Abstract
Background
Increased peak oxygen consumption (VO2peak) can reduce cardiovascular risks associated with obesity. Our aim was to analyze the effect of a weight loss program on cardiovascular fitness in overweight (W) and obese (O) subjects.
Methods
One hundred and sixty-seven subjects (77 males and 90 females), aged 18–50 years, performed a modified Bruce protocol before (pre) and after (post) a weight loss program of 24 weeks. This program combined physical training (strength, S; endurance, E; combined strength + endurance, SE; or physical activity recommendation, PA) 3 times per week, with a 25%–30% caloric restriction diet.
Results
VO2peak improved in overweight and obese males (pre and post values in L/min, respectively; W = 3.2 ± 0.6 vs. 3.7 ± 0.5, p < 0.001; O = 3.6 ± 0.6 vs. 3.8 ± 0.6, p = 0.013) as well as in overweight females (2.0 ± 0.3 vs. 2.3 ± 0.4, p < 0.001). VO2peak in the first ventilatory threshold (VT1) increased for all 4 interventions in males (p < 0.05), except for S in the obese group (1.6 ± 0.2 vs. 1.7 ± 0.3, p = 0.141). In females, it increased in E (0.9 ± 0.2 vs. 1.4 ± 0.3, p < 0.001), SE (0.9 ± 0.2 vs. 1.2 ± 0.4, p = 0.003), and PA (0.9 ± 0.1 vs. 1.2 ± 0.2, p = 0.006) in overweight groups. Time-to-exhaustion improved in all subjects except for females in PA group (15.7 ± 0.3 min vs. 15.9 ± 0.3 min, p = 0.495).
Conclusion
Our results suggest that all methods, including the recommendation of physical activity, can improve cardiovascular fitness in overweight subjects and obese males.
Keywords: Combined training, Endurance training, Obesity, Oxygen consumption, Physical activity, Strength training, Ventilatory threshold
1. Introduction
From a clinical point of view, obesity is associated with many comorbidities and represents an important health problem.1 Studies have suggested that lower aerobic fitness is also associated with a less favorable coronary or cardiovascular risk factor profile and an increase in peak oxygen consumption (VO2peak) in the amount of 1 metabolic equivalent, correlated with a 13% reduction of all-cause mortality as well as with a 15% decrease in cardiovascular risk.2, 3 In general, obese individuals have lower cardiovascular fitness than lean counterparts.4 Results suggest that increased fitness could reduce the risks associated with obesity.5
Data regarding the effect of different training methods for improving cardiovascular fitness in obese subjects are still inconsistent. Some found improvements in VO2peak only with aerobic and/or combined (aerobic/resistance) training,6, 7, 8, 9, 10 whereas others also found improvements applying resistance training.11, 12, 13, 14, 15, 16, 17 Aerobic training promotes changes in aerobic capacity, increasing mitochondrial oxidative capacities and capillary density in skeletal muscle,18 whereas resistance training increases muscle mass, which should increase maximal aerobic capacity.19 Therefore, it is reasonable to suggest that both types of training together can contribute to the improvement in cardiorespiratory fitness. In fact, many authors have found combined training to have a greater influence on cardiorespiratory response.20, 21 However, the comparison among studies is difficult owing to the different training methodologies used. It is therefore still unknown which method is the one achieving greatest enhancements.20 Along this line, and to the best of our knowledge, no study has compared the effects of different training methods applying the same volume and intensity, and therefore assuring that the distinct results were due only to the change in the type of training. In addition, there is little literature about circuit training, which has been used in many fitness centers for some years. There are also few studies reporting data regarding aerobic and anaerobic threshold changes after a weight loss program.8, 22, 23 These data could uncover interesting information about the cardiovascular fitness response of overweight and obese people following this kind of program. The improvement in these parameters is also related to a longer duration of exercise in the same intensity and consequently a longer period of fatty acid oxidation or increased intensity of exercise, which can ensure excess post-exercise oxygen consumption and contribute to weight loss.24, 25 Finally, some authors have suggested that if dietary intervention is associated with the training program, VO2peak will improve even more.26
Therefore, the main objective of this study was to analyze the effect of a weight loss program on cardiovascular fitness in overweight and obese subjects, comparing the effectiveness of isolated and combined aerobic and resistance training on VO2peak.
2. Methods
2.1. Participants
This study was performed as part of the Nutrition and Physical Activity for Obesity Study (the PRONAF study according to its Spanish initials), the aim of which was to assess the usefulness of different types of physical activity (PA) and nutrition programs for the treatment of obesity. The inclusion criteria specified adult subjects, aged 18 to 50 years, who were overweight or obese (25 kg/m2 ≤ body mass index (BMI) ≤ 34.9 kg/m2), sedentary (PA < 30 min/day), normoglycemic, and non-smoking. Only females with regular menstrual cycles were included. A total of 167 participants (77 males and 90 females) completed all the tests. Adherence to diet (80%) and exercise (90%) were included in the analysis. All participants were informed about the risks and benefits of the study and signed a document of informed consent. The PRONAF study was approved by the Human Research Review Committee of the University Hospital La Paz (HULP) (No. NCT01116856).
2.2. Study protocol
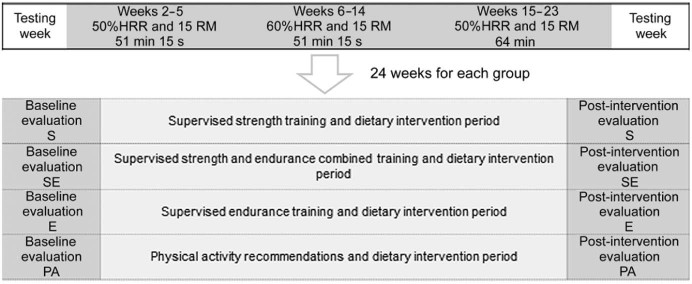
The complete methodology and the flow diagram can be found in Zapico et al.27 Briefly, subjects who fulfilled the inclusion criteria were randomly assigned to 1 of the 4 interventions detailed here, assuring a homogeneous distribution of age and gender among groups. The intervention programs lasted 22 weeks, and the assessment tests took place 1 week before (baseline) and after (post) the intervention.
2.2.1. Exercise protocols
Four different interventions were performed: strength training (S), endurance training (E), combined strength + endurance training (SE) groups followed the corresponding supervised exercise program plus the dietary intervention, and the PA group followed dietary intervention and was instructed about the general recommendations about PA from the American College of Sports Medicine (ACSM).5 The exercise of the PA group was not supervised, only registered with an accelerometer.
Subjects in the S, E, and SE groups trained 3 times per week for 22 weeks. All training sessions were carefully supervised by certified personal trainers. The exercise programs were designed according to the subject's muscle strength and heart rate reserve. Muscle strength was measured using the 15-repetition maximum testing method in the S and SE groups. Resting heart rate was calculated as the average heart rate during 10 min in a lying position, and maximal heart rate (HRmax) was obtained by means of the cardiovascular maximal effort test.
In the S group the session routine consisted of the execution of 8 scheduled exercises (i.e., shoulder press, squat, barbell row, lateral split, bench press, front split, biceps curl, and french press for triceps). For Group E, running, cycling, or elliptical (self-selected) exercises were the main components of the session routine, whereas the routine for the SE group consisted of a combination program using cycle ergometry, treadmill, or elliptical machine intercalated with squatting, rowing machine, bench press, and front split.
Both volume and intensity of the 3 training programs increased progressively (Fig. 1). The S and SE participants performed 15 repetitions of each strength exercise or 45 of aerobic exercise (only SE participants) with a rest period of 15 between them. Feedbacks of training loads were evaluated with the Rate of Perceived Exertion scale once a month, following a similar methodology used elsewhere.28
Fig. 1.
Timeline of the study. E = endurance training group; HHR = heart rate reserve; PA = physical activity recommendations group; RM = repetitions maximum; S = strength training group; SE = combined strength and endurance training group.
2.2.2. Hypocaloric diet
All groups underwent an individualized and hypocaloric diet (between 1200 and 3000 kcal) prescribed by expert dieticians in the Nutrition Department of HULP. The diet aimed for a 25% reduction of the total daily energy expenditure measured using the SenseWear Pro Armband accelerometer (BodyMedia Inc., Pittsburgh, PA, USA).
2.3. Measurements
2.3.1. Cardiovascular fitness
The test evaluating cardiovascular fitness was maximal ergospirometry following the modified Bruce protocol with a computerized treadmill (H/P/COSMOS 3PW 4.0; H/P/COSMOS Sports & Medical, Nussdorf-Traunstein, Germany). VO2peak was measured with the gas analyzer Jaeger Oxycon Pro (Erich Jaeger; Viasys Healthcare, Hoechberg, Germany). Heart response was continuously monitored with a 12-lead electrocardiogram. The effort test was maintained until exhaustion. The mean of the 3 highest measurements was used as VO2peak and HRmax.
The first and the second ventilatory thresholds (VT1 and VT2, respectively) were set at the point of maximum agreement of the most common methods of assessment published previously.29 All tests were evaluated by 2 researchers in a double-blind process. VO2peak was expressed in several values: absolute (L/min), relative to body mass (mL/kg/min), and relative to lean body mass (mL/kgLBM/min). Ventilatory thresholds were expressed in absolute (L/min) and percentage terms of VO2peak.
Subjects were classified in fitness categories according to their absolute VO2peak (L/min) and age. Subjects who scored “very poor”, “poor”, “fair”, or “average” were deemed unfit; and subjects who scored “good”, “very good”, or “excellent” were deemed fit in relation to age-specific norms.30
2.3.2. Body composition
Body composition was assessed by dual-energy X-ray absorptiometry scan (Version 6.10.029GE Encore 2002, GE Lunar Prodigy; GE Healthcare, Madison, WI, USA). Height was measured using a seca stadiometer (Quirumed, Valencia, Spain), which has a range of 80–200 cm. Body mass was measured using a TANITA BC-420MA balance (Bio Lógica Tecnología Médica S.L, Barcelona, Spain). BMI was calculated as body weight/height (kg/m2).
2.4. Statistical analysis
Data are presented as mean ± SD. Repeated three-way analysis of covariance (ANCOVA) measures were used to determine any differences among the 4 interventions (S, E, SE, and PA) and the BMI category (overweight and obese) at baseline and post-intervention. Analyses were performed in men and women separately, and age was used as covariate. The Bonferroni post hoc test was employed to locate specific differences. Differences between the PA group and the exercise groups in fitness category frequencies were calculated using a χ2 test. SPSS Statistic for Windows Version 20.0 (IBM Corp., Armonk, NY, USA) was used. The significance level was set at α = 0.05.
3. Results
Characteristics of all participants are summarized in Table 1. In both men and women, baseline characteristics were significantly different between overweight and obese subjects, except for age, height, and VO2peak values relative to body mass. No differences were observed among the groups within the same gender.
Table 1.
Characteristics at baseline (mean ± SD).
| Variable | Male |
Female |
||
|---|---|---|---|---|
| Overweight | Obese | Overweight | Obese | |
| Age (year) | 36.3 ± 8.0 | 38.6 ± 7.5 | 33.3 ± 8.5 | 38.4 ± 7.7 |
| Body weight (kg) | 87.5 ± 6.9 | 101.2 ± 7.9** | 73.5 ± 5.8 | 86.3 ± 8.5** |
| Height (cm) | 175.3 ± 6.4 | 176.7 ± 5.9 | 162.0 ± 6.1 | 163.0 ± 7.0 |
| Body mass index (kg/m2) | 28.4 ± 1.1 | 32.4 ± 1.9** | 28.0 ± 1.3 | 32.4 ± 1.9** |
| Percentage fat (%) | 33.7 ± 4.6 | 38.2 ± 4.0** | 43.3 ± 3.7 | 47.1 ± 3.6** |
| Lean body mass (kg) | 55.7 ± 5.5 | 59.8 ± 5.0* | 40.3 ± 4.2 | 44.1 ± 4.7** |
| Fat mass (kg) | 28.5 ± 5.2 | 37.2 ± 5.8** | 30.7 ± 3.7 | 39.5 ± 6.5** |
| VO2peak (L/min) | 3.2 ± 0.6 | 3.6 ± 0.6** | 2.0 ± 0.3 | 2.5 ± 0.4** |
| VO2peak (mL/kg/min) | 36.5 ± 1.1 | 36.1 ± 0.8 | 27.7 ± 0.5 | 28.2 ± 0.5 |
p < 0.05, **p < 0.001, compared with overweight.
3.1. VO2peak
Interactions between time of measurement and BMI category were found for absolute VO2peak values (F(1, 68) = 10.316, p = 0.002), VO2peak values relative to body mass (F(1, 68) = 7.714, p = 0.007), and VO2peak values relative to lean body mass (F(1, 68) = 9.911, p = 0.002) in men (Table 2). Absolute and relative (to body and to lean mass) VO2peak improved in overweight and obese men. Overweight men had greater improvement, about 12% more than obese men, in absolute VO2peak compared to baseline values. On the other hand, interactions between time and BMI category for absolute VO2peak values (F(1, 81) = 12.863, p = 0.001), relative to body mass (F(1, 81) = 12.309, p = 0.001), and VO2peak values relative to lean body mass (F(1, 81) = 12.951, p = 0.001) in women showed that being overweight increased all VO2peak values. In obese females, VO2peak values improved only relative to body mass, in general 12% less than in overweight women (Table 2). For men who improved from unfit to fit after the intervention, 43% were in the supervised training programs (S, E, and SE) and 38% were in PA, whereas in females, 29.6% were in the supervised training groups and 15.8% were in PA. However, χ2 analysis showed no significant association between type of intervention and percentage changes (Table 3).
Table 2.
Peak oxygen consumption before and after weight loss intervention (mean ± SD).
| Male |
Female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overweight | Obese | p | Observed power | η2 | Overweight | Obese | p | Observed power | η2 | |
| VO2peak (L/min) | ||||||||||
| Baseline | 3.2 ± 0.6 | 3.6 ± 0.6# |
T < 0.001 T × G 0.171 T × BMI 0.002 T × G × BMI 0.127 |
>0.99 0.43 0.89 0.48 |
0.38 0.07 0.13 0.08 |
2.0 ± 0.3 | 2.5 ± 0.4# |
T < 0.001 T × G 0.308 T × BMI 0.001 T × G × BMI 0.332 |
0.96 0.31 0.94 0.30 |
0.15 0.04 0.14 0.04 |
| Post-intervention | 3.7 ± 0.5** | 3.8 ± 0.6* | 2.3 ± 0.4** | 2.5 ± 0.4# | ||||||
| Δ | 0.5 ± 0.4 | 0.2 ± 0.5 | 0.3 ± 0.3 | −0.1 ± 0.3 | ||||||
| VO2peak (mL/kg/min) | ||||||||||
| Baseline | 37.4 ± 6.2 | 36.0 ± 5.9 |
T < 0.001 T × G 0.058 T × BMI 0.007 T × G × BMI 0.482 |
>0.99 0.62 0.78 0.22 |
0.65 0.10 0.10 0.03 |
27.7 ± 3.8 | 28.2 ± 3.3 |
T < 0.001 T × G 0.135 T × BMI 0.001 T × G × BMI 0.477 |
>0.99 0.48 0.93 0.22 |
0.55 0.07 0.13 0.03 |
| Post-intervention | 46.6 ± 5.6** | 41.4 ± 6.9#,** | 33.8 ± 5.6** | 31.0 ± 4.6#,** | ||||||
| Δ | 9.2 ± 5.7 | 5.4 ± 5.6 | 6.1 ± 4.4 | 2.8 ± 4.2 | ||||||
| VO2peak (mL/kgLBM/min) | ||||||||||
| Baseline | 57.9 ± 8.3 | 60.5 ± 8.1# |
T < 0.001 T × G 0.229 T × BMI 0.002 T × G × BMI 0.220 |
>0.99 0.37 0.87 0.38 |
0.41 0.06 0.13 0.06 |
50.7 ± 6.2 | 55.8 ± 6.1# |
T < 0.001 T × G 0.124 T × BMI 0.001 T × G × BMI 0.252 |
>0.99 0.49 0.94 0.36 |
0.22 0.07 0.14 0.05 |
| Post-intervention | 67.3 ± 6.7** | 63.9 ± 7.7** | 57.0 ± 8.4** | 56.5 ± 7.7 | ||||||
| Δ | 9.4 ± 8.1 | 3.4 ± 8.3 | 6.3 ± 6.5 | 0.7 ± 7.3 | ||||||
Note: Data in bold for the p value indicate significant difference.
Abbreviations: BMI = body mass index classification; G = intervention group; T = time; VO2peak = peak oxygen consumption.
p < 0.05, **p < 0.001, compared with baseline.
p < 0.05, compared with overweight.
Table 3.
VO2peak classified as fit and at baseline and post-intervention (%).
| Male |
Female |
|||
|---|---|---|---|---|
| PA | SUP | PA | SUP | |
| Baseline | 6.0 | 13.0 | 5.3 | 1.4 |
| Post-intervention | 44.0 | 56.0 | 21.1 | 31.0 |
| Δ | 38.0 | 43.0 | 15.8 | 29.6 |
Abbreviations: PA = physical activity group; SUP = supervised training group.
3.2. VO2 in VT1 and VT2
In males, a significant triple interaction (time–BMI category–intervention) was found for absolute VO2 in VT1 (F(3, 68) = 3.864, p = 0.013). All 4 interventions in overweight men increased these values (S: 43.0%, p < 0.001; E: 35.7%, p = 0.005; SE: 15.1%, p = 0.028; PA: 47.6%, p < 0.001). This occurred in obese men as well, except for the S group (E: 18.6%, p = 0.012; SE: 34.2%, p < 0.001; PA: 17.8%, p = 0.007). In females, interactions between time and BMI category (F(1, 80) = 16.328, p < 0.001) and between time and intervention (F(3, 80) = 7.117, p < 0.001) were found for absolute VO2 in VT1. Considering the pairwise comparison, differences between the pre- and post-measurements were found in E (+55%, p < 0.001), SE (+33%, p = 0.003), and PA (+33%, p = 0.006) groups, but only for overweight women. Concerning percentage of VO2 in VT1 with respect to VO2peak, interactions between time and BMI category (F(1, 80) = 5.991, p = 0.017) and time and intervention (F(3, 80) = 5.063, p = 0.003) were found only in females. This percentage improved in overweight women in E (38%, p < 0.001) and SE (14%, p = 0.015) groups. In males, only time (F(1, 68) = 22.377, p < 0.001) was significant. Regarding VT2, the main effect was observed in oxygen consumption for men (F(1, 68) = 33.629, p < 0.001) and for women (F(1, 80) = 8.849, p = 0.004). Percentage of VO2 in VT2 with respect to the peak did not change in any group (Table 4).
Table 4.
Oxygen consumption in VT1 and VT2 before and after weight loss intervention (mean ± SD).
| Overweight |
Obese |
p | Observed power | η2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | E | SE | PA | S | E | SE | PA | ||||
| Male | |||||||||||
| VO2peak in VT1 (L/min) |
T < 0.001 T × G 0.863 T × BMI 0.177 T × G × BMI 0.013 |
>0.99 0.10 0.27 0.80 |
0.54 0.01 0.03 0.15 |
||||||||
| Baseline | 1.2 ± 0.2 | 1.2 ± 0.1 | 1.5 ± 0.4 | 1.3 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.3 | 1.6 ± 0.2 | |||
| Post-intervention | 1.7 ± 0.2** | 1.6 ± 0.4* | 1.7 ± 0.3* | 1.9 ± 0.4** | 1.7 ± 0.3 | 1.8 ± 0.3* | 2.1 ± 0.2#,** | 1.8 ± 0.4* | |||
| Δ | 0.5 ± 0.2 | 0.4 ± 0.4 | 0.2 ± 0.3 | 0.6 ± 0.3 | 0.1 ± 0.1- | 0.3 ± 0.3 | 0.5 ± 0.3 | 0.3 ± 0.4 | |||
| VO2peak in VT1 (%) |
T < 0.001 T × G 0.619 T × BMI 0.976 T × G × BMI 0.152 |
>0.99 0.17 0.05 0.45 |
0.25 0.03 0.00 0.07 |
||||||||
| Baseline | 38.9 ± 3.1 | 37.9 ± 2.9 | 44.9 ± 2.2 | 42.6 ± 3.2 | 42.8 ± 2.1 | 46.1 ± 2.4 | 42.6 ± 2.3 | 43.7 ± 2.2 | |||
| Post-intervention | 46.1 ± 2.9 | 45.3 ± 2.7 | 44.1 ± 2.0 | 52.3 ± 2.9 | 45.9 ± 2.0 | 51.0 ± 2.2 | 50.9 ± 2.1 | 50.4 ± 2.1 | |||
| Δ | 7.2 ± 4.2 | 7.4 ± 3.9 | −0.8 ± 3.0 | 9.7 ± 4.3 | 3.1 ± 2.9 | 4.9 ± 3.3 | 8.3 ± 3.1 | 6.7 ± 3.0 | |||
| VO2peak in VT2 (L/min) |
T < 0.001 T × G 0.311 T × BMI 0.234 T × G × BMI 0.093 |
>0.99 0.31 0.22 0.54 |
0.33 0.05 0.02 0.09 |
||||||||
| Baseline | 2.6 ± 0.7 | 2.7 ± 0.3 | 2.6 ± 0.5 | 2.6 ± 0.6 | 3.2 ± 0.7 | 2.6 ± 0.4 | 2.9 ± 0.5 | 2.9 ± 0.5 | |||
| Post-intervention | 3.3 ± 0.6 | 3.0 ± 0.5 | 3.2 ± 0.9 | 2.9 ± 0.7 | 3.1 ± 0.6 | 3.0 ± 0.4 | 3.5 ± 0.6 | 3.2 ± 0.5 | |||
| Δ | 0.8 ± 0.3 | 0.3 ± 0.5 | 0.6 ± 1.0 | 0.3 ± 0.2 | −0.2 ± 0.3 | 0.4 ± 0.3 | 0.6 ± 0.6 | 0.2 ± 0.4 | |||
| VO2peak in VT2 (%) | T = 0.149 T × G 0.928 T × BMI 0.470 T × G × BMI 0.627 |
0.30 0.08 0.11 0.16 |
0.03 0.01 0.01 0.02 |
||||||||
| Baseline | 80.4 ± 4.0 | 84.6 ± 3.7 | 80.5 ± 2.8 | 83.5 ± 4.1 | 82.5 ± 2.7 | 79.0 ± 3.1 | 79.4 ± 3.0 | 81.8 ± 2.8 | |||
| Post-intervention | 86.2 ± 5.7 | 84.0 ± 5.3 | 85.0 ± 4.0 | 79.7 ± 5.8 | 83.1 ± 3.9 | 85.9 ± 4.4 | 84.6 ± 4.2 | 87.6 ± 4.0 | |||
| Δ | 5.8 ± 7.2 | −0.6 ± 6.7 | 4.5 ± 5.1 | −3.7 ± 7.3 | 0.6 ± 4.9 | 6.9 ± 5.6 | 5.2 ± 5.3 | 5.8 ± 5.1 | |||
| Female | |||||||||||
| VO2peak in VT1 (L/min) |
T < 0.001 T × G < 0.001 T × BMI < 0.001 T × G × BMI 0.146 |
>0.99 0.98 0.98 0.46 |
0.30 0.21 0.17 0.06 |
||||||||
| Baseline | 1.0 ± 0.2 | 0.9 ± 0.2# | 0.9 ± 0.2 | 0.9 ± 0.1 | 1.4 ± 0.2 | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.3 | |||
| Post-intervention | 1.1 ± 0.3 | 1.4 ± 0.3** | 1.2 ± 0.4* | 1.2 ± 0.2* | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.4 ± 0.2 | 1.2 ± 0.2 | |||
| Δ | 1 | 0.5 ± 0.3 | 0.3 ± 0.3 | 0.2 ± 0.1 | −0.1 ± 0.1 | 0.1 ± 0.3 | 0.2 ± 0.3 | 0.1 ± 0.2 | |||
| VO2peak in VT1 (%) |
T < 0.001 T × G 0.003 T × BMI 0.017 T × G × BMI 0.247 |
0.98 0.91 0.68 0.36 |
0.17 0.16 0.07 0.05 |
||||||||
| Baseline | 48.0 ± 2.2 | 43.5 ± 2.0 | 43.8 ± 2.5 | 46.7 ± 2.3 | 54.8 ± 2.2 | 49.9 ± 2.0 | 48.8 ± 2.2 | 53.7 ± 2.5 | |||
| Post-intervention | 49.2 ± 2.6 | 60.0 ± 2.4** | 50.0 ± 2.9* | 52.7 ± 2.8 | 51.0 ± 2.6 | 53.9 ± 2.3 | 54.5 ± 2.6 | 55.3 ± 2.9 | |||
| Δ | 1.2 ± 3.2 | 16.5 ± 2.8 | 6.2 ± 3.5 | 6.0 ± 3.3 | −3.8 ± 3.2 | 4.0 ± 2.8 | 5.7 ± 3.2 | 1.6 ± 3.5 | |||
| VO2peak in VT2 (L/min) |
T = 0.004 T × G 0.376 T × BMI 0.105 T × G × BMI 0.167 |
0.84 0.27 0.37 0.44 |
0.10 0.04 0.03 0.06 |
||||||||
| Baseline | 1.7 ± 0.3 | 1.6 ± 0.2 | 1.5 ± 0.2 | 1.7 ± 0.3 | 2.1 ± 0.3 | 2.1 ± 0.4 | 2.1 ± 0.3 | 1.8 ± 0.2 | |||
| Post-intervention | 1.7 ± 0.4 | 2.0 ± 0.3 | 1.8 ± 0.6 | 1.7 ± 0.3 | 2.1 ± 0.4 | 2.1 ± 0.4 | 2.2 ± 0.3 | 2.0 ± 0.3 | |||
| Δ | 0.0 ± 0.3 | 0.3 ± 0.3 | 0.2 ± 0.4 | 0.1 ± 0.4 | −0.0 ± 0.3 | −0.0 ± 0.5 | 0.1 ± 0.3 | 0.1 ± 0.1 | |||
| VO2peak in VT2 (%) | T = 0.836 T × G 0.966 T × BMI 0.251 T × G × BMI 0.464 |
0.05 0.06 0.21 0.23 |
<0.01 <0.01 0.02 0.03 |
||||||||
| Baseline | 82.5 ± 2.2 | 82.3 ± 2.0 | 77.2 ± 2.5 | 82.5 ± 2.3 | 83.7 ± 2.2 | 85.9 ± 2.0 | 80.7 ± 2.2 | 83.3 ± 2.5 | |||
| Post-intervention | 80.6 ± 2.9 | 84.6 ± 2.6 | 76.2 ± 3.2 | 78.1 ± 3.1 | 85.2 ± 2.9 | 84.8 ± 2.6 | 84.4 ± 2.9 | 86.7 ± 3.2 | |||
| Δ | −1.9 ± 3.8 | 2.3 ± 3.4 | −1.0 ± 4.2 | −4.4 ± 4.0 | 1.5 ± 3.8 | −1.1 ± 3.4 | 3.7 ± 3.8 | 3.4 ± 4.2 | |||
Note: Data in bold for the p value indicate significant difference.
Abbreviations: BMI = body mass index classification; E = endurance training group; G = intervention group; PA = physical activity recommendations group; S = strength training group; SE = combined strength + endurance training group; T = time; VO2peak = peak oxygen consumption; VT1 = aerobic ventilatory threshold; VT2 = anaerobic ventilatory threshold or respiratory compensation point physical activity.
p < 0.05, **p < 0.001, compared with baseline.
p < 0.05, compared with S group.
3.3. Time-to-exhaustion
An interaction between time and intervention was observed (F(3, 81) = 3.027, p = 0.034) in females, demonstrating a significant improvement in all supervised training programs, i.e., S (+8.9%, p < 0.001), E (+8.5%, p < 0.001), SE (+4.4%, p = 0.018), and PA (+1.3%, p = 0.495). In males, only time was significant (F(1, 68) = 63.512, p < 0.001) for this variable, i.e., S (+7.4%), E (+8.2%), SE (+10.3%), and PA (+7.1%) (Fig. 2).
Fig. 2.
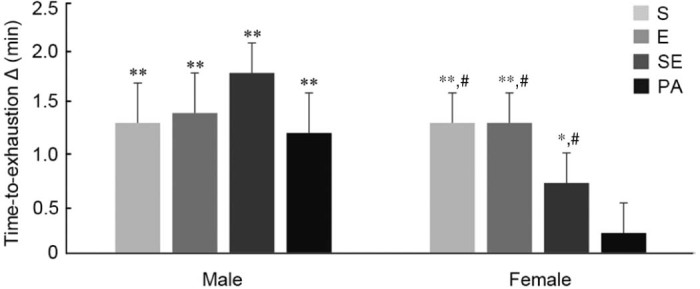
Time-to-exhaustion delta in minutes. Error bars represent one standard error of the mean. *p < 0.05, **p < 0.001, baseline–post differences; #p < 0.05, compared with PA group in female. E = endurance training group; PA = physical activity recommendation group; S = strength training group; SE = combined strength + endurance training group.
4. Discussion
The main finding of this study was that all 4 protocols were effective in improving cardiovascular fitness in overweight and obese males and overweight females.
Because age and gender have been proved to modify oxygen consumption,31, 32 we performed the analysis separately by gender and corrected for age. Another factor that influences oxygen consumption is body mass.4 Obese subjects had significantly lower estimated VO2peak relative to body mass than non-obese adults but similar VO2peak to overweight subjects.4 Our data also showed similar VO2peak relative to body mass or to lean body mass and greater absolute oxygen consumption in obese subjects compared with overweight subjects. VO2peak values were low in both categories of BMI and both genders. Compared with a Norwegian study that included 3816 subjects with heterogeneous BMI,31 our data were 20% lower in men and 25% lower in women.
Our data showed that in overweight and obese males, VO2peak relative to body mass increased by 26.8% and 16.0%, respectively, while females improved by 22.5% and 10.7%, respectively. Previous studies showed lesser improvements in relative to body mass VO2peak: between 8%33 and 17%12 for overweight and obese individuals combining aerobic exercise with a mild hypocaloric diet, or 5% for overweight males performing aerobic or resistance training.11 These differences could be due to the weight loss generated by our program, which was greater than in those studies. As reported elsewhere, the average weight loss in our sample was 9.6 kg,34 which could contribute to a considerable improvement in oxygen consumption relative to body mass. For instance, our data indicated that absolute VO2peak did not increase in obese females, whereas its relative value improved, most likely as a result of weight loss and not cardiorespiratory adaptations. Therefore, in a weight loss program, it seems to be more interesting to evaluate cardiovascular fitness by means of absolute VO2peak. Wheatley et al.35 observed that females presented limitations in cardiac performance and demonstrated that females need greater cardiac output than males to meet the same external work demand.
Another study in obese adolescents showed that gender differences exist in VO2 uptake on-kinetics during moderate exercise, indicating an enhanced potential for male subjects to deliver and/or use oxygen.36 Greater volume and intensity could be necessary to promote cardiovascular changes in obese women.
Our data also showed that all interventions were effective for improving VO2peak in overweight males and females and in obese males, which is in accordance with other studies.11, 13, 15, 16 Several studies found significant increases in VO2peak only for aerobic and/or combined training. However, in these studies, strength training involved shorter sessions6, 8, 13, 15 or fewer sessions than did aerobic or combined training.7, 14 In our study, intensity and volume were monitored to ensure the comparison among the different types of training, and the control of these parameters may have been responsible for the lack of differences. Finally, caloric restriction in our study appeared to be appropriate to allow an oxygen consumption increase, because significant increase in VO2max can be achieved only by moderate energy intake preservation.14
Although oxygen consumption is the most-used cardiovascular variable to measure the response to training, other variables may also provide important or interesting information. Few studies have assessed responses of thresholds, ventilation, and other cardiorespiratory parameters.23 PA performed at moderate intensity causes changes in VT1,37 which agrees with the training loads applied. In the same way as for VO2peak, obese women did not show an improvement in the absolute and relative values to lean mass oxygen consumption in VT1. Unlike the study by Salvadori et al.,23 which did not show changes in VT1, our data indicated that in overweight males, VO2 in VT1 improved in all interventions, as well as in overweight females and obese males, except for the S group. Strength training may lead to peripheral changes that could be considered antagonistic to aerobic power development.8 As also suggested by our data absolute and relative to lean mass VO2 in VT1 in obese people, other studies with appropriate comparison methodologies indicated increased VO2peak only for aerobic or combined training and not for strength training.9, 10 When obese men and women were analyzed together, only the combined group showed an improvement in absolute VO2peak.34 If we consider time commitments and health benefits, combined training may be more appropriate and less monotonous in inducing certain cardiorespiratory adaptations and adherence in obese people.34, 38
On the other hand, no interactions were observed for VO2peak in the VT2. Therefore, the intensity adopted does not seem enough to cause interactions in this threshold.39 Finally, the last parameter analyzed to predict improvement in cardiovascular fitness was the time-to-exhaustion on a treadmill test, which was the only parameter that improved in obese women. Even without changes in oxygen consumption, quality of oxygen utilization and neuromuscular adaptations caused by training may have led to a greater ability to tolerate high workloads (at or above the VT2) over longer periods of time before fatigue occurred.40 Our data showed improvements in time-to-exhaustion in overweight and obese females in S, SE, and E groups. However, in obese females, PA did not alter oxygen consumption or time-to-exhaustion. Interventions with exercise seemed more effective in obese women: dropout rates in PA were 68.4% for women and 31.6% for men. Moreover, within our supervised training programs, the percentage of men who improved from the unfit class to the fit class was 12.7% greater than the percentage in women. This difference between men and women increased to 23.0% in the PA group. Nevertheless, it is also important to consider the learning effect of the ergospirometry effort test, which may have influenced the findings in regard to this variable because most women in this study had never climbed on a treadmill.
Important strengths of this study include (1) the randomized design; (2) the inclusion of 4 different training programs in the same study combined with caloric restriction in all interventions; (3) the direct supervision of exercise for all training sessions; and (4) inclusion of appropriate programs of strength and combined training to provide an adequate stimulus and comparison among groups. The study is limited by the fact that it did not include a “no treatment” control group, but rather compared the intervention with previously described exercise recommendations that are broadly accepted from an ethical point of view and in clinical practice. Gains in VO2peak observed in all interventions might have been the result of the fact that the participants initially consented to participate in a study that would prescribe and counsel about exercise with the goal of increasing PA and improving fitness. Given that our supervised programs or recommendations required only minimal training and equipment, they could be implemented in a wide range of PA practices.
5. Conclusion
Interventions of exercise or recommendations of PA plus diet were effective in improving cardiovascular fitness in overweight males and females and obese males. Time-in-effort test was the only variable that increased in obese females, except for those in the PA recommendation group. Obese women seem to need greater intensity and volume of training to achieve cardiovascular changes. As practice applications, aerobic training between 50% and 60% of heart rate reserve and strength training for large muscle groups between 50% and 60% of 15 maximum repetitions, or a combination of both, 3 times per week and conducted in a circuit, may be more appropriate to induce adequate cardiorespiratory adaptations in obese people.
Authors' contributions
EAC, RC, and ABP performed the statistical analysis and drafted the manuscript; MG and MGG participated in its design and coordination and drafted the manuscript; PJB conceived of the study, and helped draft the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
The PRONAF Study takes place with the financial support of the Ministerio de Ciencia e Innovación, Convocatoria de Ayudas I + D 2008, Proyectos de Investigación Fundamental No Orientada, del VI Plan de Investigación Nacional 2008–2011 (contract: DEP2008-06354-C04-01). EAC is funded by a predoctoral grant from the Coordination for the Improvement of Higher Education Personnel (CAPES).
Footnotes
Note: Part of the text was presented at the Symposium Exernet on November 7–8, 2014, in Granada, Spain, and published in Revista Andaluza de Medicina del Deporte 2015; 8(1):25. doi: 10.1016/j.ramd.2014.10.014
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Lu Y., Hajifathalian K., Ezzati M., Woodward M., Rimm E.B., Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. The Lancet. 2014;383:970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnethon M.R., Gulati M., Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. JAMA. 2005;294:2981–2988. doi: 10.1001/jama.294.23.2981. [DOI] [PubMed] [Google Scholar]
- 3.Kodama S., Saito K., Tanaka S., Maki M., Yachi Y., Asumi M. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 4.Wang C.Y., Haskell W.L., Farrell S.W., Lamonte M.J., Blair S.N., Curtin L.R. Cardiorespiratory fitness levels among US adults 20–49 years of age: findings from the 1999–2004 National Health and Nutrition Examination Survey. Am J Epidemiol. 2010;171:426–435. doi: 10.1093/aje/kwp412. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly J.E., Blair S.N., Jakicic J.M., Manore M.M., Rankin J.W., Smith B.K. American College of Sports Medicine Position Stand. Appropriate PA intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 6.Davidson L.E., Hudson R., Kilpatrick K., Kuk J.L., McMillan K., Janiszewski P.M. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med. 2009;169:122–131. doi: 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- 7.Glowacki S.P., Martin S.E., Maurer A., Baek W., Green J.S., Crouse S.F. Effects of resistance, endurance, and concurrent exercise on training outcomes in men. Med Sci Sports Exerc. 2004;36:2119–2127. doi: 10.1249/01.mss.0000147629.74832.52. [DOI] [PubMed] [Google Scholar]
- 8.Hakkinen K., Alen M., Kraemer W.J., Gorostiaga E., Izquierdo M., Rusko H. Neuromuscular adaptations during concurrent strength and endurance training versus strength training. Eur J Appl Physiol. 2003;89:42–52. doi: 10.1007/s00421-002-0751-9. [DOI] [PubMed] [Google Scholar]
- 9.Ho S.S., Dhaliwal S.S., Hills A.P., Pal S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health. 2012;12:704. doi: 10.1186/1471-2458-12-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorge M.L., de Oliveira V.N., Resende N.M., Paraiso L.F., Calixto A., Diniz A.L. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. 2011;60:1244–1252. doi: 10.1016/j.metabol.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadizad S., Haghighi A.H., Hamedinia M.R. Effects of resistance versus endurance training on serum adiponectin and insulin resistance index. Eur J Endocrinol. 2007;157:625–631. doi: 10.1530/EJE-07-0223. [DOI] [PubMed] [Google Scholar]
- 12.Al Saif A., Alsenany S. Aerobic and anaerobic exercise training in obese adults. J Phys Ther Sci. 2015;27:1697–1700. doi: 10.1589/jpts.27.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bateman L.A., Slentz C.A., Willis L.H., Shields A.T., Piner L.W., Bales C.W. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise—STRRIDE-AT/RT) Am J Cardiol. 2011;108:838–844. doi: 10.1016/j.amjcard.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarsan A., Ardic F., Ozgen M., Topuz O., Sermez Y. The effects of aerobic and resistance exercises in obese women. Clin Rehabil. 2006;20:773–782. doi: 10.1177/0269215506070795. [DOI] [PubMed] [Google Scholar]
- 15.Willis L.H., Slentz C.A., Bateman L.A., Shields A.T., Piner L.W., Bales C.W. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol. 2012;113:1831–1837. doi: 10.1152/japplphysiol.01370.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yavari A., Najafipoor F., Aliasgarzadeh A., Niafar M., Mobasseri M. Effect of aerobic exercise, resistance training or combined training on glycaemic control and cardio-vascular risk factors in patients with type 2 diabetes. Biol Sport. 2012;29:135–143. [Google Scholar]
- 17.Schjerve I.E., Tyldum G.A., Tjonna A.E., Stolen T., Loennechen J.P., Hansen H.E. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin Sci (Lond) 2008;115:283–293. doi: 10.1042/CS20070332. [DOI] [PubMed] [Google Scholar]
- 18.Daussin F.N., Zoll J., Dufour S.P., Ponsot E., Lonsdorfer-Wolf E., Doutreleau S. Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: relationship to aerobic performance improvements in sedentary subjects. Am J Physiol Regul Integr Comp Physiol. 2008;295:R264–72. doi: 10.1152/ajpregu.00875.2007. [DOI] [PubMed] [Google Scholar]
- 19.Caruso F.C., Arena R., Phillips S.A., Bonjorno Jr J.C., Mendes R.G., Arakelian V.M. Resistance exercise training improves heart rate variability and muscle performance: a randomized controlled trial in coronary artery disease patients. Eur J Phys Rehabil Med. 2015;51:281–289. [PubMed] [Google Scholar]
- 20.Schwingshackl L., Dias S., Strasser B., Hoffmann G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: a systematic review and network meta-analysis. PLoS One. 2013;8:e82853. doi: 10.1371/journal.pone.0082853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguiar E.J., Morgan P.J., Collins C.E., Plotnikoff R.C., Callister R. Efficacy of interventions that include diet, aerobic and resistance training components for type 2 diabetes prevention: a systematic review with meta-analysis. Int J Behav Nutr Phys Act. 2014;11:2. doi: 10.1186/1479-5868-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boni E., Giustina A., Borra E., Bussi A.R., Grassi V. Cardiopulmonary adaptation to exercise after acute weight loss in severely obese subjects. Monaldi Arch Chest Dis. 1995;50:264–268. [PubMed] [Google Scholar]
- 23.Salvadori A., Fanari P., Marzullo P., Codecasa F., Tovaglieri I., Cornacchia M. Short bouts of anaerobic exercise increase non-esterified fatty acids release in obesity. Eur J Nutr. 2014;53:243–249. doi: 10.1007/s00394-013-0522-x. [DOI] [PubMed] [Google Scholar]
- 24.Burleson Jr M.A., O'Bryant H.S., Stone M.H., Collins M.A., Triplett-McBride T. Effect of weight training exercise and treadmill exercise on post-exercise oxygen consumption. Med Sci Sports Exerc. 1998;30:518–522. doi: 10.1097/00005768-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Kirk E.P., Donnelly J.E., Smith B.K., Honas J., Lecheminant J.D., Bailey B.W. Minimal resistance training improves daily energy expenditure and fat oxidation. Med Sci Sports Exerc. 2009;41:1122–1129. doi: 10.1249/MSS.0b013e318193c64e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melanson K.J., Dell'Olio J., Carpenter M.R., Angelopoulos T.J. Changes in multiple health outcomes at 12 and 24 weeks resulting from 12 weeks of exercise counseling with or without dietary counseling in obese adults. Nutrition. 2004;20:849–856. doi: 10.1016/j.nut.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Zapico A.G., Benito P.J., Gonzalez-Gross M., Peinado A.B., Morencos E., Romero B. Nutrition and PA programs for obesity treatment (PRONAF study): methodological approach of the project. BMC Public Health. 2012;12:1100. doi: 10.1186/1471-2458-12-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaibi G.Q., Cruz M.L., Ball G.D., Weigensberg M.J., Salem G.J., Crespo N.C. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc. 2006;38:1208–1215. doi: 10.1249/01.mss.0000227304.88406.0f. [DOI] [PubMed] [Google Scholar]
- 29.Rabadan M., Diaz V., Calderon F.J., Benito P.J., Peinado A.B., Maffulli N. Physiological determinants of speciality of elite middle- and long-distance runners. J Sports Sci. 2011;29:975–982. doi: 10.1080/02640414.2011.571271. [DOI] [PubMed] [Google Scholar]
- 30.O'Donovan G., Kearney E., Sherwood R., Hillsdon M. Fatness, fitness, and cardiometabolic risk factors in middle-aged white men. Metabolism. 2012;61:213–220. doi: 10.1016/j.metabol.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Loe H., Rognmo O., Saltin B., Wisloff U. Aerobic capacity reference data in 3816 healthy men and women 20–90 years. PLoS One. 2013;8:e64319. doi: 10.1371/journal.pone.0064319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rojo-Tirado M.A., Benito P.J., Atienza D., Rincon E., Calderon F.J. Effects of age, sex, and treatment on weight-loss dynamics in overweight people. Appl Physiol Nutr Metab. 2013;38:967–976. doi: 10.1139/apnm-2012-0441. [DOI] [PubMed] [Google Scholar]
- 33.Himeno E., Nishino K., Nanri H., Okazaki T., Komatsu T., Ikeda M. Evaluation of the effects of exercise and a mild hypocaloric diet on cardiovascular risk factors in obese subjects. J UOEH. 2001;23:1–12. doi: 10.7888/juoeh.23.1. [DOI] [PubMed] [Google Scholar]
- 34.Benito P.J., Bermejo L.M., Peinado A.B., Lopez-Plaza B., Cupeiro R., Szendrei B. Change in weight and body composition in obese subjects following a hypocaloric diet plus different training programs or PA recommendations. J Appl Physiol. 2015;118:1006–1013. doi: 10.1152/japplphysiol.00928.2014. [DOI] [PubMed] [Google Scholar]
- 35.Wheatley C.M., Snyder E.M., Johnson B.D., Olson T.P. Sex differences in cardiovascular function during submaximal exercise in humans. Springerplus. 2014;3:445. doi: 10.1186/2193-1801-3-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franco R.L., Bowen M.K., Arena R., Privett S.H., Acevedo E.O., Wickham E.P. Sex differences in pulmonary oxygen uptake kinetics in obese adolescents. J Pediatr. 2014;165:1161–1165. doi: 10.1016/j.jpeds.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuomainen P., Peuhkurinen K., Kettunen R., Rauramaa R. Regular physical exercise, heart rate variability and turbulence in a 6-year randomized controlled trial in middle-aged men: the DNASCO study. Life Sci. 2005;77:2723–2734. doi: 10.1016/j.lfs.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 38.Coquart J.B., Lemaire C., Dubart A.E., Luttembacher D.P., Douillard C., Garcin M. Intermittent versus continuous exercise: effects of perceptually lower exercise in obese women. Med Sci Sports Exerc. 2008;40:1546–1553. doi: 10.1249/MSS.0b013e31816fc30c. [DOI] [PubMed] [Google Scholar]
- 39.Elliott A.D., Rajopadhyaya K., Bentley D.J., Beltrame J.F., Aromataris E.C. Interval training versus continuous exercise in patients with coronary artery disease: a meta-analysis. Heart Lung Circ. 2015;24:149–157. doi: 10.1016/j.hlc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Lucia A., Hoyos J., Santalla A., Perez M., Chicharro J.L. Kinetics of VO2 in professional cyclists. Med Sci Sports Exerc. 2002;34:320–325. doi: 10.1097/00005768-200202000-00021. [DOI] [PubMed] [Google Scholar]