Highlights
-
•
Neuromuscular activity is suppressed during maximal eccentric muscle contraction in untrained subjects, evidenced by decreased electromyography signal amplitude, attenuated evoked H-reflex responses, increased autogenic motor neuron inhibition, and decreased excitability in descending corticospinal motor pathways.
-
•
Heavy-load resistance training yields marked gains in eccentric muscle strength owing to increased excitability of spinal motor neurons, decreased presynaptic or postsynaptic inhibition of spinal motor neurons, and likely also involving elevated descending motor drive from supraspinal centers.
-
•
Increased eccentric muscle strength induced by heavy-load resistance training provides an important basis for enhanced neuromuscular performance in athletes, as well as nonathletes, including older adults and clinical patients.
Keywords: Corticospinal excitability, Eccentric muscle contraction, H-reflex, Neuromuscular plasticity, Resistance training, V-wave
Abstract
Neuromuscular activity is suppressed during maximal eccentric (ECC) muscle contraction in untrained subjects owing to attenuated levels of central activation and reduced spinal motor neuron (MN) excitability indicated by reduced electromyography signal amplitude, diminished evoked H-reflex responses, increased autogenic MN inhibition, and decreased excitability in descending corticospinal motor pathways. Maximum ECC muscle force recorded during maximal voluntary contraction can be increased by superimposed electrical muscle stimulation only in untrained individuals and not in trained strength athletes, indicating that the suppression in MN activation is modifiable by resistance training. In support of this notion, maximum ECC muscle strength can be increased by use of heavy-load resistance training owing to a removed or diminished suppression in neuromuscular activity. Prolonged (weeks to months) of heavy-load resistance training results in increased H-reflex and V-wave responses during maximal ECC muscle actions along with marked gains in maximal ECC muscle strength, indicating increased excitability of spinal MNs, decreased presynaptic and/or postsynaptic MN inhibition, and elevated descending motor drive. Notably, the use of supramaximal ECC resistance training can lead to selectively elevated V-wave responses during maximal ECC contraction, demonstrating that adaptive changes in spinal circuitry function and/or gains in descending motor drive can be achieved during maximal ECC contraction in response to heavy-load resistance training.
1. Introduction
During eccentric (ECC) muscle contraction, myofibers produce force while simultaneously being lengthened that, for electrically innervated muscle preparations in vitro, results in markedly greater (≥60% increased ) contractile force and work production compared with that observed during isometric (ISO) or shortening (concentric (CONC)) contraction conditions1, 2, 3 (Fig. 1). This phenomenon was first verified (extrapolated backwards) for intact human muscle by Abbott et al.4 In terms of intact human skeletal muscles, a marked deviation (∼50% force deficit) can be observed between the shape of the contractile force–velocity relationship when obtained in vivo in untrained subjects during maximal voluntary ECC contraction conditions5, 6, 7, 8, 9, 10, 11, 12 versus that recorded for isolated muscle and myofiber preparations in situ2, 3 (Fig. 1). Notably, however, highly strength-trained individuals seem to be capable of producing substantially higher ECC muscle forces (larger joint moments) compared with untrained subjects,10 suggesting that maximal ECC muscle strength capacity is trainable.
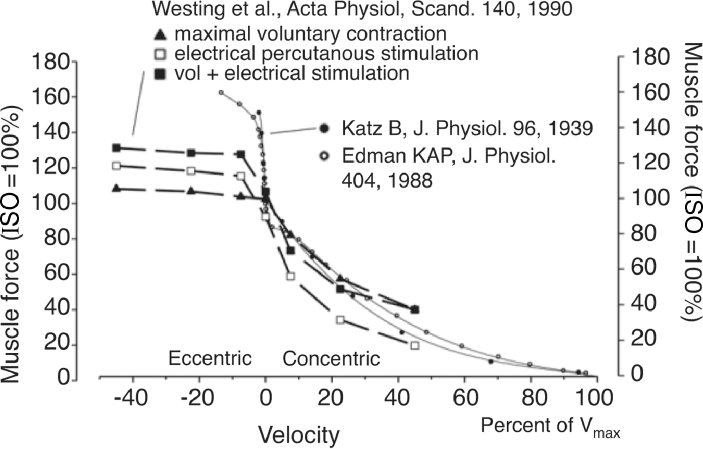
Fig. 1.
Contractile force–velocity relationships obtained for shortening (CONC) and lengthening (ECC) contractions in isolated in vitro preparations of whole muscle2 and single muscle fibres3 obtained from the frog (Rana Temporaria, m. sartorious at 11.5°C2; anterior tibialis muscle fibers at 1.4°C–1.5°C3). On the vertical axis (muscle force) a unit of 100 corresponds with a maximal ISO contraction force in vitro. On the velocity axis, 100% corresponds with Vmax. Positive and negative velocities denote CONC and ECC muscle actions, respectively. Superimposed curves show muscle strength measured in vivo during maximal voluntary activation and/or when percutaneous electrical stimulation was applied to the knee extensors.19In vivo muscle strength was obtained by use of isokinetic dynamometry as the maximal knee extensor torque generated at 60° knee joint angle (0° = full knee extension), during (a) maximal voluntary muscle activation (triangles), (b) electrical muscle stimulation (open boxes), and (c) electrical stimulation superimposed onto maximal voluntary contraction (closed boxes). To scale isokinetic knee joint angular velocity, a maximal angular velocity of 800°/s was assumed for maximal unloaded knee extension115, 116 with a force unit of 100, corresponding with the maximal voluntary ISO strength (MVC). CONC = concentric; ECC = eccentric; ISO = isometric; MVC = maximum voluntary contraction; Vmax = maximal unloaded contraction velocity. Adapted from Aagaard et al.21 with permission.
ECC contractions play a crucial role in the production and control of movement13 and have been suggested to be uniquely controlled by the central nervous system,14, 15, 16, 17 typically characterized by a more variable motor output compared with CONC contraction conditions.18 Suggesting the presence of inhibitory neural mechanism(s), electrical muscle stimulation superimposed onto maximal voluntary contractions has been observed to selectively increase active force production during ECC but not CONC muscle actions,10, 19,20 causing the resulting force–velocity relationship to more closely resemble that observed for isolated muscle or myofiber preparations21 (Fig. 1).
High levels of ECC muscle strength are required in many types of sports, because this strength provides an enhanced capacity to decelerate movements in very short time and thereby perform fast stretch–shortening cycle actions (e.g., rapid jumping),22 while also allowing rapid shifts in movement direction (e.g., fast side-cutting movements).23 Furthermore, high ECC strength in antagonist muscles provides an enhanced capacity to decelerate and break movements at the end of the range of motion, thereby potentially protecting against injury to ligaments (e.g., the anterior cruciate ligament ((ACL)) and joint capsule structures.6, 24 High ECC strength in specific antagonist muscles also plays an important role for performing rapid limb deceleration at end of the range of motion in fast ballistic movements, thereby yielding a longer time for limb acceleration and thus allowing the attainment of higher movement speeds.25 Finally, high levels of ECC muscle strength may be desirable in older individuals to decrease the risk of falls during stair descent.
Signs of nonuniform muscle activation typically can be observed during maximal voluntary ECC muscle contractions in untrained subjects (Fig. 2),7, 26 and it has been suggested that such neural strategies may serve as a protective mechanism against cytoskeletal damage induced by repetitive ECC muscle actions,7, 27 which typically is observed when more uniform patterns of myofiber recruitment are evoked by means of electrical percutaneous or motor nerve stimulation.28, 29
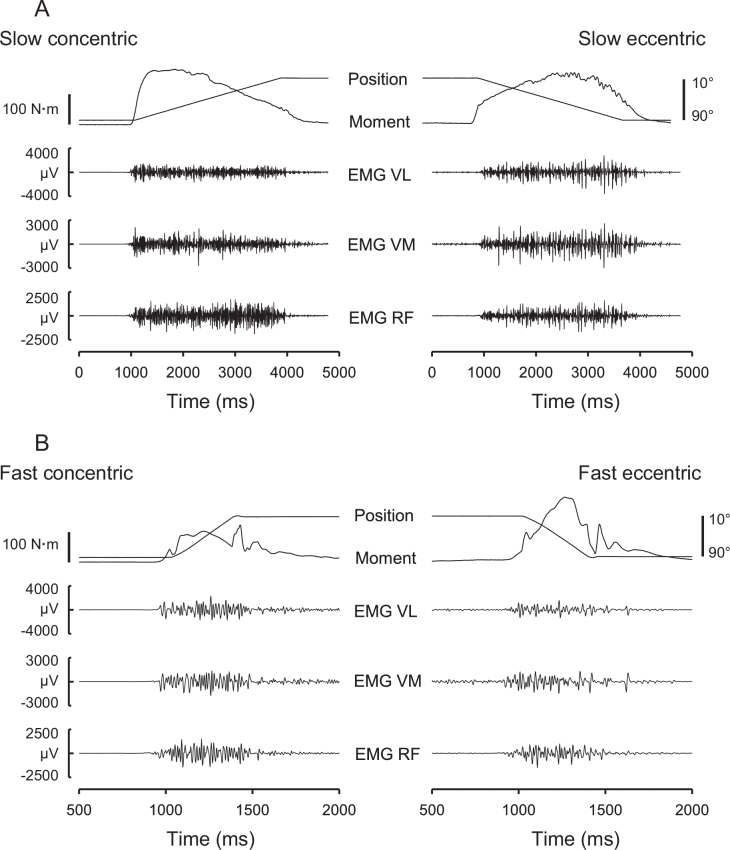
Fig. 2.
Raw tracings of isokinetic knee joint moment and (EMG signals obtained in an untrained male subject during maximal CONC (left) and ECC (right) knee extensor contraction during joint movements performed at slow (A) and fast (B) joint angular speeds (30°/s and 240°/s, respectively). Range of joint motion was from 90° to 10° during CONC contraction and from 10° to 90° during ECC contraction (0° = full knee extension). Note the appearance of large EMG amplitude spikes separated by short interspike periods of no or low neuromuscular activity during ECC contraction conditions, indicating a more nonuniform pattern of muscle activation during maximal ECC compared with CONC muscle actions in untrained individuals. CONC = concentric; ECC = eccentric; EMG = electromyography; VL = vastus lateralis, VM = vasus medialis, RF = rectus femoris. Adapted from Aagaard et al.7 with permission.
2. Mechanical muscle function during ECC muscle actions of maximal voluntary effort
Untrained individuals typically demonstrate a levelling off (plateauing) in maximal muscle strength during slow CONC or ECC muscle actions, whereas strength-trained individuals do not.5, 6 Notably, this plateauing in maximal muscle strength can be removed in response to heavy-load resistance training (HLRT).3, 30,31 Furthermore, no plateauing seems to be present in highly resistance-trained athletes exposed to years of HLRT.6, 9 Conversely, resistance training using low external loads and high contraction speeds seems to have no effect on the plateauing phenomenon,5 suggesting that heavy-load resistance exercise (>80% 1 repetition maximum) should be used to diminish or fully remove the influence of this force-inhibiting mechanism. HLRT (i.e., resistance training using exercise loads ∼80%–85% 1 repetition maximum) consistently has been reported to result in marked gains in maximal ECC muscle strength.5, 12,26,31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 Moreover, resistance training using maximal ECC muscle contractions or coupled ECC–CONC contractions (i.e., involving stretch–shortening cycle muscle actions) seems to evoke greater gains in maximal ECC muscle strength than CONC training alone.32, 33, 34, 35,42, 44 In contrast, maximal ECC muscle strength seems to remain unaffected in response to low-load resistance training,5, 33,41, 45 suggesting that the exertion of high muscle forces during training is a key stimulus to inducing adaptive changes in maximal ECC muscle strength.
3. Neuromuscular aspects related to maximal ECC muscle actions
Aagaard et al.7 reported marked increases in normalized electromyography (EMG) signal amplitudes during maximal ECC muscle actions in response to 14 weeks of HLRT, demonstrating for the first time that the suppression (“inhibition”) in neuromuscular activity that normally is observed during maximal voluntary ECC muscle actions in untrained individuals can be removed by resistance training.7 This observation helps explain the substantial gain in ECC muscle strength typically observed with HLRT,5, 6, 7,9, 26 as well as the absence of ECC moment deflection (“plateauing”) in athletes exposed to many years of HLRT.6, 9 The strong neural influence on the expression of maximal ECC muscle strength in vivo and its adaptability to training are discussed in detail herein.
4. Neural regulation of ECC muscle force
Neural regulatory mechanism(s) that limit the recruitment and/or discharge rate of motor units (MUs) have previously been suggested to exist during maximal voluntary ECC muscle contraction.7,46, 47, 48, 49 Indirect evidence of such mechanisms(s) is given by the marked suppression in neuromuscular activity (reduced EMG amplitude) often observed during ECC vs. CONC contractions of maximal voluntary effort7, 11,12, 26,46,50, 51, 52 (Fig. 2). Moreover, maximal ECC muscle force (measured as knee extensor torque) was seen to increase by ∼30% when transcutaneous electrical muscle stimulation was superimposed onto voluntary contractions in untrained individuals; similar findings failed to be observed in resistance-trained athletes,9 indicating that the suppression in motor neuron (MN) activation is modifiable by resistance training. Furthermore, using direct femoral nerve stimulation53 or superimposed muscle-twitch analysis54, 55 during maximal ECC vs. ISO contraction conditions, it was estimated that central activation was reduced by ∼20%–50% when performing ECC muscle actions of maximal voluntary effort (knee extensors), at least in untrained individuals.
Although a selective recruitment of type II muscle fibers (and de-recruitment of type I fibers) has been suggested to exist during ECC contractions,56, 57 a majority of studies have shown that type II muscle fibers are not selectively activated during ECC muscle contractions in humans and that MU recruitment generally follows the Henneman size principle during voluntary ECC muscle actions.58, 59, 60, 61, 62, 63 Thus, at least in humans, potential inhibitory mechanisms related to ECC muscle actions are unlikely to reside in a de-recruitment of type I fibers.
Based on the 3-dimensional relationship between neuromuscular activity (EMG amplitude) vs. knee extensor moment expressed as functions of knee joint angle (muscle length) and angular velocity (contraction speed) and contraction mode (ECC vs. CONC)7 (Fig. 3), respectively, it was possible to test the hypothesis that spinal MN inhibition originated owing to afferent inhibitory inflow from the ACL to the central nervous system. About 1%–3% of the ACL ligament consists of mechanoreceptors (Ruffini end organs, Pacinian corpuscles) that mainly are located at the tibial and femoral insertion sites.64, 65 Using intra-articular electrical stimulation, evidence of a reflex pathway from the ACL that modulates the activity of the knee musculature has been observed in cats,66 as well as in intact humans,67 although no afferent function of the ACL could be detected after ACL reconstruction (8 months to 12 years after surgery).68 Given that forceful quadriceps contraction can result in significant tensile stress force generation in the human ACL within the distal knee extension range of motion69, 70, 71 (see Aagaard et al.24 for a more detailed review), it was hypothesized by Aagaard et al.7 that the existence of an inhibitory reflex pathway excited by increased ACL stress forces would result in a greater suppression in neuromuscular activity during high-force contractions (assumed to result in greater ACL strain) compared with low-force contractions at more extended knee joint positions. However, the 3-dimensional EMG–angle–velocity relationships reported by Aagaard et al.7 failed to confirm this hypothesis, which prompted the authors to suggest that the mechanism(s) of neural inhibition would originate from a pathway of force-negative neural feedback from the contracting muscle itself (i.e., Ib afferent input to spinal MNs) or via other sources of spinal regulatory mechanisms (presynaptic inhibition of muscle spindle Ia afferents, postsynaptic inhibition of MNs) that theoretically could include autogenic spinal inhibition mechanisms (recurrent Renshaw inhibition).27 However, although recurrent Renshaw inhibition is considered a factor limiting the discharge frequency of spinal MNs,72, 73 discharge rates did not differ between low-intensity ECC and CONC contractions in the human tibialis anterior muscle,74 suggesting that recurrent inhibition may not play a strong role for the inhibition of spinal MN activity during ECC muscle contraction, at least during low-force contraction conditions. In a very recent study, however, Barrué-Belou et al.75 for the first time measured the magnitude of recurrent Renshaw inhibition during maximal ECC muscle actions in humans, demonstrating that autogenic recurrent Renshaw inhibition was significantly increased during maximal ECC muscle actions compared with maximal ISO and CONC contraction conditions.
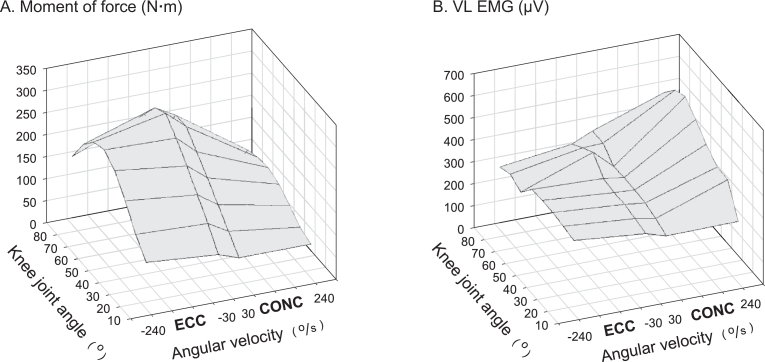
Fig. 3.
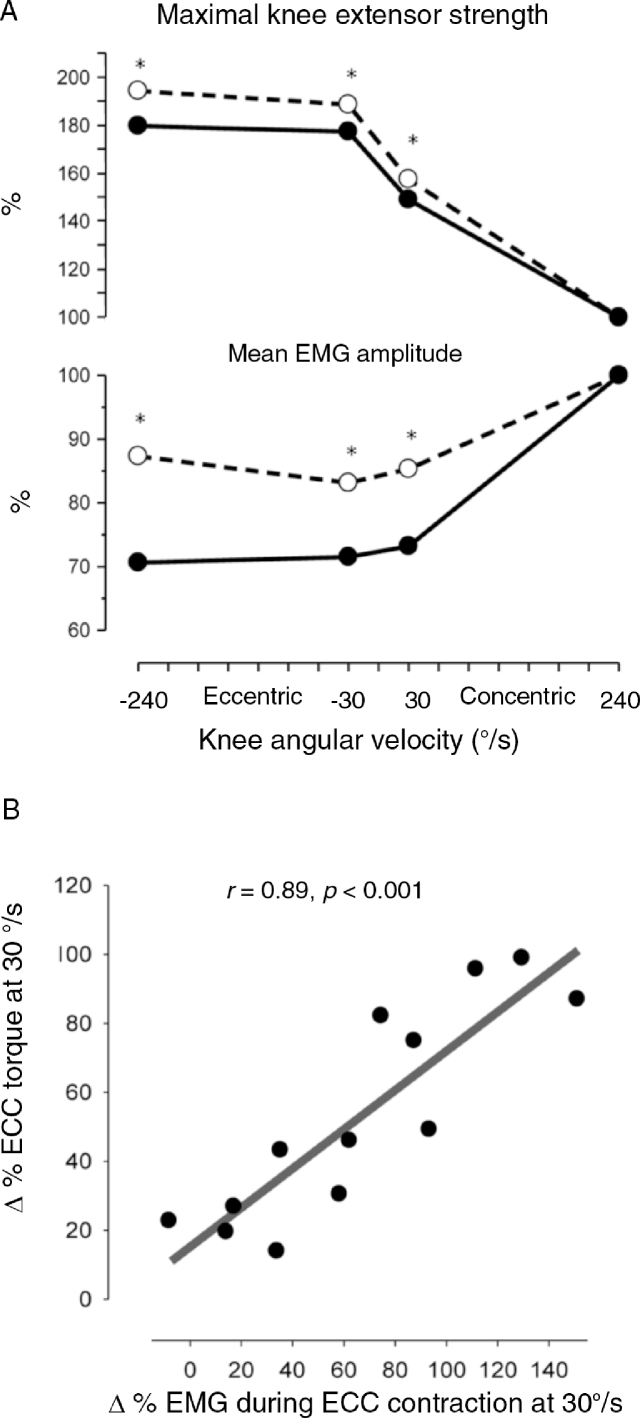
(A) Maximal CONC and ECC quadriceps muscle strength (moment of force) and (B) neuromuscular activity (VL EMG) obtained in 15 untrained subjects and displayed as a function of knee joint angle (averaged in 10° intervals) and joint angular velocity. Negative and positive velocities denote ECC and CONC muscle contraction, respectively. As seen in (B), a marked suppression in VL EMG appeared during maximal ECC and slow CONC contraction, compared with the EMG amplitudes recorded during fast CONC contraction. Thus, VL EMG was 26%–31% lower in slow ECC and CONC contraction and 47% lower in fast ECC contraction compared with fast CONC contraction when averaged at 60°–90° knee joint angle. In contrast, no suppression in EMG was observed at more extended knee joint positions, e.g., at 10°–40° joint angle. CONC = concentric; ECC = eccentric; VL EMG = vastus lateralis electromyography amplitude. Data adapted from Aagaard et al.7 with permission.
5. Neural regulation of ECC muscle force—Spinal evoked responses
The Hoffmann reflex (H-reflex) can be used to assess human spinal circuitry function in vivo,76 although the technique presents methodological advantages as well as potential limitations.77, 78, 79 Recording of evoked spinal H-reflex and V-wave (also an H-reflex variant) responses during maximal and submaximal muscle contraction have previously been used to examine the adaptive plasticity in neuromuscular function with training.50,80, 81, 82 In direct support of a neural inhibitory mechanism during ECC muscle actions in vivo, attenuated evoked spinal MN responses (reduced H-reflex amplitudes) have been observed during maximal ECC muscle contraction in untrained individuals46,48, 49, 50,83 (Fig. 4). In contrast, the size of the V-wave response that, among other factors reflects the magnitude of descending supraspinal motor drive to spinal MNs,81, 84 does not seem to differ between ECC and CONC contraction conditions,46, 50 indicating that central nervous system site(s) of inhibition are mainly of spinal origin. Owing to its relatively low intensity of peripheral nerve stimulation, the H-reflex is expected to mainly recruit the pool of spinal MNs of smaller soma size (presumably dominated by low-threshold MUs primarily innervating type I fibers),85, 86 whereas the V-wave owing to its maximal intensity of stimulation recruits both small- and large-sized MNs. Consequently, the differential modulation in H-reflex vs. V-wave amplitude during ECC muscle actions may suggest inhibitory synaptic inputs to spinal MNs to be more dominant in MUs of smaller size (as reflected by the preferential reduction in H-reflex amplitude) than in large-sized MUs (included in the V-wave response).
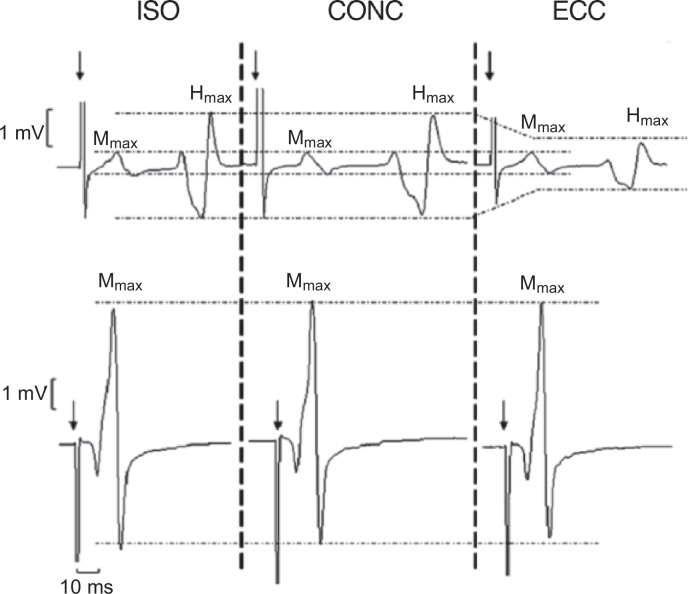
Fig. 4.
Spinal evoked H-reflex responses recorded in the soleus muscle during ISO, CONC, and ECC plantar flexor contractions of maximal voluntary effort. Note the depression in H-reflex amplitude during maximal ECC contraction, suggesting reduced spinal motorneuron excitability and/or increased presynaptic or postsynaptic inhibition. The Mmax remained unchanged across contraction modes (bottom) to verify that the depressed H-reflex response during ECC contraction was not a recording artifact. CONC = concentric; ECC = eccentric; Hmax = maximal H-wave; H-reflex = Hoffman reflex; Mmax = maximal M-wave; ISO = isometric. Adapted from Duclay and Martin46 with permission.
6. Neural regulation of ECC muscle force—Corticospinal excitability
Reflecting a decreased excitability in corticospinal pathways during ECC muscle actions, motor evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) have been reported to be decreased during maximal ECC contractions compared with CONC contractions when examined in the biceps brachii and brachioradialis muscles,47 as well as in the soleus48, 49,83 (Fig. 5). Likewise, MEP amplitudes recorded in the biceps brachii and brachioradialis muscles were 50%–70% reduced during submaximal (30% maximum voluntary contraction (MVC) ECC vs. CONC contraction conditions87 (Fig. 6). In contrast with these observations, no contraction-specific differences in MEP amplitude were observed for the medial gastrocnemius muscle, suggesting that the neural mechanisms responsible for the magnitude of maximal ECC muscle force production in vivo may differ between monoarticular (soleus) and biarticular (gastrocnemius) synergist muscles in the lower limbs.48, 49 Combined TMS and H-reflex experiments indicate a differential relative influence of cortical vs. spinal mechanisms in the modulation of neural activation during maximal ECC muscle contraction in vivo.47, 48, 49,83 For example, Gruber et al.47 observed that MEPs were diminished during maximal ECC elbow flexor contractions when elicited by TMS (MEP amplitude 16% reduced) and cervicomedullary stimulation (28% reduced), respectively. The significantly greater decrease in cervicomedullary stimulation was suggested to reflect elevated inhibition at the spinal level along with enhanced excitability at the cortical level, the latter evidenced by a significantly increased (+10%–20%) MEP/cervicomedullary stimulation ratio during maximal ECC muscle actions compared with ISO muscle actions.47 Similarly, during maximal ECC contraction conditions, H-reflex excitability seemed to be more depressed (−29%) than MEP amplitude (−19%) (soleus muscle),49 indicating that the neural control of maximal ECC muscle force production comprises a stronger modulatory influence on the excitability of the spinal pathway than on the corticospinal tract.17
Fig. 5.
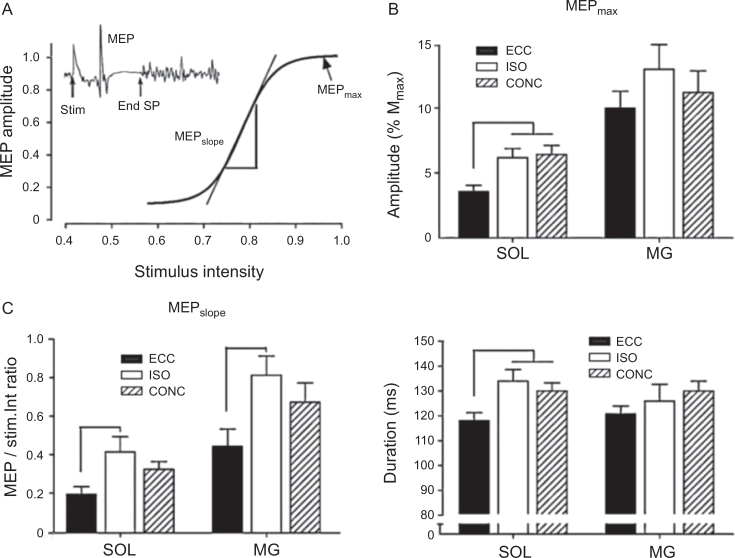
Modulation in corticospinal excitability during maximal ECC, ISO, and CONC contractions. (A) MEP and SP evoked in the soleus muscle by TMS and the associated input–output relation for 1 representative subject. The graph displays the amplitude of the MEP recorded in the target muscle expressed relative to TMS stimulus intensity. Main parameters are maximal MEP amplitude (MEPmax), the slope of the steeper part of the input–output relation (MEPslope), and the duration of the silent period (SP) recorded for the greatest stimulus intensity stim. Modulations of (B) MEPmax, (C) MEPslope, and (D) duration of SP during ECC, ISO, and CONC maximum voluntary contractions. Data are means ± SEM (n = 12) for the SOL and MG. *ECC vs. ISO (p < 0.05); ***ECC vs. ISO and CONC (p < 0.001). CONC = concentric; ECC = eccentric; ISO = isometric; MEP = motor evoked potential; MG = medial gastrocnemius; SOL = soleus; SP = silent period; stim. = stimulus artifact; TMS = transcranial magnetic stimulation. Data from Duclay et al.48 and adapted from Duchateau and Baudry16 with permission.
Fig. 6.
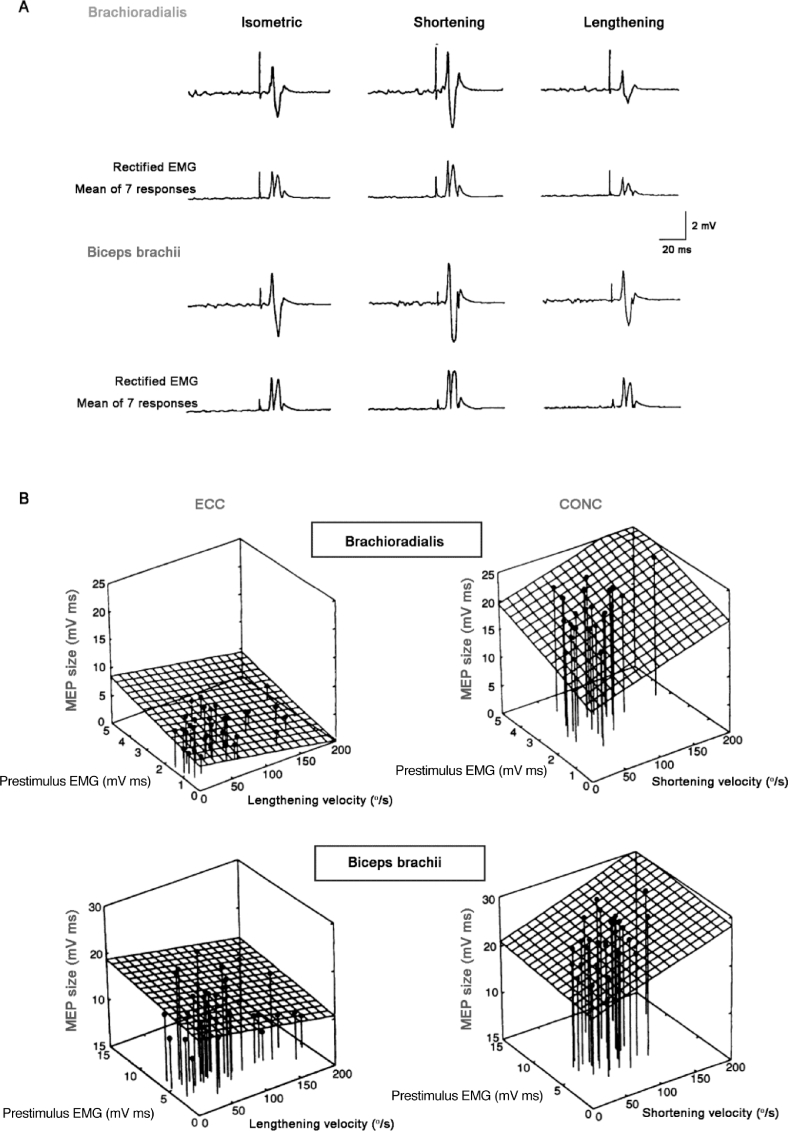
MEPs elicited in the elbow flexors by use of TMS during lengthening (ECC) and shortening (CONC) contractions performed at submaximal contraction intensity (1.5–5.0 kg loads, ∼20%–30% 1 RM). Note that MEP size is markedly decreased during ECC compared with CONC contraction conditions, even at comparable levels of prestimulus EMG activity. 1RM = 1 repetition maximum; CONC = concentric; EMG = electromyography; MEP = motor evoked potential; TMS = transcranial magnetic stimulation. Data adapted from Abbruzzese et al.87 with permission.
In addition to the above observations, poststimulus silent EMG periods induced by TMS were shortened to a greater extent during ECC contractions than during CONC contractions (compared with resting conditions) (Fig. 5), which was interpreted to reflect reduced amounts of intracortical inhibition during maximal ECC contraction conditions.48, 49 Collectively, these data suggest that the reduction in corticospinal excitability during ECC muscle contraction depend mainly on presynaptic or postsynaptic inhibitory circuitry pathways acting at the spinal level.47, 48, 49 In turn, these spinal mechanisms most likely are modulated by regulatory inputs from supraspinal pathways15, 17 (discussed in detail elsewhere in this article).
As suggested by Duclay et al.,48 the decrease in spinal excitability (suppressed H-reflex amplitude) observed with passive muscle lengthening (m. soleus) potentially could be attributed to presynaptic inhibitory mechanisms88, 89 induced by enhanced activity in muscle spindle Ia afferents themselves90 or to result from reduced spinal efficacy caused by homosynaptic postactivation depression.91 Although the spinal inhibitory mechanisms responsible for the modulation in MEP and H-reflex excitability may not be identical,92 the primary mechanism(s) that could explain the reduced H-reflex response observed during ECC muscle contractions could operate at both presynaptic and postsynaptic levels, at least for submaximal contraction intensities.74, 87 Because presynaptic inhibition seems to be lacking for corticospinal tracts synapsing onto spinal MNs,92 the lower recruitment gain of the MEP response observed during maximal ECC contraction in the soleus muscle48 could result from a decreased responsiveness of spinal MNs to the descending input.47 The observation of an ∼50% reduced MEP slope for the medial gastrocnemius muscle during maximal ECC compared with ISO MVCs (Fig. 6), despite a similar H-reflex response, suggests that at least in certain muscles spinal MN excitability is controlled also by postsynaptic inhibitory mechanisms.48 Intermuscular comparisons (soleus vs. medial gastrocnemius) of the differential modulation in MEP and H-reflex amplitude led Duclay et al.48, 49 to conclude that the suppression in corticospinal excitability observed during maximal ECC muscle contraction was mainly caused by peripheral inhibition at the spinal level induced by muscle lengthening, potentially including both presynaptic and postsynaptic inhibitory mechanisms.48, 49
Not all studies have been able to verify that corticospinal excitability is decreased during maximal ECC compared with ISO or CONC contraction conditions.93 Furthermore, signs of force plateauing and reduced surface EMG activity during ECC compared with ISO and CONC muscle actions also have failed to be observed in a number of studies. Thus maximal ECC strength (joint torque) obtained for the plantar flexors,93 adductor pollicis,94 quadriceps femoris,95 and tibialis anterior96 muscles were reported to exceed ISO MVC by ∼10%–50% and to be accompanied by comparable levels of EMG during ECC compared with ISO contraction conditions. This apparent discrepancy between studies might be caused by differences in the methodologic setup (e.g., using supine testing),93 differences in the specific training status or background of study participants, or differences in participant familiarization with the experimental procedures, among other reasons.
7. Effects of resistance training on the neural regulation of ECC muscle strength
Several studies have demonstrated that resistance training can be effective for inducing adaptive changes in neuromuscular function in both young and old adults, which include an increased efferent neural drive to myofibers (for review, see Aagaard27, 97 and Aagaard et al.98). Substantial neuroplasticity also seems to exist for the inhibitory mechanism(s) present during ECC muscle contraction. Thus, as reported by Aagaard et al.,7 the suppression in neuromuscular activity (normalized EMG amplitude) for the knee extensors measured during maximal ECC contraction conditions was partially removed (vastus lateralis: 29% reduced EMG activity before training → 21% reduced EMG activity post training = 25% removed EMG suppression; vastus medialis: 35% reduced EMG activity → 18% reduced EMG activity = 49% removed suppression) or fully abolished rectus femoris (RF): 23% reduced EMG activity → 5% reduced EMG activity = 78% removed suppression; averaged across muscles: 30% reduced EMG activity → 15% reduced EMG activity = 50% removed suppression) in response to long-term (14 weeks) HLRT, in turn resulting in a significant gain in maximal ECC muscle strength7 (Fig. 7). These findings were verified in subsequent experiments, where a strong positive relationship (r = 0.90) was observed between the increase in neuromuscular activity induced by HLRT and the corresponding gain in maximal ECC muscle strength of the knee extensors26 (Fig. 7). Similar but more moderate relationships (r = 0.50) between the gain in muscle EMG activity and maximal ECC muscle strength induced by resistance training have been observed for the shoulder abductors in patients with trapezius myalgia after 10 weeks of HLRT intervention.12 Collectively, these data indicate that the increase in the ECC strength capacity of human skeletal muscle in vivo induced by resistance training is strongly governed by a parallel gain in neuromuscular activity, altogether representing a highly important aspect of neural adaptation to exercise.
Fig. 7.
(A) Maximal contraction strength and neuromuscular activity measured during maximal ECC (negative velocities) and CONC (positive velocities) muscle contractions before (full lines) and after (broken lines) 14 weeks of HLRT. All values are normalized relative to fast CONC contraction. (*after vs. before, p < 0.05). Note that the suppression in neuromuscular activity during ECC and slow CONC contraction before training was reduced after training (more details given in text). (B) The training-induced gain in maximal ECC muscle strength is strongly related to parallel elevations in normalized neuromuscular activity (ΔEMG). CONC = concentric; ECC = eccentric; EMG = electromyography; HLRT = heavy-load resistance training. Graph adapted from Aagaard, 97 data from (A) Aagaard et al.7 and (B) Andersen et al.26 with permission.
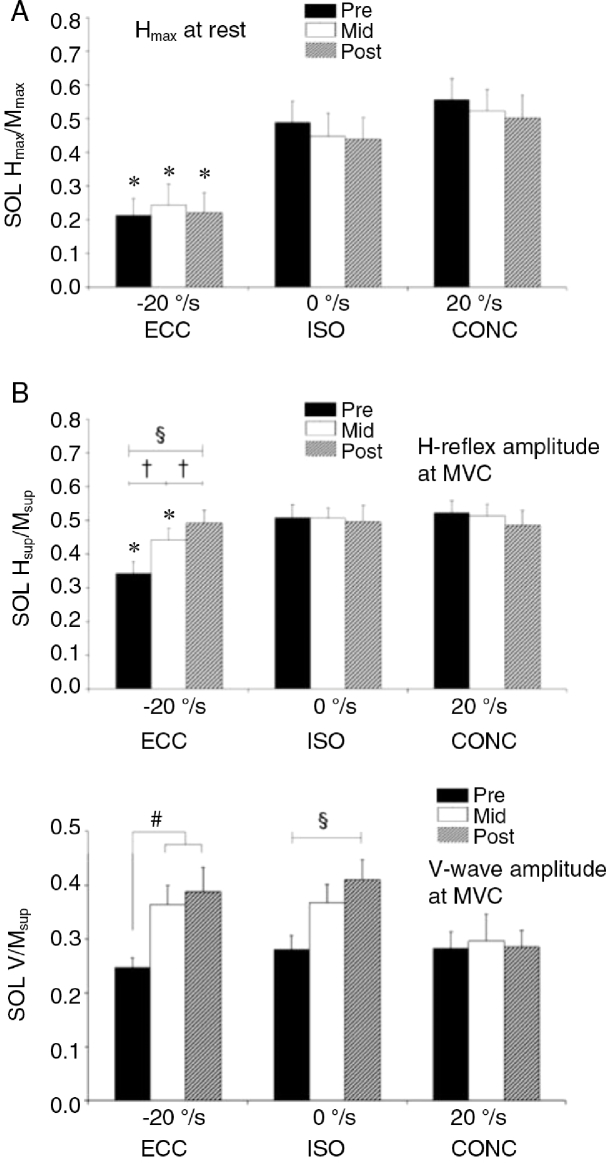
As discussed elsewhere in this article, the specific neural pathways responsible for the suppression in neuromuscular activity during ECC muscle contraction remain to be fully identified. During maximal voluntary muscle contraction, efferent motor output of spinal MNs is influenced by a multitude of synaptic inputs, including descending cortical pathways, afferent inflow from Ib Golgi organ afferents, group Ia and II muscle spindle afferents, group III muscle afferents, and recurrent Renshaw inhibition.7, 21 It has been suggested that a number of these pathways and spinal circuitry inputs affect the expression of ECC muscle strength in vivo16, 17 (cf. the Fig. 5 in Ref. 17). All of these pathways may exhibit adaptive plasticity with training.27, 99 Thus evoked V-wave and H-reflex responses recorded during maximal ECC plantar flexor contraction were found to increase (∼60% and ∼40%, respectively) after a period of HLRT conducted in form of maximal ECC resistance training50 (Fig. 8), suggesting that modulations in supraspinal and/or spinal neuronal pathways can indeed be achieved with resistance training to produce substantial gains in maximal ECC muscle strength. It is also notable that the depression in H-reflex amplitude that was present during maximal ECC vs. ISO or CONC muscle actions in untrained individuals46, 49,50, 83 was abolished in response to 7 weeks (18 sessions) of ECC resistance training50 (Fig. 8), indicating a decreased (removed) inhibitory input to spinal MNs during maximal ECC muscle contraction.
Fig. 8.
Maximal H-reflex (A, B) and V-wave responses (C) obtained before (pre), during (mid), and after (post) 7 weeks of resistance training. Data are expressed as peak-to-peak amplitude normalized to the maximal M-wave while recorded at rest (A) and during MVC (B, C). MVC conditions comprised separate ISO, CONC, and ECC plantar flexor trials. Training consisted of maximal ECC plantar flexor exercise performed in 2–3 sessions per week for 7 weeks (18 sessions in total). *ECC vs. CONC and ISO (p < 0.01); §post vs. pre (p < 0.01); †mid vs. pre and post (p < 0.01); #pre vs. mid and post (p < 0.05). Note the marked increase in H-reflex and V-wave amplitudes during ECC MVC efforts after training. Furthermore, the depression in H-reflex amplitude during ECC vs. ISO and CONC MVC trials observed at baseline (pre) was removed after the period of training (post). Also note that training-induced gains in evoked reflex responses were observed during MVC efforts only (B, C) while absent in resting conditions (A). CONC = concentric; ECC = eccentric; Hmax = H-reflex amplitude at rest; H-reflex = Hoffman reflex; Hsup = maximal H-reflex at MVC; ISO = isometric; Mmax = maximal M-wave amplitude at rest; Msup = maximal M-wave amplitude at MVC; MVC = maximum voluntary contraction; SOL = soleus; V = V-wave amplitude. Data adapted from Duclay et al.50 with permission.
Interestingly, using TMS techniques, Kidgell et al.100 recently reported that unilateral ECC HLRT resulted in increased corticospinal excitability accompanied by decreased intracortical inhibition when assessed in the contralateral untrained limb, thus revealing important neural mechanisms responsible for the cross-transfer effect with unilateral ECC resistance training.
In previous studies7, 26 it has been suggested that the removal of neural inhibition and the corresponding increase in maximal ECC muscle strength observed after resistance training could be caused by downregulated spinal inhibitory interneuron activity, possibly modulated via central descending pathways. One potential mechanism for the marked increase in ECC muscle strength induced by HLRT could be a downregulation in inhibitory interneuron input on spinal MNs from Golgi organ Ib afferents.7, 27 Also, the observation of reduced H-reflex responses during both active and passive muscle lengthening compared with shortening10, 46,50, 76,101 suggests that presynaptic inhibition of Ia afferents, potentially as a result of Golgi Ib afferent inflow,99 may be present during ECC muscle contraction, although presynaptic inhibitory input may originate from numerous other spinal and supraspinal networks as well.76, 102 In terms of potential sites for a training-induced change in postsynaptic inhibitory MN input, spinal Ib inhibitory interneurons are modulated by descending corticospinal pathways and in turn receive excitatory and inhibitory synaptic input from reticulospinal and rubrospinal tracts, respectively103, 104 (for a brief review, see Aagaard and Thorstensson21). Notably, the magnitude of Ib inhibition of homonymous MNs is reduced during voluntary contractions, likely owing to presynaptic gating from supraspinal center.104 This depression in Ib afferent inhibitory action increases with the force of contraction,104 causing the gain of the Ib force feedback to vary during voluntary contraction. Thus voluntary muscle force exertion is influenced by input from the inhibitory disynaptic Ib pathway, which in turn is the target of dynamic gating control via central descending pathways. In terms of adaptive plasticity, the possibility exists that resistance training would result in increased inhibitory input to spinal Ib interneurons as a result of increased rubrospinal activity, thereby causing a disinhibition of spinal MNs during high-force contraction conditions that would lead to increased ECC force production.7
In contrast, as elaborated by Duchateau and Enoka,17 the magnitude of modulation (suppression) of spinal and corticospinal responsiveness during ECC compared with CONC muscle contractions seems to be similar across contraction intensities (MVC vs. 50% MVC),47, 49 which argues against the hypothesis of an adaptive change in a tension-regulating inhibitory mechanism linked to Ib afferent inflow from Golgi tendon organs after resistance training. Furthermore, although a role for Golgi tendon organs in the modulation of spinal excitability during ECC contractions was not entirely excluded, Duclay et al.49 suggested that other neural mechanisms, located at both spinal and supraspinal levels, may be involved in the specific neural adjustments associated with ECC contractions. However, it may be argued that, given the greater intrinsic force capacity of single isolated myofibers during ECC vs. CONC contractions,3 it is likely that a lesser number of MUs were activated during ECC compared with CONC contractions when performed at the same absolute level of submaximal muscle force (i.e., corresponding with 50% ISO MVC)49 owing to a reduced requirement for efferent spinal MN activity in the former condition, which would be expected per se to result in reduced H-reflex and MEP responses in ECC compared with CONC contraction conditions. Thus, the observation that evoked H-reflex and MEP responses were not decreased to a greater extent between submaximal ECC vs. CONC contractions performed at fixed absolute submaximal force magnitude (corresponding with 50% ISO MVC)47, 48, 49 indirectly suggests that a reduced magnitude of inhibitory MN input (presynaptic or postsynaptic) might have been present during ECC test contractions performed at submaximal effort.
Because voluntary ECC contractions involve increased excitatory drive to spinal MNs from muscle spindle Ia afferents90, 105 and assuming that postsynaptic inhibitory modulation of MN activity by Golgi organ Ib afferent feedback plays little or no role (as discussed elsewhere in this article), spinal modulatory mechanisms during ECC contraction perhaps should be mainly attributed to the presynaptic side of MNs,17 although postsynaptic recurrent Renshaw inhibition75 also seems to play an important role (as discussed elsewhere in this article). Consistent with a significant role of presynaptic inhibition by primary afferent depolarization during ECC contractions,17 Grosprêtre et al.83 used H-reflex stimulation that was conditioned by subthreshold TMS applied to the motor cortex area to demonstrate that spinal inhibition during ECC contraction (tested at 20% MVC) was controlled by descending cortical pathways. In terms of potential changes evoked by resistance training on spinal inhibitory circuitry, postsynaptic inhibitory mechanisms may also influence spinal MN excitability48 to some extent (discussed in detail elsewhere in this article), potentially involving diminished levels of Ib inhibition from Golgi tendon organs, reciprocal inhibition, and recurrent Renshaw inhibition, respectively. In addition, it may be speculated that the neuroplasticity in ECC muscle force expression with resistance training might involve adaptive modulation in the excitatory monoaminergic drive106 vs. inhibitory serotonergic drive107 to the pool of spinal MNs, which overall would be expected to affect the relationship (specifically the gain) between synaptic MN input and efferent motor drive to active myofibers, in turn affecting the magnitude of resulting muscle force production106 (cf. the Fig. 4 in Ref. 106).
It is notable that, in using surface EMG analysis, a consistent decrease in the median power frequency during maximal ECC and CONC contractions was observed in the vastus lateralis muscle after 16 weeks of resistance training.7 Spectral (power frequency) analysis of bipolar single-surface EMG signals during MVC is not generally likely to reflect fiber-type composition108, 109 or overall MU firing frequency.110 However, given that median and mean EMG power frequency are strongly influenced by the degree of MU synchronization,111, 112, 113 it might be speculated that the observed decrease in median power frequency27 reflects a more synchronized pattern of MU firing during maximal ECC muscle contraction, potentially as a result of increased recruitment of high-threshold MUs and/or arise from an increased common synaptic input to the spinal MN pool114 owing to a decreased presynaptic inhibition of Ia afferents after training. The latter mechanism would per se contribute to the observed gains in efferent neural drive (elevated V-wave response) and maximal ECC muscle strength, respectively.
8. Conclusion
Neuromuscular activity seems to be suppressed during maximal ECC muscle contraction in untrained subjects owing to decreased levels of central activation, increased autogenic MN inhibition, and decreased MN excitability, as indicated by observations of decreased excitability in descending corticospinal motor pathways, reduced EMG amplitude, enhanced recurrent Renshaw inhibition, and diminished evoked H-reflex responses. Maximum ECC muscle strength can be effectively increased by use of HLRT, which seems to result in full or partial removal of the suppression in neuromuscular activity. Prolonged (weeks to months) HLRT results in increased H-reflex and V-wave responses during maximal ECC muscle actions, along with marked gains in maximal ECC muscle strength, indicating increased excitability of spinal MNs, decreased presynaptic or postsynaptic inhibition, and elevated descending motor drive. Notably, use of supramaximal ECC resistance training can lead to selectively elevated V-wave responses during maximal ECC contraction, demonstrating that adaptive changes in spinal circuitry function and/or gains in descending motor drive can be achieved during maximal ECC contraction in response to HLRT.
The improvement in maximal ECC muscle strength induced by resistance training has important implications, because it provides a basis for enhanced neuromotor performance both in athletes5, 6 and nontrained individuals,7 including older adults40 and clinical populations such as chronic stroke patients.43 Specifically, increased ECC muscle strength enables more rapid performance of deceleration and stretch-shortening cycle movements, side-cutting, and jumping actions. Furthermore, an increased maximal ECC strength of antagonist muscles represents an important mechanism to protect ligaments (e.g., the ACL) and other passive joint structures against excessive force and strain impacts during sports and exercise. In addition, an increased maximal ECC muscle strength in elderly individuals induced by resistance training40 is likely to allow specific activities of daily living, such as stair descent, to be performed in a safer manner, hence decreasing the risk of falls.
Competing interests
The author declares that he has no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Reference
- 1.Fenn WO. The relation between the work performed and the energy liberated in muscular contraction. J Physiol. 1924;58:373–395. doi: 10.1113/jphysiol.1924.sp002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939;96:45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edman KA. Double-hyperbolic force-velocity relation in frog muscle fibres. J Physiol. 1988;404:301–321. doi: 10.1113/jphysiol.1988.sp017291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbott BC, Bigland B, Ritchie JM. The physiological cost of negative work. J Physiol. 1952;117:380–390. doi: 10.1113/jphysiol.1952.sp004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aagaard P, Simonsen EB, Trolle M, Bangsbo J, Klausen K. Specificity of training velocity and training load on gains in isokinetic knee joint strength. Acta Physiol Scand. 1996;156:123–129. doi: 10.1046/j.1365-201X.1996.438162000.x. [DOI] [PubMed] [Google Scholar]
- 6.Aagaard P, Simonsen EB, Magnusson SP, Larsson B, Dyhre-Poulsen P. A new concept for isokinetic hamstring: quadriceps muscle strength ratio. Am J Sports Med. 1998;26:231–237. doi: 10.1177/03635465980260021201. [DOI] [PubMed] [Google Scholar]
- 7.Aagaard P, Simonsen EB, Andersen JL, Magnusson SP, Halkjær-Kristensen J, Dyhre-Poulsen P. Neural inhibition during maximal eccentric and concentric quadriceps contraction: effects of resistance training. J Appl Physiol. 2000;89:49–57. doi: 10.1152/jappl.2000.89.6.2249. [DOI] [PubMed] [Google Scholar]
- 8.Westing SH, Seger JY, Karlson E, Ekblom B. Eccentric and concentric torque-velocity characteristics of the quadriceps femoris in man. Eur J Appl Physiol Occup Physiol. 1988;58:100–104. doi: 10.1007/BF00636611. [DOI] [PubMed] [Google Scholar]
- 9.Amiridis IG, Martin A, Morlon B, Martin L, Cometti G, Pousson M. Co-activation and tension-regulating phenomena during isokinetic knee extension in sedentary and highly skilled humans. Eur J Appl Physiol Occup Physiol. 1996;73:149–156. doi: 10.1007/BF00262824. [DOI] [PubMed] [Google Scholar]
- 10.Pinniger GJ, Steele JR, Thorstensson A, Cresswell AG. Tension regulation during lengthening and shortening actions of the human soleus muscle. Eur J Appl Physiol. 2000;81:375–383. doi: 10.1007/s004210050057. [DOI] [PubMed] [Google Scholar]
- 11.Komi PV, Linnamo V, Silventoinen P, Sillanpää M. Force and EMG power spectrum during eccentric and concentric actions. Med Sci Sports Exerc. 2000;32:1757–1762. doi: 10.1097/00005768-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Andersen LL, Andersen CH, Zebis MK, Nielsen PK, Søgaard K, Sjøgaard G. Effect of physical training on function of chronically painful muscles: a randomized controlled trial. J Appl Physiol. 2008;105:1796–1801. doi: 10.1152/japplphysiol.91057.2008. [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa K. Eccentric contraction: unraveling mechanisms of force enhancement and energy conservation. J Exp Biol. 2016;219:189–196. doi: 10.1242/jeb.124057. [DOI] [PubMed] [Google Scholar]
- 14.Enoka RM. Eccentric contractions require unique activation strategies by the nervous system. J Appl Physiol. 1996;81:2339–2346. doi: 10.1152/jappl.1996.81.6.2339. [DOI] [PubMed] [Google Scholar]
- 15.Duchateau J, Enoka RM. Neural control of shortening and lengthening contractions: influence of task constraints. J Physiol. 2008;586:5853–5864. doi: 10.1113/jphysiol.2008.160747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duchateau J, Baudry S. Insights into the neural control of eccentric contractions. J Appl Physiol. 2014;116:1418–1425. doi: 10.1152/japplphysiol.00002.2013. [DOI] [PubMed] [Google Scholar]
- 17.Duchateau J, Enoka RM. Neural control of lengthening contractions. J Exp Biol. 2016;219:197–204. doi: 10.1242/jeb.123158. [DOI] [PubMed] [Google Scholar]
- 18.Christou EA, Carlton LG. Motor output is more variable during eccentric compared with concentric contractions. Med Sci Sports Exerc. 2002;34:1773–1778. doi: 10.1097/00005768-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Westing SH, Seger JY, Thorstensson A. Effects of electrical stimulation on eccentric and concentric torque-velocity relationships during knee extension in man. Acta Physiol Scand. 1990;140:17–22. doi: 10.1111/j.1748-1716.1990.tb08971.x. [DOI] [PubMed] [Google Scholar]
- 20.Seger JY, Thorstensson A. Electrically evoked eccentric and concentric torque-velocity relationships in human knee extensor muscles. Acta Physiol Scand. 2000;169:63–69. doi: 10.1046/j.1365-201x.2000.00694.x. [DOI] [PubMed] [Google Scholar]
- 21.Aagaard P, Thorstensson A. Neuromuscular aspects of exercise: adaptive responses evoked by strength training. In: Kjær M, editor. Textbook of sports medicine. Blackwell ; London: 2003. pp. 70–106. [Google Scholar]
- 22.Jakobsen MD, Sundstrup E, Randers MB, Kjær M, Andersen LL, Krustrup P. The effect of strength training, recreational soccer and running exercise on stretch-shortening cycle muscle performance during countermovement jumping. Hum Mov Sci. 2012;31:970–986. doi: 10.1016/j.humov.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Aagaard P, Bangsbo J. The muscular system: design, function and performance relationships. In: Tipton CM, Terjung RL, editors. ACSM's advanced exercise physiology. American College of Sports Medicine. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005. pp. 144–160. [Google Scholar]
- 24.Aagaard P, Simonsen EB, Andersen JL, Magnusson SP, Bojsen-Møller F, Dyhre-Poulsen P. Antagonist muscle coactivation during isokinetic knee extension. Scand J Med Sci Sports. 2000;10:58–67. doi: 10.1034/j.1600-0838.2000.010002058.x. [DOI] [PubMed] [Google Scholar]
- 25.Jarić S, Ropret R, Kukolj M, Ilić DB. Role of antagonist and antagonist muscle strength in performance of rapid movements. Eur J Appl Physiol Occup Physiol. 1995;71:464–468. doi: 10.1007/BF00635882. [DOI] [PubMed] [Google Scholar]
- 26.Andersen LL, Andersen JL, Magnusson SP, Aagaard P. Neuromuscular adaptations to detraining following resistance training in previously untrained subjects. Eur J Appl Physiol. 2005;93:511–518. doi: 10.1007/s00421-004-1297-9. [DOI] [PubMed] [Google Scholar]
- 27.Aagaard P. Training-induced changes in neural function. Exerc Sports Sci Rev. 2003;31:61–67. doi: 10.1097/00003677-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Crameri RM, Aagaard P, Qvortrup K, Langberg H, Olesen J, Kjær M. Myofibre damage in human skeletal muscle: effects of electrical stimulation versus voluntary contraction. J Physiol. 2007;583:365–380. doi: 10.1113/jphysiol.2007.128827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackey AL, Kjaer M. Connective tissue regeneration in skeletal muscle after eccentric contraction-induced injury. J Appl Physiol. 2017;122:533–540. doi: 10.1152/japplphysiol.00577.2016. [DOI] [PubMed] [Google Scholar]
- 30.Caiozzo VJ, Perrine JJ, Edgerton VR. Training-induced alterations of the in vivo force-velocity relationship of human muscle. J Appl Physiol Respir Environ Exerc Physiol. 1981;51:750–754. doi: 10.1152/jappl.1981.51.3.750. [DOI] [PubMed] [Google Scholar]
- 31.Colson SS, Martin A, Van Hoecke J. Effects of electromyostimulation versus voluntary isometric training on elbow flexor muscle strength. J Electromyogr Kinesiol. 2009;19:e311–e319. doi: 10.1016/j.jelekin.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Colliander EB, Tesch PA. Effects of eccentric and concentric muscle actions in resistance training. Acta Physiol Scand. 1990;140:31–39. doi: 10.1111/j.1748-1716.1990.tb08973.x. [DOI] [PubMed] [Google Scholar]
- 33.Duncan PW, Chandler JM, Cavanaugh DK, Johnson KR, Buehler AG. Mode and speed specificity of eccentric and concentric exercise training. J Orthop Sports Phys Ther. 1989;11:70–75. doi: 10.2519/jospt.1989.11.2.70. [DOI] [PubMed] [Google Scholar]
- 34.Higbie EJ, Cureton KJ, Warren GL, 3rd, Prior BM. Effects of concentric and eccentric training on muscle strength, cross sectional area, and neural activation. J Appl Physiol. 1996;81:2173–2181. doi: 10.1152/jappl.1996.81.5.2173. [DOI] [PubMed] [Google Scholar]
- 35.Hortobágyi T, Hill JP, Houmard JA, Fraser DD, Lambert NJ, Israel RG. Adaptive responses to muscle lengthening and shortening in humans. J Appl Physiol. 1996;80:765–772. doi: 10.1152/jappl.1996.80.3.765. [DOI] [PubMed] [Google Scholar]
- 36.Komi PV, Buskirk ER. Effect of eccentric and concentric muscle conditioning on tension and electrical activity of human muscle. Ergonomics. 1972;15:417–434. doi: 10.1080/00140137208924444. [DOI] [PubMed] [Google Scholar]
- 37.Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P. Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol. 1989;59:310–319. doi: 10.1007/BF02388334. [DOI] [PubMed] [Google Scholar]
- 38.Seger JY, Arvidson B, Thorstensson A. Specific effects of eccentric and concentric training on muscle strength and morphology in humans. Eur J Appl Physiol Occup Physiol. 1998;79:49–57. doi: 10.1007/s004210050472. [DOI] [PubMed] [Google Scholar]
- 39.Spurway NC, Watson H, McMillan K, Connolly G. The effect of strength training on the apparent inhibition of eccentric force production in voluntary activated human quadriceps. Eur J Appl Physiol. 2000;82:374–380. doi: 10.1007/s004210000221. [DOI] [PubMed] [Google Scholar]
- 40.Hortobágyi T, Tunnel D, Moody J, Beam S, DeVita P. Low- or high-intensity strength training partially restores impaired quadriceps force accuracy and steadiness in aged adults. J Gerontol Biol Sci. 2001;56:B38–B47. doi: 10.1093/gerona/56.1.b38. [DOI] [PubMed] [Google Scholar]
- 41.Holm L, Reitelseder S, Pedersen TG, Doessing S, Petersen SG, Flyvbjerg A. Changes in muscle size and MHC composition in response to resistance exercise with heavy and light loading intensity. J Appl Physiol. 2008;105:1454–1461. doi: 10.1152/japplphysiol.90538.2008. [DOI] [PubMed] [Google Scholar]
- 42.Dorgo S, Edupuganti P, Smith DR, Ortiz M. Comparison of lower body specific resistance training on the hamstring to quadriceps strength ratios in men and women. Res Q Exerc Sport. 2012;83:143–151. doi: 10.1080/02701367.2012.10599844. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Gonzalo R, Nissemark C, Åslund B, Tesch PA, Sojka P. Chronic stroke patients show early and robust improvements in muscle and functional performance in response to eccentric-overload flywheel resistance training: a pilot study. J Neuroeng Rehabil. 2014;11:150. doi: 10.1186/1743-0003-11-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norrbrand L, Fluckey JD, Pozzo M, Tesch PA. Resistance training using eccentric overload induces early adaptations in skeletal muscle size. Eur J Appl Physiol. 2008;102:271–281. doi: 10.1007/s00421-007-0583-8. [DOI] [PubMed] [Google Scholar]
- 45.Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 2000;88:2097–2106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- 46.Duclay J, Martin A. Evoked H-reflex and V-wave responses during maximal isometric, concentric, and eccentric muscle contraction. J Neurophysiol. 2005;94:3555–3562. doi: 10.1152/jn.00348.2005. [DOI] [PubMed] [Google Scholar]
- 47.Gruber M, Linnamo V, Strojnik V, Rantalainen T, Avela J. Excitability at the motoneuron pool and motor cortex is specifically modulated in lengthening compared to isometric contractions. J Neurophysiol. 2009;101:2030–2040. doi: 10.1152/jn.91104.2008. [DOI] [PubMed] [Google Scholar]
- 48.Duclay J, Pasquet B, Martin A, Duchateau J. Specific modulation of corticospinal and spinal excitabilities during maximal voluntary isometric, shortening and lengthening contractions in synergist muscles. J Physiol. 2011;589:2901–2916. doi: 10.1113/jphysiol.2011.207472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duclay J, Pasquet B, Martin A, Duchateau J. Specific modulation of spinal and cortical excitabilities during lengthening and shortening submaximal and maximal contractions in plantar flexor muscles. J Appl Physiol. 2014;117:1440–1450. doi: 10.1152/japplphysiol.00489.2014. [DOI] [PubMed] [Google Scholar]
- 50.Duclay J, Martin A, Robbe A, Pousson M. Spinal reflex plasticity during maximal dynamic contractions after eccentric training. Med Sci Sports Exerc. 2008;40:722–734. doi: 10.1249/MSS.0b013e31816184dc. [DOI] [PubMed] [Google Scholar]
- 51.Kellis E, Baltzopoulos V. Muscle activation differences between eccentric and concentric isokinetic exercise. Med Sci Sports Exerc. 1998;30:1616–1623. doi: 10.1097/00005768-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Westing SH, Cresswell AG, Thorstensson A. Muscle activation during maximal voluntary eccentric and concentric knee extension. Eur J Appl Physiol Occup Physiol. 1991;62:104–108. doi: 10.1007/BF00626764. [DOI] [PubMed] [Google Scholar]
- 53.Beltman JG., Sargeant AJ, van Mechelen W, de Haan A. Voluntary activation level and muscle fiber recruitment of human quadriceps during lengthening contractions. J Appl Physiol. 2004;97:619–626. doi: 10.1152/japplphysiol.01202.2003. [DOI] [PubMed] [Google Scholar]
- 54.Babault N, Pousson M, Ballay Y, Van Hoecke J. Activation of human quadriceps femoris during isometric, concentric, and eccentric contractions. J Appl Physiol. 2001;91:2628–2634. doi: 10.1152/jappl.2001.91.6.2628. [DOI] [PubMed] [Google Scholar]
- 55.Webber S, Kriellaars D. Neuromuscular factors contributing to in vivo eccentric moment generation. J Appl Physiol. 1997;83:40–45. doi: 10.1152/jappl.1997.83.1.40. [DOI] [PubMed] [Google Scholar]
- 56.Nardone A, Romanò C, Schieppati M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol. 1989;409:451–471. doi: 10.1113/jphysiol.1989.sp017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howell N, Fuglevand AJ, Walsh ML, Bigland-Ritchie B. Motor unit activity during isometric and concentric-eccentric contractions of the human first dorsal interosseus muscle. J Neurophysiol. 1995;74:901–904. doi: 10.1152/jn.1995.74.2.901. [DOI] [PubMed] [Google Scholar]
- 58.Søgaard K, Christensen H, Jensen BR, Finsen L, Sjøgaard G. Motor control and kinetics during low level concentric and eccentric contractions in man. Electroencephalogr Clin Neurophysiol. 1996;101:453–460. [PubMed] [Google Scholar]
- 59.Laidlaw DH, Bilodeau M, Enoka RM. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve. 2000;23:600–612. doi: 10.1002/(sici)1097-4598(200004)23:4<600::aid-mus20>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 60.Stotz PJ, Bawa P. Motor unit recruitment during lengthening contractions of human wrist flexors. Muscle Nerve. 2001;24:1535–1541. doi: 10.1002/mus.1179. [DOI] [PubMed] [Google Scholar]
- 61.Pasquet B, Carpentier A, Duchateau J. Specific modulation of motor unit discharge for a similar change in fascicle length during shortening and lengthening contractions in humans. J Physiol. 2006;577:753–765. doi: 10.1113/jphysiol.2006.117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kossev A, Christova P. Discharge pattern of human motor units during dynamic concentric and eccentric contractions. Electroencephalogr Clin Neurophysiol. 1998;109:245–255. doi: 10.1016/s0924-980x(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 63.Tax AA, Denier van der Gon JJ, Gielen CC, van den Tempel CM. Differences in the activation of m. biceps brachii in the control of slow isotonic movements and isometric contractions. Exp Brain Res. 1989;76:55–63. doi: 10.1007/BF00253623. [DOI] [PubMed] [Google Scholar]
- 64.Zimny ML, Schutte M, Dabezies E. Mechanoreceptors in the human anterior cruciate ligament. Anat Rec. 1986;214:204–209. doi: 10.1002/ar.1092140216. [DOI] [PubMed] [Google Scholar]
- 65.Schutte MJ, Dabezies EJ, Zimny ML, Happel LT. Neural anatomy of the human anterior cruciate ligament. J Bone Joint Surg Am. 1987;69:243–247. [PubMed] [Google Scholar]
- 66.Solomonow M, Baratta R, Zhou BH, Shoji H, Bose W, Beck C. The synergistic action of the anterior cruciate ligaments and thighs muscle in maintaining joint stability. Am J Sports Med. 1987;15:207–213. doi: 10.1177/036354658701500302. [DOI] [PubMed] [Google Scholar]
- 67.Dyhre-Poulsen P, Krogsgaard MR. Muscular reflexes elicited by electrical stimulation of the anterior cruciate ligament in humans. J Appl Physiol. 2000;89:2191–2195. doi: 10.1152/jappl.2000.89.6.2191. [DOI] [PubMed] [Google Scholar]
- 68.Krogsgaard MR, Fischer-Rasmussen T, Dyhre-Poulsen P. Absence of sensory function in the reconstructed anterior cruciate ligament. J Electromyogr Kinesiol. 2011;21:82–86. doi: 10.1016/j.jelekin.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 69.Draganich LF, Vahey JW. An in vitro study of anterior cruciate ligament strain induced by quadriceps and hamstring forces. J Orthop Res. 1990;8:57–63. doi: 10.1002/jor.1100080107. [DOI] [PubMed] [Google Scholar]
- 70.Beynnon B, Howe JG, Pope MH, Johnson RJ, Fleming BC. The measurement of anterior cruciate ligament strain in vivo. Int Orthop. 1992;16:1–12. doi: 10.1007/BF00182976. [DOI] [PubMed] [Google Scholar]
- 71.Hirokawa S, Solomonow M, Lu Y, Lou ZP, D'Ambrosia R. Anterior posterior and rotational displacement of the tibia elicited by quadriceps contraction. Am J Sports Med. 1992;20:299–306. doi: 10.1177/036354659202000311. [DOI] [PubMed] [Google Scholar]
- 72.Pierrot-Deseilligny E, Morin C. Evidence for supraspinal influences on Renshaw inhibition during motor activity in man. In: Desmedt JE, editor. Vol. 8 . Karger Publishers; Basel: 1980. pp. 142–169. (Progress in clinical neurophysiology). [Google Scholar]
- 73.Hultborn H, Pierrot-Deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studies by an H-reflex technique. J Physiol. 1979;297:229–251. doi: 10.1113/jphysiol.1979.sp013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petersen NT, Butler JE, Carpenter MG, Cresswell AG. Ia-afferent input to motoneurons during shortening and lengthening muscle contractions in humans. J Appl Physiol. 2007;102:144–148. doi: 10.1152/japplphysiol.00362.2006. [DOI] [PubMed] [Google Scholar]
- 75.Barrué-Belou S, Marque P, Duclay J. Recurrent inhibition is higher in eccentric compared to isometric and concentric maximal voluntary contractions. Acta Physiol (Oxf) 2018:e13064. doi: 10.1111/apha.13064. [DOI] [PubMed] [Google Scholar]
- 76.Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28:345–376. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- 77.Tucker KJ, Tuncer M, Türker KS. A review of the H-reflex and M-wave in the human triceps surae. Hum Mov Sci. 2005;24:667–668. doi: 10.1016/j.humov.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 78.Zehr EP. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol. 2002;86:455–468. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]
- 79.Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods. 2008;171:1–12. doi: 10.1016/j.jneumeth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 80.Sale DG, MacDougall JD, Upton AR, McComas AJ. Effect of strength training upon motoneuron excitability in man. Med Sci Sports Exerc. 1983;15:57–62. [PubMed] [Google Scholar]
- 81.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol. 2002;92:2309–2318. doi: 10.1152/japplphysiol.01185.2001. [DOI] [PubMed] [Google Scholar]
- 82.Vila-Chã C, Falla D, Correia MV, Farina D. Changes in H-reflex and V-wave following short-term endurance and strength training. J Appl Physiol. 2012;112:54–63. doi: 10.1152/japplphysiol.00802.2011. [DOI] [PubMed] [Google Scholar]
- 83.Grosprêtre S, Papaxanthis C, Martin A. Modulation of spinal excitability by a sub-threshold stimulation of M1 area during muscle lengthening. Neurosci. 2014;263:60–71. doi: 10.1016/j.neuroscience.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 84.Upton AR, McComas AJ, Sica REP. Potentiation of 'late' responses evoked in muscles during effort. J Neurol Neurosurg Psychiat. 1971;34:699–711. doi: 10.1136/jnnp.34.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piscione J, Grosset JF, Gamet D, Pérot C. Are H-reflex and M-wave recruitment curve parameters related to aerobic capacity? Appl Physiol Nutr Metab. 2012;37:990–996. doi: 10.1139/h2012-078. [DOI] [PubMed] [Google Scholar]
- 86.Heckman CJ, Binder MD. Computer simulations of the effects of different synaptic input systems on motor unit recruitment. J Neurophysiol. 1993;70:1827–1840. doi: 10.1152/jn.1993.70.5.1827. [DOI] [PubMed] [Google Scholar]
- 87.Abbruzzese G, Morena M, Spadavecchia L, Schieppati M. Response of arm flexor muscles to magnetic and electrical brain stimulation during shortening and lengthening tasks in man. J Physiol. 1994;481:499–507. doi: 10.1113/jphysiol.1994.sp020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pinniger GJ, Nordlund M, Steele JR, Cresswell AG. H-reflex modulation during passive lengthening and shortening of the human triceps surae. J Physiol. 2001;534:913–923. doi: 10.1111/j.1469-7793.2001.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duclay J, Robbe A, Pousson M, Martin A. Effect of angular velocity on soleus and medial gastrocnemius H-reflex during maximal concentric and eccentric muscle contraction. J Electromyogr Kinesiol. 2009;19:948–956. doi: 10.1016/j.jelekin.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 90.Burke D, Hagbarth KE, Lofstedt L. Muscle spindle activity in man during shortening and lengthening contractions. J Physiol. 1978;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of I a fibres: a study in man and the cat. J Physiol. 1987;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nielsen J, Petersen N. Is presynaptic inhibition distributed to corticospinal fibres in man? J Physiol. 1994;477:47–58. doi: 10.1113/jphysiol.1994.sp020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hahn D, Hoffman BW, Carroll TJ, Cresswell AG. Cortical and spinal excitability during and after lengthening contractions of the human plantar flexor muscles performed with maximal voluntary effort. PLoS One. 2012;7:e49907. doi: 10.1371/journal.pone.0049907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee HD, Herzog W. Force enhancement following muscle stretch of electrically stimulated and voluntarily activated human adductor pollicis. J Physiol. 2002;545:321–330. doi: 10.1113/jphysiol.2002.018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hahn D, Seiberl W, Schmidt S, Schweizer K, Schwirtz A. Evidence of residual force enhancement for multi-joint leg extension. J Biomech. 2010;43:1503–1508. doi: 10.1016/j.jbiomech.2010.01.041. [DOI] [PubMed] [Google Scholar]
- 96.Tilp M, Steib S, Herzog W. Force-time history effects in voluntary contractions of human tibialis anterior. Eur J Appl Physiol. 2009;106:159–166. doi: 10.1007/s00421-009-1006-9. [DOI] [PubMed] [Google Scholar]
- 97.Aagaard P. Wiley-Blackwell; Hoboken, NJ: 2010. Neural adaptations to resistance exercise. Strength and conditioning: biological principles and practical applications; pp. 105–124. [Google Scholar]
- 98.Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjær M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20:49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- 99.Bawa P. Neural control of motor output: can training change it? Exerc Sport Sci Rev. 2002;30:59–63. doi: 10.1097/00003677-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 100.Kidgell DJ, Frazer AK, Daly RM, Rantalainen T, Ruotsalainen I, Ahtiainen J. Increased cross-education of muscle strength and reduced corticospinal inhibition following eccentric strength training. Neurosci. 2015;300:566–575. doi: 10.1016/j.neuroscience.2015.05.057. [DOI] [PubMed] [Google Scholar]
- 101.Nordlund MM, Thorstensson A, Cresswell AG. Variations in the soleus H-reflex as a function of activation during controlled lengthening and shortening actions. Brain Res. 2002;952:301–307. doi: 10.1016/s0006-8993(02)03259-6. [DOI] [PubMed] [Google Scholar]
- 102.Gosgnach S, Quevedo J, Fedirchuk B, McCrea DA. Depression of group Ia monosynaptic EPSPs in cat hindlimb motoneurones during fictive locomotion. J Physiol. 2000;26:639–652. doi: 10.1111/j.1469-7793.2000.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Loeb GE. Neural Control of Locomotion: how do all the data fit together? BioScience. 1989;39:800–804. [Google Scholar]
- 104.Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev. 1992;72:623–666. doi: 10.1152/physrev.1992.72.3.623. [DOI] [PubMed] [Google Scholar]
- 105.Hulliger M, Nordh E, Vallbo AB. Discharge in muscle spindle afferents related to direction of slow precision movements in man. J Physiol. 1985;362:437–453. doi: 10.1113/jphysiol.1985.sp015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 107.D'Amico JM, Butler AA, Héroux ME, Cotel F, Perrier JM, Butler JE. Human motoneurone excitability is depressed by activation of serotonin 1A receptors with buspirone. J Physiol. 2017;595:1763–1773. doi: 10.1113/JP273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taylor AD, Humphries B, Smith P, Bronks R. Electrophoretic separation of myosin heavy chain isoforms in the human m. vastus lateralis: references to reproducibility and relationships with force, electromechanical delay, fibre conduction velocity, endurance and electromyography. Arch Physiol Biochem. 1997;105:10–18. doi: 10.1076/apab.105.1.10.13142. [DOI] [PubMed] [Google Scholar]
- 109.Farina D, Ferguson RA, Macaluso A, De Vito G. Correlation of average muscle fiber conduction velocity measured during cycling exercise with myosin heavy chain composition, lactate threshold, and VO2max. J Electromyogr Kinesiol. 2007;17:393–400. doi: 10.1016/j.jelekin.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 110.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 111.Yao W, Fuglevand RJ, Enoka RM. Motor-unit synchronization increases EMG amplitude and decreases force steadiness of simulated contractions. J Neurophysiol. 2000;83:441–452. doi: 10.1152/jn.2000.83.1.441. [DOI] [PubMed] [Google Scholar]
- 112.Farina D, Fattorini L, Felici F, Filligoi G. Nonlinear surface EMG analysis to detect changes of motor unit conduction velocity and synchronization. J Appl Physiol. 2002;93:1753–1763. doi: 10.1152/japplphysiol.00314.2002. [DOI] [PubMed] [Google Scholar]
- 113.Fattorini L, Felici F, Filligoi GC, Traballesi M, Farina D. Influence of high motor unit synchronization levels on non-linear and spectral variables of the surface EMG. J Neurosci Methods. 2005;143:133–139. doi: 10.1016/j.jneumeth.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 114.Farina D, Negro F, Dideriksen JL. The effective neural drive to muscles is the common synaptic input to motor neurons. J Physiol. 2014;592:3427–3441. doi: 10.1113/jphysiol.2014.273581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thorstensson A, Grimby G, Karlsson J. Force velocity relations and fibre composition in human knee extensor muscles. J Appl Physiol. 1976;40:12–16. doi: 10.1152/jappl.1976.40.1.12. [DOI] [PubMed] [Google Scholar]
- 116.Aagaard P, Simonsen EB, Trolle M, Bangsbo J, Klausen K. Moment and power generation during maximal knee extensions performed at low and high speed. Eur J Appl Physiol. 1994;69:376–381. doi: 10.1007/BF00865398. [DOI] [PubMed] [Google Scholar]