Abstract
Background
The gastrointestinal (GI) microbiota in healthy cats is altered in IBD. Little research has been performed to identify whether specific bacterial groups are associated with small cell GI lymphoma (LSA).
Hypothesis
Mucosal bacteria, including Enterobacteriaceae and Fusobacterium spp., are abundant in intestinal biopsies of cats with small cell GI LSA compared to cats with IBD.
Animals
Fourteen cats with IBD and 14 cats with small cell GI LSA.
Methods
Retrospective case control study. A search of the medical records was performed to identify cats diagnosed with IBD and with GI LSA. Bacterial groups identified by FISH in GI biopsies were compared between cohorts and correlated to CD11b+ and NF‐κB expression.
Results
Fusobacterium spp. (median; IQR bacteria/region) were higher in cats with small cell GI LSA in ileal (527; 455.5 – 661.5; P = .046) and colonic (404.5; 328.8 – 455.5; P = .016) adherent mucus, and combined colonic compartments (free mucus, adherent mucus, attaching to epithelium) (8; 0 – 336; P = .017) compared to cats with IBD (ileum: 67; 31.5 – 259; colon: 142.5; 82.3 – 434.5; combined: 3; 0 – 34). Bacteroides spp. were higher in ileal adherent mucus (P = .036) and 3 combined ileal compartments (P = .034) of cats with small cell GI LSA. There were significant correlations between Fusobacterium spp. totals and CD11b+ cell (P = .009; rs .476) and NF‐κB expression (P = .004; rs .523).
Conclusions
The bacterial alterations appreciated might be influential in development of small cell GI LSA, and should drive further studies to elucidate the effects of microbial‐mediated inflammation on GI cancer progression.
Keywords: CD11b+, Feline microbiota, Fusobacterium, Gastrointestinal lymphoma, Inflammatory bowel disease, NF‐κB
Abbreviations:
- Bacto1080
bacteroides
- CRC
colorectal cancer
- Cy‐3
cyanine dye 3
- Ebac1790
enterobacteriaceae
- Erec482
clostridium
- Eub338
eubacterium (total bacteria)
- Faecali698
faecalibacterium
- FeLV
feline leukemia virus
- FISH
fluorescence in situ hybridization
- FITC
fluorescein isothiocyanate
- FIV
feline immunodeficiency virus
- Fuso0664
fusobacterium
- GI
gastrointestinal
- H&E
hematoxylin‐eosin
- IBD
inflammatory bowel disease
- IHC
immunohistochemistry
- IQR
interquartile range
- Hel717
helicobacter
- LSA
lymphoma
- max
range maximum
- min
range minimum
- NF‐κB
nuclear factor kappa B
- PARR
PCR for antigen receptor rearrangement
- rs
spearman correlation coefficient
- T4
thyroxine
- TLR
toll like receptor
1. INTRODUCTION
Diagnosis of small cell gastrointestinal (GI) lymphoma (LSA) in cats increased six‐fold over a four‐decade period spanning from 1964 through 2004, highlighting the growing clinical importance of this disease.1 Frequent identification of the disease continues today, most notably in cats with chronic weight loss, vomiting, decreased appetite and diarrhea. Small cell GI LSA closely resembles feline inflammatory bowel disease (IBD) in its clinical presentation,2 with both diseases having poorly defined etiologies. Various risk factors including genetic and molecular alterations,3, 4, 5 diet,3, 6, 7 and chronic inflammation8, 9, 10 have been linked to the development of both disorders. Still other studies have suggested an association between chronic mucosal inflammation and the progression of IBD to pronounced dysplasia in the GI tract.11, 12, 13, 14
Although the microbiota is intimately involved in GI homeostasis and immune balance, several studies in animal models and humans with cancer incriminate bacterial‐induced chronic intestinal inflammation as promoting a local environment favorable for malignant transformation, including lymphomagenesis.11, 12, 13, 14 Increased numbers of mucosal‐adherent bacteria were observed in cats with duodenal IBD,10 while invasive bacteria were observed within blood vessels and serosa of cats with large cell lymphoma.9 These collective observations suggest that altered microbial composition (e.g. dysbiosis) is associated with development of benign mixed mucosal inflammation (lymphoplasmacytic enteritis with IBD) and GI lymphoid malignancy.9, 10, 15, 16, 17, 18 Microbial imbalances have also been implicated in the pathogenesis of approximately 20% of all human malignancies, including GI neoplasia such as colorectal adenocarcinomas and gastric carcinomas.19, 20, 21 Both chronic, high‐grade inflammation of disorders such as feline IBD, and lower grade smoldering inflammation attributable to pro‐inflammatory bacteria (e.g. Helicobacter spp., Enterobacteriaceae, Fusobacterium spp.) might drive a tumor‐permissive milieu characterized by mucosal infiltration with specialized effector cells, including CD11b+ myeloid cell derived macrophages, as observed in humans, and rodent models.9, 19, 21, 22
Differentiated CD11b+ myeloid cells play an important role in tumor progression and angiogenesis in rodent models and humans with colorectal cancer (CRC).19, 23 Nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB), a protein complex of transcription factors, is involved in immune regulation, DNA transcription, and pro‐inflammatory cytokine and chemokine production.24 This protein complex is also involved in activation of effector cells through toll‐like receptors (TLRs) in response to GI micro‐organisms. Dysregulation of the activation of NF‐κB can lead to overproduction of pro‐inflammatory cytokines and cell transformation, triggering the development of cancers in humans.25, 26, 27
We hypothesized that pro‐inflammatory bacteria, including Enterobacteriaceae and Fusobacterium spp. are abundant in the intestinal biopsies of cats with small cell GI LSA relative to cats with IBD. An objective of this study was to investigate the potential relationship between mucosal bacteria and the mucosal expression of NF‐κB and infiltrating CD11b+ immune cells in GI biopsies of cats with small cell GI LSA versus GI biopsies of cats with IBD.
2. MATERIALS AND METHODS
2.1. Study Design
This study was a retrospective study involving 3 study centers, VDx Veterinary Diagnostics Laboratory, Davis CA; the University of Camerino Veterinary Teaching Hospital, Italy and Iowa State University, Ames IA. The medical records of each facility were reviewed to identify cats having GI endoscopic or laparoscopic derived diagnosis of histologic IBD or small cell GI LSA from January 2010 to January 2015. Fourteen cats with IBD and 14 cats with small cell GI LSA were included in the study. Inclusion criteria was comprised of complete medical histories, the performance of hematologic and serum biochemical analysis, and measurement of serum T4 in all cats. Serology for Feline Leukemia Virus (FeLV) and Feline Immunodeficiency Virus (FIV) infections, diagnostic imaging, and serum cobalamin levels were also assessed in most cats. Cats were also either tested for intestinal parasites via fecal examinations, dewormed or had deworming performed in the face of negative fecal examinations before biopsies were collected. All cats in both study cohorts tested negative for intestinal parasite ova on fecal examinations. Histopathologic examination of intestinal biopsy specimens after hematoxylin‐eosin (HE) staining and immunophenotyping (e.g. IHC stains for CD21, CD79a [B‐lymphocytes] and CD3 [T‐lymphocytes]) was performed on each cat to determine a final diagnosis of IBD or small cell GI LSA. In some instances, there was overlap in immunohistopathologic features necessitating performance of polymerase chain reaction for antigen receptor rearrangement (PARR) to confirm a final diagnosis of IBD versus small cell GI LSA.
2.2. Fluorescence in Situ Hybridization (FISH)
Formalin‐fixed embedded tissue sections were mounted on glass slides and evaluated by fluorescence in situ hybridization (FISH) as previously described.8 In brief, paraffin‐embedded tissue specimens were deparaffinized using an automated system by passage through xylene (3 x 10 min), 100% alcohol (2 x 5 min), 95% ethanol (5 min) and lastly 70% ethanol (5 min). The slides were next immediately transported in deionized water to the DNA testing laboratory where they were air dried prior to hybridization. FISH probes of interest, 5'‐labeled with either Cy‐3 or FITC (Fisher Scientific, Pittsburgh, PA) were reconstituted with DNAse‐free water and diluted to a working concentration of 5ng/μL (Table 1).
Table 1.
Oligonucleotide probes used for fluorescence in situ hybridization (FISH) and bacterial quantification
| Probe | Target | Sequence (5' → 3') |
|---|---|---|
| Eub338 | Eubacteria | GCT GCC TCC CGT AGG AGT |
| Erec482 | Clostridium coccoides‐E. rectale cluster | GCT TCT TAG TCA RGT ACC G |
| Ebac1790 | Enterobacteriaceae | CGT GTT TGC ACA GTG CTG |
| Bacto1080 | Bacteroides spp.Prevotella spp. | GCA CTT AAG CCG ACA CCT |
| Hel717 | Helicobacter spp. | AGG TCG CCT TCG CAA TGA GTA |
| Faecali698 | Faecalibacterium spp. | GTG CCC AGT AGG CCG CCT TC |
| Fuso0664 | Fusobacterium spp. | CTT GTA GTT CCG CYT ACC TC |
For total bacterial counts, Eub338‐FITC was used.28 For other analyses, specific probes targeting Clostridium spp. (Erec482),29 Bacteroides‐Prevotella group (Bacto1080),30 Fusobacterium spp. (Fuso0664),31 Enterobacteriaceae (Ebac1790),32 Helicobacter spp. (Hel717),33 and Faecalibacterium spp. (Faecali698),34 were labeled with Cy‐3 and were applied simultaneously with the universal bacterial probe Eub338‐FITC. This probe array was selected to identify specific bacterial groups and individual bacterial species previously shown to be relevant in the pathogenesis of intestinal cancers in humans, rodents, and dogs.9, 14, 19, 22, 23, 27, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54
Tissue sections were bathed in 30μL of DNA‐probe‐hybridization buffer mix in a hybridization chamber maintained at 54°C overnight (12 hours). Buffer 1 (20mM Tris, 0.9M NaCl, 0.1% SDS, 20% Formamide, 10% Dextran sulfate) was used for the hybridization of Eub338, Erec482, Bacto1080, Fuso0664, Ebac1790 and Faecali698. Buffer 2 (20mM Tris, 0.9M NaCl, 0.1% SDS, 40% Formamide) was used for the hybridization of Hel717. Washing was then performed using wash buffer (hybridization buffer without SDS), the slides were rinsed with sterile water, then allowed to air‐dry, and mounted with SlowFade Gold mounting media (Life Technologies, Carlsbad, CA) and 22x22‐1 glass cover slip (Fisher Scientific, Pittsburgh, PA). Probe specificity was confirmed in pilot studies by combining the irrelevant probe non‐Eub338‐FITC with Eub338‐Cy‐3, and through hybridization experiments with pure bacterial isolates to screen for non‐selective hybridization.
2.3. In Situ Quantification of Mucosal Bacteria Associated with Biopsy Specimens
Bacteria were visualized and quantified using an Eclipse TE2000‐E fluorescence microscope (Nikon Instruments, Melville, NY). Images were captured with a CoolSnap EZ camera (Photometrics Scientific, Tuscon, AZ) and bacterial enumeration was performed using MetaMorph software (Molecular Devices, LLC., Sunnyvale, CA). A minimum of 3 separate hybridized intestinal tissue sections were evaluated for their bacterial content. Bacterial quantification was performed in 10 representative mucosal fields of each hybridized tissue section of formalin‐fixed, paraffin embedded biopsy specimens. A hybridized tissue section was first visualized at 200x magnification to locate the center of each tissue section. Then surrounding fields of bacterial populations were subsequently selected, and quantified using a 60x Plan Apo oil objective with an optional 1.5x multiplier lens. Bacterial counts were obtained randomly in 3 mucosal fields in 2 of the 3 hybridized tissue sections, and randomly in 4 views on the third hybridized tissue section, accounting for 10 views for bacterial quantification in total. The ten fields counted included bacteria located within 4 well‐defined mucosal compartments: (1) bacteria found within free mucus, (2) bacteria localized within adherent mucus, (3) bacteria attached to the surface epithelium, and (4) bacteria invasive within the mucosa (Figure 1).
Figure 1.
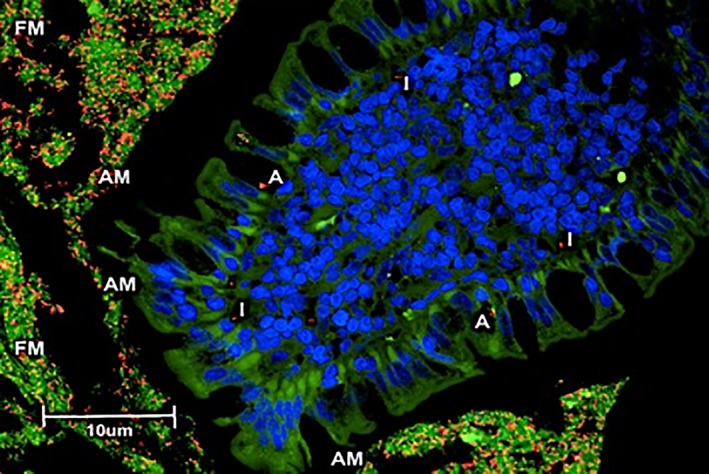
Mucosal compartments for bacterial quantification. FM, bacteria within free mucus within the GI lumen; AM, bacteria within adherent mucus adhered to the epithelia; A, bacteria directly attaching to surface epithelia; I, bacteria invasive within the mucosa. Three‐color fluorescence in situ hybridization (FISH) of Bacteroides spp. identified in ileal biopsies of a cat with small cell GI LSA. Cy‐3‐labeled Bacteroides spp. (labeled orange) localized within an ileal biopsy specimen (FM; AM; A; and I). The free (FM) and adherent mucus (AM) are also occupied by other bacteria (total bacteria labeled green with FITC‐Eub). The dark blue structures are epithelial cell nuclei stained with DAPI
2.4. Immunohistochemical Assessment of the Number of CD11b+ Cells and Mucosal NF‐κB Expression
The number of mucosal CD11b+ myeloid cells, and nuclear factor – kappa B (NF‐κB) expression were quantified in intestinal tissues of all 28 cats. This included 14 cats diagnosed with IBD and 14 cats diagnosed with small cell GI LSA. For IHC evaluation, paraffin embedded tissue sections were rehydrated and neutralized for endogenous peroxidases with 3% hydrogen peroxide for 5 minutes followed by rinsing for 5 minutes in distilled water. For antigen retrieval, the heat‐induced epitope retrieval (HIER) method was selected, on the basis of previous experiences showing better results with antigens, such as CD11b+, that might require more efficient breakage of methylene bridges formed during formalin fixation and allowing antibody to bind. In brief, slides were incubated in an universal antigen retrieval buffer (Universal Heat‐Mediated Antigen Retrieval Reagent kit) (ABCAM, San Francisco, CA), used in a water bath set to 140°F and allowing the slides to incubate in retrieval solution overnight. Non‐specific binding was blocked by incubation of slides for 10 minutes with a protein‐blocking agent (Dako Products, Carpinteria, CA) before application of the primary antibody. Slides were then incubated overnight in a moist‐chamber with the following primary antibodies: canine cross‐reactive monoclonal antibody against NF‐κB (p65) (BD Transduction Laboratories, Lexington, KY)55 diluted 1 : 50, and a goat polyclonal to CD11b+ (anti‐CD11b+ antibody ‐ ref. N° ab62817) (ABCAM, San Francisco, CA) diluted 1 : 50. The immunoreaction with streptavidin–immunoperoxidase (Black & Decker, Towson, MD) was visualized with 3,3′‐diaminobenzidine substrate (Vector Laboratories, Burlingame, UK). Tissues were counterstained with Mayer's hematoxylin (Figure 2). For negative IHC controls, the primary antibodies were omitted. Sections of feline and canine spleen and tonsil served as positive control tissues for both NF‐κB (p65) and CD11b+ cell staining. Finally, isotype controls were used as negative controls to help differentiate eventual non‐specific background signal from specific antibody signal, by the use of primary antibodies lacking specificity to the target, but matching the class and type of the primary antibody used in the IHC studies.
Figure 2.
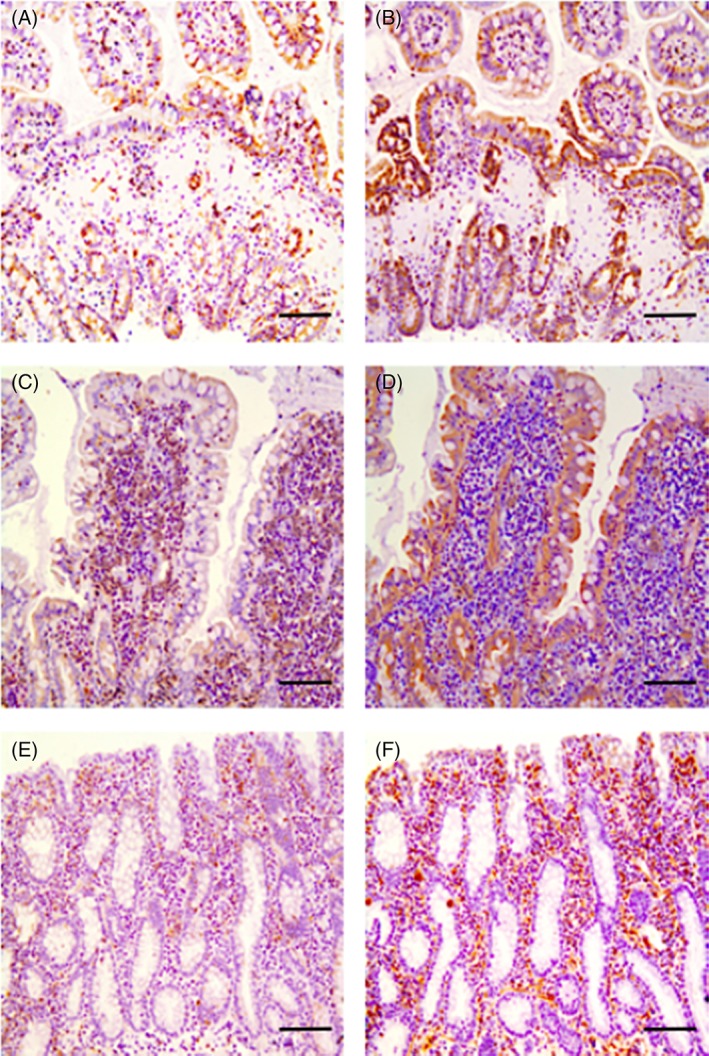
IHC staining for CD11b+ and NF‐κB primary antibodies in ileal and colonic biopsies of cats with IBD and small cell GI LSA. (A) CD11b+myeloid cell staining in the ileum of a cat with IBD (cat 1). Cross sectional view of ileal villi after IHC staining with a goat polyclonal antibody to CD11b+ as represented by the brown chromophore staining amongst the epithelium and in the lamina propria. (B) NF‐κB staining in the ileum of a cat with IBD (cat 1). Cross sectional view of ileal villi after IHC staining with a canine cross‐reactive monoclonal antibody against NF‐κB (p65) as represented by the brown chromophore staining amongst the epithelium and in the lamina propria. (C) CD11b+ staining in the ileum of a cat with small cell GI LSA (cat 2). Longitudinal view of ileal villi after IHC staining with a goat polyclonal antibody to CD11b+ as represented by the brown chromophore staining amongst the epithelium and in the lamina propria. (D) NF‐κB staining in the ileum of a cat with small cell GI LSA (cat 2). Longitudinal view of ileal villi after IHC staining with a canine cross‐reactive monoclonal antibody against NF‐κB (p65) as represented by the brown chromophore amongst the epithelium and in the lamina propria. (E) CD11b+ staining in the colon of a cat with small cell GI LSA (cat 3). Longitudinal and cross sectional views of colonic mucosa and lamina propria after IHC staining with a goat polyclonal antibody to CD11b+ as represented by the brown chromophore staining amongst the epithelium and in the lamina propria. (F) NF‐κB staining in the colon of a cat with small cell GI LSA (cat 3). Longitudinal and cross sectional views of colonic mucosa and lamina propria after IHC staining with a canine cross‐reactive monoclonal antibody against NF‐κB (p65) as represented by the brown chromophore staining amongst the epithelium and in the lamina propria. All scale bars, 200 μm
For assessment of intestinal NF‐κB (p65) and CD11b+ cells, the cells were quantified in select compartments of the GI tract biopsies (small intestine: villi, basal crypt area, villus‐crypt junction; large intestine: apical crypt area, basal crypt area). All cellular types were evaluated using a light microscope (Carl Zeiss) (Carl Zeiss Jena GmbH, Germany), a × 40 objective, a × 10 eyepiece, and a square eyepiece graticule (10 × 10 squares, having a total area of 62,500 μm2). Ten appropriate fields were chosen for each compartment and arithmetic means were calculated for each intestinal region. Results were expressed as IHC positive cells per 62,500 μm2. Cells on the margins of the tissue sections were not considered for evaluation to avoid inflation of positive cell numbers.
The numbers of mucosal NF‐κB (p65) expressing cells and CD11b+ cells were quantified using an image‐analysis system consisting of the previously mentioned light microscope (Carl Zeiss) (Carl Zeiss Jena GmbH, Germany) attached to a Javelin JE3462 high‐resolution camera and a personal computer equipped with a Coreco‐Oculus OC‐TCX frame grabber and high‐resolution monitor. Computerized color‐image analysis was performed by using Image‐Pro Plus software (Media Cybernetics, Inc., Rockville, MD). The area of each biopsy in all cross sections in every cat was recorded. For each cat, the total bioptic area was calculated as the sum of the areas of all fields in all bioptic cross sections on one slide. The NF‐κB (p65) expressing cells and CD11b+ cells were counted per section, and stained cell densities were expressed as the number of mononuclear cells per square millimeter of analyzed bioptic area.56
2.5. Statistical Analysis
Tabular data were organized by probe, organ, mucosal compartment and disease group. Mean, median, standard deviation, minimum, and maximum values were calculated from the absolute bacterial counts. Normality of data was assessed visually by histograms and Q‐Q plots. If data were not found to be normally distributed, they were log‐transformed and reassessed. Median values were compared among groups for significance using the Wilcoxon rank sum test. Analyses were considered significant at P < .05. All graphs were constructed using non‐log‐transformed, actual raw data.
Associations between bacterial numbers (identified by FISH) and the numbers of CD11b+ cells and NF‐κB expressing cells were assessed using Spearman's rank correlation and tested for significance. Significance was determined as P < .05. Sample size for enrollment was calculated after consultation with a statistician and was based on the desired power and variability in the number of CD11b+ cells and NF‐κB expressing cells observed in IBD versus small cell GI LSA feline cohorts. Randomization of 12 cats per group would give a power of 80% to detect differences in expression of CD11b+ cells and NF‐κB expressing cells between cohorts at the .05 significance level. All analyses were made using SAS 9.4 (SAS Institute, Cary, NC).
3. RESULTS
3.1. Final Histopathologic Diagnoses
Fourteen cats were diagnosed with IBD based on histopathologic assessment of the mucosal cellular infiltrate (lymphoplasmacytic) after H&E, immunophenotypic staining and PARR as an adjunct diagnostic in equivocal cases. GI biopsies in 9 of 14 of these cats exhibited a 30% to 60% heterogenous population of small to intermediate lymphocytes and plasma cells invading the lamina propria, with mild but varying numbers of neutrophilic and eosinophilic cellular infiltrates per any given 400x magnification field assessed after H&E staining via microscopy. The diagnosis of IBD in these cats was supported by IHC showing relatively evenly mixed CD3 and CD21 or CD79a cellular positivity without immunophenotypic evidence of clustering of either T‐cell or B‐cell populations. Sparse numbers of intraepithelial lymphocytes (i.e. approximately 0 to 20 per 100 villous enterocytes) were also appreciated in limited villous epithelium and less commonly in crypt epithelium. Additionally, PARR was performed on intestinal biopsies from the remaining 5 of these 14 cats to confirm diagnosis of a polyclonal lymphocyte mucosal population, as these biopsies were assessed as having areas of mild to moderate villous blunting and fusion with crypt effacement on histopathologic assessment, greater numbers of intraepithelial lymphocytes in both villous and crypt epithelium and rare, patchy areas of moderately heavy monomorphous lymphoid infiltrates of small to intermediate‐sized CD3 staining lymphocytes .
Fourteen cats were diagnosed with small cell GI LSA based on H&E stains and immunophenotyping, with 1 cat requiring PARR to confirm a final diagnosis. Thirteen of these 14 cats showed histopathologic evidence of moderate to marked villous blunting and fusion with crypt effacement after H&E staining. Moderate to marked intraepithelial villous and crypt lymphocytes (i.e. more than 40 per 100 villous enterocytes) and diffuse heavy effacement of the lamina propria with a heavy monomorphous lymphoid infiltrate of small to intermediate‐sized CD3 staining lymphocytes, occasionally with sparse CD21/CD79a positive cells, were also appreciated after IHC staining. The occasional sparse CD21/CD79a positive cells were suspected to represent a mild inflammatory background. The 1 cat that required PARR to confirm the diagnosis of small cell GI LSA was assessed as having similar villous and crypt histopathologic features but exhibited a mild number of eosinophils and neutrophils infiltrating the lamina propria, minimal intraepithelial lymphocytes and patchy areas of monomorphous lymphoid infiltrate with varying areas of mixed T and B‐cell populations, suggesting possible polyclonality.
3.2. Demographics for Cats with IBD and Small Cell GI LSA
Of the 14 cats diagnosed with IBD, 7 were castrated male and 7 were spayed female. Nine of these cats were domestic shorthairs, and there was 1 cat each of domestic longhair, Lynx Point, Savannah, Siamese, and Ocicat. Of the 14 cats diagnosed with small cell GI LSA, 6 were castrated male, 7 were spayed female, and sex and neuter statuses were not reported in the medical record for 1 cat. Eleven of these cats were domestic shorthairs, and there was 1 cat each of domestic longhair, Himalayan, and Maine Coon. Histories were available for all 28 cats. Duration of clinical signs for each cat was reported as at least 3 weeks prior to presentation. Clinical signs of vomiting (n = 24) and weight loss (n = 13) were noted most frequently with some cats also exhibiting variable hyporexia (n = 9), small bowel diarrhea, large bowel diarrhea, or both (n = 10) and hematochezia (n = 2). In general, cats with small cell GI LSA were older than cats with IBD (small cell GI LSA: mean 10.9, standard deviation 3.35; IBD: mean 7.4, standard deviation 3.68; P = .009). Serum T4 measurements were within reference range for all cats (n = 28). Cats in which FeLV and FIV status were assessed (10 cats with IBD, and 14 cats with small cell GI LSA) were all negative. Serum cobalamin measurements were available for 11 cats (5 with IBD and 6 with small cell GI LSA). One of the 5 cats with IBD with reported serum cobalamin concentrations had a cobalamin level less than 300ng/L and 4 of the 6 small cell GI LSA cats with reported serum cobalamin concentrations had cobalamin measurements less than 300ng/L. Abdominal ultrasound findings were available in 22 of the 28 cats (10 cats with IBD and 12 cats with small cell GI LSA). The remaining 6 cats either did not have abdominal imaging performed or the report was excluded from the medical records. Of the cats with abdominal ultrasounds performed, 10 had normal gastrointestinal wall thickness (7 cats with IBD and 3 cats with small cell GI LSA) and 12 had diffuse thickening of the GI muscularis (3 cats with IBD and 9 cats with small cell GI LSA). Five of the cats with IBD and 9 of the cats with small cell GI LSA had enlarged mesenteric lymph nodes. In the remaining cats with abdominal imaging performed, lymph nodes were noted as normal in size.
3.3. Quantification and Spatial Distribution of Mucosal Bacteria Determined by FISH
Only few bacteria were identified as invasive within the intestinal mucosal biopsies. Therefore, bacterial quantification was performed in the 3 other mucosal compartments (free mucus, adherent mucus, attaching to surface epithelia) (Table 2) and separately in adherent mucus (Table 3), in both the ileal and the colonic biopsies. Gastrointestinal biopsies of cats with small cell GI LSA had significantly increased numbers of total Fusobacterium spp. bacteria in the 3 combined mucosal compartments of the colon (P = .017) compared to GI biopsies of cats with IBD (Table 2). Total Fusobacteria spp. bacteria in the 3 combined mucosal compartments of ileal biopsies of cats with small cell GI LSA was not statisticaly significanct (P = .059) compared to ileal biopsies of cats with IBD (Table 2). Gastrointestinal biopsies of cats with small cell GI LSA had significantly increased numbers of total Fusobacteria spp. bacteria in the adherent mucus compartments of the ileum (P = .046) and colon (P = .016) versus GI biopsies of cats with IBD (Table 3). There was a significantly increased number of Bacteroides spp. bacteria in the 3 combined ileal mucosal compartments (P = .034) (Table 2) and in the ileal adherent mucus (P = .036) (Table 3) of GI biopsies of cats with small cell GI LSA compared to GI biopsies of cats with IBD. The numbers of total bacteria, Clostridium spp., Enterobacteriaceae, Helicobacter spp. and Faecalibacterium spp. were not significantly different when comparing the colonic and ileal biopsies of cats with IBD relative to the biopsies of cats with small cell GI LSA.
Table 2.
Total bacteria in 3 combined compartments (free mucus, adherent mucus, attaching to surface epithelia). data has been logged transformed to control for variability, and are reported as mean and standard deviation of the transformed data
| Probe | Group | Ileum | Colon |
|---|---|---|---|
| Eub338 | IBD | 5.52 ± 1.92 | 6.56 ± .65 |
| LSA | 6.75 ± .37 | 6.55 ± .39 | |
| Erec482 | IBD | 4.95 ± 2.13 | 5.59 ± 1.23 |
| LSA | 6.19 ± .77 | 5.86 ± .78 | |
| Ebac1790 | IBD | 4.36 ± 2.47 | 4.33 ± 1.87 |
| LSA | 3.98 ± 1.75 | 4.8 ± 1.14 | |
| Bacto1080 | IBD | 5.64 ± 1.01 | 5.91 ± 1.59 |
| LSA | 6.6 ± .38a | 6 ± 1.17 | |
| Hel717 | IBD | 1.54 ± 1.6 | 1.97 ± 1.82 |
| LSA | 2.07 ± 1.07 | 2.27 ± 1.71 | |
| Faecali698 | IBD | 4.62 ± 2.13 | 5.12 ± 1.71 |
| LSA | 5.95 ± .75 | 5.35 ± 1.27 | |
| Fuso0664 | IBD | 4.6 ± 2.14 | 5.14 ± 1.27 |
| LSA | 6.32 ± .52 | 6.14 ± .66b |
IBD, inflammatory bowel disease cats; LSA, small cell gastrointestinal lymphoma cats. (total = 28 cats; IBD = 14 cats; small cell GI LSA = 14 cats).
Mean Bacteroides spp. bacterial count in ileal biopsies of small cell GI LSA cats significantly higher than in ileal biopsies of cats with IBD (P = .034).
Mean Fusobacterium spp. bacterial count in colonic biopsies of small cell GI LSA cats significantly higher than in colonic biopsies of cats with IBD (P = .017).
Eub338, total bacteria; Erec482, Clostridium spp.; Ebac1790, Enterobacteriaceae; Bacto1080, Bacteroides‐Prevotella group; Hel717, Helicobacter spp.; Faecali698, Faecalibacterium spp.; Fuso0664, Fusobacterium spp.
Table 3.
Total bacteria in adherent mucus. Data has been logged transformed to control for variability, and are reported as mean and standard deviation of the transformed data
| Probe | Group | Ileum | Colon |
|---|---|---|---|
| Eub338 | IBD | 5.33 ± 2.04 | 6.51 ± .66 |
| LSA | 6.72 ± .38 | 6.5 ± .41 | |
| Erec482 | IBD | 4.9 ± 2.11 | 5.51 ± 1.33 |
| LSA | 6.09 ± .97 | 5.8 ± .81 | |
| Ebac1790 | IBD | 4.34 ± 2.46 | 4.25 ± 1.91 |
| LSA | 3.67 ± 2.13 | 4.72 ± 1.18 | |
| Bacto1080 | IBD | 5.43 ± 1.23 | 5.76 ± 1.86 |
| LSA | 6.57 ± .38a | 5.95 ± 1.25 | |
| Hel717 | IBD | 1.45 ± 1.53 | 1.86 ± 1.7 |
| LSA | 1.99 ± 1.1 | 2.04 ± 1.82 | |
| Faecali698 | IBD | 4.57 ± 2.13 | 5.03 ± 1.72 |
| LSA | 5.94 ± .74 | 5.2 ± 1.59 | |
| Fuso0664 | IBD | 4.4 ± 2.18 | 5.06 ± 1.29 |
| LSA | 6.28 ± .56b | 6.1 ± .7c |
IBD, inflammatory bowel disease cats; LSA, small cell gastrointestinal lymphoma cats. (total = 28 cats; IBD = 14 cats; small cell GI LSA = 14 cats).
Mean Bacteroides spp. bacterial count in ileal biopsies of small cell GI LSA cats significantly higher than in ileal biopsies of cats with IBD (P = .036).
Mean Fusobacterium spp. bacterial count in ileal biopsies of small cell GI LSA cats significantly higher than in ileal biopsies of cats with IBD (P = .046).
Mean Fusobacterium spp. count in colonic biopsies of small cell GI LSA cats significantly higher than in colonic biopsies of cats with IBD (P = .016).
Eub338, total bacteria; Erec482, Clostridium spp.; Ebac1790, Enterobacteriaceae; Bacto1080, Bacteroides‐Prevotella group; Hel717, Helicobacter spp.; Faecali698, Faecalibacterium spp.; Fuso0664, Fusobacterium spp..
The amount of each species of bacteria evaluated were subjectively observed to be more prevalent in the adherent mucus layer versus the free mucus, attached to the surface epithelia, and invasive within the mucosa, including for Fusobacterium spp. and Bacteroides spp. bacteria (Figure 3 and 4).
Figure 3.
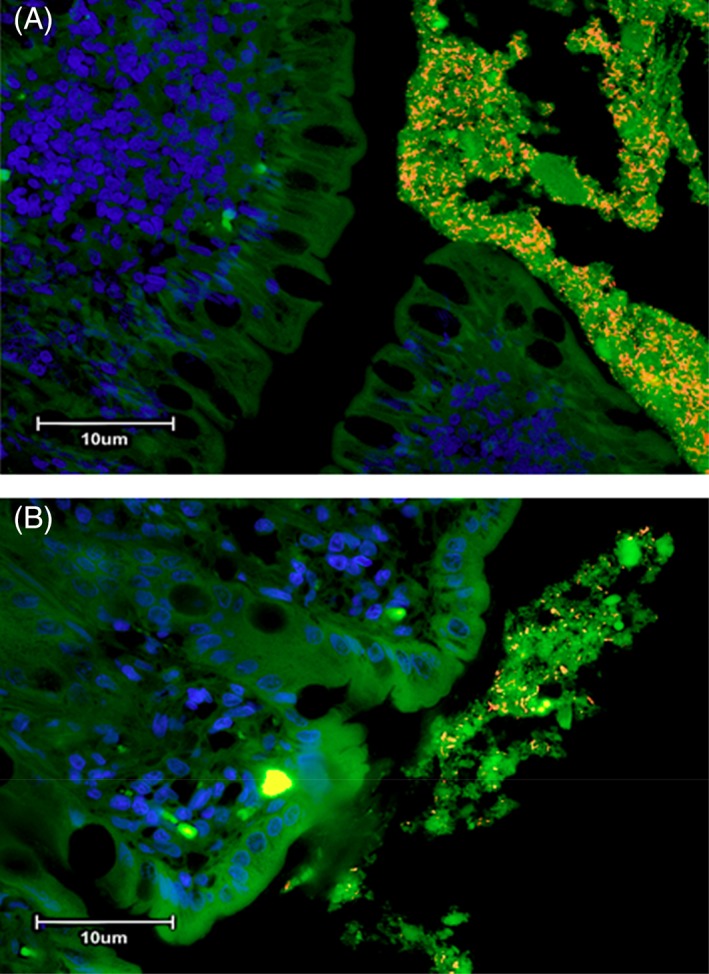
(A) Three‐color fluorescence in situ hybridization (FISH) of Fusobacterium spp. identified in colonic biopsies of a cat with small cell GI LSA. Cy‐3‐labeled Fusobacterium spp. (labeled orange) localized within adherent mucus of a colonic biopsy specimen. The mucus is also occupied by other bacteria (total bacteria labeled green with FITC‐Eub). The dark blue structures are epithelial cell nuclei stained with DAPI. (B) Three‐color fluorescence in situ hybridization (FISH) of Fusobacterium spp. identified in colonic biopsies of a cat with IBD. Cy‐3‐labeled Fusobacterium spp. (labeled orange) localized within adherent mucus of a colonic biopsy specimen. The mucus is also occupied by other bacteria (total bacteria labeled green with FITC‐Eub). The dark blue structures are epithelial cell nuclei stained with DAPI
Figure 4.
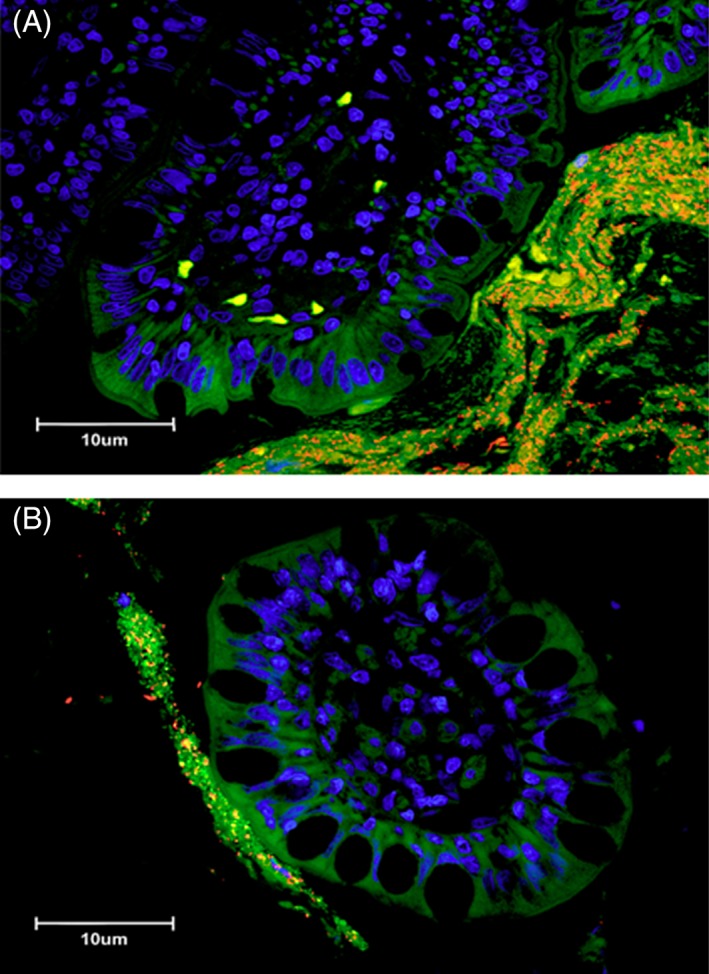
(A) Three‐color fluorescence in situ hybridization (FISH) of Bacteroides spp. identified in ileal biopsies of a cat with small cell GI LSA. Cy‐3‐labeled Bacteroides spp. (labeled orange) localized within adherent mucus of an ileal biopsy specimen. The mucus is also occupied by other bacteria (total bacteria labeled green with FITC‐Eub). The dark blue structures are epithelial cell nuclei stained with DAPI. (B) Three‐color fluorescence in situ hybridization (FISH) of Bacteroides spp. identified in ileal biopsies of a cat with IBD. Cy‐3‐labeled Bacteroides spp. (labeled orange) localized within adherent mucus of an ileal biopsy specimen. The mucus is also occupied by other bacteria (total bacteria labeled green with FITC‐Eub). The dark blue structures are epithelial cell nuclei stained with DAPI
3.4. Number of Mucosal CD11b+ Myeloid Cells and NF‐κB Expression
Cats with small cell GI LSA had significantly increased numbers of CD11b+ cells within the lamina propria of both ileal (median = 503; min 30, max 1985; P = .012) and colonic biopsies (median = 77; min 13, max 2858; P < .0001) versus cats with IBD (Figure 5). Cats with small cell GI LSA also had significantly increased expression of NF‐κB in the lamina propria of both ileal (median = 750; min 69, max 2227; P = .0055) and colonic biopsies (median = 154; min 40, max 3581; P < .0001) when compared to cats with IBD (Figure 6). Additionally, data analysis showed a positive correlation between the total numbers of Fusobacterium spp. bacteria and the total numbers of CD11b+ cells (P = .009; rs .476) as well as cells expressing NF‐κB (P = .004; rs .523) as assessed by spearman correlation coefficients.
Figure 5.
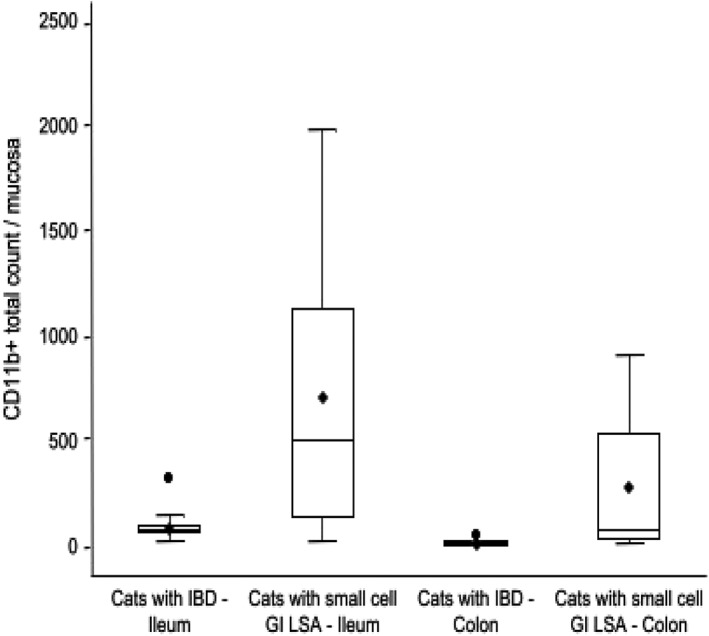
Mucosal CD11b+ cellular expression in GI biopsies of cats with IBD compared to cats with small cell GI LSA in the ileum and colon (total = 28 cats; IBD = 14 cats; small cell GI LSA = 14 cats). Boxes show lowest, median, and upper quartiles. Whiskers represent 1.5 of the interquartile range, with means denoted by the black rhombi and outliers denoted by the black circles. Median mucosal CD11b+ myeloid cell counts are significantly higher in the ileal (median = 503; P = .012) and colonic (median = 77; P < .0001) biopsies of cats with small cell GI LSA compared to the ileal (median = 82.5) and colonic (median = 15) biopsies of cats with IBD
Figure 6.
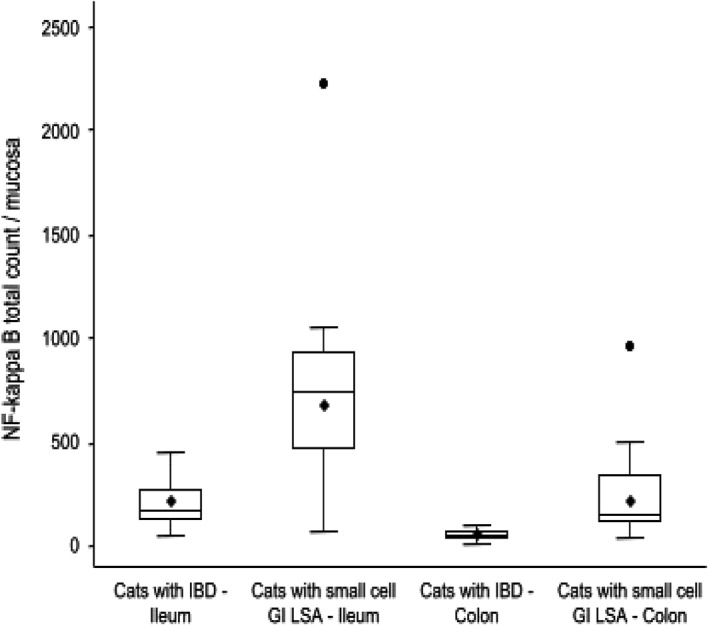
Mucosal NF‐κB cellular expression in GI biopsies of cats with IBD compared to cats with small cell GI LSA in the ileum and colon (total = 28 cats; IBD = 14 cats; small cell GI LSA = 14 cats). Boxes show lowest, median, and upper quartiles. Whiskers represent 1.5 of the interquartile range, with means denoted by the black rhombi and outliers denoted by the black circles. Median NF‐κB mucosal expression is significantly higher in the ileal (median = 750; P = .0055) and colonic (median = 154; P < .0001) biopsies of cats with small cell GI LSA when compared to the ileal (median = 169) and colonic (median = 49.5) biopsies of cats with IBD
4. DISCUSSION
Major bacterial phyla identified in feces of healthy cats include Enterobacteriaceae, Bacteroides spp., Helicobacter spp., Fusobacterium spp. and members of Firmicutes, including Clostridium spp. clusters IV and XIVa, and Faecalibacterum spp.37, 39, 49, 57 Compared to earlier studies performed in healthy cats, we chose to investigate the spatial distribution of the mucosal microbiota of ileal and colonic biopsies using FISH techniques in 2 important and clinically overlapping disease cohorts, cats with IBD and cats with small cell GI LSA. Our results indicate that the numbers of mucosa‐associated Fusobacterium spp. and Bacteroides spp. were increased in the ileal biopsies of cats with small cell GI LSA compared to the ileal biopsies of cats with IBD, while the numbers of mucosa‐associated Fusobacterium spp. bacteria were also increased in the colonic biopsies of cats with small cell GI LSA compared to cats with IBD. Furthermore, the numbers of Fusobacteria spp. in adherent mucus were strongly associated with increased numbers of CD11b+ cells and up‐regulated mucosal expression of NF‐κB in GI biopsies of cats with small cell GI LSA compared to GI biopsies of cats with IBD.
In humans, some Fusobacterium spp., including Fusobacterium nucleatum, have been associated with an increased risk for colorectal and pancreatic cancers, as well as some oral squamous cell carcinomas.38, 42, 44, 52 Fusobacterium nucleatum numbers are significantly correlated with tumor size, and shortened survival times in a population of human Japanese patients with later stage CRC compared to earlier stages.53 These findings, as well as additional observations in rodent models and humans with CRC suggest that chronic inflammatory disease of the GI tract is a potential risk factor for the development of additional channels for amplified inflammatory processes, aneuploidy, high‐grade dysplasia, metaplasia and loss of cellular proliferative checkpoints, all of which are processes that can be appreciated in malignancy.13, 19, 54, 58, 59 The increased numbers of mucosal Fusobacterium spp. bacteria in GI biopsies of cats with GI LSA relative to GI biopsies of cats with IBD noted in the current study, although possibly just an effect of the GI malignancy, could also be associated with a potential progression from high grade chronic lymphoplasmacytic inflammation to inflammation‐mediated lymphoid intestinal cancer.
Although incompletely understood, GI inflammation‐mediated progression to cancer development might involve bacterial toxin production, release of alarmins, gut biofilm modification, dysregulation of gut barrier function, suppression of anti‐inflammatory mediators and transition through an element of high grade dysplasia to cell damage and aneuploidy.13, 19, 35, 40, 60, 61, 62 Fusobacterium spp. is pro‐inflammatory, exhibits adhesive and invasive properties via virulence factors (i.e., FadA, fusobacterium autotransporter protein 2 and fusobacterial outer membrane protein A), and promotes pro‐oncogenic features which might contribute to accelerated tumor growth and tumor burden.38, 47, 54 Increased Fusobacterium nucleatum is associated with an increase in adenoma formation and accelerated small intestinal adenocarcinoma development in rodent models of GI neoplasia.41 Coupled with this was an expansion of CD11b+ myeloid cells (i.e., dendritic cells, macrophages, and myeloid‐derived suppressor cells) in the neoplastic tissues and an NF‐κB‐driven pro‐inflammatory response.41 Similarly, our results also indicated increases in CD11b+ and NF‐κB expressing cells in GI biopsies of cats with small cell GI LSA, and there was a positive correlation between increased Fusobacterium spp. numbers and both CD11b+ and NF‐κB expressing cells. Furthermore, CD11b+ myeloid cells might contribute to development of a tumor microenvironment,19, 63 and Fusobacterium spp. might induce toll like receptors (TLR), to stimulate NF‐κB through the TLR4/MYD88 pathway.54 Importantly, TLRs are found on both immune and non‐immune cells, and can be expressed on tumors including human mantle cell lymphoma.21, 64
There were also increased numbers of the Bacteroides‐Prevotella bacteria in the ileal biopsies of the cats with small cell GI LSA compared to ileal biopsies of the cats with IBD. Prior studies have shown that increased Bacteroides spp. can be seen in healthy cats versus cats with IBD.65, 66 This suggests that progressive disease might be associated with a decreased Bacteroides spp. load. This difference in findings might be explained by the different sampling sites (mucosal versus fecal) that were used for Bacteroides spp. quantification,67 or might be due to the different cohorts compared, considering that quantification of GI Bacteroides spp. in cats with GI small cell LSA, to the authors' knowledge, has not been previously evaluated. Also, as the Bacteroides spp. incorporates a number of different subspecies, there is also the possibility that cats with small cell GI LSA harbor increased numbers of enterotoxigenic Bacteroides spp. as compared to those with IBD, as occurs in humans.68, 69
The additional 5 probes evaluated using FISH included Clostridium spp. (Erec482), Enterobacteriaceae (Ebac1790), Helicobacter spp. (Hel717), Faecalibacterium spp. (Faecali896) and total bacteria (Eub338). Clostridium spp. population and diversity have been shown to be decreased while Enterobacteriaceae bacteria is increased in humans, dogs and cats with chronic intestinal inflammation.16, 17, 65, 70, 71, 72 Utilizing 16S rRNA sequencing in cats with chronic enteropathies, Faecalibacterium spp. bacteria were decreased while Enterobacteriaceae numbers were increased in cats with diarrhea.66 This variation of bacterial species also occurs in dogs with IBD.72, 73 Therefore, different inflammatory states are characterized by differences in the microbiota. The current study did not show any significant differences in Enterobacteriaceae, Faecalibacterium spp. and total bacteria (Eub338) in GI biopsies of cats with small cell GI LSA compared to GI biopsies of cats with IBD.
Helicobacter spp. induces host responses influencing risk of oncogenesis, and promotes chronic gut inflammation in humans and mice.46 In a recent study, FISH showed that the presence of enterohepatic helicobacter was significantly associated with poorly differentiated large intestinal adenocarcinoma in cats.48 Our results showed no significant difference in Helicobacter spp. bacterial counts in GI biopsies of cats with small cell GI LSA compared to GI biopsies of cats with IBD.
It should be noted that in this study the majority of bacteria evaluated were located in the adherent mucus of the GI biobsies, a finding consistent with a prior study.10 Less bacteria was appreciated in the free mucus and attaching to the surface epithelium, although still subjectively substantial. In a number of the biopsies there was very minimal to no bacteria appreciated invasively in the mucosa.
Although there is clinical relevance in determining the short and long‐term outcomes and response to treatment of the underlying enteropathy in cats with alterations in the GI microbiota, we were unable to fully assess the significance of the relationship of Fusobacterium spp. quantification and outcome due to the variation of treatments and the limitation on follow up for a number of these cats. This is worth assessing in future studies, as in human studies and rodent models, overabundance of Fusobacterium spp. in patients or rodents with CRC does appear to have a correlation with poorer prognostic outcome.36, 51, 54
There are a number of potential limitations to this study. These would include the retrospective study design using medical records completed by different clinicians and the small population of cats evaluated. As diagnosis of IBD and small cell GI LSA rely as much on prior history as diagnostic findings the authors included, at a minimum, the most pertinent historical findings that would be needed to make these diagnoses in light of findings associated with diagnostic testing performed. The administration of antibiotics that could have affected the microbiota would be another potential limitation. The authors attempted to include cats with no history of antibiotic administration 2 weeks prior to the acquisition of GI biopsies but this information was not always reported in the medical records. Processing, including formalin‐fixation and paraffin embedding of the biopsies, might have also played a role in the finding of bacteria contained in the adherent mucus compartment, but this is consistent with a prior study10 and should not have caused a discrepancy in any 1 cat in the study relative to the study population as a whole. Another potential limitation was the inability to perform PARR on the formalin‐fixed, paraffin embedded tissues of all cats. The authors acknowledge that both PARR and IHC, when utilized together could have reduced the underestimation of small cell GI LSA in this population and might have allowed for a more sensitive means of screening for small cell GI LSA amongst the cohorts.2, 74 Unfortunately, tissue specimens were sometimes too limited per each feline endoscopic biopsy sample to allow for this additional diagnostic, due to the extensive tissue requirement for both hybridization for FISH and CD11b+ and NF‐κB immunohistochemical assessment. Also, the expense of performing PARR on all samples in this study would have been beyond the scope of funds available for the study. The authors did attempt to circumvent this limitation by performing PARR on any sample that exhibited questionable lymphoid monomorphism. Furthermore, it has been suggested that although PARR is valuable as a differentiating diagnostic test, it should be considered as an adjunct to immunohistochemical and histologic diagnostics, as it also does have its limitations relative to false negatives.2, 74 It has been recommended that PARR be considered as part of a stepwise diagnostic strategy, indicating the importance of more advanced testing only as required in a true clinical setting.2, 74
In conclusion, we showed that Fusobacterium spp. bacteria are increased in the ileum and colon of GI biopsies of cats with small cell GI LSA relative to GI biopsies of cats with IBD. We also showed that increased Fusobacterium spp. bacteria are positively correlated to increased CD11b+ and NF‐κB expression in neoplastic ileal and colonic biopsies versus ileal and colonic biopsies of cats with lymphoplasmacytic ileitis and colitis (idiopathic IBD). Future studies would be needed to attempt to further clarify whether increased Fusobacterium spp is a consequence of small cell GI LSA in cats or whether it plays a role in the development of the disease.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
ACKNOWLEDGMENTS
The authors acknowledge the contributions of Curtis Mosher, Associate Scientist, Carver Lab for Ultrahigh Resolution Biological Microscopy; and Todd Atherly, Research Associate, Veterinary Clinical Sciences; Iowa State University, Ames IA. This study was performed at Iowa State University College of Veterinary Medicine, Veterinary Clinical Sciences, Ames, IA, and supported by the Comparative Gastroenterology Society Waltham Research Grant and Iowa State University Veterinary Clinical Science Research Incentive Grant. Previous abstract presentation: Results were presented in part in an oral abstract at the 2017 ACVIM Forum, National Harbor, MD.
Garraway K, Johannes CM, Bryan A, et al. Relationship of the mucosal microbiota to gastrointestinal inflammation and small cell intestinal lymphoma in cats. J Vet Intern Med. 2018;32:1692–1702. 10.1111/jvim.15291
From the Department of Veterinary Clinical Sciences, College of Veterinary Medicine, Department of Internal Medicine, Department of Veterinary Pathology, and Veterinary Diagnostic and Production Animal Medicine, Iowa State University College of Veterinary Medicine, Ames IA, USA; College of Agriculture and Life Sciences, Genetics, Development and Cell Biology, Molecular Biology, Iowa State University, Ames IA, USA; VDx Veterinary Diagnostics and Preclinical Research Services, Davis CA, USA; School of Bioscience and Veterinary Medicine, University of Camerino, Camerino, Italy.
Funding information
Comparative Gastroenterology Society, Grant/Award Number: Waltham Research Grant; Iowa State University Veterinary Clinical Sciences, Grant/Award Number: Research Incentive Grant
[Correction added after first online publication 31 August 2018: Footnoted information moved within text throughout]
REFERENCES
- 1. Rissetto K, Villamil JA, Selting KA, et al. Recent Trends in Feline Intestinal Neoplasia: an Epidemiologic Study of 1,129 Cases in the Veterinary Medical Database from 1964 to 2004. J Am Anim Hosp Assoc. 2011;47:28‐36. [DOI] [PubMed] [Google Scholar]
- 2. Kiupel M, Smedley RC, Pfent C, et al. Diagnostic Algorithm to Differentiate Lymphoma From Inflammation in Feline Small Intestinal Biopsy Samples. Vet Pathol. 2011;48:212‐222. [DOI] [PubMed] [Google Scholar]
- 3. Barko PC, McMichael MA, Swanson KS, et al. The Gastrointestinal Microbiome: A Review. J Vet Intern Med. 2018;32:9‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gress V, Wolfesberger B, Fuchs‐Baumgartinger A, et al. Characterization of the T‐cell receptor gamma chain gene rearrangements as an adjunct tool in the diagnosis of T‐cell lymphomas in the gastrointestinal tract of cats. Res Vet Sci. 2016;107:261‐266. [DOI] [PubMed] [Google Scholar]
- 5. Hammer SE, Groiss S, Fuchs‐Baumgartinger A, et al. Characterization of a PCR‐based lymphocyte clonality assay as a complementary tool for the diagnosis of feline lymphoma. Vet Comp Oncol. 2017;15:1354‐1369. [DOI] [PubMed] [Google Scholar]
- 6. Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Clin Nutr. 2014;100:394S‐S398. [DOI] [PubMed] [Google Scholar]
- 7. Ramadan Z, Xu H, Laflamme D, et al. Fecal Microbiota of Cats with Naturally Occurring Chronic Diarrhea Assessed Using 16S rRNA Gene 454‐Pyrosequencing before and after Dietary Treatment. J Vet Intern Med. 2014;28:59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cassmann E, White R, Atherly T, et al. Alterations of the Ileal and Colonic Mucosal Microbiota in Canine Chronic Enteropathies. PLoS One. 2016;11:e0147321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoehne SN, McDonough SP, Rishniw M, et al. Identification of Mucosa‐Invading and Intravascular Bacteria in Feline Small Intestinal Lymphoma. Vet Pathol. 2017;54:234‐241. [DOI] [PubMed] [Google Scholar]
- 10. Janeczko S, Atwater D, Bogel E, et al. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet Microbiol. 2008;128:178‐193. [DOI] [PubMed] [Google Scholar]
- 11. Matnani R, Ganapathi KA, Lewis SK, et al. Indolent T‐ and NK‐cell lymphoproliferative disorders of the gastrointestinal tract: a review and update. Hematol Oncol. 2017;35:3‐16. [DOI] [PubMed] [Google Scholar]
- 12. Perry AM, Warnke RA, Hu Q, et al. Indolent T‐cell lymphoproliferative disease of the gastrointestinal tract. Blood. 2013;122:3599‐3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsai JH, Rabinovitch PS, Huang D, et al. Association of Aneuploidy and Flat Dysplasia With Development of High‐Grade Dysplasia of Colorectal Cancer in Patients With Inflammatory Bowel Disease. Gastroenterology. 2017;153:1492‐1495. [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto ML, Schiestl RH. Intestinal Microbiome and Lymphoma Development. Cancer J. 2014;20:190‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allenspach K, House A, Smith K, et al. Evaluation of mucosal bacteria and histopathology, clinical disease activity and expression of Toll‐like receptors in German shepherd dogs with chronic enteropathies. Vet Microbiol. 2010;146:326‐335. [DOI] [PubMed] [Google Scholar]
- 16. Suchodolski JS, Dowd SE, Wilke V, et al. 16S rRNA Gene Pyrosequencing Reveals Bacterial Dysbiosis in the Duodenum of Dogs with Idiopathic Inflammatory Bowel Disease. PLoS One. 2012;7:e39333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suchodolski JS, Xenoulis PG, Paddock CG, et al. Molecular analysis of the bacterial microbiota in duodenal biopsies from dogs with idiopathic inflammatory bowel disease. Vet Microbiol. 2010;142:394‐400. [DOI] [PubMed] [Google Scholar]
- 18. Xenoulis PG, Palculict B, Allenspach K, et al. Molecular‐phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol Ecol. 2008;66:579‐589. [DOI] [PubMed] [Google Scholar]
- 19. Brennan CA, Garrett WS. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu Rev Microbiol. 2016;70:395‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akin H, Tozun N. Diet, Microbiota, and Colorectal Cancer. J Clin Gastroenterol. 2014;48:S67‐S69. [DOI] [PubMed] [Google Scholar]
- 21. Wang L, Zhao Y, Qian J, et al. Toll‐Like Receptor‐4 Signaling in Mantle Cell Lymphoma: Effects on Tumor Growth and Immune Evasion. Cancer. 2013;119:782‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bridgeford EC, Marini RP, Feng Y, et al. Gastric Helicobacter species as a cause of feline gastric lymphoma: A viable hypothesis. Vet Immunol Immunopathol. 2008;123:106‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCoy AN, Araújo‐Pérez F, Azcárate‐Peril A, et al. Fusobacterium Is Associated with Colorectal Adenomas. PLoS One. 2013;8:e53653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monaco C, Andreakos E, Kiriakidis S, et al. Canonical pathway of nuclear factor κB activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci USA. 2004;101:5634‐5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beinke S, Ley SC. Functions of NF‐κB1 and NF‐κB2 in immune cell biology. Biochem J. 2004;382:393‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153‐169. [DOI] [PubMed] [Google Scholar]
- 27. Normark S, Nilsson C, Normark BH, et al. Persistent infection with Helicobacter pylori and the development of gastric cancer. Adv Cancer Res. 2003;90:63‐89. [DOI] [PubMed] [Google Scholar]
- 28. Amann RI, Binder BJ, Olson RJ, et al. Combination of 16S rRNA‐Targeted Oligonucleotide Probes with Flow Cytometry for Analyzing Mixed Microbial Populations. Appl Environ Microbiol. 1990;56:1919‐1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Franks AH, Harmsen HJ, Raangs GC, et al. Variations of Bacterial Populations in Human Feces Measured by Fluorescent In Situ Hybridization with Group‐Specific 16S rRNA‐Targeted Oligonucleotide Probes. Appl Environ Microbiol. 1998;64:3336‐3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doré J, Sghir A, Hannequart‐Gramet G, et al. Design and Evaluation of a 16S rRNA‐Targeted Oligonucleotide Probe for Specific Detection and Quantification of human Faecal Bacteroides Populations. Syst Appl Microbiol. 1998;21:65‐71. [DOI] [PubMed] [Google Scholar]
- 31. Gmür R, Wyss C, Xue Y, et al. Ginigival crevice microbiota from Chinese patients with gingivitis or necrotizing ulcerative gingivitis. Eur J Oral Sci. 2004;112:33‐41. [DOI] [PubMed] [Google Scholar]
- 32. Poulsen LK, Lan F, Kristensen CS, et al. Spatial Distribution of Escherichia coli in the Mouse Large Intestine Inferred from rRNA In Situ Hybridization. Infect Immun. 1994;62:5191‐5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chan V, Crocetti G, Grehan M, et al. Visualization of Helicobacter species within the murine cecal mucosa using specific fluorescence in situ hybridization. Helicobacter. 2005;10:114‐124. [DOI] [PubMed] [Google Scholar]
- 34. Garcia‐Mazcorro JF, Dowd SE, Poulsen J, et al. Abundance and short‐term temporal variability of fecal microbiota in healthy dogs. Microbiologyopen. 2012;1:340‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bui FQ, Johnson L, Roberts J, et al. Fusobacterium nucleatum infection of gingival epithelial cells leads to NLRP3 inflammasome‐dependent secretion of IL‐1ß and the danger signals ASC and HMGB1. Cell Microbiol. 2016;18:970‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Desai AR, Musil KM, Carr AP, et al. Characterization and quantification of feline fecal microbiota using cpn60 sequence‐based methods and investigation of animal‐to‐animal variation in microbial population structure. Vet Microbiol. 2009;137:120‐128. [DOI] [PubMed] [Google Scholar]
- 38. Gholizadeh P, Eslami H, Kafil HS. Carcinogenesis mechanisms of Fusobacterium nucleatum. Biomed Pharmacother. 2017;89:918‐925. [DOI] [PubMed] [Google Scholar]
- 39. Honneffer JB, Minamoto Y, Suchodolski JS. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. Wjg. 2014;20:16489‐16497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson CH, Dejea CM, Edler D, et al. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015;21:891‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor immune microenvironment. Cell Host Microbe. 2013;14:207‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mitsuhashi K, Nosho K, Sukawa Y, et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotargert. 2015;6:7209‐7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mueller A, O'Rourke J, Chu P, et al. The Role of Antigenic Drive and Tumor‐Infiltrating Accessory Cells in the Pathogenesis of Helicobacter‐Induced Mucosa‐Associated Lymphoid Tissue Lymphoma. Am J of Pathol. 2005;167:797‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagy KN, Sonkodi I, Szöke I, et al. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34:304‐308. [PubMed] [Google Scholar]
- 45. Nosho K, Sukawa Y, Adachi Y, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. Wjg. 2016;22:557‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E‐cadherin/ß‐catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Swennes AG, Parry NM, Feng Y, et al. Enterohepatic Helicobacter spp. in cats with non‐haematopoietic intestinal carcinoma: a survey of 55 cases. J Med Microbiol. 2016;65:814‐820. [DOI] [PubMed] [Google Scholar]
- 49. Tizard IR, Jones SW. The Microbiota Regulates Immunity and Immunologic Diseases in Dogs and Cats. Vet Clin North Am Small Anim PractEpub: Nov 29, 2017; ( 10.1016/j.cvsm.2017.10.008). [DOI] [PubMed]
- 50. Wang LL, Yu XJ, Zhan SH, et al. Participation of microbiota in the development of gastric cancer. World J Gastroenterol. 2014;20:4948‐4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wei Z, Cao S, Liu S, et al. Could gut microbiota serve as a prognostic biomarker associated with colorectal cancer patients' survival? A pilot study on relevant mechanism. Oncotarget. 2016;7:46158‐46172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Whitmore SE, Lamont RJ. Oral Bacteria and Cancer. PLoS Pathog. 2014;10:e1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yamaoka Y, Suehiro Y, Hashimoto S, et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J Gastroenterol 2018;53:517 Epub: Aug 19, ( 10.1007/s00535-017-1382-6). [DOI] [PubMed] [Google Scholar]
- 54. Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll‐Like Receptor 4 Signaling to Nuclear Factor‐ κB, and Up‐regulating Expression of MicroRNA‐21. Gastroenterology. 2017;152:851‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mkaouar L, Endo Y, Jun HX, et al. Relationship between NF‐κB Expression and Malignancy of Canine Mammary Gland Tumor Tissues. J Vet Med Sci. 2012;74:713‐718. [DOI] [PubMed] [Google Scholar]
- 56. Engel AG, Arahata K. Mononuclear cells in myopathies: quantitation of functionally distinct subsets, recognition of antigen‐specific cell‐mediated cytotoxicity in some diseases, and implications for the pathogenesis of the different inflammatory myopathies. Hum Pathol. 1986;17:704‐721. [DOI] [PubMed] [Google Scholar]
- 57. Ritchie LE, Steiner JM, Suchodolski JS. Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis. FEMS Microbiol Ecol. 2008;66:590‐598. [DOI] [PubMed] [Google Scholar]
- 58. Kappelman MD, Farkas DK, Long MD, et al. Risk of Cancer in Patients with Inflammatory Bowel Diseases: a Nationwide Population‐Based Cohort Study with 30 Years of Follow Up. Clin Gastroenterol Hepatol. 2014;12:265‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Navaneethan U, Jegadeesan R, Gutierrez NG, et al. Progression of low‐grade dysplasia to advanced neoplasia based on the location and morphology of dysplasia in ulcerative colitis patients with extensive colitis under colonoscopic surveillance. J Crohns Colitis. 2013;7:e684‐e691. [DOI] [PubMed] [Google Scholar]
- 60. Bojesen RD, Riis LB, Høgdall E, et al. Inflammatory Bowel Disease and Small Bowel Cancer Risk, Clinical Characteristics, and Histopathology: A Population‐Based Study. Clin Gastroenterol Hepatol. 2017;15:1900‐1907. [DOI] [PubMed] [Google Scholar]
- 61. Dejea CM, Sears CL. Do biofilms confer a pro‐carcinogenic state? Gut Microbes. 2016;7:54‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rook GA, Dalgleish A. Infection, immunoregulation, and cancer. Immunol Rev. 2011;240:141‐159. [DOI] [PubMed] [Google Scholar]
- 63. Zhang QQ, Hu XW, Liu YL, et al. CD11b deficiency suppresses intestinal tumor growth by reducing myeloid cell recruitment. Sci Rep. 2015;5:15948 10.1038/srep15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wolska A, Lech‐Maranda E, Robak T. Toll‐Like Receptors and their Role in Hematologic Malignancies. Curr Mol Med. 2009;9:324‐335. [DOI] [PubMed] [Google Scholar]
- 65. Inness VL, McCartney AL, Khoo C, et al. Molecular characterization of the gut microflora of healthy and inflammatory bowel disease cats using fluorescence in situ hybridization with special reference to Desulfovibrio spp. J Anim Physiol Anim Nutr Nutr. 2007;91:48‐53. [DOI] [PubMed] [Google Scholar]
- 66. Suchodolski JS, Foster ML, Sohail MU, et al. The Fecal Microbiome in Cats with Diarrhea. PLoS One. 2015;10:e0127378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gerritsen J, Smidt H, Rijkers GT, et al. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Purcell RV, Pearson J, Aitchison A, et al. Colonization with enterotoxigenic Bacteroides fragilis is associated with early‐stage colorectal neoplasia. PLoS One. 2017;12:e0171602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wexler HM. Bacteroides: the Good, the Bad, and the Nitty‐Gritty. Clin Microbiol Rev. 2007;20:593‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baumgart M, Dogan B, Rishniw M, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Chron's disease involving the ileum. Isme J. 2007;1:403‐418. [DOI] [PubMed] [Google Scholar]
- 71. Frank DN, St Amand AL, Feldman RA, et al. Molecular‐phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780‐13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Suchodolski JS, Markel ME, Garcia‐Mazcorro JF, et al. The Fecal Microbiome in Dogs with Acute Diarrhea and Idiopathic Inflammatory Bowel Disease. PLoS One. 2012;7:e51907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vázquez‐Baeza Y, Hyde ER, Suchodolski JS, et al. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat Microbiol. 2016;1:16177 10.1038/nmicrobiol.2016.177. [DOI] [PubMed] [Google Scholar]
- 74. Sabattini S, Bottero E, Turba ME, et al. Differentiating feline inflammatory bowel disease from alimentary lymphoma in duodenal endoscopic biopsies. J Small Anim Pract. 2016;57:396‐401. [DOI] [PubMed] [Google Scholar]


