Abstract
Background
Pancreatic enzyme supplements for the treatment of exocrine pancreatic insufficiency (EPI) in dogs can be uncoated or enteric coated. Enteric coated supplements might be advantageous.
Hypothesis/Objectives
Enteric coated enzyme supplements are superior to uncoated supplements in dogs with clinical EPI.
Animals
Eleven dogs with naturally occurring EPI that were apparently free from other diseases.
Methods
Randomized, blinded, controlled cross‐over clinical trial comparing a novel micro‐encapsulated enteric coated enzyme supplement to a commercially available uncoated product in dogs with clinical EPI. Search of serum canine serum trypsin‐like immunoreactivity concentration ≤ 2.5 µg/L in the Gastrointestinal Laboratory database was used to identify dogs with EPI.
Results
There was no difference −4.46% (95% CI: −7.97%‐–0.96%; P = .15) in the % acid hydrolysis fecal fat (primary outcome) between the enteric coated formulation (median: 11.8%; range 6.4%‐17.0%) and the uncoated pancreatic enzyme replacement product (median: 17.5%; range: 5.2%‐24.9%) in the 11 dogs that completed the study. Other variables did not differ between treatments.
Conclusions and Clinical Importance
This study, which had low statistical power, did not detect a difference between formulations.
Keywords: enteric coating, EPI, randomized clinical trial
Abbreviations
- AH fat
acid hydrolysis fat
- BCS
body condition score
- CP
crude protein
- cTLI
canine trypsin‐like immunoreactivity
- DM
dry matter
- DMB
dry matter basis
- EPI
exocrine pancreatic insufficiency
- GCP
good clinical practices
- MMA
methyl malonic acid
- OM
organic matter
1. INTRODUCTION
Exocrine pancreatic insufficiency (EPI) is a common disorder in dogs that is characterized by inadequate synthesis and secretion of pancreatic digestive enzymes. Exocrine pancreatic insufficiency clinically manifests itself with signs of maldigestion and malabsorption.1 Traditionally, EPI was diagnosed by measurement of canine serum trypsin‐like immunoreactivity (cTLI) concentration by a radioimmunoassay (RIA), which is both highly sensitive and specific for canine EPI.2 A serum TLI concentration of ≤2.5 µg/L is considered diagnostic for EPI in dogs.2 The primary treatment of EPI is to provide digestive enzymes with each meal. Preparations of pancreatic enzymes include enteric coated capsules or granules, uncoated enzyme powder, or raw pancreas.3, 4 Abnormally low serum cobalamin concentrations occur in more than 80% of dogs with EPI, and low concentrations of cobalamin have been associated with poor clinical response and outcome.
Reportedly, the causes of death in dogs with EPI are euthanasia because of the cost of treatment, the need for lifelong treatment, or failure to respond to treatment.4 One study reported an adequate response to treatment in 92% of the dogs evaluated.4 However, this was determined by death from unrelated causes at 6–24 month follow up. The same study showed that response to initial treatment was good, partial, or poor, in 64, 19, and 17% of dogs (n = 140). A more recent study, showed that response to initial treatment was good in 60% of treated dogs, partial in 17%, and poor in 23% (n = 178).5
In some dogs, pancreatic enzyme replacement has been associated with gingival bleeding, but this adverse effect can often be managed by decreasing the dose of the pancreatic enzyme supplement.6, 7 In certain animals, the oral mucosa might be overly sensitive to pancreatic enzyme supplements resulting in oral bleeding. The use of enteric coated enzymes would be expected to reduce this adverse effect. Moreover, enteric coating also reduces inactivation of enzymes in the stomach by gastric acid and proteases. An earlier study suggested that uncoated preparations were more efficacious than enteric coated ones,4 while, a more recent study suggested otherwise.5 Recently, a randomized clinical trial comparing uncoated and enteric coated enzyme preparations in dogs suggested that dogs treated with the enteric coated enzyme preparation might show a better treatment response.8 However, in that study the control treatment was an enzyme formulation that is no longer commercially available.
This study aimed to compare the clinical efficacy of a new enteric coated micro‐pelleted formulation of a pancreatic enzyme replacement product (Primazym, dogs 40000 Ph. Eur. U. Capsules for Dogs, Eurovet Animal Health B.V., Bladel, the Netherlands, subsidiary of Dechra Pharmaceuticals plc) to a commercially available uncoated pancreatic enzyme replacement product (Tryplase, MSD Animal Health, Buckinghamshire [Intervet]).
2. MATERIALS AND METHODS
The study was designed as per Guidance for Industry, Good Clinical Practice (GCP), International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH), GL9 guidelines (https://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM052417.pdf).
2.1. Animals
The Gastrointestinal Laboratory, Texas A&M University database was used to identify potential study subjects, which consisted of dogs diagnosed with EPI based on a serum cTLI concentration ≤ 2.5 µg/L. In addition, owners were contacted with the help of the EPI4 dogs website [http://www.epi4dogs.com/]. Dogs with a serum cTLI concentration ≤2.5 µg/L and clinical signs of EPI (including one or more of polyphagia, weight loss, steatorrhea, loose feces, voluminous feces, or malodorous feces) were recruited into the study. Dogs had to be at least 1 year of age, not pregnant or lactating, and free from any clinically apparent comorbidities.
Exclusion criteria included changes on complete blood count or serum biochemistry panel evaluated at baseline that were considered clinically relevant and suggestive of a concurrent disease process, such as impairment of renal function, diabetes mellitus, etc.
Serum cobalamin concentrations were measured and if they were <400 ng/L, cobalamin was supplemented according to a standard parenteral supplementation protocol (http://vetmed.tamu.edu/gilab/research/cobalamin-information). Dogs could only be enrolled into the study once serum cobalamin concentration had been normalized. Parenteral cobalamin supplementation was permitted if indicated during the course of the study.
Informed owner consent was obtained for all dogs enrolled. The consent form had been reviewed and approved by the Clinical Research Review Committee at Texas A&M University and the study protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Texas A&M University (AUP 2011‐84 & IACUC 2014‐0094 CA).
The study was coordinated by the investigators located at the Gastrointestinal Laboratory at Texas A&M University. The study sites incorporated various homes of privately owned dogs and veterinary practices across the United States.
2.2. Trial design
The study was conducted using a cross over design. Eligible dogs were randomly assigned to treatment, using a blocked design. A block size of 4 was used and before initiation of the study block patterns were randomly selected using a random number generator in a spreadsheet program (Microsoft Excel, Microsoft Corporation, Redmond, Washington). Ten block patterns were selected and assignments were placed in sequentially numbered envelopes by an investigator that did not participate in enrollment. In addition to the block pattern described above, the blocks were further stratified by sex and body weight of the dogs. The stratification was as follows male and <= 10 kg, male and > 10 kg, female and <= 10 kg, and female and > 10 kg. Stratification was employed to ensure that the groups were comparable in case there was a carry‐over effect or substantial enrollment losses occurred once the study had started.
Veterinarians and owners only had knowledge of the designation of “treatment A” and “treatment B” at all times and did not have access to the protocol in its entire format. In addition, veterinarians and owners were not told the identity of “treatment A” or “treatment B,” even after the completion of the study. The laboratory personnel performing the analysis of serum, blood, and fecal samples only had access to the dog's unique ID. Additionally, the investigator who analyzed the data was blinded to the coding of the treatments.
2.3. Study protocol for each dog
There was a 3‐day assessment period before starting the study [baseline]. During the assessment periods the owner collected a fecal sample (that then was kept frozen until analysis) on 3 consecutive days and also evaluated the dog in terms of attitude, appetite, drinking, feces volume, color, and consistency, frequency of defecation, and evidence of borborygmus, flatulence, or coprophagy using a standardized form (supporting information, S1). After collection of the last fecal sample, food was withheld for at least 12 hours and then examined by a veterinarian. Special emphasis was placed on examination of the gingiva. At that time, the veterinarian also collected whole blood and serum samples. These clinical samples were sent for analysis to ensure all inclusion criteria were satisfied. Once all inclusion criteria were met, the owner received either the enteric coated product (treatment A) or the uncoated control product (treatment B) from the veterinarian and the treatment period of 3 weeks was started. The dosage guidelines for the study were based on evaluation of the summary of product characteristics of both products (supporting information, S2).
The capsule(s) were opened and the contents were mixed with the food immediately before offering the food to the dog. Owners were informed that enzyme replacement should be added to every meal and that in‐between meals and snacks without enzyme supplementation should be avoided. Dogs were allowed to remain on their regular diet; however, owners signed a statement that no changes in the dog's diet or environment could take place during the course of the study period (∼6 weeks).
The owner collected a fecal sample on each of the last 3 days of the first 3‐week treatment period. The dog was again examined by a veterinarian after food had been withheld for at least 12 hours and blood and serum samples were again collected. The dog was then switched to the other treatment for a second treatment period of 3 weeks. At the end of the second 3‐week treatment period the owner collected another set of 3 fecal samples on each of the last 3 days. The dog was once again evaluated by a veterinarian and another set of blood and serum samples were collected. As an incentive for completing the study, each owner received a 6‐week supply of the supplement of their choice (ie, either “treatment A” or “treatment B”) after successfully completing the study and turning in all samples and questionnaire forms.
2.4. Post‐admission withdrawals and concomitant treatment
Permitted concomitant treatment included cobalamin supplementation based on laboratory assessment. Development of other diseases/abnormalities such as increased serum folate concentration suggestive of small intestinal dysbiosis was ascertained by laboratory evaluation at 3 week intervals. Each case was evaluated separately and a decision concerning study continuation was made in cooperation with the dog owner, the primary care veterinarian, and the investigators. Routine preventative medications, such as those for external and internal parasites that were being used before enrollment, such as monthly heartworm prevention or prophylactic tick and flea medication administered orally or as spot on were permitted.
2.5. Adverse events and compliance monitoring
Owners were instructed to report any adverse events. Data related to the incidence, severity, and duration of local and systemic adverse events were compiled during the study.
To assess owner compliance the discrepancy between the number of capsules provided and those returned to the veterinarian where compared.
2.6. Laboratory testing
The blood and serum samples were submitted for the respective assays at the earliest time after arrival of the shipment. Samples were temporarily stored at 4°C before testing. Complete blood counts were performed at the Texas Veterinary Medical Diagnostic Laboratory, College Station. Serum chemistry profiles and measurements of serum concentrations of cobalamin, folate, cTLI, and methylmalonic acid (MMA) were performed at the Gastrointestinal Laboratory, Texas A&M University, College Station. A clinical chemistry analyzer (SIRRUS Clinical Chemistry Analyzer, Stanbio Laboratory, Boerne, Texas) was used to measure serum chemistry profiles, chemiluminescence immunoassays (IMMULITE 2000, Siemens, Illinois) were used to measure serum cobalamin and folate concentrations, a double antibody RIA (Canine TLI Double Antibody Radioimmunoassay, Siemens Healthcare Diagnostics Inc, Deerfield, Illinois) was used to measure cTLI concentrations, and MMA was measured using a stable isotope dilution gas chromatography/mass spectrometry in‐house assay. Serum MMA concentrations were measured in batches at a later date.
Collected feces samples from 3 consecutive days were pooled, aliquoted, and stored at −80°C until analysis. At the end of the clinical phase of the study, all fecal samples were shipped for analysis of fat, moisture, and nitrogen at the Animal Sciences Laboratory, Department of Animal Sciences and Division of Nutritional Sciences, University of Illinois.
2.7. Assessment of outcomes
Percent fecal fat was used as the primary outcome of interest.9, 10 Secondary outcomes of interest were: clinical signs as reported by the owner (see supporting information), laboratory analysis of hematological and biochemical variables, and fecal characteristics (percent of moisture and nitrogen in the feces samples) for the 3‐day assessment during both treatment periods.
2.8. Sample size
The sample size for the study was estimated using percent fecal fat as the outcome and assuming that a difference of 1.5% or more between products would be considered superior. A minimum of 16 dogs was required to complete the study to provide a statistical test with 80% power to detect a difference of 1.5% fecal fat between dogs before the start of treatment and after treatment with either enzyme supplement assuming that the standard deviation of fecal fat was also 1.5%. To allow for possible exclusion of dogs based on problems identified after completion of the study, an additional 2 dogs (n = 18) were expected to be needed to complete the study. It was estimated that a total of 38 dogs would be required for initial enrollment to account for dropouts during the course of the study.
2.9. Statistical analysis
Data were evaluated for normality by graphical assessment and by performing a Shapiro‐Wilk's test. Data were analyzed nonparametrically because of the small sample size and the apparent violation of the normality assumption in most outcome variables. Quantitative outcomes were compared among baseline, treatment A, and treatment B time points using Friedman tests followed by post‐hoc pairwise Wilcoxon signed‐rank tests incorporating Bonferroni correction of P values (3 pairwise comparisons; P < .017 each). Qualitative data were compared between treatment groups using McNemar chi‐square tests. Statistical analyses were performed using a commercial software package (IBM SPSS Statistics Version 22, International Business Machines Corp, Armonk, New York) package and statistical significance was set at P < .05 for 2‐tailed tests.
3. RESULTS
During the trial, the comparison product became no longer commercially available and only 11 dogs completed the study. There were no major deviations from the study design in any of the dogs.
A total of 322 dogs were screened for potential eligibility for enrollment. From these, a total of 52 dogs were enrolled and assessed for eligibility. However, 37 dogs had to be excluded; 19 dogs had lower than desired serum cobalamin concentrations (<400 ng/L), 1 dog had a cTLI concentration >2.5 ug/L, 7 owners decided not to participate after enrolling in the study or were lost to follow up, 2 dogs were euthanized after owners preliminarily expressed interest in the study, and a total of 8 dogs could not participate because the comparison product was no longer available. A total of 15 dogs started the trial, but 4 of these dogs did not complete the study; 1 dog developed seizures, 1 developed a urinary tract infection that required antibiotics, and in the case of 2 dogs they failed to improve as expected on the enzymes so the owner or veterinarian decided to withdraw from the study (Figure 1). Six male and 5 female dogs completed the study.
Figure 1.

Case flow indicating how the study was executed
3.1. Assessment of fecal fat
In the 11 dogs that completed the study, the median % acid hydrolysis (AH) fecal fat at the time of enrolment was 13.4% (range: 8.0%‐21.2%). However, only 2 dogs were enrolled into the study without receiving previous enzyme supplementation (Figure 2). Nine of the 11 dogs completing the trial had a lower % AH fecal fat in the study period when they were treated with the enteric coated formulation product when compared with the uncoated product. In 2 dogs, however the % AH fecal fat was lower during treatment with the uncoated product, than during treatment with the enteric coated product. In these 2 dogs, the % AH fecal fat was 8.1% and 5.2% during treatment with the uncoated product and 10.6% and 6.4% during treatment with the enteric coated formulation. This fat percentage was similar to what would be expected in healthy dogs (∼8.8%‐10.5% fecal fat, depending on diet11). There was no significant difference −4.46% (95% CI: −7.97% to −0.96%; P = .15) in the median % AH fecal fat between the enteric coated formulation (median: 11.8%; range 6.4%‐17.0%) and the uncoated pancreatic enzyme replacement product (median: 17.5%; range: 5.2%‐24.9%) in the 11 dogs that completed the study. However, the statistical test had low power because of the smaller than desired sample size.
Figure 2.
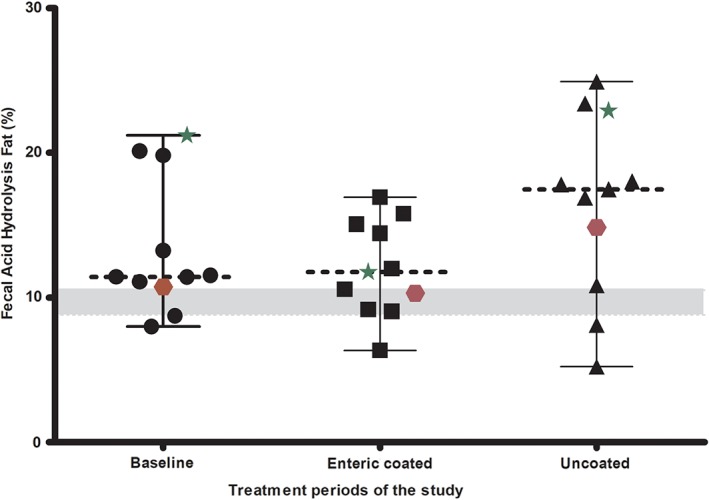
Percentage AH fecal fat in the 11 dogs during the baseline (enrollment) and 2 periods of enzyme replacement with enteric coated and uncoated treatments. The star and hexagon points represent dogs with EPI who had no previous pancreatic enzyme supplement before enrollment into the study. The dotted line represents the median for that period
3.2. Assessment of other variables
The results of CBC, serum chemistry profile, serum concentrations of cTLI, cobalamin, folate, and MMA, body condition scores, fecal characteristics (dry matter, organic matter, crude protein, and AH fat), and owner assessments during the trial are shown in Tables 1, 2, 3, 4. There were no significant differences among any of the evaluated variables between baseline, treatment with the enteric coated product, and treatment with the uncoated product, except for absolute white cell counts, total serum calcium concentrations, and body condition score. The absolute white cell counts (ie, the sum of neutrophils, lymphocytes, monocytes, and eosinophils) and the calcium concentrations were within the reference intervals established for the laboratory and did not prompt further investigation. The body condition score of dogs increased from baseline regardless of treatment group. None of the dogs that completed the study were supplemented with cobalamin.
Table 1.
Comparison of hematological variables
| Baseline | Treatment A enteric coated | Treatment B uncoated | |||
|---|---|---|---|---|---|
| Variable | Reference interval | Median (min, max) | Median (min, max) | Median (min, max) | P valuea |
| Total WBC | 6000‐17 000/μL | 8980 (6700, 14 900) | 8520 (5710, 16 100) | 9060 (5330, 12 500) | .70 |
| Neutrophils | 3000‐11 500/μL | 5298a (3216, 11 952) | 4565b (3335, 9982) | 5255b (2825, 8750) | <.001 |
| Lymphocytes | 1000–4800/μL | 1886a (1474, 3386) | 2279a (949, 3864) | 1964b (1469, 3430) | <.001 |
| Monocytes | 150–1350/μL | 508a (144, 1586) | 472a (119, 907) | 394b (85, 1250) | <.001 |
| Eosinophils | 100–1250/μL | 915a (144, 1467) | 788a (343, 2093) | 634b (216, 1459) | <.001 |
| Red blood cells | 5.5–8.5 M/μL | 6.69 (4.45, 8.01) | 7.13 (4.68, 8.21) | 7.00 (5.68, 7.63) | .44 |
| Hemoglobin | 12–18 g/dL | 15.1 (10.1, 19.3) | 17.1 (13.5, 19.5) | 16.6 (12.6, 19.3) | .36 |
| HCT | 32%‐55% | 47.3 (30.8, 57.9) | 51.4 (36.0, 60.6) | 49.6 (40.0, 53.5) | .16 |
| MCV | 60–77 fL | 70.3 (64.7, 76.9) | 72.6 (65.4, 82.0) | 70.4 (67.2, 75.9) | .70 |
| MCH | 19.5–24.5 pg | 23.5 (21.0, 25.4) | 24.3 (21.3, 28.8) | 24.0 (22.2, 27.5) | .53 |
| MCHC | 32–36 g/dL | 33.2 (31.2, 34.8) | 33.3 (29.3, 37.5) | 33.5 (31.5, 41.0) | .81 |
| Platelet estimate | 200–500 K/μL | 286 (14, 574) | 217 (146, 389) | 279 (150, 384) | .15 |
Based on Friedman tests. Medians with superscripts in common are significantly different (P < .05) based on post‐hoc pairwise Wilcoxon signed‐rank tests with Bonferroni correction of P values.
Table 2.
Comparison of serum chemistry and other serum variables
| Baseline | Treatment A enteric coated | Treatment B uncoated | |||
|---|---|---|---|---|---|
| Variable | Median (min, max) | Median (min, max) | Median (min, max) | P valuea | |
| Glucose | 60–120 mg/dL | 92 (61, 118) | 96 (72, 113) | 95 (78, 105) | .34 |
| BUN (Blood urea nitrogen) | 8–30 mg/dL | 13 (6, 23) | 14 (9, 22) | 14 (8, 21) | .84 |
| Creatinine | 0.5‐1.4 mg/dL | 0.8 (0.5, 1.0) | 0.8 (0.6, 1.1) | 0.9 (0.7, 1.3) | .20 |
| Calcium | 9.3–11.8 mg/dL | 9.7a (9.0, 10.5) | 10.2b (9.9, 10.6) | 10.1a,b (9.7, 10.9) | .03 |
| Phosphorous | 2.9‐6.2 mg/dL | 3.2 (2.7, 5.2) | 3.7 (3.3, 4.8) | 3.7 (3.0, 5.5) | .07 |
| Albumin | 2.4‐3.6 g/dL | 2.9 (2.4, 3.4) | 3.1 (2.8, 3.3) | 3.0 (2.7, 3.3) | .16 |
| Total protein | 5.7‐7.8 g/dL | 5.5 (4.8, 6.5) | 5.8 (5.2, 6.4) | 5.5 (5.1, 6.6) | .16 |
| Total bilirubin | 0‐0.4 mg/dL | 0.1 (0.0, 0.3) | 0.1 (0.0, 0.3) | 0.2 (0.0, 0.2) | .25 |
| ALP (alkaline phosphatase) | 12–122 U/L | 56 (23, 224) | 63 (17, 143) | 55 (24, 118) | .91 |
| ALT (alanine transaminase) | 13–79 U/L | 56 (33, 189) | 45 (29, 138) | 42 (30, 81) | .37 |
| AST (aspartate aminotransferase) | 13–52 U/L | 35 (28, 48) | 30 (12, 47) | 29 (22, 47) | .16 |
| GGT (gamma‐glutamyl transferase) | 0–25 U/L | 5 (2, 7) | 4 (2, 14) | 4 (1, 21) | .30 |
| Cholesterol | 124–335 mg/dL | 178 (124, 326) | 227 (184, 344) | 213 (161, 281) | .50 |
| Triglycerides | 26–108 mg/dL | 52 (40, 411) | 69 (28, 140) | 61 (37, 148) | .91 |
| Globulin | 1.5‐4.5 g/dL | 2.6 (2.0, 3.1) | 2.9 (2.0, 3.3) | 2.6 (2.1, 3.3) | .40 |
| Cobalamin | 251–908 ng/L | 499 (404, 1000) | 558 (316, 1000) | 461 (291, 1000) | .76 |
| Folate | 7.7–24.4 μg/L | 20.4 (9.3, 72.1) | 17.4 (5.4, 64.7) | 17.1 (8.0, 72.1) | .70 |
| Trypsin‐Like Immunoreactivity (TLI) | 5.7–45.2 μg/L | 0.5 (0.2, 2.4) | 0.6 (0.1, 1.4) | 0.4 (0.1, 2.2) | .91 |
| MMA | 415–1193 nmol/L | 741 (566, 2376) | 870 (588, 1550) | 847 (512, 7914) | .18 |
Based on Friedman tests. Medians with superscripts in common are significantly different (P < .05) based on post‐hoc pairwise Wilcoxon rank sum tests with Bonferroni correction of P values.
Table 3.
Comparison of quantitative outcomes; dry matter, organic matter, crude protein, and AH fat
| Baseline | Treatment A enteric coated | Treatment B uncoated | ||
|---|---|---|---|---|
| Variable | Median (min, max) | Median (min, max) | Median (min, max) | P valuea |
| %DM (57°C) | 31.4 (27.5, 35.8) | 31.8 (26.5, 74.6) | 31.9 (27.8, 49.6) | .70 |
| %DM (105°C) | 96.3 (94.9, 97.0) | 96.1 (94.8, 97.2) | 96.0 (94.7, 96.9) | .91 |
| %DM (Abs) | 29.8 (26.5, 34.7) | 30.8 (25.2, 72.1) | 30.9 (26.7, 48.1) | .76 |
| % Ash (DMB) | 14.0 (10.1, 27.1) | 15.7 (9.2, 28.7) | 16.8 (9.3, 25.8) | .53 |
| %OM (DMB) | 86.0 (72.9, 89.9) | 84.3 (71.3, 90.8) | 83.2 (74.2, 90.7) | .53 |
| % CP (DMB) | 34.9 (24.5, 42.5) | 39.4 (26.4, 50.0) | 36.8 (26.1, 42.4) | .23 |
| % AH fat (DMB) | 11.4 (8.0, 21.2) | 11.8 (6.4, 17.0) | 17.5 (5.2, 24.9) | .15 |
| Body weight | 56.2 (12.2, 77.5) | 60.7 (12.7, 95.0) | 62.0 (12.5, 94.1) | .61 |
| BCS | 4a (2, 6) | 5a (3, 7) | 5a (3, 7) | .032 |
Abbreviations: AH fat, acid hydrolysis fat; BCS, body condition score; CP, crude protein; DM, dry matter; DMB, dry matter basis; OM, organic matter.
Based on Friedman tests. Medians with superscripts in common are significantly different (P < .05) based on post‐hoc pairwise Wilcoxon rank sum tests with Bonferroni correction of P values.
Table 4.
Comparison of categorical outcomes based on responses reported by the owners
| Treatment A enteric coated | Treatment B uncoated | ||
|---|---|---|---|
| Variable | Proportion (95% CI) | Proportion (95% CI) | P valuea |
| Attitude (=1) | 0.73 (0.42, 0.93) | 0.91 (0.63, 1.0) | .62 |
| Drinking (=1) | 1.0 (0.76, 1.0) | 0.91 (0.63, 1.0) | 1.0 |
| Defecation frequency (=3) | 0.27 (0.07, 0.58) | 0.55 (0.26, 0.81) | .45 |
| Volume of feces (>0) | 0.18 (0.03, 0.48) | 0.00 (0.00, 0.24) | .48 |
| Consistency of feces (=2) | 0.27 (0.07, 0.58) | 0.27 (0.07, 0.58) | .62 |
| Color of feces (=1) | 0.64 (0.34, 0.87) | 0.73 (0.42, 0.93) | 1.0 |
| Coprophagia (=1) | 0.09 (0.00, 0.37) | 0.00 (0.00, 0.24) | 1.0 |
| Borborygmus (=1) | 0.00 (0.00, 0.24) | 0.00 (0.00, 0.24) | 1.0 |
| Flatulence (=1) | 0.27 (0.07, 0.58) | 0.18 (0.03, 0.48) | 1.0 |
Owner response scales.
Attitude ‐ 0 = more quiet or lethargic, 1 = normal, 2 = overly active or playful.
Drinking ‐ 0 = less than normal, 1 = normal, 2 = more than normal.
Defecation frequency ‐ 0 = zero, 1 = one, 2 = two, 3 = three, 4 = four, 5 = five or more.
Volume of feces ‐ 0 = normal, 1 = copious, 2 = very copious.
Consistency of feces ‐ 0 = hard, 1 = normal, 2 = pulpy, 3 = loose, watery.
Color of feces‐ 0 = darker, than normal, 1 = normal, 2 = gray, 3 = yellowish.
Coprophagia ‐ 0 = no, 1 = yes.
Borborygmus ‐ 0 = no, 1 = some, 2 = frequent.
Flatulence‐ 0 = no, 1 = some, 2 = frequent.
Abbreviation: CI, confidence interval.
Based on McNemar's chi square test comparing the 2 treatments.
3.3. Compliance
The median (range) percentage of capsules returned was 8.7% (0%, 26%) and 6.0% (0%, 41%) for the enteric coated formulation and the uncoated pancreatic enzyme replacement product, respectively (P = .878). Of the 11 dogs, 6 wanted the uncoated product as their 6 week incentive, and 5 wanted the enteric coated product for their 6 week incentive.
3.4. Adverse events
Adverse events were reported in 5 enrolled dogs at 6 different events. Only 1 of these 5 dogs went on to complete the study. Most events were nonspecific signs of gastrointestinal disease and the owners, veterinarians, or both decided to remove the dog from the study. However, 1 dog had a seizure episode, which would appear to be coincidental and unrelated to the enzyme supplementation. As the episodes were not life threatening and probably unrelated, the blinding was not broken at any time. None of the dogs demonstrated any evidence of gingival or oral bleeding for the duration of the study.
4. DISCUSSION
The objective of the study was to assess the clinical efficacy of a new enteric coated micro‐pelleted formulation of a pancreatic enzyme replacement product compared to a commercially available uncoated pancreatic enzyme replacement product. In our study, the cross over design employed and the use of fecal characteristics, particularly percent fecal fat as the primary outcome measure of efficacy of enzyme replacement treatment, provided more comparable study groups as individual dogs might respond differently to enzyme supplementation in general. However, we were unable to detect any differences between coated and uncoated enzyme preparations in our study. This might be because of the low power, and large confidence intervals observed, and hence larger studies are necessary to test this finding.
Studies done in the United Kingdom reported mixed results regarding the efficacy of enteric‐coated versus uncoated enzyme products in dogs with EPI.4, 5 An earlier observational study showed that 2/3rd of veterinarians prescribed nonenteric coated products and dogs had a better response to this treatment.4 Another more recent questionnaire‐based study from the United Kingdom showed that about 2/3rd of veterinarians now used enteric coated products.5 Authors of a previous randomized clinical trial concluded that dogs receiving an enteric coated pancreatic enzyme supplement responded better than those receiving an identical uncoated product, with respect to magnitude of weight gain. However, their primary outcome was change in body weight. Our study had 2 treatment groups and dogs were randomized to receive 1 of 2 enzyme replacement products either the test (coated) or the control (uncoated) product.8
We considered it inappropriate and unethical to discontinue enzyme replacement treatment before starting the study on dogs already receiving enzyme replacement treatment, hence previous enzyme replacement treatment was not a reason for exclusion. For our study, we did not employ a washout period between the 2 products or employ a placebo control, because omission of enzyme replacement treatment in dogs with EPI would lead to compromised health, nutritional status, and significant weight loss. Instead, an uncoated veterinary product was used as the comparison treatment. This decision not to use a placebo treatment or washout period is consistent with clinical trials in humans where another enzyme replacement treatment was used as the standard of comparison.12, 13, 14 Based on the fact that pancreatic enzymes have to be given with every meal, we considered it unlikely for a residual effect to be present after at least 19 days of treatment with the enteric coated or the uncoated product, at which point fecal samples were collected for laboratory analysis.
The goal of our study was to use a time frame that would help us evaluate the efficacy reliably. Studies in the human literature, where cross over design using different pancreatic enzyme supplements for cystic fibrosis have employed 1,12 2,13, 14 or 413 weeks duration. Dietary trials in dogs with EPI15 have also used a 3 week period, hence we decided to use a 3 week period for each treatment arm in our study.
Fecal composition, including the microbiome16 remains altered in dogs with naturally occurring EPI even after enzyme supplementation,17 and fecal fat content is believed to be the most difficult variable to return to normal in people treated for EPI, hence we used percent fecal fat as the primary outcome in our study. While no significant difference was observed in the percent fecal fat between the 2 treatments, this could be a result of small number of dogs that went on to complete the study or an actual lack of effect. In humans with EPI who received enzyme replacement treatment an improvement of the coefficient of fat malabsorption was observed. However, fat malabsorption did not normalize despite enzyme supplementation.18 The dogs were not all being fed the same diet and this could have led to a variation in the fecal fat percentage. However, this effect was probably minimal in our study because of the crossover design and the fact that dogs were required to stay on the same diet for the entire duration of the study.
The secondary measures of assessment, namely clinical signs, might have improved as reported by the dogs’ owners but they were not significantly different between the enteric coated and the uncoated product. A similar observation was made in the earlier clinical trial.8
As expected, the body condition score was improved in dogs during both treatment periods compared to baseline similar to what was reported in earlier studies.8
The change in serum calcium concentration was significant from baseline. However, serum calcium concentrations remained within the reference interval established for the laboratory. The reference interval for the laboratory for serum calcium in dogs is 9.3–11.8 mg/dL. To the best of our knowledge, this finding has not previously been investigated or reported in dogs with EPI. Decreased serum concentrations of vitamin D metabolites and a low bone mass occur in human patients with chronic pancreatitis and steatorrhea; however, these individuals had normal serum calcium concentrations.19
While no adverse events were reported in the previous study comparing enteric coated and uncoated pancreatic enzyme replacement products in dogs,8 we did observe 1 dog to have self‐limiting nonspecific signs including restlessness, dull mentation, lack of appetite, and possibly a tender abdomen that resolved with time. Another dog failed to respond to either the enteric coated or the uncoated product. Overall, no major adverse events were recorded during the course of the study. Long term and larger studies are necessary to identify less common events such as gingival bleeding.
In addition to the dog that developed seizures after starting the enzyme supplementation, which was probably coincidental, and the dog that did poorly on both enzyme supplements from consideration, 2 dogs while on the uncoated product were removed compared with 1 dog while on the enteric coated supplement. However, it is difficult to infer too much from this finding as owner and veterinarian opinions might have influenced which of the dogs were removed from the study, and these opinions might not have been objective.
Meticulous adherence to the GCP guidelines is cumbersome; however, it adds to the validity of the results. The major limitation of our study was the small number of dogs that completed the study and the resulting low power of the statistical test comparing the %AH fecal fat between groups. The study was completed using expired uncoated enzyme supplement so the lipase activity was measured 2 weeks after the trial was finished to ensure sufficient lipase activity was present despite expiration. Ideally, a single batch of both coated and uncoated enzyme supplements should have been used for the entire study. Another limitation of the study was that it was only designed to assess short term outcomes. Blinding might have been compromised since the products were grossly different in appearance and there was a difference in the number of capsules required per meal. Similar to the earlier trial,8 we experienced study withdrawals that interfered with our ability to obtain the desired number of dogs completing the study. At the time of designing the study, the other clinical trial of an enteric coated pancreatic enzyme supplement8 had not been published and no dosage guidelines for the test product were available. Therefore, our dosages for the enteric coated product were based on calculation of the metabolizable energy, the range of daily energy requirements from the National Research Council for average adult dogs, and manufacturer recommendations for the enzyme replacement products. No changes to number of capsules per meal were permitted, hence our study was not designed to identify an increased requirement of enteric coated enzymes over time, which has been reported in the earlier clinical trial.8 Longer duration and larger scale trials are needed to establish if this dose regimen is appropriate, and if the dosages of enteric coated supplements have to be increased with time, or can be tapered off to the “least effective possible dose” similar to uncoated preparations.
Based on the number of capsules returned, 3 dogs could have been excluded based on a discrepancy in the number of capsules sent and returned (difference exceeded 20%). However, all 11 dogs were retained in the analysis after approval from the study monitor. A restricted statistical analysis based only on the 8 dogs that completed the study with adequate compliance did not change the reported findings. Given the cross‐over design of the study and the fact that in 2 of the 3 dogs where many capsules remained unaccounted for happened during both periods, this effect is expected to be minimal. For the 3rd dog, there was only a minimal increase of unaccounted capsules (21.8% versus 20.0%) than the typical cutoff of 20%.
Within limitations, our study did not identify important differences between the 2 evaluated formulations of pancreatic enzymes at the recommended dosages. However, the absence of a clinical difference between the pancreatic enzymes cannot be concluded, as the study was too small to have adequate power. Both preparations were well tolerated with minimal adverse effects. However, larger clinical trials are needed to confirm these findings and to determine the optimal dose for enteric coated micro‐pelleted enzyme products.
Off‐Label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Institutional Animal Care and Use Committee (IACUC) or Other Approval Declaration
Authors declare no IACUC or other approval was needed.
ConFLict of Interest
Our study was funded by Dechra Veterinary Products, the manufacturer of the enteric coated micropelleted formulation of a pancreatic enzyme supplement tested in our study. Drs Parambeth, Steiner, Suchdolski, and Lidbury are affiliated with the Gastrointestinal Laboratory, Texas A&M University that offers measurement of canine trypsin‐like immunoreactivity on a fee‐for‐service basis.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors acknowledge the referring veterinarians, their clinical staff, and the owners of all dogs who participated in the study. The study was funded by Eurovet Animal Health BV (#11.001). Dechra Veterinary Products is a trading business of Eurovet Animal Health BV, Handelsweg 25, 5531 AE Bladel, The Netherlands. This work was completed at the Gastrointestinal Laboratory, Department of Small Animal Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, Texas.
Parambeth JC, Fosgate GT, Suchodolski JS, Lidbury JA, Steiner JM. Randomized placebo controlled clinical trial of an enteric coated micro‐pelleted formulation of a pancreatic enzyme supplement in dogs with exocrine pancreatic insufficiency. J Vet Intern Med. 2018;32:1591–1599. 10.1111/jvim.15235
Funding information Eurovet Animal Health BV, Grant/Award Number: 11.001
REFERENCES
- 1. Westermarck E, Wiberg M. Exocrine pancreatic insufficiency in the dog: Historical background, diagnosis, and treatment. Top Companion Anim Med. 2012;27:96–103. [DOI] [PubMed] [Google Scholar]
- 2. Williams DA, Batt RM. Sensitivity and specificity of radioimmunoassay of serum trypsin‐like immunoreactivity for the diagnosis of canine exocrine pancreatic insufficiency. J Am Vet Med Assoc. 1988;192:195–201. [PubMed] [Google Scholar]
- 3. Wiberg ME, Lautala HM, Westermarck E. Response to long‐term enzyme replacement treatment in dogs with exocrine pancreatic insufficiency. J Am Vet Med Assoc. 1998;213:86–90. [PubMed] [Google Scholar]
- 4. Hall EJ, Bond PM, McLean C, Batt RM, McLean L. A survey of the diagnosis and treatment of canine exocrine pancreatic insufficiency. J Small Anim Pract. 1991;32:613–619. [Google Scholar]
- 5. Batchelor DJ, Noble PJ, Cripps PJ, et al. Breed associations for canine exocrine pancreatic insufficiency. J Vet Intern Med. 2007;21:207–214. [DOI] [PubMed] [Google Scholar]
- 6. Rutz GM, Steiner JM, Williams DA. Oral bleeding associated with pancreatic enzyme supplementation in three dogs with exocrine pancreatic insufficiency. J Am Vet Med Assoc. 2002;221:1716–1718. [DOI] [PubMed] [Google Scholar]
- 7. Snead E. Oral ulceration and bleeding associated with pancreatic enzyme supplementation in a German shepherd with pancreatic acinar atrophy. Can Vet J. 2006;47:579–582. [PMC free article] [PubMed] [Google Scholar]
- 8. Mas A, Noble PJ, Cripps PJ, Batchelor DJ, Graham P, German AJ. A blinded randomised controlled trial to determine the effect of enteric coating on enzyme treatment for canine exocrine pancreatic efficiency. BMC Vet Res. 2012;8:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pairent FW, Trapnell JE, Howard JM. The treatment of pancreatic exocrine insufficiency. III. The effects of pancreatic ductal ligation and oral pancreatic enzyme supplements on fecal lipid excretion in the dog. Ann Surg. 1969;170:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarner M. Treatment of pancreatic exocrine deficiency. World J Surg. 2003;27:1192–1195. [DOI] [PubMed] [Google Scholar]
- 11. Merritt AM, Burrows CF, Cowgill L, et al. Fecal fat and trypsin in dogs fed a meat‐base or cereal‐base diet. J Am Vet Med Assoc 1979;174:59–51. [PubMed] [Google Scholar]
- 12. Wooldridge JL, Heubi JE, Amaro‐Galvez R, et al. EUR‐1008 pancreatic enzyme replacement is safe and effective in patients with cystic fibrosis and pancreatic insufficiency. J Cyst Fibros. 2009;8:405–417. [DOI] [PubMed] [Google Scholar]
- 13. Patchell CJ, Desai M, Weller PH, et al. Creon® 10000 Minimicrospheres™ vs. Creon® 8000 microspheres—an open randomised crossover preference study. J Cyst Fibros. 2002;1:287–291. [DOI] [PubMed] [Google Scholar]
- 14. Robinson PJ, Olinsky A, Smith AL, Chitravanshi SB. High compared with standard dose lipase pancreatic supplement. Arch Dis Child. 1989;64:143–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westermarck E, Wiberg ME. Effects of diet on clinical signs of exocrine pancreatic insufficiency in dogs. J Am Vet Med Assoc 2006;228(2):225–229. [DOI] [PubMed] [Google Scholar]
- 16. Isaiah A, Parambeth JC, Steiner JM, Lidbury JA, Suchodolski JS. The fecal microbiome of dogs with exocrine pancreatic insufficiency. Anaerobe. 2017;45:50–58. [DOI] [PubMed] [Google Scholar]
- 17. Parambeth JC, Isaiah A, Lidbury JA, Suchodolski JS, Steiner JM. Fecal composition in dogs with naturally occurring exocrine pancreatic insufficiency. J Vet Intern Med. 2017;31:1293. [Google Scholar]
- 18. Waljee AK, Dimagno MJ, Wu BU, Schoenfeld PS, Conwell DL. Systematic review: Pancreatic enzyme treatment of malabsorption associated with chronic pancreatitis. Aliment Pharmacol Ther. 2009;29:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haaber AB, Rosenfalck AM, Hansen B, Hilsted J, Larsen S. Bone mineral metabolism, bone mineral density, and body composition in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Int J Pancreatol. 2000;27:21–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


