bilateral cases, 5 left eyes and 7 right eyes totalling 38 eyes. The mean age of the patients was 62 (SD 13.25). Two patients had passed away during the 9-month period. On review of these follow-up patients, 77% were female, 24% were male and 88% were classified as white British.
Out of the 25 patients, 48% had received 1 g IVMP for 3 days as their initial treatment and 44% received 500 mg IVMP for 3 days. One patient received no treatment and 1 patient received IVMP 250 mg for 3 days as initial treatment. The follow-up questionnaire reviewed associated side effects from initial treatments and found that 2 patients had poor diabetic control after their intravenous steroids and 1 patient had significant gastrointestinal upset. After initial treatment 36% of patients were treated with a reducing regime of oral steroids.
One patient received rituximab, 1 patient received azathioprine and 2 patients received orbital radiotherapy. The average dose of steroid over 9 months was 4.5 g (range 1.3–8 g, median 4.5 g) and 30% of patients were taking selenium.
Out of the 38 eyes with follow-up 47% received an orbital decompression within 9 months. All of those patients who had a decompression had at least their medial wall decompressed. Of the medial wall decompression, 83% were completed by an ophthalmologist and the remaining 17% had their medial wall decompressed by an ENT surgeon. When reviewing the type of decompression which was performed, 33% of patients had just their medial wall decompressed, 55% had their medial and lateral wall decompressed and 12% had their medial, lateral and floor decompressed. The mean wait for an orbital decompression post diagnosis of DON was two and half months. One patient had visual reduction to perception of light due to a significant intraoperative haemorrhage during decompression surgery and another patient had intractable diplopia post decompression.
The mean visual acuity gain after 9 months of follow-up for all patients was 0.25 LogMAR (SD = 0.57). The mean visual acuity gain after just medical therapy was 0.25 LogMAR (SD = 0.37)and after both medical therapy and orbital decompression it was 0.24 LogMAR (SD = 0.73) After 9 months, 21% of patients were reported to have significant visual field loss. Proptosis reduced by 4 mm on average in the right eye and 2.5 mm on average in the left eye. Eyes which had been treated with both medical therapy and decompression on average had a reduction in proptosis of 4.5 mm. Those treated with just medical therapy alone had on average a reduction of 2.5 mm in proptosis. After 9 months 21% of treated eyes had an RAPD, 24% had optic atrophy and 32% of patients had a visual field defect due to DON. No patients had been registered as sight impaired or severely sight impaired after 9 months follow-up.
Discussion
This prospective BOSU study into DON is the first in the United Kingdom. The annual incidence of DON is estimated to be 0.96 cases per million population per annum [6]. Assuming the UK population is 65 million we can extrapolate that there should be ~62 cases of DON in the UK per annum. The BOSU study captured 49 patients with DON which is 79% of all estimated cases in the UK, therefore, we can deduce that the sample was representative. The incidence rate of DON from this BOSU study in the UK is 0.75 per million population per annum.
The study has made it possible to review the distribution of DON utilising the first half of the patients post code. To get a sense of how DON directly relates to population density, Fig. 1 shows the number of cases superimposed on a map from the 2011 census. As expected the number of DON cases broadly corresponds to the most densely populated areas, with some hotspots and some notable areas of no cases of DON. For instance, there is a large triangular area in the map that includes the counties of East Riding, Lincolnshire, Nottinghamshire, Leicestershire, Northamptonshire, Cambridgeshire, Suffolk, Buckinghamshire, Oxfordshire, Herefordshire, Gloucestershire, and Wiltshire. This area has a population of 7.5 million and the expected annual incidence of DON is ~7 instead of 0. There are also wide differences in the incidence of DON by country within the UK, with the incidence being highest in Scotland (2.7/million/annum), followed by Wales (1/million/annum), England (0.7/million/annum), and Northern Ireland (0.55/million/annum). The distribution of DON in the UK does not match that of smoking incidence (Fig. 2).
Fig. 2.
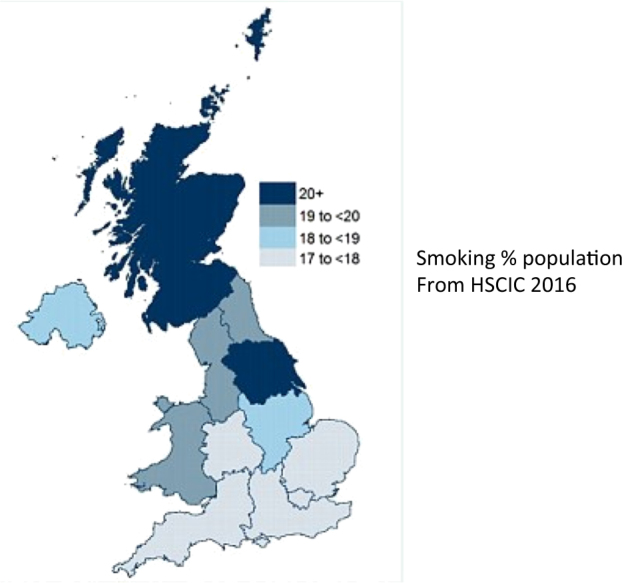
This figure shows that the distribution of DON in the UK does not match that of smoking incidence
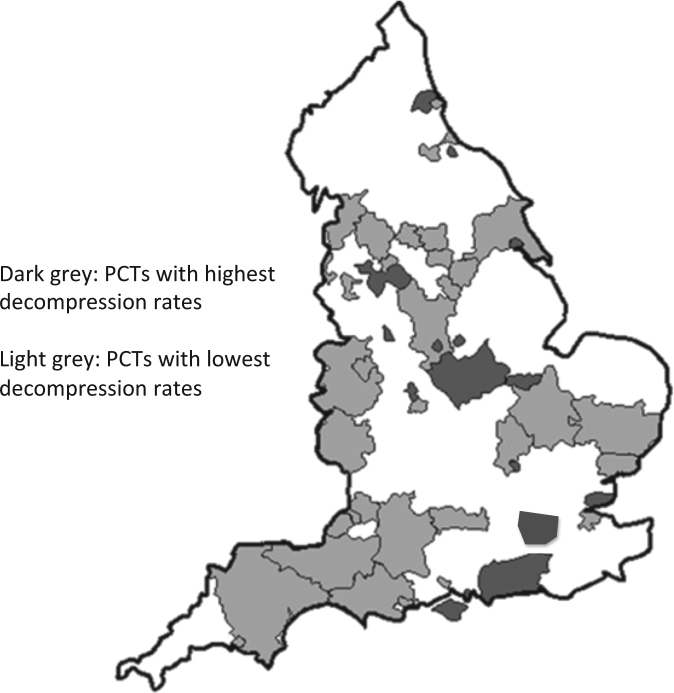
The reasons for the apparent uneven distribution are unclear. They may be explained on the basis of a very low incidence and small sample size, thus resulting in misleading trends. The above observations could also be explained on the basis of cases not being reported through BOSU, or not being detected. The distribution of DON in this study does resemble that of surgical orbital decompressions (Fig. 3) performed, which in turn correlated to proximity to a major orbital centre [7, 8].
Fig. 3.
The distribution of DON in this study does resemble that of surgical orbital decompressions
This study agrees with the current literature that older, hyperthyroid, smoking patients are at the highest risk of DON [9]. The study however does have a higher percentage of female patients affected in comparison to Mckeag et al. [10]. Forty-five percent of cases are bilateral and this study highlights the importance of looking carefully for bilateral disease and not relying on a RAPD. The most common presenting feature is blurred vision and the most common examination finding is restricted upgaze with the majority of patients having reduced colour vision.
Patients presented with a wide range of visual acuity (0–2 logMAR) and it is important to consider DON when vision may appear normal. There was also a wide range in proptosis measurements (16–32 mm) and clinicians must be aware that a tight orbit can result in optic nerve compression without proptosis [11]. The presence of an RAPD and optic disc swelling are indicative of DON however in this study it has been reported to occur without either sign. If optic neuropathy is challenging to diagnose visual evoked potentials (VEP) can be useful in supporting a diagnosis of DON [12, 13]. Only 14% of patient had a VEP which may imply that either they were not necessary to make the diagnosis of DON or that VEPs are not readily available in ophthalmology units. The study also did not find an association between choroidal folds and DON.
When reviewing treatments, 85% of patients were commenced on initial medical treatment which followed the European Group on Graves’ Orbitopathy (EUGOGO) protocol [14]. Only one patient had a primary decompression and 6% of patients were treated with oral steroids. The patients treated with oral steroids were in hospitals which do not provide orbital decompression surgery. Six patients had their steroid regime altered due to their diabetes. It is important to consider the potential side effects of high dose IV steroids and diabetics should have their blood sugars closely monitored throughout treatment.
The 9-month follow-up questionnaires had a 51% response rate from the initial cohort of patients. This dropout rate is commonly experienced with questionnaire based prospective studies and clearly the BOSU study would have been more powerful if this was lower. The two patients who passed away during follow-up died of cardiac disease. It is not known whether the treatment with steroids were directly related to the patients’ cardiovascular disease however it is well known that steroids can induce hyperglycaemia, hypertension, dyslipidaemia and central obesity which all contribute to cardiovascular disease [15]. Within the follow-up cohort 92% of patients had received treatment in concordance with the EUGOGO guidelines on the management of DON. A third of patients received a reducing regime of oral steroids after their intravenous therapy. There is no current consensus on oral steroids regimes post intravenous steroids for DON, however, their use has been described and a clinical decision must be made depending on the patients initial response to IV steroids [16]. We do know that IV steroids are more effective with less side effects than oral steroids for active thyroid eye disease [19].
Treatments with further immunomodulatory drugs are typically commenced after treatment with steroids for DON however recent encouraging publications on Teprotumumab for active GO may result in a change of practise in the future [18, 19]. Only a third of patients were taking selenium at 9 months. There is currently a broad range of evidence supporting selenium supplementation for 6 months and its role in improving the course of GO in patients with mild active disease [20]. This should be actively promoted when managing GO patients and further education of clinicians may facilitate this. Only two patients received orbital radiotherapy after their initial treatment for DON. This would appear reasonable as we know orbital radiotherapy has a delayed effect in GO and initially may induce further inflammation after treatment, which could be detrimental in DON [21]. If however the patient has had a good response to initial DON treatment and they remain active with no evidence of optic neuropathy, then orbital radiotherapy could be considered however recent studies have questioned its benefits [22].
Almost half of all patients treated for DON had an orbital decompression within 9 months. Those patients who did not receive a decompression in the study either developed a significant improvement in their vision after medical treatment or were being managed in a unit which did not provide orbital surgery. It is important to also consider that those patients may have not progressed on to decompression if the clinician did not feel there would be significant benefit or if the patient declined surgery. This BOSU study highlights the variety of treatments available for DON. Due to the complexity of the condition we should aim to streamline patients who have diagnosed DON to ensure that they receive appropriate initial therapy and are subsequently managed in an orbital centre so that any intervention can be performed when necessary. If a medial wall decompression is necessary this can either be completed by an orbital surgeon or an ENT surgeon with ophthalmology follow-up.
In this BOSU study, patients who were treated with either IVMP or IVMP and orbital decompression on average had a visual improvement of 0.25 LogMAR. This figure shows that visual loss from DON is somewhat reversible with treatment. Those patients who present with only minimal visual loss retain better vision post treatment than those who presented with significant visual loss. This would imply that an early presentation and treatment when there has not been significant visual decline would result in patients retaining as much vision as possible. Only 20% of patient had a visual field defect due to DON after follow-up and no patients had been registered as sight impaired however one patient’s vision at follow-up did meet the criteria.
Previous publications have shown that proptosis improves in active GO patients treated with IVMP [23, 24]. In our cohort those patients who received medical treatment alone had on average a 2.5 mm reduction in proptosis, which is 2 mm less than those treated both medically and surgically. Within the group of patients who had an orbital decompression a third of these only had their medial wall decompressed. The removal of the medial wall is most important for relieving pressure on the optic nerve, however, further reduction in proptosis can be achieved by completing a balanced medial and lateral wall orbital decompression [24]. Orbital decompression has potentially serious complications and patients should be well counselled before undergoing surgery as one of our patients had significantly reduced vision post surgery and another had intractable diplopia.
DON is a rare condition in the UK (0.75 cases per million) which is a challenge to both diagnose and treat. In the UK, DON is well managed, however, improvements could be made by ensuring that all patients have streamlined access to their regional thyroid eye clinic and orbital service.
Electronic supplementary material
Acknowledgements
We thank the British Ophthalmological Surveillance Unit (BOSU), part of the Royal College of Ophthalmologists, for the award of the Red bursary in 2014 and their continued support throughout the study. We also thank and acknowledge the input of the ophthalmologists throughout the UK who reported case of dysthyroid optic neuropathy through the BOSU yellow card; and also the input and feedback on the manuscript from members of TEAMeD (Thyroid Eye Disease Amsterdam Declaration Implementation Group).
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflict of interest.
Footnotes
The initial results have been presented at the British Oculoplastic Surgery Society congress in 2017.
Electronic supplementary material
The online version of this article (10.1038/s41433-018-0144-x) contains supplementary material, which is available to authorized users.
References
- 1.Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, et al. Prevalence and natural history of Graves’ orbitopathy in a large series of patients with newly diagnosed graves’ hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013;98:1443–9. doi: 10.1210/jc.2012-3873. [DOI] [PubMed] [Google Scholar]
- 2.Perros, et al. PREGO (presentation of Graves’ orbitopathy) study: changes in referral patterns to European Group On Graves’ Orbitopathy (EUGOGO) centres over the period from 2000 to 2012. Br J Ophthalmol. 2015;99:1531–5. doi: 10.1136/bjophthalmol-2015-306733.. [DOI] [PubMed] [Google Scholar]
- 3.Laurberg, et al. Incidence and clinical presentation of moderate to severe graves’ orbitopathy in a Danish population before and after iodine fortification of salt. J Clin Endocrinol Metab. 2012;97:2325–32. doi: 10.1210/jc.2012-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits M, Perros P, Boboridis K, Boschi A, Currò N, Daumerie C, Kahaly GJ, Krassas GE, Lane CM, Lazarus JH, Marinò M, Nardi M, Neoh C, Orgiazzi J, Pearce S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, Wiersinga WM. European Group on Graves’ Orbitopathy (EUGOGO). Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Thyroid. 2008;18:281–2. doi: 10.1089/thy.2007.0315. [DOI] [PubMed] [Google Scholar]
- 5.Perros, et al. A questionnaire survey on the management of Graves’ orbitopathy in Europe. Eur J Endocrinol August. 2006;1:155207–11. doi: 10.1530/eje.1.02201. [DOI] [PubMed] [Google Scholar]
- 6.Perros P, Hegedüs L, Bartalena L, Marcocci C, Kahaly GJ, Baldeschi L, Salvi M, Lazarus JH, Eckstein A, Pitz S, Boboridis K, Anagnostis P, Ayvaz G, Boschi A, Brix TH, Currò N, Konuk O, Marinò M, Mitchell AL, Stankovic B, Törüner FB, von Arx G, Zarković M, Wiersinga WM. Graves’ orbitopathy as a rare disease in Europe: a European Group on Graves’ Orbitopathy (EUGOGO) position statement. Orphanet J Rare Dis. 2017;12:72. doi: 10.1186/s13023-017-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dharmasena A, Keenan TD, Goldacre MJ. Orbital decompression for thyroid-associated orbitopathy in England: trends over time and geographical variation. Orbit. 2014;33:109–14. doi: 10.3109/01676830.2013.851707. [DOI] [PubMed] [Google Scholar]
- 8.Perros P, Chandler T, Dayan CM, Dickinson AJ, Foley P, Hickey J, MacEwen CJ, Lazarus JH, McLaren J, Rose GE, Uddin JM, Vaidya B. Orbital decompression for Graves’ orbitopathy in England. Eye. 2012;26:434–7. doi: 10.1038/eye.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon Chan, Shin JaeHo, Woo KyungIn, Kim YoonDuck. Clinical profile and visual outcomes after treatment in patients with dysthyroid optic neuropathy. Korean J Ophthalmol. 2012;26:73–9. doi: 10.3341/kjo.2012.26.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeag D, Lane C, Lazarus JH, Baldeschi L, Boboridis K, Dickinson AJ, Hullo AI, Kahaly G, Krassas G, Marcocci C, Marinò M, Mourits MP, Nardi M, Neoh C, Orgiazzi J, Perros P, Pinchera A, Pitz S, Prummel MF, Sartini MS, Wiersinga WM. European Group on Graves’ Orbitopathy (EUGOGO), Clinical features of dysthyroid optic neuropathy: a European Group on Graves’ Orbitopathy (EUGOGO) survey. Br J Ophthalmol. 2007;91:455–8. doi: 10.1136/bjo.2006.094607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rootman J. Retrobulbar pressures in dysthyroid optic neuropathy. Br J Ophthalmol. 1996;80:1034. doi: 10.1136/bjo.80.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsaloumas MD, Good PA, Burdon MA, Misson GP. Flash and pattern visual evoked potentials in the diagnosis and monitoring of dysthyroid optic neuropathy. Eye. 1994;8:638–45. doi: 10.1038/eye.1994.159. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosio G, Ferrara G, Vitale R, De Marco R. Visual evoked potentials in patients with Graves’ ophthalmopathy complicated by ocular hypertension and suspect glaucoma or dysthyroid optic neuropathy. Doc Ophthalmol. 2003;106:99–104. doi: 10.1023/A:1022561530782. [DOI] [PubMed] [Google Scholar]
- 14.Bartalena Luigi, Baldeschi Lelio, Boboridis Kostas, Eckstein Anja, Kahaly George J., Marcocci Claudio, Perros Petros, Salvi Mario, Wiersinga Wilmar M. The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves' Orbitopathy. European Thyroid Journal. 2016;5(1):9–26. doi: 10.1159/000443828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol. 2000;16:505–11. [PubMed] [Google Scholar]
- 16.Guy JR, Fagien S, Donovan JP, Rubin ML. Methyl prednisolone pulse therapy in severe dysthyroid optic neuropathy. Ophthalmology. 1989;96:1048–52. doi: 10.1016/S0161-6420(89)32784-9. [DOI] [PubMed] [Google Scholar]
- 17.Kahaly J, et al. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005;90:5234–40. doi: 10.1210/jc.2005-0148. [DOI] [PubMed] [Google Scholar]
- 18.Terry JS, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376:1748–61. doi: 10.1056/NEJMoa1614949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcocc C, et al. Claudio Marcocc, selenium and the course of mild Graves’ orbitopathy. N Engl J Med. 2011;364:1920–31. doi: 10.1056/NEJMoa1012985. [DOI] [PubMed] [Google Scholar]
- 20.Bartalena L, Marcocci C, Tanda ML, Rocchi R, Mazzi B, Barbesino G, Pinchera A. Orbital radiotherapy for Graves’ ophthalmopathy. Thyroid. 2002;12:245–50. doi: 10.1089/105072502753600223. [DOI] [PubMed] [Google Scholar]
- 21.Tambe K, Bhargava J, Tripathi A, Gregory M, Burns J, Sampath R. The role of intravenous methyl prednisolone immunosuppression in the management of active thyroid eye disease. Orbit. 2010;29:227–31. doi: 10.3109/01676831003660663. [DOI] [PubMed] [Google Scholar]
- 22.Rajendram Rathie, Taylor Peter N, Wilson Victoria J, Harris Nicola, Morris Olivia C, Tomlinson Marjorie, Yarrow Sue, Garrott Helen, Herbert Helen M, Dick Andrew D, Cook Anne, Gattamaneni Rao, Jain Rajni, Olver Jane, Hurel Steven J, Bremner Fion, Drummond Suzannah R, Kemp Ewan, Ritchie Diana M, Rumsey Nichola, Morris Daniel, Lane Carol, Palaniappan Nachi, Li Chunhei, Pell Julie, Hills Robert, Ezra Daniel G, Potts Mike J, Jackson Sue, Rose Geoffrey E, Plowman Nicholas, Bunce Catey, Uddin Jimmy M, Lee Richard W J, Dayan Colin M. Combined immunosuppression and radiotherapy in thyroid eye disease (CIRTED): a multicentre, 2 × 2 factorial, double-blind, randomised controlled trial. The Lancet Diabetes & Endocrinology. 2018;6(4):299–309. doi: 10.1016/S2213-8587(18)30021-4. [DOI] [PubMed] [Google Scholar]
- 23.Cirić J, Zarković M, Stojanović M, Pepenezić Z, Randjelović G, Gligorović M, Trbojević B, Drezgić M, Nesović M. Treatment of Grave’s ophthalmopathy with high doses of corticosteroids. Srp Arh Celok Lek. 2000;128:179–83. [PubMed] [Google Scholar]
- 24.Baldeschi L, MacAndie K, Hintschich C, Wakelkamp IM, Prummel MF, Wiersinga WM. The removal of the deep lateral wall in orbital decompression: its contribution to exophthalmos reduction and influence on consecutive diplopia. Am J Ophthlmol. 2005;140:642–7. doi: 10.1016/j.ajo.2005.04.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.