Abstract
Background
Fluoroquinolones have been widely used for a variety of Gram-positive and Gram-negative infections, and by 2002 they had become the most commonly prescribed class of antibiotics for adults in the United States. With widespread use, the class has become associated with a range of adverse events. Delafloxacin is a fluoroquinolone approved in the United States for the treatment of adults with acute bacterial skin and skin structure infections (ABSSSIs). Delafloxacin is differentiated from other fluoroquinolones due to structural differences and in its activity against methicillin-resistant Staphylococcus aureus, including quinolone-resistant strains. This paper reviews the safety profile of delafloxacin across clinical studies with an emphasis on the incidence of adverse events of special interest that are associated with fluoroquinolones.
Methods
Data from 2 completed phase III studies of delafloxacin for the treatment of ABSSSIs were pooled and are the primary focus of this paper. Additional support from the full safety analysis set (30 completed phase I to phase III clinical studies) is included where applicable.
Results
Fewer patients in the pooled delafloxacin group had AESIs than in the comparator group (7.0% vs 9.2%, respectively). Delafloxacin had a low rate of discontinuations due to treatment-related adverse events (<1%). Serious adverse events occurred at similar rates in patients treated with delafloxacin vs comparators.
Conclusions
Serious adverse events occurred at similar rates in patients treated with delafloxacin vs nonquinolone comparators used to treat ABSSSIs.
Clinicaltrials.gov identifier
Keywords: adverse events, delafloxacin, fluoroquinolone, MRSA, safety
Fluoroquinolones (FQs), originally considered a generally well-tolerated class of antibiotics, have been widely used in mild outpatient infections, which has resulted in both resistance and safety issues. Although warnings of joint pathology were in the norfloxacin and ciprofloxacin labels by 1987, a Boxed Warning was added to fluoroquinolones by the Food and Drug Administration (FDA) in July of 2008 for increased risk of tendinitis and tendon rupture. In February 2011, the risk of worsening symptoms for those with myasthenia gravis was added to the Boxed Warning. In August 2013, updates to the labels described the potential for irreversible peripheral neuropathy. More recently, in July 2016, the FDA again required changes to the label for all fluoroquinolones, including a boxed warning related to disabling and potentially permanent side effects involving the tendons, muscles, joints, nerves, and central nervous system [1]. As part of their review, the FDA concluded for mild outpatient infections that do not require routine antibiotics (acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections) that “fluoroquinolones should be reserved for use in patients who have no other treatment options” [2]. Finally, in July of 2018, new label changes directed by the FDA added that hypoglycemia can lead to coma and makes the mental health side effects (disorientation, agitation, nervousness, memory impairment, delirium) more prominent and more consistent across the systemic fluoroquinolone drug class [3].
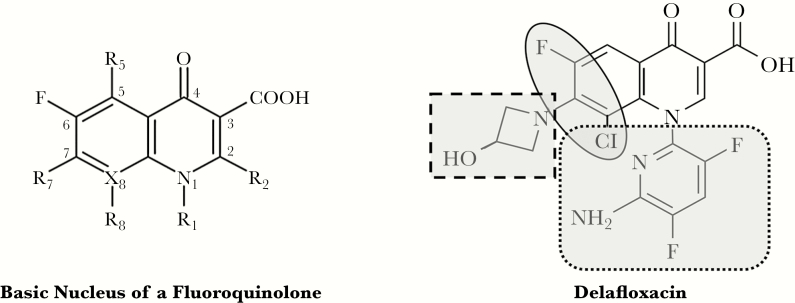
Delafloxacin is a recently approved FQ in the United States for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSIs). It is also currently under development for the treatment of adult patients with community-acquired bacterial pneumonia (NCT02679573). Although fluoroquinolones have not traditionally been among the firstline options for ABSSSIs, unique structural and chemical characteristics, which result in enhanced potency and intracellular penetration under acidic conditions, distinguish delafloxacin from other fluoroquinolones [4]. It is highly active against methicillin-resistant Staphylococcus aureus, including quinolone-resistant strains. Unlike other fluoroquinolones, delafloxacin is mainly anionic at a physiologic pH but is uncharged at a slightly acidic pH (≤5.5), allowing delafloxacin to accumulate in bacteria as the pH of the local environment becomes more acidic [5, 6]. Due to its unique structure, delafloxacin also has the potential to minimize some of the known safety concerns of fluoroquinolones, particularly central nervous system events and phototoxicity (Figure 1) [5, 7–9].
Figure 1.
Structure activity relationships lead to unique features. Large and heavily substituted N1 (dotted square) and unique polarity (oval) offer photo safety regardless of presence of a halogen. Anionic nature (dashed square) and bulky molecule at N1 (dotted square) lower central nervous system toxicity.
Clinicians should base use of any antibiotic upon individual patient and institutional needs, in concert with appropriate stewardship, in order to select patients most likely to benefit from the agent while minimizing collateral damage. In an effort to inform this decision-making process of weighing the risks and benefits of available therapeutic options, the intent of this paper is to review the current safety database of delafloxacin with a special emphasis on the incidence of adverse events of special interest (AESIs) across clinical studies involving delafloxacin conducted to date. Those AESIs are found in Table 1 and are associated with members of the the fluoroquinolone class to varying degrees. Delafloxacin FDA labeling has the fluoroquinolone class Box Warning regarding potential for tendinitis, tendon rupture, peripheral neuropathy, central nervous system effects, and exacerbation of myasthenia gravis.
Table 1.
Adverse Events of Special Interest Associated With Fluoroquinolones, With MedDRA-Associated Search Terms
| Medical Topics | Potential SMQ | Search Criteria |
|---|---|---|
| Potential myopathy | Rhabdomyolysis/myopathy SMQ | Rhabdomyolysis/myopathy SMQ broad |
| C. difficile diarrhea | Pseudomembranous colitis SMQ | Pseudomembranous colitis SMQ narrow |
| Convulsions | Convulsions SMQ | Convulsion SMQ narrow |
| Potential peripheral neuropathy | Peripheral neuropathy SMQ | Peripheral neuropathy SMQ broad |
| Potential tendon disorder | NA | HLT tendon disorders |
| Potential QT prolongation | Torsade de pointes/QT prolongation SMQ | Torsade de pointes/QT prolongation SMQ broad |
| Potential phototoxicity | NA | Preferred term “photosensitivity reaction” |
| Hyperglycemia | Hyperglycemia SMQ | Narrow SMQ “hyperglycemia/new-onset diabetes” |
| Hypoglycemia | NA | HLT “hypoglycemic conditions NEC” plus preferred terms “blood glucose abnormal” and “blood glucose fluctuation” |
| Hepatic-related events | Sub-SMQ–drug-related hepatic disorders comprehensive | SMQ “cholestasis and jaundice of hepatic origin, narrow” and SMQ “hepatic failure, fibrosis, and cirrhosis and other liver damage–related conditions, narrow” and SMQ “hepatitis, noninfectious, narrow” and SMQ “liver-related investigators, signs and symptoms, narrow” |
Source: Review of MedDRA Introductory Guide for SMQs, Version 16.1.
Abbreviations: HLT, MedDRA high-level term; MedDRA, Medical Dictionary for Regulatory Activities; NEC, not elsewhere classified; SMQ, standardized MedDRA queries.
METHODS
To date, delafloxacin has been evaluated in 30 completed phase I to phase III clinical studies, comprising a total of 2658 delafloxacin-treated subjects (Supplementary Table 1).
As results of the phase I and II studies have been reported elsewhere, this review will focus primarily on pooled data from the 2 phase III ABSSSI studies, which used multiday dosing and used the final formulation and dosing approved by the FDA [10–13]. However, for completeness, the AESIs seen in the phase II studies are noted if they occurred. The phase III studies encompass 1510 patients, in which delafloxacin-treated patients received 5–14 days of delafloxacin 300 mg intravenously (IV) and/or 450 mg orally every 12 hours plus a 30-day post-treatment observation period (Supplementary Table 1). In these studies, delafloxacin was compared with IV vancomycin 15 mg/kg (actual body weight) plus aztreonam dosed every 12 hours [7, 8]. The pooled phase III safety analysis set consisted of 1492 patients who were randomized and received at least 1 dose of the study drug.
Safety was assessed by the collection of adverse event (AE) reports and routine scheduled laboratory testing. Patients were asked a nondirected question by the investigator to elicit any medically related changes in their well-being or medications. The investigator assessed the “relatedness” of the event to the treatments, and also assessed the severity and seriousness. These events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) 16.1, which allows standardization in classification and coding into organ classes. In addition, adverse event reports potentially related to the AESIs were identified by medical review and use of Standardized MedDRA queries (SMQs) where possible. The SMQs are validated, standard sets of MedDRA terms used to support signal detection, to group similar medical entities and concepts for medical monitoring, and to assess and evaluate a variety of safety topics of regulatory interest [14]. The SMQs cast a wide net over multiple adverse event terms that could potentially be associated with a medical event of interest. For example, the SMQ for potential QT interval prolongation includes events as specific as torsade de pointes and events as general as syncope (Table 1). This conservative approach allows a view of many events across body systems, in order to evaluate signals that may otherwise be difficult to assess.
RESULTS
The demographics and baseline characteristics of subjects are shown in the Supplementary Data (Supplementary Table 2). In the pooled phase III clinical studies, the median duration of therapy was 6.0 days for both studies, with a range of 0.5–14.0 days and 0.5–14.5 days for the delafloxacin and vancomycin/aztreonam groups, respectively. The most commonly reported adverse events for delafloxacin were diarrhea, nausea, vomiting, and headache. Most AEs were mild to moderate in intensity. Relative to IV delafloxacin, oral delafloxacin was not associated with an increase in gastrointestinal events, and the pattern of treatment-emergent adverse events was similar to those of the IV formulation in the 2 phase III trials [7, 8]. Delafloxacin had a low rate of discontinuations due to treatment-related adverse events (TRAEs; <1%).
The incidence of serious adverse events (SAEs) was similar between delafloxacin and comparators (Table 2). No significant differences in safety were identified in patient subgroups by age, gender, race, ethnicity, body mass index, diabetes status, renal impairment, or history of infectious hepatitis B or C [7, 8, 15–19]. Laboratory changes were also similar between delafloxacin-treated patients and the comparator group. When such laboratory changes were seen, they were rarely reported by the investigator to be adverse events related to treatment. The rates of the AESIs associated with fluoroquinolones were lower in the delafloxacin group vs the comparator group (Table 3).
Table 2.
Overall Summary of Treatment-Emergent Adverse Events: Pooled Phase III
| Pooled Phase III Skin | ||
|---|---|---|
| Delafloxacin (n = 741), No. (%) | VAN/AZ (n = 751), No. (%) | |
| Total number of TEAEs | 775 | 879 |
| Patients with any TEAE | 334 (45.1) | 358 (47.7) |
| Patients with any related TEAE | 164 (22.1) | 196 (26.1) |
| Patients with any TEAE leading to premature study drug discontinuation | 13 (1.8) | 26 (3.5) |
| Patients with any related TEAE leading to premature study drug discontinuation | 6 (0.8) | 18 (2.4) |
| Patients with any TEAE of special interest, all cause | 52 (7.0) | 69 (9.2) |
| Patient with any serious TEAE | 27 (3.6) | 26 (3.5) |
| Patient with any related serious TEAE | 2 (0.3) | 4 (0.5) |
| Subjects with at least 1 related TEAE with incidence of ≥2% | ||
| Gastrointestinal disorders | 81 (10.9) | 45 (6.0) |
| Nausea | 45 (6.1) | 32 (4.3) |
| Diarrhea | 45 (6.1) | 15 (2.0) |
| Skin and subcutaneous tissue disorders (pruritus, urticaria, dermatitis, rash) | 7 (0.9) | 35 (4.7) |
A treatment-emergent adverse event was defined as an adverse event with (1) start date/time on or after the date/time of first study drug administration and before or on the date/time of last study medication administration + 28 days or (2) start date/time before the date/time of first study drug administration and worsening on or after the date/time of first study drug administration and before or on the date/time of last study medication administration + 28 days. Percentages are calculated as 100 × (n/N). The total number of TEAEs counts all TEAEs for patients. At each level of patient summarization, a patient with 1 or more reported events was counted only once. “Related” includes “possibly related,” “probably related,” “related,” and “definitely related.” Adverse events were coded using MedDRA, Version 16.1.
Abbreviations: MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment-emergent adverse event; VAN/AZ, vancomycin/aztreonam.
Table 3.
Treatment-Emergent Adverse Events of Special Interest (All Cause and Treatment-Related): Pooled Phase III
| AESI (All Causea) |
AESI (Related) |
|||
|---|---|---|---|---|
| Special Interest Preferred Term | Delafloxacinb (n = 741), No. (%), No. (%) |
VAN/AZb (n = 751), No. (%) |
Delafloxacinb (n = 741), No. (%) |
VAN/AZb (n = 751), No. (%) |
| Subjects with at least 1 TEAE of special interest | 52 (7.0) | 69 (9.2) | 25 (3.4) | 43 (5.7) |
| Hepatic-related events | 23 (3.1) | 30 (4.0) | 16 (2.2) | 20 (2.7) |
| Increased ALT | 14 (1.9) | 14 (1.9) | 10 (1.3) | 10 (1.3) |
| Increased AST | 10 (1.3) | 14 (1.9) | 6 (0.8) | 10 (1.3) |
| Increased transaminases | 3 (0.4) | 5 (0.7) | 3 (0.4) | 2 (0.3) |
| Increased hepatic enzyme | 2 (0.3) | 2 (0.3) | 1 (0.1) | 2 (0.3) |
| Liver function test abnormal | 0 | 2 (0.3) | 0 | 2 (0.3) |
| Hypertransaminasaemia | 2 (0.3) | 1 (0.1) | 1 (0.1) | 1 (0.1) |
| Increased gamma-glutamyltransferase | 1 (0.1) | 1 (0.1) | 0 | 0 |
| Hepatic cirrhosis | 0 | 1 (0.1) | 0 | 0 |
| Potential myopathy | 15 (2.0) | 34 (4.5) | 7 (0.9) | 20 (2.7) |
| Increased blood creatinine phosphokinase | 8 (1.1) | 15 (2.0) | 3 (0.4) | 7 (0.9) |
| Increased blood creatinine | 2 (0.3) | 4 (0.5) | 1 (0.1) | 4 (0.5) |
| Myalgia | 1 (0.1) | 2 (0.3) | 0 | 1 (0.1) |
| Renal impairment | 2 (0.3) | 1 (0.1) | 2 (0.3) | 0 |
| Renal failure, acute | 1 (0.1) | 7 (0.9) | 1 (0.1) | 3 (0.4) |
| Musculoskeletal pain | 1 (0.1) | 2 (0.3) | 0 | 1 (0.1) |
| Renal failure | 0 | 3 (0.4) | 0 | 3 (0.4) |
| Decreased creatinine renal clearance | 0 | 1 (0.1) | 0 | 1 (0.1) |
| Hyperglycemia | 6 (0.8) | 4 (0.5) | 2 (0.3) | 1 (0.1) |
| Hyperglycemia | 2 (0.3) | 2 (0.3) | 2 (0.3) | 1 (0.1) |
| Diabetes mellitus | 3 (0.4) | 1 (0.1) | 0 | 0 |
| Type 2 diabetes mellitus | 1 (0.1) | 1 (0.1) | 0 | 0 |
| Potential peripheral neuropathy | 4 (0.5) | 3 (0.4) | 1 (0.1) | 2 (0.3) |
| Paraesthesia | 4 (0.5) | 1 (0.1) | 1 (0.1) | 1 (0.1) |
| Hypoaesthesia | 1 (0.1) | 1 (0.1) | 0 | 1 (0.1) |
| Neuropathy peripheral | 0 | 1 (0.1) | 0 | 0 |
| Potential QT prolongation | 2 (0.3) | 1 (0.1) | 0 | 1 (0.1) |
| Syncope | 2 (0.3) | 0 | 0 | 0 |
| Loss of consciousness | 0 | 1 (0.1) | 0 | 1 (0.1) |
| Potential tendon disorder | 3 (0.4) | 1 (0.1) | 0 | 0 |
| Tendonitis | 3 (0.4) | 0 | 0 | 0 |
| Trigger finger | 0 | 1 (0.1) | 0 | 0 |
| Hypoglycemia | 2 (0.3) | 3 (0.4) | 1 (0.1) | 2 (0.3) |
| C. difficile diarrhea | 1 (0.1) | 0 | 1 (0.1) | 0 |
| Convulsions | 0 | 1 (0.1) | 0 | 1 (0.1) |
| Potential phototoxicity | 0 | 0 | 0 | 0 |
Adverse events of special interest were selected based on medical issues of interest for the fluoroquinolone class of antibiotics and include C. difficile diarrhea, convulsions, hepatic-related events, hyperglycemia, hypoglycemia, potential myopathy, potential peripheral neuropathy, potential phototoxicity, potential tendon disorder, and potential QT prolongation.
If a special interest adverse event did not occur, it is not presented in the table. At each level of subject summarization, a subject is counted once if the subject reported 1 or more TEAEs of special interest. Adverse events were coded using MedDRA, Version 16.1.
Abbreviations: AESI, adverse event of special interest; AZ, aztreonam; TEAE, treatment-emergent adverse event; VAN, vancomycin.
aPotentially related, as assessed by the investigator.
bDelafloxacin 300 mg IV/450 mg oral Q12h; VAN 15 mg/kg (actual body weight) + AZ 1–2 g IV q12h.
INDIVIDUAL ADVERSE EVENTS OF SPECIAL INTEREST
Blood Glucose Disturbances
The pooled analysis showed a similar incidence of hypoglycemia and hyperglycemia among those treated with delafloxacin (n = 741; hyperglycemia 0.8%, hypoglycemia 0.3%) and comparators (n = 751; hyperglycemia 0.5%, hypoglycemia 0.4%). Hyperglycemia was mild or moderate in severity. Rates of treatment-related hyperglycemia (0.3% and 0.1%) and hypoglycemia (0.1% and 0.3%) were also similar between delafloxacin and comparator treatment groups, respectively. In 1 of the phase III studies (Study 302), intensive glucose monitoring for 12 hours postdose in patients who were also undergoing pharmacokinetic (PK) testing did not show differences between the 2 treatment groups (Supplementary Figure 1) [3]. No treatment discontinuations or serious adverse events were attributed to hyperglycemia or hypoglycemia in any patients treated with delafloxacin.
Clostridium difficile Diarrhea
In the safety analysis set, 1 patient (0.1%) in the delafloxacin group had C. difficile diarrhea compared with none in the comparator group. The patient entered the study as a prior treatment failure with sulfamethoxazole/trimethoprim and clindamycin. The C. difficile diarrhea was judged to be related to delafloxacin, was mild in severity, and resolved with treatment with oral metronidazole. It should be noted that 1 case was seen in 1 patient who received delafloxacin in a phase II ABSSSI study. The 83-year-old patient had enrolled in the trial with 1 prior dose each of amoxicillin/clavulanate, amoxicillin, and ceftriaxone. She was reported to have mild C. difficile diarrhea 16 days after the completion of delafloxacin that resolved spontaneously without treatment.
Convulsions
Patients with ongoing treatment for seizures or untreated history of seizures were excluded from the phase III studies. No patients in the delafloxacin group (n = 741) had convulsions, whereas 1 patient in the comparator group (n = 751) had treatment-related convulsions. There were no treatment discontinuations or serious events related to convulsions.
In a pooled analysis of 2 phase II studies in patients with ABSSSIs treated for 5 to 14 days, 1 patient in the treatment group (delafloxacin 300 mg IV twice daily, n = 127) experienced a convulsion, but none in the comparator group did (n = 221). The patient had a history of convulsion, and the event was not considered related to study treatment.
Hepatic Events
Rates of hepatic events (hypertransaminasaemia, increased transaminases, LFT increases, and increased ALT and AST) were similar between patients in the delafloxacin (n = 741) and comparator (n = 751) groups (3.1% and 4.0%, respectively), as were rates of treatment-related hepatic events (2.2% and 2.7%, respectively) in a pooled analysis of 2 phase III studies in patients with ABSSSIs treated for 5 to 14 days. There were no premature discontinuations of delafloxacin due to a hepatic event. In evaluation of laboratory testing, regardless of baseline values, 8 patients in the delafloxacin treatment group reported ALT >5 times the ULN result at any time in the study, whereas 13 patients in the VAN/AZ treatment groups reported ALT >5 times the ULN results at any time in the study. There were no patients in either group who met Hy’s Law (Table 4). In the single delafloxacin-treated patient who experienced a serious adverse event of increased ALT/AST, the event was deemed possibly related to the study drug by the investigator. A medical monitor deemed the event likely related to the patient’s hepatitis C. The baseline hepatitis C antibody testing was negative, but it was positive on day 38 and confirmed positive on day 73. The event of AST increase resolved on day 38, and the events of ALT and blood creatine phosphokinase increase resolved on day 73.
Table 4.
Incidence of Elevated Transaminases and Total Bilirubin: Pooled Phase III Safety Analysis Set
| Parameter Criterion (Patients Reporting at Least 1 Incident) | Delafloxacin (n = 741), No. (%) |
All Comparators (n = 751), No. (%) |
|---|---|---|
| ALT >3 × ULN, U/L | ||
| Day 3 | 5 (0.7) | 12 (1.6) |
| Day 7 | 2 (0.3) | 7 (0.9) |
| End of treatment | 12 (1.6) | 20 (2.7) |
| Follow-up | 15 (2.0) | 13 (1.7) |
| Late follow-up | 16 (2.2) | 7 (0.9) |
| Overall worst postbaseline | 36 (4.9) | 41 (5.5) |
| ALT >5 × ULN, U/L | ||
| Day 3 | 0 | 1 (0.1) |
| Day 7 | 0 | 2 (0.3) |
| End of treatment | 3 (0.4) | 6 (0.8) |
| Follow-up | 3 (0.4) | 4 (.05) |
| Late follow-up | 5 (0.7) | 1 (0.1) |
| Overall worst postbaseline | 8 (1.1) | 13 (1.7) |
| AST >3 × ULN, U/L | ||
| Day 3 | 8 (1.1) | 7 (0.9) |
| Day 7 | 3 (0.4) | 5 (0.7) |
| End of treatment | 14 (1.9) | 12 (1.6) |
| Follow-up | 12 (1.6) | 10 (1.3) |
| Late follow-up | 9 (1.2) | 6 (0.8) |
| Overall worst postbaseline | 32 (4.3) | 26 (3.5) |
| AST >5 × ULN, U/L | ||
| Day 3 | 0 | 1 (0.1) |
| Day 7 | 0 | 0 |
| End of treatment | 3 (0.4) | 2 (0.3) |
| Follow-up | 4 (0.5) | 2 (0.3) |
| Late follow-up | 2 (0.3) | 1 (0.1) |
| Overall worst postbaseline | 6 (0.8) | 4 (0.5) |
| Total bilirubin >2 × ULN, mcmol/L | ||
| Day 3 | 3 (0.4) | 0 |
| Day 7 | 1 (0.1) | 0 |
| End of treatment | 1 (0.1) | 0 |
| Follow-up | 3 (0.4) | 0 |
| Late follow-up | 3 (0.4) | 1 (0.1) |
| Overall worst postbaseline | 7 (0.9) | 1 (0.1) |
For “overall worst postbaseline,” all laboratory assessments including those obtained from unscheduled visits are included, and the patient is included in the numerator if he/she met the criterion at least once postbaseline.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal.
Potential Myopathy
The events reported in the SMQs for potential myopathy were blood creatinine phosphokinase increase, blood creatinine increase, myalgia, musculoskeletal pain, and myositis and included renal impairment/failure and decreased creatinine clearance. The rates of potential myopathy in the pooled phase III ABSSSI studies were 2.0% (15/741) for delafloxacin and 4.5% (34/751) for comparators, and events were generally mild or moderate in severity. Rates of potential myopathy judged treatment-related by the investigator were lower in patients treated with delafloxacin vs comparators (0.9% vs 2.7%, respectively). Rates of potential myopathy lasting longer than 30 days were lower in patients treated with delafloxacin vs comparators (0.5% vs 1.3%, respectively). No treatment discontinuations or serious adverse events were attributed to myopathy in patients treated with delafloxacin.
Potential Peripheral Neuropathy
The events reported in the SMQs for potential peripheral neuropathy were hypoesthesia, burning sensation, and paresthesia. Rates of potential peripheral neuropathy in the pooled phase III ABSSSI studies were 0.5% (4/741) and 0.4% (3/751) for delafloxacin and vancomycin/aztreonam, respectively. These events were mild or moderate in severity. Those judged by the investigator to be related to treatment occurred in 0.1% (1/741) of delafloxacin patients and 0.3% (2/751) of vancomycin/aztreonam patients. The incidence of potential peripheral neuropathy lasting longer than 30 days was similar between patients in the delafloxacin (1/741, 0.1%) and comparator groups (2/751, 0.3%). There were no treatment-related discontinuations or serious adverse events attributed to delafloxacin.
Phototoxicity
There were no patients either in the delafloxacin group (n = 741) or the comparator group (n = 751) who reported potential phototoxicity. These findings are consistent with those of a phase I, single-blind, randomized study of 52 healthy adults who received 200 mg or 400 mg/d of oral delafloxacin, or 400 mg/d oral lomefloxacin (active control), or placebo for 6 days. Delafloxacin at both doses did not demonstrate a phototoxic effect (ie, no change from baseline in minimal erythema dose [MED] over the wavelength range of 295–430 nm), whereas lomefloxacin exhibited moderate phototoxicity at Ultraviolet-A (UVA) wavelengths of 335 nm and 365 nm [20].
Potential for QT Prolongation
A 12-lead electrocardiograph (ECG) was performed at screening and, if clinically indicated by the investigator, after screening. The events reported in the SMQs for potential QT prolongation were syncope and loss of consciousness. There were no reports of torsade de pointes, nor were there serious events or treatment discontinuations in this category. A pooled analysis of 2 phase III studies in patients with ABSSSIs treated for 5 to 14 days reported that 2 patients (0.3%) in the delafloxacin group (n = 741) experienced syncope, whereas 1 patient (0.1%) in the comparator group (n = 751) experienced loss of consciousness. The events in the delafloxacin group were considered unrelated to treatment, whereas the event in the comparator group was considered related to treatment [21].
Potential Tendon Disorder
The events reported in the SMQs of tendon disorder were tendinitis and trigger finger. A pooled analysis of 2 phase III studies in patients with ABSSSIs showed that tendinitis occurred in 3 (0.4%) patients receiving delafloxacin (n = 741) and no patients receiving vancomycin/aztreonam (n = 751). No patients receiving delafloxacin experienced trigger finger, which occurred in 1 patient (0.1%) receiving vancomycin/aztreonam. All events were mild or moderate in severity. No treatment discontinuations or serious adverse events were attributed to tendon disorders in patients receiving delafloxacin, and no cases of tendon rupture were reported. In a pooled analysis of 2 phase II studies in patients with ABSSSIs treated for 5 to 14 days, there were no cases of tendon rupture with delafloxacin 300 mg IV twice daily (n = 127) or comparators (n = 221) [10, 11]. In addition, there were no cases in clinical trials meeting the definition of fluoroquinolone-associated disability, as defined by the FDA (patients who were previously healthy, prescribed a fluoroquinolone, and developed an adverse event in 2 or more of the following body systems: peripheral nervous system, neuropsychiatric, musculoskeletal, senses, cardiovascular, or skin; the events had to last for more than 30 days after the FQ was discontinued and had to have a reported outcome of disability) [22].
DISCUSSION
Widely used for a variety of Gram-positive and Gram-negative hospital- and community-acquired infections, by 2002, fluoroquinolones had become the most commonly prescribed class for adults in the United States [23]. Their widespread and inappropriate use has led to the emergence of fluoroquinolone and multidrug-resistant pathogens, and a range of class-associated adverse events and risks became recognized [24, 25]. Clinicians should base use of any antibiotic upon individual patient and institutional needs, in concert with antimicrobial stewardship, to select the most appropriate therapy in order to maximize patient benefit while minimizing potential collateral damage [25].
Extensive clinical experience with fluoroquinolone-identified AESIs guided the safety assessment of delafloxacin in clinical trials that included 2658 delafloxacin-treated subjects. Findings here are based upon nonclinical and clinical work to date. However, the delafloxacin database is currently small relative to the size required to see some of the less frequently occurring adverse events, and time in the study was limited to a 30-day post-treatment observation period.
Overall, the most common AEs (seen in ≥5% of subjects regardless of causality) reported for IV delafloxacin were gastrointestinal events, headache, and infusion site pain (3.7%). Delafloxacin had a low rate of discontinuations due to treatment-related adverse events (<1%). There was 1 death in patients treated with delafloxacin, which was considered unrelated to delafloxacin. Serious adverse events occurred at similar rates in patients treated with delafloxacin vs comparators, including antibiotics used to treat serious skin infections. Fewer patients in the pooled delafloxacin group had AESIs than in the comparator group (7.0% vs 9.2%, respectively).
Additional findings merit note, as they lend credence to the findings from the phase III studies. A phase I study demonstrated no potential for phototoxicity, and no phototoxicity was seen in the phase III trials [20, 26]. No effects on muscle were observed in nonclinical studies with delafloxacin, and no treatment discontinuations or serious adverse events were attributed to myopathy in patients treated with delafloxacin in the phase III studies [21].
In contrast to other fluorinated FQs that cross the blood–brain barrier [27], delafloxacin showed little to no brain penetration during animal radiolabeled distribution studies. No direct neurotoxic effects (central nervous system or peripheral nervous system) were seen with delafloxacin in animal toxicology studies, and there was no histopathological evidence of neurotoxicity [21, 28]. Convulsions have not been observed with delafloxacin in any animal study, including high-dose toxicology studies [21]. In addition, at clinically relevant plasma levels, radiolabeled delafloxacin did not bind to GABA, benzodiazepine, NMDA, or adenosine receptors, targets implicated in the neurological symptoms of some marketed fluoroquinolones [29–31].
Fluoroquinolones are associated with mild, transient elevations in aminotransferase levels in approximately 1% to 3% of patients, but serious, potentially life-threatening, acute liver injury appears to occur, albeit rarely, with currently available FQ agents (approximately 1:100 000 persons exposed) [32–34]. Animal studies in rats and dogs administered delafloxacin showed no microscopic findings of liver injury [21]. A pooled safety analysis of phase I studies (n = 814) showed that hepatic-related events occurred in 0.9% of subjects receiving delafloxacin, and the incidence did not increase with increased dose. The phase III trials included hypertransaminasaemia, increased transaminases, and increased ALT and AST in reported transaminase elevations of 3.1%.
Another concern with fluoroquinolones, which was recently updated in FQ class product labeling, is dysglycemia [35, 36]. Gatifloxacin was withdrawn from the market in 2006 due to concerns of severe glucose disturbances [35]. Studies of moxifloxacin, levofloxacin, and ciprofloxacin have yielded conflicting results in terms of their relative risks for both hyper- and hypoglycemia [35–40]. Dysglycemias were not observed in preclinical studies of delafloxacin. In 1 phase II study, as part of the routine chemistry review, low serum glucose levels were found in 2 out of 49 subjects receiving delafloxacin 300 mg q12h, but both patients were asymptomatic [11]. Subsequently, intensive glucose monitoring for 12 hours postdose in patients in 1 of the phase III studies did not show differences between the delafloxacin and vancomycin/aztreonam groups [7].
Fluoroquinolone-associated QT interval prolongation is another potential concern and is caused by inhibition of potassium channels encoded by the human ether-a-go-go-related (hERG) gene [41–43]. The degree of cardiac potassium channel blockade and, consequently, QT prolongation, varies among individual fluoroquinolones, with a 100-fold difference in in vitro potency against hERG between sparfloxacin (most potent) and ofloxacin (least potent). Fluoroquinolones associated with prolongation of the QTc interval in humans also have been shown to prolong the QTc interval in the anesthetized dog model, prolong action potential duration in canine Purkinje fibers, and/or inhibit the delayed rectifier potassium (hERG) current [43]. Delafloxacin had no significant effect on several markers of QT prolongation in preclinical studies, no effect on QTc prolongation in instrumented anesthetized dogs (up to the highest dose tested, 30 mg/kg IV), and no inhibition of hERG channel at delafloxacin concentrations up to 185 µM [21, 41]. With these negative findings indicating a lack of signal for QTc prolongation, there was no requirement by the FDA to directly measure QTc intervals in the phase III trials [44]. The 2 events reported in the SMQs for potential QT prolongation were syncope and loss of consciousness and were not considered related to treatment. As a result, there is no warning for QTc prolongation in the delafloxacin label.
Most fluoroquinolones produce degenerative changes in articular cartilage and arthropathy in skeletally immature animals [45]. However, the full battery of delafloxacin studies conducted with adult and juvenile animals failed to demonstrate similar findings, with the exception of minimal focal articular changes seen in a single adult high-dose (480 mg/kg/d) dog [21]. Although delafloxacin has the potential to bind magnesium ions, it does not bind to NMDA receptors, which have been hypothesized to be related to tendinopathies [46]. There was no increase in tendon events in phase II or phase III in delafloxacin-treated patients vs comparators, but these studies have included few patients with factors known to increase the risk [7, 8, 10, 11, 47–49]. Although it is difficult to predict whether delafloxacin may or may not induce tendinitis and tendon rupture in adult humans based on the available animal data, the delafloxacin prescribing information contains the fluoroquinolone class Box Warning on tendinitis and tendon rupture, and patients should be monitored appropriately.
There were several limitations to this safety analysis of delafloxacin. The database for delafloxacin is limited both in terms of number of subjects and study duration. The phase III study treatment period of up to 14 days plus a 30-day post-treatment observation period is the longest observation period in this review. In contrast, the FDA cited data ranging from 31.6 to 33.2 million fluoroquinolone retail prescriptions (82% of FQ use) annually from 2010 to 2014. This prescribing volume elucidated adverse events that otherwise would be difficult to see. For example, trovafloxacin hepatic-related injury was recognized soon after the drug became available for general use, but only after approximately 2.5 million courses of therapy were administered [50]. Such big data supported the FDA conclusion that for mild outpatient infections that do not require routine antibiotics, fluoroquinolones should be reserved for use in patients who have no other treatment options. For more severe infections, such as more complicated cases of ABSSSIs where delafloxacin received an indication from the FDA, the overall number of prescriptions will be significantly lower.
Vancomycin and aztreonam (V/A) were used as comparators in the phase III studies. Although aztreonam is not an agent likely to be used to treat ABSSSIs in the clinic, it has become a standard Gram-negative agent in clinical trials enabling use of either a vancomycin or linezolid comparator and de-escalation of Gram-negative coverage as necessary. Using non-FQ comparators in phase III trials not associated with these AESIs allows a comparison with the basal population rate, but characterizing the risk of some AESIs in the general population can be difficult. This difficulty may lead to underreporting and variability in diagnostic validity [51]. However, analysis of available data indicates that delafloxacin does not appear to be associated with an increased risk of adverse events of special interest associated with other fluoroquinolones when compared with V/A in its phase III trials.
Preclinical testing, phase I–III trials, and postmarketing surveillance all contribute to but have limitations in their ability to identify true quinolone-related toxicities. Alone, no data source can reliably quantify risk assessment. Despite their inadequacies, data from all these sources may be used to strengthen the assessment of the risks associated with antimicrobial therapy for formulary status or individual patient treatment decisions. Understanding FQ history and monitoring use in the clinic will be essential, and future analysis of larger groups is warranted.
CONCLUSION
Analysis of available data indicates that delafloxacin does not appear to be associated with an increased risk of adverse events of special interest associated with other fluoroquinolones when compared with vancomcycin/aztreonam in its phase III trials. Due to the limited nature of the current delafloxacin safety database, it is clinically prudent to monitor for potential adverse events observed with other fluoroquinolones when using delafloxacin, and future analysis should be undertaken.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. S.C. contributed to the design, interpretation of original phase III data, overall data review, and review of this manuscript. D.H., T.L., and R.C. contributed to the data review process and review of the manuscript.
Financial support. All research was funded by Melinta Therapeutics, Inc. Editorial assistance for this manuscript was provided by Strategic Healthcare Communications, Hillsborough, New Jersey, funded by Melinta Therapeutics.
Potential conflicts of interest. T.L. reports personal fees from Melinta for consulting and a speakers’ bureau. G.R.C. reports personal fees from Arsanis, Basilea, Bayer, Bio2 Medical, Cempra, Contrafect, Medtronic (Study Design Group), Meiji Seika Pharma Co., Melinta, Motif, Novella (Adjudication Committee Member), Paratek, Pfizer (Mortality Board Member), Quintiles, Regeneron, Tetraphase, The Medicines Company, and Theravance for consulting. D.H. reports personal fees from Melinta, Macrolide Pharmaceuticals, The Medicines Company, Selux Diagnostics, and Danaher-Cepheid for advisory services and scientific advisory board membership. S.C. is employed by Melinta Therapeutics. All authors have submitted the ICMJE Form for Disclosure of Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. US Food and Drug Administration. FDA briefing document. Joint meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. 2015. https://www.fda.gov/downloads/%20AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugs%20AdvisoryCommittee/UCM467383.pdf. Accessed 9 March 2017. [Google Scholar]
- 2. US Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. 2016. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM513019.pdf. Accessed 8 March 2017. [Google Scholar]
- 3. US Food and Drug Administration. FDA drug safety communication: FDA reinforces safety information about serious low blood sugar levels and mental health side effects with fluoroquinolone antibiotics; requires label changes. 2018. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM612834.pdf. Accessed 31 July 2018. [Google Scholar]
- 4. Jorgensen SCJ, Mercuro NJ, Davis SL, Rybak MJ. Delafloxacin: place in therapy and review of microbiologic, clinical and pharmacologic properties. Infect Dis Ther 2018; 7:197–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Bambeke F. Delafloxacin, a non-zwitterionic fluoroquinolone in phase III of clinical development: evaluation of its pharmacology, pharmacokinetics, pharmacodynamics and clinical efficacy. Future Microbiol 2015; 10:1111–23. [DOI] [PubMed] [Google Scholar]
- 6. Lemaire S, Tulkens PM, Van Bambeke F. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pullman J, Gardovskis J, Farley B, et al. ; PROCEED Study Group Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: a phase 3, double-blind, randomized study. J Antimicrob Chemother 2017; 72:3471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Riordan W, McManus A, Teras J, et al. A comparison of the efficacy and safety of intravenous followed by oral delafloxacin with vancomycin plus aztreonam for the treatment of acute bacterial skin and skin structure infections: a phase 3, multinational, double-blind, randomized study. Clin Infect Dis 2018; 67:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kocsis B, Domokos J, Szabo D. Chemical structure and pharmacokinetics of novel quinolone agents represented by avarofloxacin, delafloxacin, finafloxacin, zabofloxacin and nemonoxacin. Ann Clin Microbiol Antimicrob 2016; 15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother 2016; 71:821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Riordan W, Mehra P, Manos P, et al. A randomized phase 2 study comparing two doses of delafloxacin with tigecycline in adults with complicated skin and skin-structure infections. Int J Infect Dis 2015; 30:67–73. [DOI] [PubMed] [Google Scholar]
- 12. Longcor J, Winkler M, Bukofzer S, Lawrence L.. A phase 2 study of the safety and efficacy of oral delafloxacin (DLX) in community acquired pneumonia (CAP) [Poster 1069]. Poster presented at: IDWeek 2012;October 17–21, 2012; San Diego, CA. [Google Scholar]
- 13. Longcor J, Hopkins S, Winkler M, Bukofzer S, Lawrence L.. A phase 2 study of the safety and efficacy of oral delafloxacin (DLX) in subjects with acute bacterial exacerbation of chronic bronchitis (ABECB) [Poster 1071]. Poster presented at: IDWeek 2012; October 17–21, 2012; San Diego, CA. [Google Scholar]
- 14. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Understanding MedDRA: The Medical Dictionary for Regulatory Activities. 2013. http://www.meddra.org/sites/default/files/page/documents/meddra2013. Accessed December 1, 2018. [Google Scholar]
- 15. Hansen E, Ungureanu S, Ninov B, et al. Comparison of delafloxacin (DLX) and vancomycin (VAN) in the treatment of acute bacterial skin and skin structure infections (ABSSSI) by age and gender in two phase 3 trials [Poster 1153]. Poster presented at: IDWeek 2016; October 26–30, 2016; New Orleans, LA. [Google Scholar]
- 16. Giordano P, Nseir W, Lawrence L, et al. Delafloxacin (DLX) is effective and well-tolerated in treatment of diabetic (DM) patients with acute bacterial skin and s AQ10 kin structure infections (ABSSSI) versus vancomycin/aztreonam (VAN/AZ) [Poster P1353]. Poster presented at: ECCMID 2017; April 22–25, 2017; Vienna, Austria. [Google Scholar]
- 17. Shah S, Baynton B, Lawrence L, et al. Delafloxacin (DLX) is effective and well-tolerated in treatment of obese patients with acute bacterial skin and skin structure infections (ABSSSI) versus vancomycin/ aztreonam (VAN/AZ) [Poster 1354]. Poster presented at: ECCMID 2017; April 22–25, 2017; Vienna, Austria. [Google Scholar]
- 18. Beasley R. Delafloxacin (DLX is effective and well-tolerated in treatment of patients with renal impairment with acute bacterial skin and skin structure infections (AGBSSSI) versus vancomycin/aztreonam (VAN/AZ). Poster P1355. Poster presented at: ECCMID 2017; April 22–25, 2017; Vienna, Austria. [Google Scholar]
- 19. Tien A. Delafloxacin (DLX) is effective and well-tolerated compared to vancomycin/aztreonam (VAN/AZ) in treatment of paitnes with acute bacterial skin and skin structure infections (ABSSSI) and history of infectious hepatitis. Poster 235. Paper presented at: ASM Microbe 2017; June 1–5, 2017; New Orleans, LA. [Google Scholar]
- 20. Dawe RS, Ferguson J, Ibbotson S, et al. Lack of phototoxicity potential with delafloxacin in healthy male and female subjects: comparison to lomefloxacin. Photochem Photobiol Sci 2018; 17:773–80. [DOI] [PubMed] [Google Scholar]
- 21. FDA. Summary basis of approval – Baxdela™ (delafloxacin) application number 208610Orig1s000. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208610Orig1s000,208611Orig1s000TOC.cfm. Accessed October 1, 2018. [Google Scholar]
- 22. Food and Drug Administration. FDA briefing document: joint meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee, November 5, 2015. 2015. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 27, 2017. [Google Scholar]
- 23. Linder JA, Huang ES, Steinman MA, et al. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am J Med 2005; 118:259–68. [DOI] [PubMed] [Google Scholar]
- 24. Werner NL, Hecker MT, Sethi AK, Donskey CJ. Unnecessary use of fluoroquinolone antibiotics in hospitalized patients. BMC Infect Dis 2011; 11:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaye KS, Auwaerter P, Bosso JA, et al. Strategies to address appropriate fluoroquinolone use in the hospital. Hosp Pharm 2010; 45:844–53. [Google Scholar]
- 26. Domagala JM. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J Antimicrob Chemother 1994; 33:685–706. [DOI] [PubMed] [Google Scholar]
- 27. Hayashi N, Nakata Y, Yazaki A. New findings on the structure-phototoxicity relationship and photostability of fluoroquinolones with various substituents at position 1. Antimicrob Agents Chemother 2004; 48:799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Francis JK, Higgins E. Permanent peripheral neuropathy: a case report on a rare but serious debilitating side-effect of fluoroquinolone administration. J Investig Med High Impact Case Rep 2014; 2:2324709614545225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dodd PR, Davies LP, Watson WE, et al. Neurochemical studies on quinolone antibiotics: effects on glutamate, GABA and adenosine systems in mammalian CNS. Pharmacol Toxicol 1989; 64:404–11. [DOI] [PubMed] [Google Scholar]
- 30. De Sarro A, De Sarro G. Adverse reactions to fluoroquinolones. An overview on mechanistic aspects. Curr Med Chem 2001; 8:371–84. [DOI] [PubMed] [Google Scholar]
- 31. Sousa J, Alves G, Fortuna A, Falcão A. Third and fourth generation fluoroquinolone antibacterials: a systematic review of safety and toxicity profiles. Curr Drug Saf 2014; 9:89–105. [DOI] [PubMed] [Google Scholar]
- 32. US National Library of Medicine, National Institutes of Health. Ciprofloxacin. LiverTox: clinical and research information on drug-induced liver injury. 2017. https://livertox.nih.gov/Ciprofloxacin.htm. Accessed 14 March 2017. [Google Scholar]
- 33. Van Bambeke F, Tulkens PM. Safety profile of the respiratory fluoroquinolone moxifloxacin: comparison with other fluoroquinolones and other antibacterial classes. Drug Saf 2009; 32:359–78. [DOI] [PubMed] [Google Scholar]
- 34. Orman ES, Conjeevaram HS, Vuppalanchi R, et al. ; DILIN Research Group Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol 2011; 9:517–23.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aspinall SL, Good CB, Jiang R, et al. Severe dysglycemia with the fluoroquinolones: a class effect?Clin Infect Dis 2009; 49:402–8. [DOI] [PubMed] [Google Scholar]
- 36. Chou HW, Wang JL, Chang CH, et al. Risk of severe dysglycemia among diabetic patients receiving levofloxacin, ciprofloxacin, or moxifloxacin in Taiwan. Clin Infect Dis 2013; 57:971–80. [DOI] [PubMed] [Google Scholar]
- 37. Kabbara WK, Ramadan WH, Rahbany P, Al-Natour S. Evaluation of the appropriate use of commonly prescribed fluoroquinolones and the risk of dysglycemia. Ther Clin Risk Manag 2015; 11:639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. LaPlante KL, Mersfelder TL, Ward KE, Quilliam BJ. Prevalence of and risk factors for dysglycemia in patients receiving gatifloxacin and levofloxacin in an outpatient setting. Pharmacotherapy 2008; 28:82–9. [DOI] [PubMed] [Google Scholar]
- 39. Mohr JF, McKinnon PS, Peymann PJ, et al. A retrospective, comparative evaluation of dysglycemias in hospitalized patients receiving gatifloxacin, levofloxacin, ciprofloxacin, or ceftriaxone. Pharmacotherapy 2005; 25:1303–9. [DOI] [PubMed] [Google Scholar]
- 40. Park-Wyllie LY, Juurlink DN, Kopp A, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med 2006; 354:1352–61. [DOI] [PubMed] [Google Scholar]
- 41. Litwin JS, Benedict MS, Thorn MD, et al. A thorough QT study to evaluate the effects of therapeutic and supratherapeutic doses of delafloxacin on cardiac repolarization. Antimicrob Agents Chemother 2015; 59:3469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Owens RC Jr, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43:1603–11. [DOI] [PubMed] [Google Scholar]
- 43. Kang J, Wang L, Chen XL, et al. Interactions of a series of fluoroquinolone antibacterial drugs with the human cardiac K+ channel HERG. Mol Pharmacol 2001; 59:122–6. [DOI] [PubMed] [Google Scholar]
- 44. Safety Pharmacology Studies Assessing the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals. International Conference on Harmonization S7B Document Washington, DC: US Food and Drug Administration; 2002. [Google Scholar]
- 45. Burkhardt JE, Hill MA, Carlton WW, Kesterson JW. Histologic and histochemical changes in articular cartilages of immature beagle dogs dosed with difloxacin, a fluoroquinolone. Vet Pathol 1990; 27:162–70. [DOI] [PubMed] [Google Scholar]
- 46. Schizas N, Weiss R, Lian O, et al. Glutamate receptors in tendinopathic patients. J Orthop Res 2012; 30:1447–52. [DOI] [PubMed] [Google Scholar]
- 47. Wise BL, Peloquin C, Choi H, et al. Impact of age, sex, obesity, and steroid use on quinolone-associated tendon disorders. Am J Med 2012; 125:1228.e23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsai WC, Yang YM. Fluoroquinolone-associated tendinopathy. Chang Gung Med J 2011; 34:461–7. [PubMed] [Google Scholar]
- 49. Bidell MR, Lodise TP. Fluoroquinolone-associated tendinopathy: does levofloxacin pose the greatest risk?Pharmacotherapy 2016; 36:679–93. [DOI] [PubMed] [Google Scholar]
- 50. Mandell L, Tillotson G. Safety of fluoroquinolones: an update. Can J Infect Dis 2002; 13:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hayashi PH, Chalasani NP. Liver injury in the elderly due to fluoroquinolones: should these drugs be avoided?CMAJ 2012; 184:1555–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.