Abstract
Background
The effects of sacubitril/valsartan (S/V) on the renin‐angiotensin‐aldosterone system (RAAS) in dogs with cardiomegaly secondary to myxomatous mitral valve disease (MMVD) are currently unknown.
Objectives
To determine the pharmacodynamic effects of S/V on the RAAS, natriuretic peptide concentrations, systolic arterial pressure (SAP), tests of renal function, and serum electrolyte concentrations in dogs with cardiomegaly secondary to MMVD.
Animals
Thirteen client‐owned dogs weighing 4‐15 kg with American College of Veterinary Internal Medicine (ACVIM) Stage B2 MMVD.
Methods
Prospective, randomized, double‐blind, placebo‐controlled pilot study of S/V in dogs with ACVIM Stage B2 MMVD.
Results
Thirteen dogs were recruited: S/V (n = 7) and placebo (n = 6). The median percentage increase in urinary aldosterone to creatinine ratio (UAldo : C) between day 0 and day 30 was significantly lower in the S/V group (12%; P = .032) as compared with the placebo group (195%). The median percentage decrease of NT‐proBNP concentration from day 0 to day 30 was not statistically different between groups (P = .68). No statistical differences were seen in echocardiographic, thoracic radiographic, SAP, or serum biochemical test results measured at any time point between groups. No adverse events were observed for dogs in either group.
Conclusion and Clinical Importance
Sacubitril/valsartan may provide a new pharmaceutical method to effectively inhibit the RAAS in dogs with ACVIM Stage B2 MMVD.
Keywords: aldosterone breakthrough, heart, progression
Abbreviations
- ABT
aldosterone breakthrough
- ACE
angiotensin‐converting enzyme
- ACEI
angiotensin‐converting enzyme inhibitors
- ATII
angiotensin II
- CHF
congestive heart failure
- MMVD
myxomatous mitral valve disease
- MR
mitral regurgitation
- NEP
neprilysin
- NT‐proBNP
N‐terminal pro‐brain natriuretic peptide
- PH
pulmonary hypertension
- RAAS
renin‐angiotensin‐aldosterone system
- SAP
systolic arterial pressure
- S/V
sacubitril/valsartan
- TR
tricuspid regurgitation
- UAldo: C
urinary aldosterone to creatinine ratio
1. INTRODUCTION
Chronic activation of the renin‐angiotensin‐aldosterone system (RAAS) contributes to the development of congestive heart failure (CHF) and is associated with increased morbidity and mortality in human patients and dogs with cardiac disease.1, 2, 3 Angiotensin converting enzyme inhibitors (ACEI) are commonly used to suppress this system and are considered standard of care in dogs for treatment of CHF secondary to myxomatous mitral valve disease (MMVD).4 Angiotensin converting enzyme (ACE) activity is present in plasma and tissues, with the majority of activity being present in tissues. Angiotensin converting enzyme inhibitors are effective at decreasing plasma ACE activity but less so at decreasing tissue ACE activity, and most of the angiotensin II (ATII) production in tissues occurs via non‐ACE‐mediated pathways, rendering ACEI ineffective.5, 6, 7 The lack of effectiveness of ACEI to suppress ACE‐dependent and non‐ACE‐dependent tissue ATII production, along with local production of aldosterone, is thought to drive the phenomenon of aldosterone breakthrough (ABT).8 Aldosterone breakthrough has been documented in multiple studies in dogs, with a prevalence of 32% in dogs with MMVD treated for CHF.9, 10, 11, 12, 13, 14 These findings, with evidence that mineralocorticoid receptor antagonists showed prognostic benefit in CHF treatment, further suggest the need for improved mitigation of ABT.15
Angiotensin II receptor blockers (ARB) are used to inhibit the effects of tissue ATII production from non‐ACE‐dependent pathways. Despite the beneficial effects of ARB on the inhibition of tissue RAAS, ABT still occurs.16, 17, 18 The natriuretic peptide (NP) system is activated with cardiovascular disease and antagonizes the RAAS.19, 20, 21 Neprilysin (NEP) is an enzyme that naturally cleaves NPs, and increased NEP concentrations are positively associated with cardiovascular death and hospitalization in humans with heart failure.22, 23 A combinational first‐in‐class angiotensin receptor blocker/NEP inhibitor was developed (sacubitril/valsartan [S/V], Entresto; Novartis Pharmaceuticals, Basel, Switzerland) to decrease the cardiovascular stimulus for RAAS activation, inhibit RAAS hormones, decrease breakdown of endogenous NPs, and decrease the occurrence of ABT.19 Sacubitril/valsartan showed an overwhelming benefit compared with enalapril in humans with symptomatic heart failure, and it lowered plasma aldosterone concentrations in healthy dogs with diet‐induced RAAS activation better than benazepril and valsartan.24, 25
The aim of our pilot study was to determine the effects of S/V on the RAAS and NP system in dogs with ACVIM Stage B2 MMVD by evaluating urinary aldosterone‐to‐creatinine ratio (UAldo : C) and plasma NT‐proBNP concentrations, respectively, compared with placebo. A second aim of the study was to determine the safety of S/V in dogs with preclinical MMVD by evaluating systolic arterial pressure (SAP) and serum blood urea nitrogen (BUN), creatinine, and electrolyte concentrations. We hypothesized that S/V would have an inhibitory effect on the RAAS compared with placebo in dogs with preclinical MMVD by demonstrating more reduction in the UAldo : C over time, as well as lower plasma NT‐proBNP concentrations because of a decrease in cardiovascular stimulation of NP release, without causing adverse effects.
2. MATERIALS AND METHODS
2.1. Animals and study timeline
Our study was a prospective, randomized, double‐blind, placebo‐controlled study. The study was conducted in accordance with the guidelines of the Animal Care and Use Committee of Auburn University (2016‐2986) and with informed consent of the owners.
[Correction added after first online publication 31 August 2018: Next to last line removed unneeded text]
2.2. Inclusion and exclusion criteria
Our be eligible for inclusion, dogs had to be ≥6 years of age with body weight of ≥4.0 kg and ≤15 kg, and had to be in ACVIM Stage B2 MMVD.3 Each patient had to show all of the following echocardiographic and thoracic radiographic criteria to be classified in Stage B2: classic myxomatous lesions of the mitral valve apparatus, mitral regurgitation (MR) on color Doppler interrogation, left atrial enlargement based on a left atrial‐to‐aortic root ratio (LA : Ao on the right parasternal short axis view) ≥ 1.6,26 left ventricular enlargement based on an internal diameter in diastole indexed to body weight (iLVIDd)27 ≥ 1.7, and radiographic evidence of cardiomegaly (vertebral heart score [VHS]28 > 10.5). Each dog was required to be on pimobendan (0.5‐0.6 mg/kg/day PO in divided doses; Boehringer Ingelheim, Ingelheim am Rhein, Germany) for at least 7 days before baseline sampling (day 0) as well as to continue pimobendan until the completion of the study.29
Each dog underwent a screening evaluation before inclusion, which included a physical examination, echocardiography, thoracic radiographs (TXR), SAP, and routine hematology and serum biochemistry profile. Day 0 consisted of echocardiography, TXR, SAP, a serum renal profile (BUN, creatinine, electrolytes), blood collection for measuring plasma NT‐proBNP concentrations, and urine collection for UAldo : C. If screening and day 0 sampling were not performed on the same day (ie, the dog was screened, eligible for enrollment, but not yet on pimobendan), then day 0 sampling must have been conducted within 10 days after initial screening. Dogs were required to start either S/V or placebo on the morning after day 0 sampling. Enrolled dogs were re‐evaluated 7 days after beginning S/V or placebo to monitor for adverse effects on SAP or renal profile parameters that could occur secondary to the trial drug administration. Dogs were subsequently evaluated 30 days (day 30) after initiating S/V or placebo treatment. Day 30 diagnostic tests included repeat echocardiography, TXR, SAP, a serum renal profile, blood collection for plasma NT‐proBNP concentrations, and urine collection for UAldo : C. Heart rate and heart murmur intensity were documented at each visit.
Dogs were excluded from the study if they had congenital cardiac disease or acquired cardiac disease other than MMVD, clinically relevant arrhythmias, or echocardiographic evidence of clinically relevant pulmonary hypertension (PH) defined as a right ventricular‐to‐right atrial pressure gradient (RV : RA PG) > 65 mm Hg.29 Dogs were excluded if they had previous or current evidence of left‐sided CHF. Congestive heart failure was defined as radiographic evidence of cardiogenic pulmonary edema denoted by an interstitial or alveolar pulmonary pattern in the caudodorsal lung fields, as well as contemporaneous clinical signs (eg, tachypnea, dyspnea, cough, and increased sleeping respiratory rate) that would require furosemide treatment. Dogs were excluded if they were being treated with ACEI, other cardiovascular medication(s) other than pimobendan, or non‐cardiovascular medications with known effects on the RAAS, such as corticosteroids.29 Dogs with clinically relevant diseases, or diseases suspected to affect the RAAS (ie, chronic renal disease, hyperadrenocorticism) were excluded. Dogs that were pregnant or lactating were not eligible for enrollment.
2.3. Randomization, blinding, and trial medication
Investigators, veterinary technicians handling study participants, and owners were blinded to treatment allocation. The pharmacists at the Auburn University Veterinary Teaching Hospital held the blinding code for the treatment groups until the clinical trial was completed. At the time of enrollment, the attending pharmacist randomly allocated each dog to either the placebo group or the treatment (S/V) group. Sacubitril/valsartan (24/26, 49/51, and 97/103 mg tablets; Entresto, Novartis Pharmaceuticals, Basel, Switzerland) was enclosed in a gelatin‐coated capsule for the purpose of blinding and administered PO at a target dosage of 20 mg/kg q12h based on a previous study.25 Lactose powder as a placebo control was packaged in a capsule and administered PO q12h.30 The S/V and placebo in the capsule were similar in weight and visually indistinguishable. The dose of S/V was not adjusted during the study. Owners were instructed to administer pimobendan and trial drug (S/V or placebo) concurrently with morning and evening meals. Diets were not standardized among patients, but owners were instructed to keep the type and timing of feedings (including treats) consistent throughout the study period to decrease the effect of diet variability on daily fluctuation in RAAS hormone concentrations.31
2.4. Systolic arterial pressure measurement
Dogs were allowed a 10‐minutes acclimation to the clinic environment, and indirect blood pressure measurement was the first diagnostic test performed on each sampling day based on previously described guidelines.32 Dogs were gently restrained in right lateral recumbency and measurements were obtained using a Doppler sphygmomanometry method (Doppler Flow Detector, Parks Medical Electronics, Inc, Aloha, Oregon) with a cuff size approximately 40% of the limb circumference on the left pelvic limb. Blood pressure results were determined by discarding the first reading and averaging 5 consecutive SAP readings.
2.5. Diagnostic imaging
Right lateral projections were used to calculate the VHS as previously described.28 The pulmonary parenchyma was evaluated for evidence of pulmonary edema based on the presence of an interstitial or alveolar pattern and contemporaneous clinical signs.
Echocardiography was performed in all dogs without sedation using an ultrasound unit (Vivid E9, General Electric, Boston, Massachusetts) with a 6 MHz phased array transducer. All echocardiographic evaluations were performed by a cardiology resident (Daniel K. Newhard) under direct supervision of a board‐certified veterinary cardiologist (S.W. Jung and R.L. Winter). The structures of the mitral valve apparatus were assessed using both the right parasternal 4‐chamber long‐axis view and the left apical 4‐chamber view. Left atrial‐to‐aortic root ratios were obtained via the right parasternal short‐axis view as previously described.26 M‐mode echocardiography was used to obtain the left ventricular internal diameter at end‐diastole (LVIDd) and the left ventricular internal diameter at end‐systole (LVIDs) using the right‐parasternal short‐axis view.33 Left ventricular internal diameter at end‐diastole indexed to body weight was calculated using the formula (iLVIDd) = LVIDd (cm)/[BW (kg)]0.294 and LVIDs indexed to body weight (iLVIDs) was calculated with the formula (iLVIDs) = LVIDs (cm)/[BW (kg)]0.315.27 The tricuspid valve was assessed for regurgitation (TR) with color Doppler interrogation, and if present, the instantaneous peak systolic tricuspid regurgitant velocity was measured using continuous‐wave Doppler interrogation. The modified Bernoulli equation was applied to the peak systolic tricuspid regurgitant velocity to calculate the instantaneous RV : RA PG in mm Hg. Other echocardiographic parameters evaluated included peak velocity of early diastolic transmitral flow (MV E Vel), mitral annular tissue Doppler interrogation E′ velocity (MV E′ Vel), MV E Vel to E′ Vel ratio (E/E′), and left ventricular fractional shortening using the formula [(LVIDd – LVIDs)/LVIDd × 100].34
[Correction added after first online publication 31 August 2018: Updated initials to name in fourth line]
2.6. Sample collection and analysis
Blood collection time was standardized among patients at each visit by collecting blood 3–4 hours after each patient's morning meal, which included pimobendan and trial drug administration. Sample collection was performed with all patients in the standing position to decrease the effect of variability of body positioning on RAAS hormone concentrations.35 Blood was collected into ethylenediamine tetra‐acetic acid (EDTA) tubes for hematologic evaluation (Advia 120, Siemens Healthcare, Erlangen, Germany) and serum was collected into lithium heparin tubes for biochemistry evaluation (Cobas c111, Roche Professional Diagnostics, Rotkreuz, Switzerland). For NT‐proBNP measurement, whole blood was collected into EDTA tubes and immediately centrifuged at 1500g for 15 minutes at room temperature to separate and isolate plasma. Plasma was stored in 1.2 mL aliquots in polypropylene tubes. All plasma samples were aliquoted within 30 minutes of blood collection and stored in −80°C until batch analysis. Samples were packed frozen on dry ice and shipped overnight to IDEXX Laboratories (IDEXX Laboratories, Westbrook, Maine) and NT‐proBNP concentrations were measured using the 2nd‐generation canine Cardiopet proBNP test (Cardiopet proBNP second‐generation Test‐Canine, IDEXX Laboratories, Westbrook, Maine).
Midstream voided first‐morning urine samples were collected at home by the owners on the morning of day 0 and day 30, and subsequently brought to each visit. Urine was collected and brought to the hospital on each sampling day within 4 hours of collection. During each visit on day 0 and day 30, an additional 5 mL of urine were collected either by voiding or cystocentesis. Two and one‐half mL of the first‐morning urine sample (brought by the owner) and 2.5 mL of the urine sample collected at the visit were pooled together36 and centrifuged at 1500g for 5 minutes. Three milliliter of the supernatant were collected into polypropylene tubes and stored in −80°C until batch analysis. Urine creatinine concentrations were measured using a standard colorimetric assay and urine aldosterone concentrations were measured contemporaneously from the same urine samples (Aldosterone, Active RIA, Beckman‐Coulter, Brea, California) to calculate the UAldo : C.36 Both tests to determine the urine creatinine and urine aldosterone concentrations were performed by a veterinary diagnostic laboratory (Michigan State University Center for Population and Animal Health, Lansing, Michigan).
2.7. Statistical analysis
Assessment of data distribution in regard to normality was determined using the Shapiro‐Wilk test. Normally distributed variables were expressed as mean ± standard deviation. Central tendency and dispersion for nonparametric data were expressed as median and interquartile range (IQR). A Student's t‐test with the Bonferroni correction was used to analyze differences in continuous variables between the 2 groups (placebo and S/V) at day 0 and day 30. A Chi‐square test was used to identify a significant relationship between 2 categorical variables. For UAldo : C and NT‐proBNP data, a percentage change (%) from day 0 to day 30 was calculated using the formula [(day 30 – day 0)/day 0 × 100]. A Mann‐Whitney test was used to analyze the difference of percentage changes between 2 groups. A commercial software package (GraphPad Prism 6, La Jolla, California) was used for all statistical analysis, and a P‐value < .05 was set to indicate statistical significance.
3. RESULTS
Thirteen dogs were enrolled in the study, with 7 dogs in the S/V group and 6 dogs in the placebo group. All dogs enrolled completed the study without adverse effects. The S/V group was composed of 2 Pomeranian and 2 mixed breed dogs with 1 each of Bichon Frise, miniature Schnauzer, and Maltese. The placebo group was composed of 1 each of Bichon Frise, Dachshund, miniature Schnauzer, Jack Russell terrier, Pekingese, and Beagle. The S/V group was composed of 4 neutered male dogs and 3 female spayed dogs, and the placebo group was composed of 4 female spayed dogs and 2 neutered male dogs. Two dogs in the S/V group were on noncardiac medications, with 1 dog receiving cephalexin, hydroxyzine, oclacitinib (APOQUEL, Zoetis, Parsippany, New Jersey), clomipramine, hyposensitization injections, and topical aural nonsteroidal irrigation, and the other dog receiving carprofen, cephalexin, oclacitinib, and phenylpropanolamine. One dog in the placebo group was on non‐cardiac medications, including ophthalmologic cyclosporine and ketorolac. Baseline demographic characteristics and prescribed pimobendan dosage were not statistically different between groups (Table 1). The median number of days taking pimobendan before day 0 in the S/V group and the placebo group was 8 days (range, 8–146 days) and 9 days (range, 8–36 days), respectively. The median dosage of S/V in the S/V group was 36 mg/kg/day (range, 36–42.4 mg/kg/day).
Table 1.
Baseline characteristics of both groups at day 0
| Variables | Placebo (n = 6) | S/V (n = 7) | P‐value | |
|---|---|---|---|---|
| Demographics | Age (months) | 141.8 ± 14.11 | 141.1 ± 21.12 | .95 |
| Sex (M/F; %) | (2/4)(33/67) | (3/4)(43/57) | 1 | |
| Physical examination variable | Body weight (kg) | 8.23 ± 1.20 | 8.28 ± 1.92 | .97 |
| Heart rate (bpm) | 127.7 ± 23.28 | 147.6 ± 21.30 | .45 | |
| SAP (mm Hg) | 163.0 ± 12.47 | 164.4 ± 11.25 | .94 | |
| Heart murmur intensity (moderate [grade 3–4]/severe [grade 5–6]) (%) | (3/3)(50/50) | (4/3)(57/43) | .9 | |
| Medication dosage | pimobendan (mg/kg/day) | 0.44 ± 0.12 | 0.39 ± 0.11 | .57 |
| S/V (mg/kg/day) | NA | 36.88 ±1.8 | NA | |
| Diagnostic imaging variables | VHS | 11.03 ± 0.51 | 11.17 ± 0.41 | .68 |
| iLVIDd | 1.78 ± 0.06 | 1.73 ± 0.17 | .65 | |
| iLVIDs | 0.75 ± 0.05 | 0.87 ± 0.12 | .15 | |
| LA/Ao | 2.11 ± 0.16 | 1.85 ± 0.14 | .21 | |
| MV E Vel (m/s) | 1.05 ± 0.22 | 0.97 ± 0.14 | .58 | |
| E/E′ | 12.07 ± 4.13 | 11.73 ± 2.35 | .89 | |
| Laboratory variables | Na (mmol/L) | 151.2 ± 0.91 | 147.7 ± 1.79 | .50 |
| K (mmol/L) | 4.41 ± 0.56 | 4.17 ± 0.20 | .40 | |
| BUN (mg/dL) | 20.43 ± 4.99 | 21.59 ± 4.16 | .75 | |
| Creatinine (mg/dL) | 0.65 ± 0.15 | 0.72 ± 0.04 | .30 | |
Abbreviations: Ao, aorta; BUN, blood urea nitrogen; E/E′, peak velocity of early diastolic transmitral flow to parietal mitral annular tissue Doppler interrogation E′ velocity ratio; iLVIDd, left ventricular internal diameter at end‐diastole indexed to body weight; K, potassium; LA, left atrium; LVIDs, left ventricular internal diameter at end‐systole indexed to body weight; MV E Vel, peak velocity of early diastolic transmitral flow; Na, sodium; S/V, sacubitril/valsartan; VHS, vertebral heart score.
Continuous variables are reported as mean and SD. Categorical variables are reported as number (%).
The physical examination findings, echocardiographic parameters, VHS, SAP, serum BUN, creatinine, and electrolyte concentrations, plasma NT‐proBNP concentrations, and UAldo : C for each group at day 0 and day 30 are summarized in Tables 1 and 2, respectively. There were no significant differences in any of these parameters between groups on either day. Each patient's BUN, creatinine, and electrolyte concentrations were within the reference ranges for both groups at day 0, on the 7‐day recheck, and at day 30.
Table 2.
Variable comparison between groups at day 30
| Variables | Placebo (n = 6) | S/V (n = 7) | P‐value | |
|---|---|---|---|---|
| Physical examination variable | Heart rate (bpm) | 118.3 ± 20.35 | 123.7 ± 21.32 | .73 |
| SAP (mm Hg) | 169.7 ± 7.43 | 149.6 ± 14.67 | .31 | |
| Heart murmur intensity (moderate [grade 3–4]/severe [grade 5–6]) (%) | (3/3)(50/50) | (4/3)(57/43) | .9 | |
| Diagnostic imaging variables | VHS | 10.83 ± 0.56 | 10.74 ± 0.54 | .82 |
| iLVIDd | 1.80 ± 0.12 | 1.71 ± 0.16 | .44 | |
| iLVIDs | 0.83 ± 0.04 | 0.81 ± 0.11 | .85 | |
| LA/Ao | 2.13 ± 0.23 | 1.74 ± 0.19 | .29 | |
| MV E Vel (m/s) | 1.09 ± 0.24 | 1.00 ± 0.16 | .56 | |
| E/E′ | 11.76 ± 2.14 | 13.38 ± 3.81 | .52 | |
| Laboratory variables | Na (mmol/L) | 148.3 ± 1.1 | 147.6 ± 2.1 | .61 |
| K (mmol/L) | 4.2 ± 0.2 | 4.1 ± 0.2 | .49 | |
| BUN (mg/dL) | 23.52 ± 5.43 | 19.40 ± 3.86 | .38 | |
| Creatinine (mg/dL) | 0.68 ± 0.12 | 0.82 ± 0.16 | .21 | |
Abbreviations: Ao, aorta; BUN, blood urea nitrogen; E/E′, peak velocity of early diastolic transmitral flow to parietal mitral annular tissue Doppler interrogation E′ velocity ratio; iLVIDd, left ventricular internal diameter at end‐diastole indexed to body weight; K, potassium; LA, left atrium; LVIDs, left ventricular internal diameter at end‐systole indexed to body weight; MV E Vel, peak velocity of early diastolic transmitral flow; Na, sodium; S/V, sacubitril/valsartan; VHS, vertebral heart score.
Continuous variables are reported as mean and SD. Categorical variables are reported as number (%).
Six dogs in the S/V group and 4 dogs in the placebo group had TR at the time of enrollment. The RV : RA PG was estimated according to the maximal TR velocity (TR V max). The median RV : RA PG in the S/V group and the placebo group was 40.1 mm Hg (IQR, 30.6–45.4 mm Hg) and 43.9 mm Hg (IQR, 35.2–48.4 mm Hg), respectively. There was no difference in the median RV : RA PG between groups at day 0 (P = .5243). Using a cut‐off RV : RA PG of ≥ 36 mm Hg (TR V max of 3.0 m/sec) for the diagnosis of PH,37 2 dogs in the S/V group and 3 dogs in the placebo group had mild PH, with none of the dogs having an RV : RA PG > 50 mm Hg.
The plasma NT‐proBNP concentrations and UAldo : C for each group at day 0 and day 30 are shown in Table 3. The mean UAldo : C (pmol/mmol) was not significantly different between day 0 and day 30 in both groups (Table 3), but the mean UAldo : C percentage increase (%) from day 0 to day 30 was significantly lower in the S/V group (12%; P = .032) when compared with the control group (195%; Figure 1). The mean plasma NT‐proBNP concentration was significantly lower in the S/V group compared with the placebo group (S/V, 930 pmol/L; placebo, 1535 pmol/L; P = .049) on day 0 and on day 30 (S/V, 536 pmol/L; placebo: 1089 pmol/L; P = .026). However, no statistical differences were seen in the NT‐proBNP concentrations between day 0 and day 30 within each group (Table 3). The mean percentage decrease (%) of NT‐proBNP concentrations from day 0 to day 30 was not significantly different between groups (S/V, −35%; placebo, −28%; P = .68; Figure 2).
Table 3.
Changes in UAldo : C (pmol/mmol) and NT‐proBNP (pmol/L) concentrations from day 0 to day 30 in both groups
| Placebo | S/V (Entresto) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 30 | P‐value | Day 0 | Day 30 | P‐value | |
| UAldo : C (pmol/mmol) | 281.5 ± 113.42 | 695.8 ± 381.1 | 0.06 | 335.1 ± 94.23 | 332.3 ± 68.02 | 0.96 |
| NT‐proBNP (pmol/L) | 1535 ± 418.2 | 1089 ± 366.0 | 0.13 | 930.9 ± 340.7 | 536.6 ± 251.5 | 0.09 |
Abbreviations: NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; S/V, sacubitril/valsartan; UAldo: C, urinary aldosterone to creatinine ratio.
Continuous variables are reported as mean and SD.
Figure 1.
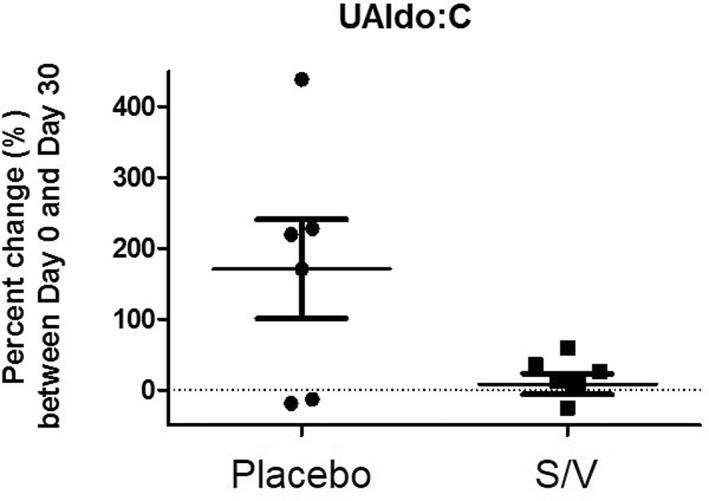
Urinary aldosterone to creatinine ratio percent change from day 0 to day 30 in both groups. Asterisk (*) denotes statistical significance in S/V group compared with placebo, P = .032. UAldo : C: urinary aldosterone to creatinine ratio, S/V: sacubitril/valsartan
Figure 2.
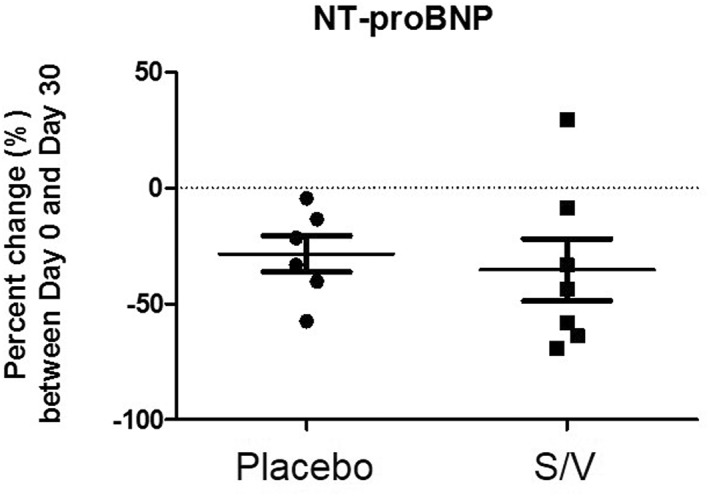
N‐terminal pro‐brain natriuretic peptide percent change from day 0 to day 30 in both groups. Means were not statistically different between groups, P = .68. NT‐proBNP: N‐terminal pro‐brain natriuretic peptide, S/V: sacubitril/valsartan
4. DISCUSSION
In our study, the mean percentage increase of UAldo : C from day 0 to day 30 was significantly lower for the S/V group compared with placebo (Figure 1), showing that S/V has a beneficial inhibitory effect on RAAS activation in dogs in ACVIM Stage B2 MMVD. Mean plasma NT‐proBNP concentrations decreased from day 0 to day 30 in both groups, but neither the absolute difference nor the percentage decrease was statistically significant in either group. For the 30‐day trial, no adverse effects were noted with SAP or BUN, creatinine, and electrolyte concentrations.
Urinary aldosterone concentrations have been used to evaluate the terminal cascade of the RAAS in dogs and the UAldo : C has been validated as an accurate measure of 24‐hours urinary aldosterone secretion in dogs.36, 38 The effect of S/V to decrease aldosterone concentrations is multifactorial. Valsartan inhibits the ATII‐dependent production and secretion of aldosterone, and has been shown to have an effect on tissue RAAS by normalizing myocardial ATII concentrations in dogs with experimentally induced MR.39 Sacubitril prevents enzymatic degradation of circulating NPs, sustaining their plasma concentrations. Natriuretic peptides prevent hormonal cascade through the RAAS by inhibiting renin secretion and blocking renal aldosterone receptors.19 Natriuretic peptide receptors are also present in the zona glomerulosa of the adrenal glands, suggesting the ability of NPs to directly inhibit aldosterone production and release from the adrenal glands.40
We evaluated the effects of S/V on the NP system by measuring plasma NT‐proBNP concentrations in both groups. Sacubitril/valsartan is 2 times more likely to cause a clinically meaningful decrease in NT‐proBNP concentrations compared to enalapril in humans, and this reduction has been shown to have a prognostic benefit in those taking S/V.24, 41 Sacubitril/valsartan prevents NP enzymatic degradation via NEP inhibition, leading to sustained concentrations of biologically active NPs. Although BNP concentrations increase with S/V treatment, NT‐proBNP concentrations can decrease because NT‐proBNP is not a substrate for NEP degradation and is cleared by different mechanisms.41 Sacubitril/valsartan has been shown to decrease left atrial volume and dimensions in humans, improving hemodynamics and decressing the stimulus for NP release, which can lead to decreased BNP, and subsequently decreased NT‐proBNP, concentrations.42 Although the mean plasma NT‐proBNP concentration decreased from day 0 to day 30 in both groups, neither the absolute difference nor the percentage decrease was statistically significant in either group. Multiple explanations may be invoked for the lack of a statistically significant difference in the decrease in NT‐proBNP concentrations. High biological variability occurs in NT‐proBNP concentrations in dogs with MMVD.43 Low statistical power (sample size, small effects or both) and duration of S/V administration could have contributed to the insignificant change in NT‐proBNP concentrations. Neprilysin‐dependent degradation is species and tissue‐specific, and NP resistance may occur in human patients with cardiovascular disease.23, 44, 45 The consequences of these factors are unknown in dogs. A decrease in NT‐proBNP concentrations in the S/V group can be explained by the reasons described above, but these would not explain the decrease seen in the placebo group. Limited published information is available on the effect of pimobendan on plasma NT‐proBNP concentrations, but a study in dogs with MMVD and PH showed a significant decrease in plasma NT‐proBNP concentrations after short‐term pimobendan administration.46 Therefore, pimobendan potentially could have caused the decrease in plasma NT‐proBNP concentration in both groups. N‐terminal pro‐brain NP concentrations were used as a marker of NP system activity in this study, but measuring additional markers of the NP system, such as atrial NP, N‐terminal pro‐atrial NP, and BNP, may be indicated in future studies to obtain a more complete evaluation of the NP system.
Sacubitril/valsartan administration over 30 days did not cause any adverse effects on SAP or BUN, creatinine, or electrolyte concentrations in the dogs of this study. Although S/V can cause these derangements in humans, it does so to a lesser extent than does enalapril.24 On the other hand, clinically irrelevant systemic hypotension was seen more frequently in human patients taking S/V than in those taking enalapril, but S/V did not significantly alter SAP over time in the dogs of our study.24 Sacubitril/valsartan and related drugs have been shown to be a safe and effective treatment for systemic hypertension in humans and have been shown to delay the progression of chronic and proteinuric kidney diseases.46, 47, 48, 49, 50 Increased NEP concentrations are present in human patients with end‐stage renal dysfunction, suggesting an additional indication for use of S/V in patients with renal disease.51 Because of these beneficial effects that the NPs have on the cardiovascular and renal systems, drugs such as S/V may have important implications for cardiovascular‐renal disorders in dogs.19, 52 The addition of exogenous NPs to furosemide administration causes more profound diuresis and natriuresis and has the potential to allow for decreases in diuretic dosages, while preventing furosemide‐induced RAAS activation.40, 46, 53, 54 Future studies evaluating the concurrent use of S/V and furosemide are indicated in dogs.
Our study had several limitations. The first is small sample size but ours was a pilot study to determine the safety and effectiveness of S/V in dogs with ACVIM Stage B2 MMVD. Another limitation is the potential effects of chronobiology and diet on the RAAS and NP system. Circadian fluctuations of RAAS hormones and dietary sodium content could have affected our results, but intrapatient variables (feeding times, food types, sample collection times) were held constant for the duration of the study period.55, 56 Pooled urine samples also were used to minimize the potential effect of these variables on urinary aldosterone concentrations. First‐morning urine samples were used, but those samples may not have been representative of overnight urine pooling (ie, the patient may have urinated overnight), which could affect the UAldo : C. We evaluated UAldo : C as a marker of RAAS activation, but there is evidence that early cardiac disease does not lead to increased circulating RAAS hormones in dogs, and RAAS activation may be dependent on duration and severity of cardiac disease.57, 58, 59 This may not have a relevant impact on the results of our study because each patient was used as its own control over the 30 day‐trial and all study dogs had moderate to severe cardiomegaly. Sacubitril/valsartan may have a stronger impact on biomarker concentrations in patients with more profound RAAS activation. Pimobendan was not expected to have an effect on RAAS hormone concentrations,60 but dogs in our study were receiving other noncardiac medications the effects of which on RAAS hormones are unknown. Lastly, the dosage of S/V used in our study was extrapolated from a previous study using S/V in healthy dogs.25 Although biological effects were seen at the current dosage used, a higher or lower dosage may produce different results.
5. CONCLUSION
Sacubitril/valsartan is effective at lowering urine aldosterone concentrations in asymptomatic dogs with cardiomegaly secondary to MMVD. Using S/V is safe in these patients, with no deleterious effects on BUN, creatinine, and electrolyte concentrations, or SAP. Additional studies are warranted to evaluate long‐term (>30 days) administration of S/V, compare the effects of S/V to ACEI, and evaluate the use of S/V in dogs treated for CHF.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Study approved by Auburn University IACUC (2016–2986).
ACKNOWLEDGMENTS
The authors are grateful for the assistance provided by Drs Marisa Ames, Jonathan Mochel, Melissa Beall, and Jesse Buch. The authors would thank Drs Starr Miller and Freda Chancy for blinding and trial drug preparation, as well as Keri Harrelson, Jennifer Pannell, and Kellye Coffman for their assistance in data collection. This study was conducted at the Auburn University Veterinary Teaching Hospital, Auburn, AL and supported by the ACVIM Cardiology Pacemaker Grant and an internal grant at Auburn University.
Newhard DK, Jung S, Winter RL, Duran SH. A prospective, randomized, double‐blind, placebo‐controlled pilot study of sacubitril/valsartan (Entresto) in dogs with cardiomegaly secondary to myxomatous mitral valve disease. J Vet Intern Med. 2018;32:1555–1563. 10.1111/jvim.15240
Funding information ACVIM Cardiology Pacemaker Grant
REFERENCES
- 1. Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990;82:1730–1736. [DOI] [PubMed] [Google Scholar]
- 2. Girerd N, Pang PS, Swedberg K, et al. Serum aldosterone is associated with mortality and re‐hospitalization in patients with reduced ejection fraction hospitalized for acute heart failure: Analysis from the EVEREST trial. Eur J Heart Fail. 2013;15:1228–1235. [DOI] [PubMed] [Google Scholar]
- 3. Hezzell MJ, Boswood A, Elliott J. Relationships between serum and urinary aldosterone, ventricular remodeling and outcome in dogs with mitral valve disease. 2010 American College of Veterinary Internal Medicine (ACVIM) Forum Research Abstract Program. JVet Intern Med, 2010;24 10.1111/j.1939-1676.2010.0521 [DOI] [Google Scholar]
- 4. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. JVet Intern Med. 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 5. Hamlin RL, Nakayama T. Comparison of some pharmacokinetic parameters of 5 angiotensin‐converting enzyme inhibitors in normal beagles. JVet Intern Med. 1998;12:93–95. [DOI] [PubMed] [Google Scholar]
- 6. Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II‐forming enzyme in the human heart. JBiol Chem. 1990;265:22348–22357. [PubMed] [Google Scholar]
- 7. Dell'Italia LJ, Meng QC, Balcells E, et al. Increased ACE and chymase‐like activity in cardiac tissue of dogs with chronic mitral regurgitation. Am J Physiol. 1995;269:H2065–H2073. [DOI] [PubMed] [Google Scholar]
- 8. Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–492. [DOI] [PubMed] [Google Scholar]
- 9. Lantis AC, Ames MK, Werre S, Atkins CE. The effect of enalapril on furosemide‐activated renin‐angiotensin‐aldosterone system in healthy dogs. JVet Pharmacol Therap. 2015;38:513–517. [DOI] [PubMed] [Google Scholar]
- 10. Ames MK, Atkins CE, Lantis AC, zum Brunnen J. Evaluation of subacute change in RAAS activity (as indicated by urinary aldosterone:creatinine, after pharmacologic provocation) and the response to ACE inhibition. JRenin Angiotensin Aldosterone Syst. 2016;17:147032031663389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lantis AC, Ames MK, Atkins CE, DeFrancesco TC, Keene BW, Werre SR. Aldosterone breakthrough with benazepril in furosemide‐activated renin‐angiotensin‐aldosterone system in normal dogs. JVet Pharmacol Therap. 2015;38:65–73. [DOI] [PubMed] [Google Scholar]
- 12. Ames MK, Atkins CE, Eriksson A, Hess AM. Aldosterone breakthrough in dogs with naturally occurring myxomatous mitral valve disease. JVet Cardiol. 2017;19:218–227. [DOI] [PubMed] [Google Scholar]
- 13. Haggstrom J, Hansson K, Karlberg BE, Kvart C, Madej A, Olsson K. Effects of long‐term treatment with enalapril or hydralazine on the renin‐angiotensin‐aldosterone system and fluid balance in dogs with naturally acquired mitral valve regurgitation. Am J Vet Res. 1996;57:1645–1652. [PubMed] [Google Scholar]
- 14. Sakatani A, Miyagawa Y, Takemura N. Evaluation of the effect of an angiotensin‐converting enzyme inhibitor, alacepril, on drug‐induced renin‐angiotensin‐aldosterone system activation in normal dogs. JVet Cardiol. 2016;18:248–254. [DOI] [PubMed] [Google Scholar]
- 15. Bernay F, Bland JM, Haggstrom J, et al. Efficacy of spironolactone on survival in dogs with naturally occurring mitral regurgitation caused by myxomatous mitral valve disease. JVet Intern Med. 2010;24:331–341. [DOI] [PubMed] [Google Scholar]
- 16. Cohn JN, Tognoni G;Valsartan Heart Failure Trial I. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. [DOI] [PubMed] [Google Scholar]
- 17. Pitt B, Segal R, Martinez FA, et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet. 1997;349:747–752. [DOI] [PubMed] [Google Scholar]
- 18. Bomback AS, Rekhtman Y, Klemmer PJ, Canetta PA, Radhakrishnan J, Appel GB. Aldosterone breakthrough during aliskiren, valsartan, and combination (aliskiren + valsartan) therapy. JAm Soc Hypertens. 2012;6:338–345. [DOI] [PubMed] [Google Scholar]
- 19. Volpe M. Natriuretic peptides and cardio‐renal disease. Int J Cardiol. 2014;176:630–639. [DOI] [PubMed] [Google Scholar]
- 20. van Kimmenade RR, Januzzi JL Jr. The evolution of the natriuretic peptides ‐ Current applications in human and animal medicine. JVet Cardiol. 2009;11:S9–21. [DOI] [PubMed] [Google Scholar]
- 21. Oyama MA, Singletary GE. The use of NT‐proBNP assay in the management of canine patients with heart disease. Vet Clin North Am Small Anim Pract. 2010;40:545–558. [DOI] [PubMed] [Google Scholar]
- 22. Bayes‐Genis A, Barallat J, Galan A, et al. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. JAm Coll Cardiol. 2015;65:657–665. [DOI] [PubMed] [Google Scholar]
- 23. Miller WL, Burnett JC Jr, Hartman KA, Henle MP, Burritt MF, Jaffe AS. Lower rather than higher levels of B‐type natriuretic peptides (NT‐pro‐BNP and BNP) predict short‐term mortality in end‐stage heart failure patients treated with nesiritide. Am J Cardiol. 2005;96:837–841. [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJ, Packer M, Desai AS, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 25. Mochel J, Burkey BF, Fink M, et al. First‐in‐class angiotensin receptor neprilysin inhibitor LCZ696 modulates the dynamics of the renin cascade and natriuretic peptides system with significant reduction of aldosterone exposure [abstract]. JAm Coll Cardiol. 2014;63 Suppl 1:A806. [Google Scholar]
- 26. Rishniw M, Erb HN. Evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. JVet Intern Med. 2000;14:429–435. [DOI] [PubMed] [Google Scholar]
- 27. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. JVet Intern Med. 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 28. Buchanan JW, Bucheler J. Vertebral scale system to measure canine heart size in radiographs. JAm Vet Med Assoc. 1995;206:194–199. [PubMed] [Google Scholar]
- 29. Boswood A, Haggstrom J, Gordon SG, et al. Effect of Pimobendan in Dogs with Preclinical Myxomatous Mitral Valve Disease and Cardiomegaly: The EPIC Study‐A Randomized Clinical Trial. JVet Intern Med. 2016;30:1765–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schwarz K, Singh S, Parasuraman SK, et al. A randomized double‐blind placebo‐controlled crossover trial of sodium nitrate in patients with stable angina INAS. Future Cardiol 2016;12:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mochel JP, Fink M, Bon C, et al. Influence of feeding schedules on the chronobiology of renin activity, urinary electrolytes and blood pressure in dogs. Chronobiol Int. 2014;31:715–730. [DOI] [PubMed] [Google Scholar]
- 32. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. JVet Intern Med. 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 33. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. JVet Intern Med. 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 34. della Torre PK, Kirby AC, Church DB, Malik R. Echocardiographic measurements in greyhounds, whippets and Italian greyhounds–dogs with a similar conformation but different size. Aust Vet J. 2000;78:49–55. [DOI] [PubMed] [Google Scholar]
- 35. Cohen EL, Conn JW, Rovner DR. Postural augmentation of plasma renin activity and aldosterone excretion in normal people. JClin Invest. 1967;46:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gardner SY, Atkins CE, Rausch WP, DeFrancesco TC, Chandler DW, Keene BW. Estimation of 24‐h aldosterone secretion in the dog using the urine aldosterone:creatinine ratio. JVet Cardiol. 2007;9:1–7. [DOI] [PubMed] [Google Scholar]
- 37. Borgarelli M, Abbott J, Braz‐Ruivo L, et al. Prevalence and prognostic importance of pulmonary hypertension in dogs with myxomatous mitral valve disease. JVet Intern Med. 2015;29:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Atkins CE, Lantis AC, Ames MK, Gardner SY. Utility of urinary aldosterone measurement in quantitating RAAS activation. JVet Pharmacol Ther. 2012;35:512–515. author reply 516–518. [DOI] [PubMed] [Google Scholar]
- 39. Perry GJ, Wei CC, Hankes GH, et al. Angiotensin II receptor blockade does not improve left ventricular function and remodeling in subacute mitral regurgitation in the dog. JAm Coll Cardiol. 2002;39:1374–1379. [DOI] [PubMed] [Google Scholar]
- 40. Martin FL, Stevens TL, Cataliotti A, et al. Natriuretic and antialdosterone actions of chronic oral NEP inhibition during progressive congestive heart failure. Kidney Int. 2005;67:1723–1730. [DOI] [PubMed] [Google Scholar]
- 41. Zile MR, Claggett BL, Prescott MF, et al. Prognostic Implications of Changes in N‐Terminal Pro‐B‐Type Natriuretic Peptide in Patients With Heart Failure. JAm Coll Cardiol. 2016;68:2425–2436. [DOI] [PubMed] [Google Scholar]
- 42. Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double‐blind randomised controlled trial. Lancet. 2012;380:1387–1395. [DOI] [PubMed] [Google Scholar]
- 43. Winter RL, Saunders AB, Gordon SG, Buch JS, Miller MW. Biologic variability of N‐terminal pro‐brain natriuretic peptide in healthy dogs and dogs with myxomatous mitral valve disease. JVet Cardiol. 2017;19:124–131. [DOI] [PubMed] [Google Scholar]
- 44. Baerts L, Gomez N, Vanderheyden M, De Meester I, Mc Entee K. Possible mechanisms for brain natriuretic peptide resistance in heart failure with a focus on interspecies differences and canine BNP biology. Vet J. 2012;194:34–39. [DOI] [PubMed] [Google Scholar]
- 45. Cataliotti A, Boerrigter G, Costello‐Boerrigter LC, et al. Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide‐induced aldosterone activation in experimental heart failure. Circulation. 2004;109:1680–1685. [DOI] [PubMed] [Google Scholar]
- 46. Atkinson KJ, Fine DM, Thombs LA, Gorelick JJ, Durham HE. Evaluation of pimobendan and N‐terminal probrain natriuretic peptide in the treatment of pulmonary hypertension secondary to degenerative mitral valve disease in dogs. JVet Intern Med. 2009;23:1190–1196. [DOI] [PubMed] [Google Scholar]
- 47. Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP. Blood‐pressure reduction with LCZ696, a novel dual‐acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double‐blind, placebo‐controlled, active comparator study. Lancet. 2010;375:1255–1266. [DOI] [PubMed] [Google Scholar]
- 48. Kario K, Sun N, Chiang FT, et al. Efficacy and safety of LCZ696, a first‐in‐class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: A randomized, double‐blind, placebo‐controlled study. Hypertension 2014;63:698–705. [DOI] [PubMed] [Google Scholar]
- 49. Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002;106:920–926. [DOI] [PubMed] [Google Scholar]
- 50. Bodey F, Hopper I, Krum H. Neprilysin inhibitors preserve renal function in heart failure. Int J Cardiol. 2015;179:329–330. [DOI] [PubMed] [Google Scholar]
- 51. Deschodt‐Lanckman M, Michaux F, De Prez E, Abramowicz D, Vanherweghem JL, Goldman M. Increased serum levels of endopeptidase 24.11 ('enkephalinase') in patients with end‐stage renal failure. Life Sci. 1989;45:133–141. [DOI] [PubMed] [Google Scholar]
- 52. Pouchelon JL, Atkins CE, Bussadori C, et al. Cardiovascular‐renal axis disorders in the domestic dog and cat: a veterinary consensus statement. JSmall Anim Pract. 2015;56:537–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ayalasomayajula S, Schuehly U, Pal P, et al. Effect of the angiotensin receptor neprilysin inhibitor sacubitril/valsartan on pharmacokinetics and pharmacodynamics of a single‐dose of furosemide. Br J Clin Pharmacol. 2018;84(5):926–936. 10.1111/bcp.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hormann SM, Davis LE, Pogge EK. From Theory to Practice: The Diuretic Potential of Sacubitril/Valsartan: A Tale of 2 Patients. JCardiovasc Nurs. 2018;33:104–110. [DOI] [PubMed] [Google Scholar]
- 55. Mochel JP, Fink M, Peyrou M, et al. Chronobiology of the renin‐angiotensin‐aldosterone system in dogs: Relation to blood pressure and renal physiology. Chronobiol Int. 2013;30:1144–1159. [DOI] [PubMed] [Google Scholar]
- 56. Mochel JP, Danhof M. Chronobiology and pharmacologic modulation of the renin‐angiotensin‐aldosterone system in dogs: What have we learned? Rev Physiol Biochem Pharmacol. 2015;169:43–69. [DOI] [PubMed] [Google Scholar]
- 57. Haggstrom J, Hansson K, Kvart C, Karlberg BE, Vuolteenaho O, Olsson K. Effects of naturally acquired decompensated mitral valve regurgitation on the renin‐angiotensin‐aldosterone system and atrial natriuretic peptide concentration in dogs. Am J Vet Res. 1997;58:77–82. [PubMed] [Google Scholar]
- 58. Oyama MA. Neurohormonal activation in canine degenerative mitral valve disease: implications on pathophysiology and treatment. JSmall Anim Pract. 2009;50:3–11. [DOI] [PubMed] [Google Scholar]
- 59. Fujii Y, Orito K, Muto M, Wakao Y. Modulation of the tissue reninangiotensin‐aldosterone system in dogs with chronic mild regurgitation through the mitral valve. Am J Vet Res. 2007;68:1045–1050. [DOI] [PubMed] [Google Scholar]
- 60. Sayer MB, Atkins CE, Fujii Y, Adams AK, DeFrancesco TC, Keene BW. Acute effect of pimobendan and furosemide on the circulating renin‐angiotensin‐aldosterone system in healthy dogs. JVet Intern Med. 2009;23:1003–1006. [DOI] [PubMed] [Google Scholar]


