Abstract
Background
The inward rectifier inhibitor pentamidine analogue 6 (PA‐6) is effective in cardioversion of goats with persistent rapid pacing induced atrial fibrillation (AF) and is not proarrhythmic in dogs with experimental chronic 3rd‐degree AV block. Efficacy and safety in the clinical setting are unknown.
Hypothesis
That PA‐6 would be effective in converting AF to sinus rhythm (SR) in dogs with naturally occurring AF, without the presence of overt adverse effects.
Animals
Ten client‐owned large and giant breed dogs.
Methods
Animals with persistent or permanent AF were recruited for our prospective study. PA‐6 was administered IV as a bolus of 2.5 mg/kg 10 min−1 followed by a maintenance infusion of 0.04 mg/kg min−1 for a maximum of 50 minutes in conscious dogs. Standard 6 lead limb ECG was recorded during the infusion. Visible and audible signs of adverse effects were scored during the entire procedure.
Results
PA‐6 did not induce changes in QRS duration (54.7 ± 4.6 versus 56.7 ± 6.1 ms, P = .42), QTc interval (241.1 ± 19.5 versus 258.7 ± 19.8 ms, P = .061) or RR interval (363.4 ± 84.6 versus 440.8 ± 96.3 ms, P = .072) at the end of the bolus. No cardioversion to SR was observed in any dog. Three dogs displayed no adverse effects. Five dogs had premature ventricular depolarizations during PA‐6 infusion on the ECG. Respiratory distress with laryngeal stridor, subtle muscle twitching, and mild generalized muscular weakness were noncardiac adverse effects observed in 5 dogs. Adverse effects resolved spontaneously.
Conclusions and Clinical importance
Chronic naturally occurring AF in large and giant breed dogs could not be cardioverted to SR by PA‐6.
Keywords: adverse effects, arrhythmia, inward rectifier current, plasma, respiratory distress, stridor
Abbreviations
- AF
atrial fibrillation
- cAVB
chronic 3rd degree atrial‐ventricular block
- DCM
dilated cardiomyopathy
- IK1
potassium inward rectifier current
- IKACh
acetylcholine sensitive rectifier current
- PA‐6
pentamidine analogue 6
- QTc
heart rate corrected QT interval of the ECG
- SR
sinus rhythm
1. INTRODUCTION
Atrial fibrillation (AF) is one of the most prevalent arrhythmia in dogs,1, 2, 3, 4 affecting mainly large and giant breeds, and associates with high case fatality rates.1, 2, 3, 4 Dogs with lone AF have a better survival than do those with concomitant heart disease,3, 5 but the arrhythmia is strongly associated with dilated cardiomyopathy.6 Currently, several drugs and procedures exist to treat AF in dogs.7, 8, 9 Treatment focusses on controlling ventricular response rate rather than on rhythm control.4, 6 Unfortunately, the usefulness of antiarrhythmic drugs is limited by both modest antiarrhythmic efficacy and detrimental adverse effects.10 For example, amiodarone treatment in 17 dogs with AF resulted in cardioversion and maintenance of sinus rhythm (SR) in 6 dogs, whereas important adverse effects including bradycardia and increased serum activity of liver‐derived enzymes were shown in 1 and 4 animals, respectively.11
The inward rectifier potassium current (I K1) plays an important role to maintain the resting membrane potential and it contributes to phase 3 repolarization.12 In a dog experimental model of AF, I K1 was upregulated in the atria. Hence, I K1 current inhibitors have been suggested as a new antiarrhythmic option for rhythm control in AF treatment.13 A specific and efficient I K1 inhibitor, pentamidine analogue 6 (PA‐6) was developed based on the antiprotozoal drug pentamidine.14 PA‐6 prolonged action potential duration without proarrhythmia in isolated canine ventricular cardiomyocytes.14 Recently, cardiac safety and anti‐AF efficacy of the PA‐6 was evaluated in vivo.15 Within 20 minutes after the start of PA‐6 administration to anesthetized goats with rapid pacing induced persistent AF, cardioversion to SR occurred in 5 out of 6 AF goats.15 The PA‐6 prolonged AF cycle length (a measure of fibrillatory rate and a surrogate marker for local refractoriness) and decreased AF complexity (number of waves per fibrillatory cycle, wave size, number of breakthrough wave per cycle and fractionation index). To investigate PA‐6 safety, dogs with SR were treated with PA‐6 under general anesthesia resulting in <7% QTc lengthening and no effect on QRS or PQ duration.15 PA‐6 infused in anesthetized dogs with chronic 3rd degree atrioventricular block (cAVB), a sensitive experimental model of drug induced cardiac proarrhythmia,16 induced QT prolongation but no arrhythmias.15 Given the efficacy of PA‐6 to inhibit I K1 channels in vitro, to cardiovert AF to SR in vivo and its safety profile in cAVB dogs, our study aimed to investigate cardioversion in awake dogs with naturally occurring AF.
2. MATERIALS AND METHODS
2.1. Ethics statement
This study was approved by the experimental committee of the authors’ institution (Utrecht University, the Netherlands) and signed owner consent was obtained for all dogs in the investigation.
2.2. Study population, treatment protocol, and analysis
Dogs diagnosed with AF were searched in the clinic's data base and their owners were contacted via the telephone to ask whether they would participate in the study. At the same time, owners who visited the clinic and whose dogs were diagnosed with AF, were also asked whether they would like to participate in the study. In 9 dogs, AF was detected by coincidence in the course of a clinical examination because of complaints like anorexia, hyperthermia, weight loss, otitis externa, neurological signs, during presurgical examination, or routine echocardiography in a predisposed breed. None of these animals displayed signs of AF. One dog showed signs of AF upon initial presentation, having exercise intolerance, ascites and lethargy. To enroll in this pilot study, dogs had to show AF on a 6‐lead surface ECG. AF was classified as lone AF or AF with an underlying heart disease based on echocardiographic findings. None of the dogs had undergone earlier attempts of cardioversion. All interventions were performed under awake condition. Continuous bipolar 3‐lead ECG (I, II, III) was recorded by a digital ECG equipment (PC‐ECG 2000, Eickemeyer, Tuttlingen, Germany). ECG electrodes were attached to the animal's skin with adhesive sticks on a shaved region on the palmar and plantar surface of the distal limbs.17
Pentamidine analogue 6 (PA‐6) was dissolved in distilled water, filter sterilized, at the final concentration of 10 mg/mL. The PA‐6 (2.5 mg/kg) was infused (via an IV secured cannula in a peripheral limb vein), using an infusion pump (Pilote Delta NL, Fresenius Vial, Brezin, France) over 10 minutes as a bolus, which was immediately followed by a maintenance infusion of 0.04 mg/kg for 5–50 minutes. The duration of maintenance infusion depended on the presence of adverse clinical response in the dogs. From dogs 6–10, blood samples (3 mL each collection) were collected in lithium‐heparin tubes at baseline (ie, before PA‐6 infusion) and after PA‐6 bolus administration (t = 10 minutes), and stored on ice immediately. Tubes were centrifuged at 2000g for 15 minutes at 4°C. Plasma samples were stored at −80°C until analysis for PA‐6 content was performed by the use of ultra‐performance liquid chromatography–tandem mass spectrometry (UPLC‐MS/MS) as described elsewhere15 with 2 modifications (injection volume increased from 2 to 5 μL and the calibration curve ranged from 0.005 to 5 μM).
Surface ECG variables (RR‐, QT‐, QRS‐intervals) were determined in lead II on the digitally recorded ECGs using (PC‐ECG 2000, Eickemeyer, Tuttlingen, Germany). ECG variables were quantified at baseline, at the beginning and at the end of the bolus, and the end of the maintenance infusion. For each time point, RR‐, QT‐, and QRS‐intervals were averaged from 30 successive beats. The heart rate‐corrected QT‐interval (QTC) was calculated by Van de Water's formula QTC = QT‐0.087* (RR‐1000).18
2.3. Statistics
All statistical analyses were carried out by a commercially available software package (SPSS version 21). All values are expressed as mean ± standard deviation (SD). Statistical differences among groups were evaluated using one‐way ANOVA with Tukey's HSD post‐hoc or Bonferroni test. Differences were considered as statistically significant if P < .05.
3. RESULTS
Ten client owned large‐ and giant‐breed dogs (8 males and 2 females; 56.7 ± 16.6 kg; 7.4 ± 2.6 year) presenting with naturally occurring persistent or permanent AF were enrolled in this study between May 2015 and December 2015. Based upon clinical examination, the mean duration between initial AF detection and PA‐6 administration was 5.8 ± 6.5 month (range 1–19 month). Five dogs had no comorbidities, 3 dogs were diagnosed with dilated cardiomyopathy, 1 with mitral dysplasia and with tricuspid dysplasia. Four dogs received no prior medication, whereas Digoxin (6 dogs), Pimobendan (4), Furosemide (2), Benazepril (2), Diltiazem (1), and Meloxicam (1) were given in various combinations to the remaining 6 dogs.
After PA‐6 administration (2.5 mg/kg/10 minutes), no significant changes in QRS duration 54.7 ± 4.6 versus 56.7 ± 6.1 ms, P = .42), QTc interval (241.1 ± 19.5 versus 258.7 ± 19.8 ms, P = .061), or RR interval (363.4 ± 84.6 ms versus 440.8 ± 96.3, P = .072) were observed (Figure 1). There was a slight increase in QTc‐interval at the end of infusion (241.1 ± 19.5 versus 260.4 ± 21.0 ms, P = .047). No dogs cardioverted to SR during PA‐6 administration at the end of bolus, nor at the end of the maintenance infusion time.
Figure 1.
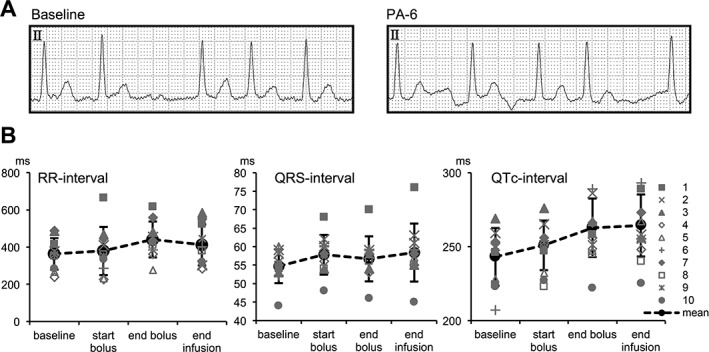
Electrophysiological variables in AF dogs (n = 10) at baseline, at the start and at the end of PA‐6 bolus (2.5 mg/kg/10 min) infusion, and at the end of PA‐6 maintenance infusion (0.04 mg/kg/min). RR‐, QRS‐, and QTc‐intervals are depicted in ms for individual animals (1–10; gray symbols) and as the mean ± SD (black symbols and black dotted line)
Because of adverse effects including generalized muscle weakness and respiratory distress during the maintenance infusion in the 2nd dog, the maintenance time (original set for 50 minutes) was reduced in the remaining 8 dogs (dog #2: 40 minutes; #3: 20 minutes; #4,5: 20 minutes; #6–10: 5 minutes). Premature ventricular depolarizations were observed on the ECG in 5 of the 10 dogs. Laryngeal stridor (2 dogs) and pharyngeal stertor (1 dog) were heard and was accompanied by severe respiratory distress in 1 dog. Muscular weakness was observed in 2 dogs. Three dogs did not present adverse effects. All adverse effects disappeared spontaneously and completely within several hours. At follow up, 5 dogs were doing well (#1 at 23 months follow up; #2 at 9 months; #6 at 10 days; #9 at 19 months; #10 at 13 months), dog #3 developed shock and diarrhea 17 days after PA‐6 infusion, dog #4 was euthanized 6 months after PA‐6 infusion because of anorexia and lethargy. No follow up information was available for dogs #5, #7, and #8.
At the study design a total number of 15 dogs were planned to enroll. Because of the absence of positive effects and the occurrence of adverse effects, the investigators did not find ethical justification to enroll further dogs after the 10th.
Free plasma concentrations of PA‐6 at completion of the bolus infusion were determined in 6 dogs (#5‐#10) and varied between 0.41 and 2.53 μM (average 1.46 ± 0.89 μM). No correlation between plasma concentrations and age (R2 = 0.001, P = .95), bodyweight (R2 = 0.007, P = .87), or differences in average plasma concentration and use of comedication (P = .37) or the presence of adverse effects (P = .55) could be found. A weak positive correlation between PA‐6 plasma concentration and QTc‐prolongation was observed (R2 = 0.37, P = .20).
4. DISCUSSION
This study focused on PA‐6 application in dogs with naturally occurring chronic AF. Previous in vivo were performed under general anesthesia, and therefore information on PA‐6 application in awake animals was lacking. The main finding of the present study is that cardioversion in response to PA‐6 was absent in all of the 10 enrolled dogs, in contrast to anesthetized goats with rapid pacing induced AF.15 Furthermore, awake dogs showed adverse effects including ectopic cardiac activity, muscle weakness, and some of them suffered from severe respiratory distress during treatment. ECG analysis has been performed to evaluate electrophysiological effects of PA‐6. In contrast to anesthetized experimental cAVB dogs,15 in our study no QTc lengthening was observed immediately after bolus administration (P = .061); however, QTc duration was increased (P = .047) at the end of PA‐6 infusion. QRS duration and RR interval at the end of bolus were not affected. Our ECG recordings under awake conditions did not allow us to extract data for analysis of atrial fibrillatory variables. We therefore cannot exclude changes in atrial electrophysiology upon PA‐6 administration, although apparently insufficient to a degree that would result in cardioversion.
There are 4 main differences between our study and the previous study in goats15 that might explain the lack of cardioversion in the dogs. Firstly, species difference might cause different responses. Between distinct species, different pathophysiological mechanisms leading to AF can exist that could require different pharmacological approach.19 Whereas in human AF, disease progression associates with increased I K1 density,20 no published data are available whether the same applies to the goat model of rapid atrial pacing induced AF. However, increased expression of KIR2.1 mRNA and protein (underlying cardiac I K1) in a dog model of atrial tachypacing induced AF was demonstrated,21 providing evidence that in canines the I K1 channels can be a plausible target for AF pharmacotherapy. It might be worthwhile to assess I K1 function and underlying KIR2.x expression in atria from healthy dogs and dogs with AF to further validate the potential of I K1 inhibition as a treatment for canine AF. In addition, comparison between dogs with lone AF, DCM associated AF and AF resulting from atrial dilatation or other etiology might be informative in specifying the potential benefits of I K1 inhibition in different forms of canine AF.
Secondly, the absence of anesthesia in our study, in contrast to the in vivo goat study, might affect the response rate to PA‐6. Besides a role of I K1 in AF, also constitutive I KACh activity has been associated with AF in humans22 and was subsequently suggested as a potential target in AF treatment.23 Indeed, I KACh inhibition in dogs with rapid atrial pacing induced AF results in prolongation of the effective refractory period and cardioversion to SR.24 Currently, there is no evidence that PA‐6 inhibits I KACh,15 but previous studies reported that I KACh action is reduced by some anesthetic agents.25, 26 Under awake condition, the activity of I KACh is higher and thereby counteracts the blocking effect of I K1 compared with conditions under anesthesia. For future studies, a cotreatment of I K1 and IKACh inhibitors could be more effective to restore SR.
Thirdly, structural atrial remodeling can affect response rate. Atrial structural remodeling is present in diverse canine models of AF.27, 28 An inverse relationship between the chronicity of AF and the duration of SR after successful transthoracic cardioversion occurs in dogs with naturally occurring AF.29 Furthermore, the presence of underlying heart disease, for example, dilated cardiomyopathy or degenerative mitral valve disease, had a negative effect on the maintenance of SR.7, 29 Although underlying heart disease had no effect on electrical cardioversion,7 its efficacy is difficult to compare with pharmacological cardioversion. Previous studies in goats report a decrease in efficacy of some class IC and III anti‐arrhythmic drugs during AF progression in the goat model.30, 31 The cardioversion efficacy of flecainide and cibenzoline gradually declined from 60 and 80% after 1 week to 17 and 63% after 16 weeks of AF, respectively.31 In another study, SR could be restored in 80% of the goats after 1 month of AF by dofetilide and AVE0118.30 However, the efficacy of cardioversion decreased to 50% after 3 months and reached 0% efficacy from month 4 of AF onwards.30 In a recent study, goats with induced persistent AF for 3 weeks, PA‐6 treatment could restore SR.15 In human AF patients, which are likely to resemble dogs even better than experimental animals with pacing induced AF, duration of AF inversely correlates with efficacy of pharmacological cardioversion too. For example, cardioversion efficacy of flecainide drops from 86 to 22% for patients in AF for less or more than 10 days, respectively.32 Similarly, cardioversion efficacy of vernakalant decreased significantly for patients in AF for 8–45 days (11.6%) compared with those in AF for <7 days (50.9%).33 Qualitative similar observations were made for amiodarone (51.7%, <12 months in AF versus 5.6%, >12 months in AF),34 Ibutilide (46%, <7 days in AF versus 18%, ≥7 days in AF),35 and propofenone (74%, <2 weeks in AF versus 21%, >2 weeks in AF).36 In dogs the onset of AF is rarely known.4 Also in dogs treated here, the time point of AF onset was unclear and time between AF detection and PA‐6 application was at least 3 weeks. For this reason, we hypothesize that chronic atrial remodeling processes, including structural changes, might have contributed to the lack of efficacy for PA‐6 to promote successful cardioversion.
Finally, the free plasma concentrations of PA‐6 might be insufficient to cardiovert the animals to SR. On average, lower plasma levels were achieved in the currently studied dogs than in the dogs of the previous experimental study (1.46 μM ± 0.87 versus 5.54 ± 0.87 μM, P < .01),15 although an identical bolus infusion dosing scheme was used. In the previous experimental study, dogs were anesthetized with IV pentobarbital and inhaled isoflurane.15 In dogs, pentobarbital increases the fibrinogen receptor antagonist L‐734,217 plasma concentrations by ∼2‐fold immediately after bolus infusion compared with awake dogs,37 whereas isoflurane increases verapamil plasma levels ∼3‐fold after a 10 minutes infusion period compared with awake animals.38 Although not all drugs display increased plasma concentrations upon anesthesia,39 the absence of anesthesia in our investigation may cause the lower PA‐6 plasma levels observed here compared with the previous experimental study.15 Furthermore, we observed high variability of PA‐6 plasma levels in contrast to the previous study. Obviously, the dogs in the current study had a larger variation in body weight and age than the goats and dogs in the experimental study (56.7 ± 16.6 kg; 7.4 ± 2.6 year) versus 56.7 ± 6.2 kg; 1.8 ± 0.2 year (goats) and 24.3 ± 2.3 kg; 2.6 ± 0.5 years [dogs]), which might have caused the higher variability. Furthermore, 6 of 10 of the currently treated dogs used comedication and 5 had comorbidities, which hinders direct comparison of the groups.
Absence of QRS widening and mild QTc prolongation are in accordance with the previous finding in studies in dogs at SR.15 In that study however, no effect on cardiac cycle length was found in dogs, in contrast to AF goats.
Only 3 dogs did not show any adverse effects. Adverse effects resemble to some extent barium infusion in experimental dogs, but unlike ectopy and respiratory distress, pharyngeal stertor, or laryngeal stridor was not reported specifically upon barium.40 Barium is a potent inhibitor of inward rectifier channels of the KIR family, but not confined to KIR2.x channels only. With respect to ECG analysis, premature ventricular contractions were seen in 5 animals treated with PA‐6 and presented the only arrhythmic events observed. I K1 channels contribute to resting membrane potential stability of the cardiomyocyte and its inhibition might therefore result in ventricular ectopy.41 Respiratory and muscular dysfunction were the other major class of adverse effects observed during PA‐6 treatment. These might be explained by the role of I K1 in skeletal muscle, which includes the respiratory muscles, where I K1 stabilizes the resting membrane potential, its absolute value and contributes to repolarization. Muscle twitch and weakness are likely the result of skeletal muscle I K1 dysfunction.
5. CONCLUSIONS
In awake large and giant dog breeds presenting with naturally occurring chronic AF, PA‐6 lacks efficacy for cardioversion. Furthermore, PA‐6 is not well tolerated in awake conditions and induces solitary premature ventricular depolarizations on ECG and respiratory distress. We propose that subsequent clinical studies for PA‐6 efficacy are being performed in anesthetized and artificially ventilated patients, which might circumvent main adverse effects and potentially allows for increasing dose of PA‐6.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was approved by the experimental committee of the authors' institution and signed owner consent was obtained for all dogs in the investigation.
ACKNOWLEDGMENTS
The authors thank Dr S. Zeemering (Maastricht University) for technical advice. Clinical work was performed at the Department of Clinical Sciences of Companion Animals, Faculty of Veterinary Medicine, Utrecht University, Utrecht, The Netherlands. Drug preparation and data analysis was performed at the Department of Medical Physiology, Division of Heart & Lungs, University Medical Center Utrecht, Utrecht, The Netherlands. Plasma sample analysis was performed at the Department of Pharmaceutical Chemistry, School of Pharmacy, The University of Kansas, Lawrence, KS, USA. This study was supported by an Advances in Veterinary Research (AVR) grant (#AVR‐15‐05) from the department of Clinical Sciences of Companion Animals, Utrecht University, The Netherlands.
Szatmári V, Ji Y, Herwijnen Bianca van, et al. Efficacy of pentamidine analogue 6 in dogs with chronic atrial fibrillation. J Vet Intern Med. 2018;32:1549–1554. 10.1111/jvim.15242
Funding information
Advances in Veterinary Research (AVR), Grant/Award Number: #AVR‐15‐05; The Department of Clinical Sciences of Companion Animals, Utrecht University, The Netherlands
REFERENCES
- 1. Brownlie SE. An electrocardiographic survey of cardiac rhythm in Irish wolfhounds. Vet Rec. 1991;129:470–471. [DOI] [PubMed] [Google Scholar]
- 2. Guglielmini C, Chetboul V, Pietra M, et al. Influence of left atrial enlargement and body weight on the development of atrial fibrillation: retrospective study on 205 dogs. Vet J. 2000;160:235–241. [DOI] [PubMed] [Google Scholar]
- 3. Menaut P, Bélanger MC, Beauchamp G, et al. Atrial fibrillation in dogs with and without structural or functional cardiac disease: a retrospective study of 109 cases. JVet Cardiol. 2005;7:75–83. [DOI] [PubMed] [Google Scholar]
- 4. Saunders A, Gordon S, Miller M. Canine atrial fibrillation. Compend Contin Educ Vet. 2009;31:E1–E9. [PubMed] [Google Scholar]
- 5. Westling J, Westling W, Pyle RL. Epidemiology of atrial fibrillation in the dog. Intern J Appl Res Vet Med. 2008;6:151–154. [Google Scholar]
- 6. Gelzer AR, Kraus MS. Management of atrial fibrillation. Vet Clin North Am Small Anim Pract. 2004;34:1127–1144. [DOI] [PubMed] [Google Scholar]
- 7. Bright JM, Martin JM, Mama K. A retrospective evaluation of transthoracic biphasic electrical cardioversion for atrial fibrillation in dogs. JVet Cardiol. 2005;7:85–96. [DOI] [PubMed] [Google Scholar]
- 8. Oyama MA, Prosek R. Acute conversion of atrial fibrillation in two dogs by intravenous amiodarone administration. JVet Intern Med. 2006;20:1224–1227. [DOI] [PubMed] [Google Scholar]
- 9. Gelzer AR, Kraus MS, Rishniw M, et al. Combination therapy with digoxin and diltiazem controls ventricular rate in chronic atrial fibrillation in dogs better than digoxin or diltiazem monotherapy: a randomized crossover study in 18 dogs. JVet Intern Med. 2009;23:499–508. [DOI] [PubMed] [Google Scholar]
- 10. Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 2012;125:381–389. [DOI] [PubMed] [Google Scholar]
- 11. Saunders AB, Miller MW, Gordon SG, Van de Wiele CM. Oral amiodarone therapy in dogs with atrial fibrillation. JVet Intern Med. 2006;20:921–926. [DOI] [PubMed] [Google Scholar]
- 12. Hibino H, Inanobe A, Furutani K, et al. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. [DOI] [PubMed] [Google Scholar]
- 13. Van der Heyden MAG,Sánchez‐Chapula JA. Toward specific cardiac I(K1) modulators for in vivo application: old drugs point the way. Heart Rhythm. 2011;8:1076–1080. [DOI] [PubMed] [Google Scholar]
- 14. Takanari H, Nalos L, Stary‐Weinzinger A, et al. Efficient and specific cardiac IK1 inhibition by a new pentamidine analogue. Cardiovasc Res. 2013;99:203–214. [DOI] [PubMed] [Google Scholar]
- 15. Ji Y, Varkevisser R, Opacic D, et al. The inward rectifier current inhibitor PA‐6 terminates atrial fibrillation and does not cause ventricular arrhythmias in dedicated goat and dog models. Br J Pharmacol. 2017;174:2576–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oros A, Beekman JD, Vos MA. The canine model with chronic, complete atrio‐ventricular block. Pharmacol Ther. 2008;119:168–178. [DOI] [PubMed] [Google Scholar]
- 17. Anderson E. Electrocardiography In: Ettinger SJ, Feldman EC, Côté E, eds. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat. 8th ed St. Louis, MO: Elsevier; 2017:390–392. [Google Scholar]
- 18. Van de Water A,Verheyen J, Xhonneux R, Reneman RS. An improved method to correct the QT interval of the electrocardiogram for changes in heart rate. JPharmacol Methods. 1989;22:207–217. [DOI] [PubMed] [Google Scholar]
- 19. Dosdall DJ, Ranjan R, Higuchi K, et al. Chronic atrial fibrillation causes left ventricular dysfunction in dogs but not goats: experience with dogs, goats, and pigs. Am J Physiol Heart Circ Physiol. 2013;305:H725–H731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veldhuis M, Ji Y, Van der Heyden MAG. A Little Too Much: Cardiac Electrophysiological Effects of Elevated Inward Rectifying Current Carried by the KIR2.1 Ion Channel Protein. Adaptive Med. 2015;7:1–8. [Google Scholar]
- 21. Luo X, Pan Z, Shan H, et al. MicroRNA‐26 governs profibrillatory inward‐rectifier potassium current changes in atrial fibrillation. JClin Invest. 2013;123:1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dobrev D, Friedrich A, Voigt N, et al. The G protein‐gated potassium current I(K,ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–3706. [DOI] [PubMed] [Google Scholar]
- 23. Ehrlich JR, Nattel S. Novel approaches for pharmacological management of atrial fibrillation. Drugs. 2009;69:757–774. [DOI] [PubMed] [Google Scholar]
- 24. Walfridsson H, Anfinsen OG, Berggren A, et al. Is the acetylcholine‐regulated inwardly rectifying potassium current a viable antiarrhythmic target? Translational discrepancies of AZD2927 and A7071 in dogs and humans. Europace. 2015;17:473–482. [DOI] [PubMed] [Google Scholar]
- 25. Magyar J, Szabo G. Effects of Volatile Anesthetics on the G Protein‐Regulated Muscarinic Potassium Channel. Mol Pharmacol. 1996;50:1520–1528. [PubMed] [Google Scholar]
- 26. Yamakura T, Lewohl JM, Harris RA. Differential effects of general anesthetics on G protein‐coupled inwardly rectifying and other potassium channels. Anesthesiology. 2001;95:144–153. [DOI] [PubMed] [Google Scholar]
- 27. Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. [DOI] [PubMed] [Google Scholar]
- 28. Everett TH IV,Li H, Mangrum JM, et al. Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation. 2000;102:1454–1460. [DOI] [PubMed] [Google Scholar]
- 29. Bright JM, ZumBrunnen J. Chronicity of atrial fibrillation affects duration of sinus rhythm after transthoracic cardioversion of dogs with naturally occurring atrial fibrillation. JVet Intern Med. 2008;22:114–119. [DOI] [PubMed] [Google Scholar]
- 30. Verheule S, Tuyls E, van Hunnik A, et al. Fibrillatory conduction in the atrial free walls of goats in persistent and permanent atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:590–599. [DOI] [PubMed] [Google Scholar]
- 31. Eijsbouts S, Ausma J, Blaauw Y, et al. Serial cardioversion by class IC Drugs during 4 months of persistent atrial fibrillation in the goat. JCardiovasc Electrophysiol. 2006;17:648–654. [DOI] [PubMed] [Google Scholar]
- 32. Borgeat A, Goy JJ, Maendly R, et al. Flecainide versus quinidine for conversion of atrial fibrillation to sinus rhythm. Am J Cardiol. 1986;58:496–498. [DOI] [PubMed] [Google Scholar]
- 33. Stiell IG, Roos JS, Kavanagh KM, Dickinson G. A multicenter, open‐label study of vernakalant for the conversion of atrial fibrillation to sinus rhythm. Am Heart J. 2010;159:1095–1101. [DOI] [PubMed] [Google Scholar]
- 34. Galperín J, Elizari MV, Chiale PA, et al. Efficacy of amiodarone for the termination of chronic atrial fibrillation and maintenance of normal sinus rhythm: a prospective, multicenter, randomized, controlled, double blind trial. JCardiovasc Pharmacol Ther. 2001;6:341–350. [DOI] [PubMed] [Google Scholar]
- 35. Stambler BS, Wood MA, Ellenbogen KA, et al. Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation. Ibutilide Repeat Dose Study Investigators. Circulation. 1996;94:1613–1621. [DOI] [PubMed] [Google Scholar]
- 36. Mattioli AV, Lucchi GR, Vivoli D, Mattioli G. Propafenone versus procainamide for conversion of atrial fibrillation to sinus rhythm. Clin Cardiol. 1998;21:763–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prueksaritanont T, Stranieri MT, Hand EL, et al. Effects of pentobarbital on pharmacokinetics and pharmacodynamics of a potent fibrinogen receptor antagonist, L‐734,217, in dogs. Biopharm Drug Dispos. 1997;18:649–663. [DOI] [PubMed] [Google Scholar]
- 38. Chelly JE, Hysing ES, Abernethy DR, et al. Effects of inhalational anesthetics on verapamil pharmacokinetics in dogs. Anesthesiology. 1986;65:266–271. [PubMed] [Google Scholar]
- 39. Boucher M, Dubray C, Li JH, et al. Influence of pentobarbital and chloralose anesthesia on quinidine‐induced effects on atrial refractoriness and heart rate in the dog. JCardiovasc Pharmacol. 1991;17:199–206. [DOI] [PubMed] [Google Scholar]
- 40. Roza O, Berman LB. The pathophysiology of barium: hypokalemic and cardiovascular effects. JPharmacol Exp Ther. 1971;177:433–439. [PubMed] [Google Scholar]
- 41. Van der Heyden MA,Jespersen T. Pharmacological exploration of the resting membrane potential reserve: Impact on atrial fibrillation. Eur J Pharmacol. 2016;771:56–64. [DOI] [PubMed] [Google Scholar]


